Surgical Management of Adrenocortical Carcinoma: Current Highlights
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Laparoscopic Adrenalectomy vs. Open Surgery
3.2. The Potential Role of Robotic Approach
3.3. Extension of Surgical Resection and Lymphnode Dissection
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abiven, G.; Coste, J.; Groussin, L.; Anract, P.; Tissier, F.; Legmann, P.; Dousset, B.; Bertagna, X.; Bertherat, J. Clinical and Biological Features in the Prognosis of Adrenocortical Cancer: Poor Outcome of Cortisol-Secreting Tumors in a Series of 202 Consecutive Patients. J. Clin. Endocrinol. Metab. 2006, 91, 2650–2655. [Google Scholar] [CrossRef] [PubMed]
- Kerkhofs, T.M.A.; Verhoeven, R.H.A.; Bonjer, H.J.; Van Dijkum, E.J.N.; Vriens, M.R.; De Vries, J.; Van Eijck, C.H.; Bonsing, B.A.; Van De Poll-Franse, L.V.; Haak, H.R.; et al. Surgery for adrenocortical carcinoma in The Netherlands: Analysis of the national cancer registry data. Eur. J. Endocrinol. 2013, 169, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; de Krijger, R.R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018, 179, G1–G46. [Google Scholar] [CrossRef]
- Fassnacht, M.; Assie, G.; Baudin, E.; Eisenhofer, G.; de la Fouchardiere, C.; Haak, H.R.; de Krijger, R.; Porpiglia, F.; Terzolo, M.; Berruti, A. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Kerkhofs, T.; Verhoeven, R.; Van der Zvan, J.-M.; Dieleman, J.; Kerstens, M.; Links, T.; Van de Poll-France, L.; Haak, H. Adrenocortical carcinoma: A population-based study on incidence and survival in the Netherlands since 1993. Endocr. Abstr. 2013, 32. [Google Scholar] [CrossRef]
- Chandrasekar, T.; Goldberg, H.; Klaassen, Z.; Wallis, C.J.D.; Woon, D.T.S.; Herrera-Caceres, J.O.; Kulkarni, G.S.; Fleshner, N.E. The who, when, and why of primary adrenal malignancies: Insights into the epidemiology of a rare clinical entity. Cancer 2018, 125, 1050–1059. [Google Scholar] [CrossRef]
- Baudin, E. Adrenocortical carcinoma. Endocrinol. Metab. Clin. N. Am. 2015, 44, 411–434. [Google Scholar] [CrossRef] [PubMed]
- Else, T.; Kim, A.C.; Sabolch, A.; Raymond, V.M.; Kandathil, A.; Caoili, E.M.; Jolly, S.; Miller, B.S.; Giordano, T.J.; Hammer, G.D. Adrenocortical Carcinoma. Endocr. Rev. 2013, 35, 282–326. [Google Scholar] [CrossRef] [Green Version]
- Lerario, A.; Moraitis, A.; Hammer, G.D. Genetics and epigenetics of adrenocortical tumors. Mol. Cell. Endocrinol. 2014, 386, 67–84. [Google Scholar] [CrossRef] [Green Version]
- Soon, P.S.H.; McDonald, K.L.; Robinson, B.G.; Sidhu, S.B. Molecular Markers and the Pathogenesis of Adrenocortical Cancer. Oncologist 2008, 13, 548–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedullà, G.; Crocetti, D.; Paliotta, A.; Tarallo, M.R.; De Gori, A.; Cavallaro, G.; De Toma, G. Surgical treatment of pheochromocytoma in MEN 2. Ann. Ital. Chir. 2014, 85. [Google Scholar]
- Ichijo, T.; Ueshiba, H.; Nawata, H.; Yanase, T. A nationwide survey of adrenal incidentalomas in Japan: The first report of clinical and epidemiological features. Endocr. J. 2020, 67, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Kostiainen, I.; Hakaste, L.; Kejo, P.; Parviainen, H.; Laine, T.; Löyttyniemi, E.; Pennanen, M.; Arola, J.; Haglund, C.; Heiskanen, I.; et al. Adrenocortical carcinoma: Presentation and outcome of a contemporary patient series. Endocrine 2019, 65, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Boland, G.W. Adrenal Imaging: From Addison to Algorithms. Radiol. Clin. N. Am. 2011, 49, 511–528. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.J.; McDermott, S.; Blake, M.A. Adrenal Imaging: Magnetic Resonance Imaging and Computed Tomography. Imaging Endocr. Disord. 2016, 45, 55–69. [Google Scholar] [CrossRef]
- Kiernan, C.M.; Lee, J.E. Minimally Invasive Surgery for Primary and Metastatic Adrenal Malignancy. Surg. Oncol. Clin. N. Am. 2019, 28, 309–326. [Google Scholar] [CrossRef]
- Ilias, I.; Sahdev, A.; Reznek, R.H.; Grossman, A.B.; Pacak, K. The optimal imaging of adrenal tumours: A comparison of different methods. Endocr. Relat. Cancer 2007, 14, 587–599. [Google Scholar] [CrossRef] [Green Version]
- Lattin, G.E.; Sturgill, E.D.; Tujo, C.A.; Marko, J.; Sanchez-Maldonado, K.W.; Craig, W.D.; Lack, E.E. From the Radiologic Pathology Archives: Adrenal Tumors and Tumor-like Conditions in the Adult: Radiologic-Pathologic Correlation. Radiographics 2014, 34, 805–829. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Perrier, N.; Grubbs, E.; Sircar, K.; Ye, Z.; Lee, J.; Ng, C. CT features and quantification of the characteristics of adrenocortical carcinomas on unenhanced and contrast-enhanced studies. Clin. Radiol. 2012, 67, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Korobkin, M.; Brodeur, F.J.; Francis, I.R.; Quint, L.E.; Dunnick, N.R.; Goodsitt, M. Delayed enhanced CT for differentiation of benign from malignant adrenal masses. Radiology 1996, 200, 737–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaujoux, S.; Weinandt, M.; Bonnet, S.; Reslinger, V.; Bertherat, J.; Dousset, B. Surgical treatment of adrenal carcinoma. J. Visc. Surg. 2017, 154, 335–343. [Google Scholar] [CrossRef]
- Sinclair, T.J.; Gillis, A.; Alobuia, W.M.; Wild, H.; Kebebew, E. Surgery for adrenocortical carcinoma: When and how? Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101408. [Google Scholar] [CrossRef]
- Caruso, D.; Zerunian, M.; De Santis, D.; Biondi, T.; Paolantonio, P.; Rengo, M.; Bellini, D.; Ferrari, R.; Ciolina, M.; Lucertini, E.; et al. Magnetic Resonance of Rectal Cancer Response to Therapy: An Image Quality Comparison between 3.0 and 1.5 Tesla. BioMed Res. Int. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Balachandran, A.; Habra, M.A.; Phan, A.T.; Bassett, R.L., Jr.; Macapinlac, H.A.; Chuang, H.H. Impact of ¹⁸F-FDG PET/CT on the management of adrenocortical carcinoma: Analysis of 106 patients. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2066–2073. [Google Scholar] [CrossRef] [PubMed]
- Dickson, P.V.; Kim, L.; Yen, T.W.F.; Yang, A.; Grubbs, E.G.; Patel, D.; Solórzano, C.C. Evaluation, Staging, and Surgical Management for Adrenocortical Carcinoma: An Update from the SSO Endocrine and Head and Neck Disease Site Working Group. Ann. Surg. Oncol. 2018, 25, 3460–3468. [Google Scholar] [CrossRef]
- Johanssen, S.; Hahner, S.; Saeger, W.; Quinkler, M.; Beuschlein, F.; Dralle, H.; Haaf, M.; Kroiss, M.; Jurowich, C.; Langer, P.; et al. Deficits in the Management of Patients With Adrenocortical Carcinoma in Germany. Dtsch. Aerzteblatt Online 2010, 107, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.R.; Hammer, G.D.; Else, T. Transcutaneous biopsy of adrenocortical carcinoma is rarely helpful in diagnosis, potentially harmful, but does not affect patient outcome. Eur. J. Endocrinol. 2014, 170, 829–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanis, K.N.; Kanthan, R. Diagnostic and prognostic features in adrenocortical carcinoma: A single institution case series and review of the literature. World J. Surg. Oncol. 2015, 13, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bilimoria, K.Y.; Shen, W.T.; Elaraj, D.; Bentrem, D.J.; Winchester, D.J.; Kebebew, E.; Sturgeon, C. Adrenocortical carcinoma in the United States: Treatment utilization and prognostic factors. Cancer 2008, 113, 3130–3136. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux, S.; Mihai, R.; Carnaille, B.; Dousset, B.; Fiori, C.; Porpiglia, F.; Hellman, P.; Iacobone, M.; Kraimps, J.-L.; Donatini, G.; et al. European Society of Endocrine Surgeons (ESES) and European Network for the Study of Adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. J. Br. Surg. 2017, 104, 358–376. [Google Scholar] [CrossRef] [Green Version]
- Ayala-Ramirez, M.; Jasim, S.; Feng, L.; Ejaz, S.; Deniz, F.; Busaidy, N.; Waguespack, S.G.; Naing, A.; Sircar, K.; Wood, C.G.; et al. Adrenocortical carcinoma: Clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur. J. Endocrinol. 2013, 169, 891–899. [Google Scholar] [CrossRef]
- Lughezzani, G.; Sun, M.; Perrotte, P.; Jeldres, C.; Alasker, A.; Isbarn, H.; Budäus, L.; Shariat, S.F.; Guazzoni, G.F.; Montorsi, F.; et al. The European Network for the Study of Adrenal Tumors staging system is prognostically superior to the international union against cancer-staging system: A North American validation. Eur. J. Cancer 2010, 46, 713–719. [Google Scholar] [CrossRef]
- Miller, B.S.; Gauger, P.G.; Hammer, G.D.; Giordano, T.J.; Doherty, G.M. Proposal for modification of the ENSAT staging system for adrenocortical carcinoma using tumor grade. Langenbecks Arch. Surg. 2010, 395, 955–961. [Google Scholar] [CrossRef]
- Fassnacht, M.; Johanssen, S.; Quinkler, M.; Bucsky, P.; Willenberg, H.S.; Beuschlein, F.; Terzolo, M.; Mueller, H.H.; Hahner, S.; Allolio, B. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: Proposal for a Revised TNM Classification. Cancer 2009, 115, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Calcatera, N.A.; Hsiung-Wang, C.; Suss, N.R.; Winchester, D.J.; Moo-Young, T.A.; Prinz, R.A. Minimally Invasive Adrenalectomy for Adrenocortical Carcinoma: Five-Year Trends and Predictors of Conversion. World J. Surg. 2017, 42, 473–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurice, M.J.; Bream, M.J.; Kim, S.P.; Abouassaly, R. Surgical quality of minimally invasive adrenalectomy for adrenocortical carcinoma: A contemporary analysis using the National Cancer Database. BJU Int. 2016, 119, 436–443. [Google Scholar] [CrossRef]
- Lee, C.W.; Salem, A.I.; Schneider, D.F.; Leverson, G.E.; Tran, T.B.; Poultsides, G.A.; Postlewait, L.M.; Maithel, S.K.; Wang, T.S.; Hatzaras, I.; et al. Minimally Invasive Resection of Adrenocortical Carcinoma: A Multi-Institutional Study of 201 Patients. J. Gastrointest. Surg. 2016, 21, 352–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastelan, D.; Knezevic, N.; Tomsic, K.Z.; Alduk, A.; Kakarigi, L.; Coric, M.; Skoric-Polovina, T.; Solak, M.; Kraljevic, I.; Balasko, A.; et al. Open vs laparoscopic adrenalectomy for localized adrenocortical carcinoma. Clin. Endocrinol. 2020, 93, 404–408. [Google Scholar] [CrossRef]
- Brix, D.; Allolio, B.; Fenske, W.; Agha, A.; Dralle, H.; Jurowich, C.; Langer, P.; Mussack, T.; Nies, C.; Riedmiller, H.; et al. Laparoscopic Versus Open Adrenalectomy for Adrenocortical Carcinoma: Surgical and Oncologic Outcome in 152 Patients. Eur. Urol. 2010, 58, 609–615. [Google Scholar] [CrossRef]
- Donatini, G.; Caïazzo, R.; Cao, C.D.; Aubert, S.; Zerrweck, C.; El-Kathib, Z.; Gauthier, T.; Leteurtre, E.; Wemeau, J.-L.; Vantyghem, M.C.; et al. Long-Term Survival After Adrenalectomy for Stage I/II Adrenocortical Carcinoma (ACC): A Retrospective Comparative Cohort Study of Laparoscopic Versus Open Approach. Ann. Surg. Oncol. 2013, 21, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Fosså, A.; Røsok, B.I.; Kazaryan, A.M.; Holte, H.J.; Brennhovd, B.; Westerheim, O.; Marangos, I.P.; Edwin, B. Laparoscopic versus open surgery in stage I–III adrenocortical carcinoma—A retrospective comparison of 32 patients. Acta Oncol. 2013, 52, 1771–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardi, C.P.; Raffaelli, M.; De Crea, C.; Boniardi, M.; De Toma, G.; Marzano, L.A.; Miccoli, P.; Minni, F.; Morino, M.; Pelizzo, M.R.; et al. Open versus endoscopic adrenalectomy in the treatment of localized (stage I/II) adrenocortical carcinoma: Results of a multiinstitutional Italian survey. Surgery 2012, 152, 1158–1164. [Google Scholar] [CrossRef]
- Zheng, G.Y.; Li, H.Z.; Deng, J.H.; Zhang, X.B.; Wu, X.C. Open adrenalectomy versus laparoscopic adrenalectomy for adrenocortical carcinoma: A retrospective comparative study on short-term oncologic prognosis. OncoTargets Ther. 2018, 11, 1625–1632. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Liu, Z.; Liang, J.; Tang, Y.; Zou, Z.; Zhou, C.; Zhang, F.; Lu, Y. Laparoscopic versus open adrenalectomy for localized (stage 1/2) adrenocortical carcinoma: Experience at a single, high-volumecenter. Surgery 2018, 164, 1325–1329. [Google Scholar] [CrossRef]
- Vanbrugghe, C.; Lowery, A.; Golffier, C.; Taieb, D.; Sebag, F. Adrenocortical carcinoma surgery—Surgical extent and approach. Langenbeck’s Arch. Surg. 2016, 401, 991–997. [Google Scholar] [CrossRef]
- Mir, M.C.; Klink, J.C.; Guillotreau, J.; Long, J.A.; Miocinovic, R.; Kaouk, J.H.; Simmons, M.N.; Klein, E.; Krishnamurthi, V.; Campbell, S.C.; et al. Comparative Outcomes of Laparoscopic and Open Adrenalectomy for Adrenocortical Carcinoma: Single, High-Volume Center Experience. Ann. Surg. Oncol. 2012, 20, 1456–1461. [Google Scholar] [CrossRef]
- Cooper, A.B.; Habra, M.A.; Grubbs, E.G.; Bednarski, B.K.; Ying, A.K.; Perrier, N.D.; Lee, J.E.; Aloia, T.A. Does laparoscopic adrenalectomy jeopardize oncologic outcomes for patients with adrenocortical carcinoma? Surg. Endosc. 2013, 27, 4026–4032. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.S.; Ammori, J.B.; Gauger, P.G.; Broome, J.T.; Hammer, G.D.; Doherty, G.M. Laparoscopic Resection is Inappropriate in Patients with Known or Suspected Adrenocortical Carcinoma. World J. Surg. 2010, 34, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.S.; Gauger, P.G.; Hammer, G.D.; Doherty, G.M. Resection of adrenocortical carcinoma is less complete and local recurrence occurs sooner and more often after laparoscopic adrenalectomy than after open adrenalectomy. Surgery 2012, 152, 1150–1157. [Google Scholar] [CrossRef]
- Leboulleux, S.; Deandreis, D.; Al Ghuzlan, A.; Aupérin, A.; Goéré, D.; Dromain, C.; Elias, D.; Caillou, B.; Travagli, J.P.; de Baere, T.; et al. Adrenocortical carcinoma: Is the surgical approach a risk factor of peritoneal carcinomatosis? Eur. J. Endocrinol. 2010, 162, 1147–1153. [Google Scholar] [CrossRef] [Green Version]
- Porpiglia, F.; Fiori, C.; Daffara, F.; Zaggia, B.; Bollito, E.; Volante, M.; Berruti, A.; Terzolo, M. Retrospective Evaluation of the Outcome of Open Versus Laparoscopic Adrenalectomy for Stage I and II Adrenocortical Cancer. Eur. Urol. 2010, 57, 873–878. [Google Scholar] [CrossRef]
- Autorino, R.; Bove, P.; De Sio, M.; Miano, R.; Micali, S.; Cindolo, L.; Greco, F.; Nicholas, J.; Fiori, C.; Bianchi, G.; et al. Open Versus Laparoscopic Adrenalectomy for Adrenocortical Carcinoma: A Meta-analysis of Surgical and Oncological Outcomes. Ann. Surg. Oncol. 2015, 23, 1195–1202. [Google Scholar] [CrossRef]
- Stefanidis, D.; Goldfarb, M.; Kercher, K.W.; Hope, W.W.; Richardson, W.; Fanelli, R.D. SAGES guidelines for minimally invasive treatment of adrenal pathology. Surg. Endosc. 2013, 27, 3960–3980. [Google Scholar] [CrossRef]
- Mpaili, E.; Moris, D.; Tsilimigras, D.I.; Oikonomou, D.; Pawlik, T.M.; Schizas, D.; Papalampros, A.; Felekouras, E.; Dimitroulis, D. Laparoscopic Versus Open Adrenalectomy for Localized/Locally Advanced Primary Adrenocortical Carcinoma (ENSAT I–III) in Adults: Is Margin-Free Resection the Key Surgical Factor that Dictates Outcome? A Review of the Literature. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 408–414. [Google Scholar] [CrossRef]
- Hu, X.; Yang, W.-X.; Shao, Y.-X.; Dou, W.-C.; Mm, S.-C.X.; Li, X. Minimally Invasive Versus Open Adrenalectomy in Patients with Adrenocortical Carcinoma: A Meta-analysis. Ann. Surg. Oncol. 2020, 27, 3858–3869. [Google Scholar] [CrossRef] [PubMed]
- Ağcaoğlu, O.; Aliyev, S.; Karabulut, K.; Mitchell, J.; Siperstein, A.; Berber, E. Robotic Versus Laparoscopic Resection of Large Adrenal Tumors. Ann. Surg. Oncol. 2012, 19, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Nordenström, E.; Westerdahl, J.; Hallgrimsson, P.; Bergenfelz, A. A prospective study of 100 roboticallyassisted laparoscopic adrenalectomies. J. Robot. Surg. 2011, 5, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Perivoliotis, K.; Baloyiannis, I.; Sarakatsianou, C.; Tzovaras, G. Comparing the efficacy and safety of laparoscopic and robotic adrenalectomy: A meta-analysis and trial sequential analysis. Langenbeck’s Arch. Surg. 2020, 405, 125–135. [Google Scholar] [CrossRef]
- Margonis, G.A.; Kim, Y.; Prescott, J.D.; Tran, T.B.; Postlewait, L.M.; Maithel, S.K.; Wang, T.S.; Evans, D.B.; Hatzaras, I.; Shenoy, R.; et al. Adrenocortical Carcinoma: Impact of Surgical Margin Status on Long-Term Outcomes. Ann. Surg. Oncol. 2015, 23, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulick, R.D.; Brennan, M.F. Long-Term Survival After Complete Resection and Repeat Resection in Patients with Adrenocortical Carcinoma. Ann. Surg. Oncol. 1999, 6, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Reibetanz, J.; Jurowich, C.; Erdogan, I.; Nies, C.; Rayes, N.; Dralle, H.; Behrend, M.; Allolio, B.; Fassnacht, M. Impact of Lymphadenectomy on the Oncologic Outcome of Patients with Adrenocortical Carcinoma. Ann. Surg. 2012, 255, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Gerry, J.M.; Tran, T.B.; Postlewait, L.M.; Maithel, S.K.; Prescott, J.D.; Wang, T.S.; Glenn, J.A.; Phay, J.E.; Keplinger, K.; Fields, R.C.; et al. Lymphadenectomy for Adrenocortical Carcinoma: Is There a Therapeutic Benefit? Ann. Surg. Oncol. 2016, 23 (Suppl. 5), 708–713. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.S.; Doherty, G. Regional Lymphadenectomy for Adrenocortical Carcinoma. Ann. Surg. 2013, 257, e13–e14. [Google Scholar] [CrossRef] [PubMed]
- Deschner, B.W.; Do, Z.E.S.; DeLozier, O.M.; Drake, J.A.; Tsao, M.; Glazer, E.S.; Do, J.L.D.; Yakoub, D.; Dickson, P.V. Critical analysis of lymph node examination in patients undergoing curative-intent resection for adrenocortical carcinoma. J. Surg. Oncol. 2020. [Google Scholar] [CrossRef]
- Schteingart, D.E.; Doherty, G.; Gauger, P.G.; Giordano, T.; Hammer, G.D.; Korobkin, M.; Worden, F.P. Management of patients with adrenal cancer: Recommendations of an international consensus conference. Endocr. Relat. Cancer 2005, 12, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux, S.; Brennan, M.F. Recommendation for standardized surgical management of primary adrenocortical carcinoma. Surgery 2012, 152, 123–132. [Google Scholar] [CrossRef]
- Gagner, M.; Lacroix, A.; Bolté, E. Laparoscopic adrenalectomy in Cushing’s syndrome and pheochromocytoma. N. Engl. J. Med. 1992, 327, 1033. [Google Scholar]
- Sroka, G.; Slijper, N.; Shteinberg, D.; Mady, H.; Galili, O.; Matter, I. Laparoscopic adrenalectomy for malignant lesions: Surgical principles to improve oncologic outcomes. Surg. Endosc. 2013, 27, 2321–2326. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H.; Newell-Price, J.; Sahdev, A.; Tabarin, A.; Terzolo, M.; Tsagarakis, S.; Dekkers, O. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2016, 175, G1–G34. [Google Scholar] [CrossRef] [Green Version]
- Cavallaro, G.; Crocetti, D.; Paliotta, A.; De Gori, A.; Tarallo, M.R.; Letizia, C.; De Toma, G. Cystic adrenal lesions: Clinical and surgical management. The experience of a referral centre. Int. J. Surg. 2015, 13, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Tarallo, M.; Crocetti, D.; Fiori, E.; Sapienza, P.; Letizia, C.; De Toma, G.; Cavallaro, G. Criticism of learning curve in laparoscopic adrenalectomy: A systematic review. Clin. Ter. 2020, 171, e178–e182. [Google Scholar]
- Zafar, S.S.; Abaza, R. Robot-Assisted Laparoscopic Adrenalectomy for Adrenocortical Carcinoma: Initial Report and Review of the Literature. J. Endourol. 2008, 22, 985–990. [Google Scholar] [CrossRef]
- Yiannakopoulou, E. Robotic assisted adrenalectomy: Surgical techniques, feasibility, indications, oncological outcome and safety. Int. J. Surg. 2016, 28, 169–172. [Google Scholar] [CrossRef]
- Ludwig, A.T.; Wagner, K.R.; Lowry, P.S.; Papaconstantinou, H.T.; Lairmore, T.C. Robot-Assisted Posterior Retroperitoneoscopic Adrenalectomy. J. Endourol. 2010, 24, 1307–1314. [Google Scholar] [CrossRef]
- Zeiger, M.A.; Thompson, G.B.; Duh, Q.-Y.; Hamrahian, A.H.; Angelos, P.; Elaraj, D.; Fishman, E.; Kharlip, J.; Garber, J.R.; Mechanick, J.I.; et al. American Association Of Clinical Endocrinologists And American Association Of Endocrine Surgeons Medical Guidelines For The Management Of Adrenal Incidentalomas. Endocr. Pract. 2009, 15, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Icard, P.; Louvel, A.; Chapuis, Y. Survival rates and prognostic factors in adrenocortical carcinoma. World J. Surg. 1992, 16, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Bellantone, R.; Ferrante, A.M.R.; Boscherini, M.; Lombardi, C.P.; Crucitti, P.; Crucitti, F.; Favia, G.; Borrelli, D.; Boffi, L.; Capussotti, L.; et al. Role of reoperation in recurrence of adrenal cortical carcinoma: Results from 188 cases collected in the Italian National Registry for Adrenal Cortical Carcinoma. Surgery 1997, 122, 1212–1218. [Google Scholar] [CrossRef]
- Miller, B.S.; Doherty, G.M. Surgical management of adrenocortical tumours. Nat. Rev. Endocrinol. 2014, 10, 282–292. [Google Scholar] [CrossRef]
- Laan, D.; Thiels, C.A.; Glasgow, A.; Wise, K.B.; Thompson, G.B.; Richards, M.L.; Farley, D.R.; Truty, M.J.; McKenzie, T.J. Adrenocortical carcinoma with inferior vena cava tumor thrombus. Surgery 2017, 161, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Crucitti, F.; Bellantone, R.; Ferrante, A.M.R.; Boscherini, M.; Crucitti, P. The Italian registry for adrenal cortical carcinoma: Analysis of a multiinstitutional series of 129 patients. Surgery 1996, 119, 161–170. [Google Scholar] [CrossRef]
- Mirallié, E.; Blanchard, C.; Caillard, C.; Rodien, P.; Briet, C.; Mucci, S.; Drui, D.; Hamy, A. Adrenocortical carcinoma: Impact of surgical treatment. Ann. Endocrinol. 2019, 80, 308–313. [Google Scholar] [CrossRef] [PubMed]
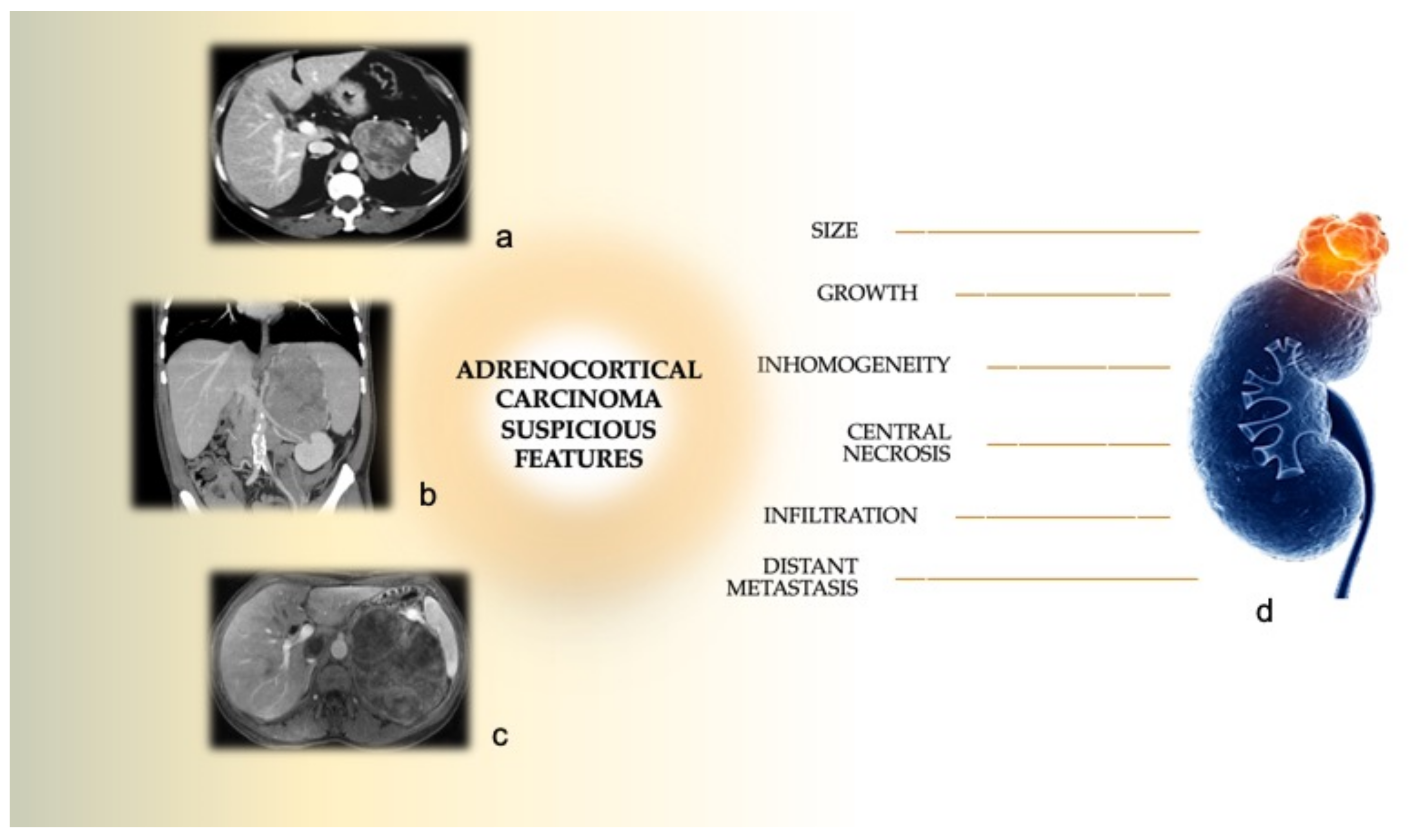

| ENSAT Stage | TNM | Description |
|---|---|---|
| I | T1 N0 M0 | The tumor has 5 cm or less of major diameter (T1), it has not spread to nearby lymph nodes (N0) or distant sites (M0). |
| II | T2 N0 M0 | The tumor is greater than 5 cm and it has no grown into tissues outside the adrenal gland (T2), it has not spread to nearby lymph nodes (N0) or distant sites (M0). |
| III | T1 N1 M0 | The tumor has 5 cm or less of major diameter and it has not grown into tissues outside the adrenal gland (T1). The cancer has spread to nearby lymph nodes (N1) but not to distant sites (M0). |
| T2 N1 M0 | The tumor is greater than 5 cm and it has not grown into tissues outside the adrenal gland (T2). The cancer has spread to nearby lymph nodes (N1) but not to distant sites (M0). | |
| T3 Any N M0 | The tumor is growing in the fat surrounding the adrenal gland. The tumor can be any size (T3). It might or might not have spread to nearby lymph nodes (Any N0). It has not spread to distant sites (M0). | |
| T4 Any N M0 | The tumor is growing into nearby organs (kidney, pancreas, spleen and liver or large blood vessels such as renal vein or vena cava). The tumor can be any size (T4). It might or might not have spread to nearby lymph nodes (Any N). It has not spread to distant organs (M0). | |
| IV | Any T Any N M1 | The cancer has spread to distant sites like the liver or lungs (M1). It can be any size (Any T) and may or may not have spread to nearby tissues (Any T) or lymph nodes (Any N). |
| Study | Study Period | Study Design | Minors Score | Patients n | Median Age (Year) | Gender F M n, (%) | Hormon Secretion n, (%) OA, LA | Surgical Approach n, (%) | Conversion n (%) | Median Follow-Up Months, OA: MIS |
|---|---|---|---|---|---|---|---|---|---|---|
| Kastelan et al., (2020) [41] | 2004–2018 | Retrospective | 18 | 46 | 48 (18–74) | 32 (69) 14 (31) | 11:10 (48:43) | 0A 23 (50) LA 23 (50) | 0 | 52 |
| Zheng et al., (2018) [46] | 2013–2015 | Retrospective | 19 | 42 | 46 (40–54) | 23 (55) | 13:11 | 0A 22 (52) LA 20 (48) | 0 | Maximum 36 |
| Wu et al., (2018) [47] | 2009–2017 | Retrospective | 20 | 44 | 45 (2–74) | 27 (61) 17 (39) | 9:11 (39:52) | 0A 23 (52) LA 21 (48) | 1 | 34 |
| Calcatera et al., (2018) [38] | 2010–2014 | Retrospective | 17 | 588 | 54 | 360 (61) 228 (39) | 0A 388, (66) MIS 200, (34) | 38 (19) | ||
| Maurice et al., 2017 [39] | 2010–2013 | Retrospective | 17 | 481 | OA 56 (43–67) LA 61 (50–69) | 302 (63) 179 (37) | OA 320, (67) MIS 161, (33) | 24 (15) | 25:23.6 | |
| Lee et al., 2017 [40] | 1994–2014 | Retrospective | 17 | 201 | 52 (11–87) | 131 (65) 70 (35) | 58:11 40:25 | 0A 154, (77) MIS 47, (23) | 9 (19) | 60 |
| Vanbrugghe et al., 2016 [48] | 2002–2013 | Retrospective | 18 | 25 | 47 (22–77) | 15 (60) 10 (40) | 3:4 (33:25) | 0A 9, (36) LA 16, (64) | 0 | 52.9:36.4 |
| Donatini et al., 2014 [43] | 1985–2011 | Retrospective | 21 | 34 | 45 | 26 (76) 8 (24) | 8:3 (38:23) | 0A 21, (61)L A 13, (39) | 0 | 66 |
| Mir et al., 2013 [49] | 1993–2011 | Retrospective | 18 | 44 | 49 (40–65) | 22 (50) 22 (50) | 0A 26, (59) LA 18, (41) | 5 (24) | 26 | |
| Fossa et al., 2013 [44] | 1998–2011 | Retrospective | 17 | 32 | 48 (29–75) | 23 (72) 9 (28) | 6:13 | 0A 15, (47) LA 17, (53) | 2 (11) | 29.1 |
| Cooper et al., 2013 [50] | 1993–2012 | Retrospective | 17 | 302 | 45 | 196 (65) 106 (35) | 0A 256, (85) LA 46, (15) | - | 34.4 | |
| Miller et al., 2012 [52] | 2005–2011 | Retrospective | 18 | 156 | 47 (10–80) | 64 (41) 92 (59) | OA 110, (70) LA 46, (30) | - | 29.5:19 | |
| Lombardi et al., 2012 [45] | 2003–2010 | Retrospective | 21 | 156 | 47 (10–81) | 100 (64) 56 (36) | 62 | 0A 126, (80) LA 30, (20) | 0 | 42 |
| Porpiglia et al., 2010 [54] | 2002–2008 | Retrospective | 19 | 43 | 43 (24–68) | 26 (60) 17 (40) | 14:11 (56:61) | 0A 25, (58) LA 18, (42) | - | 35 |
| Miller et al., 2010 [51] | 2003–2008 | Retrospective | 16 | 88 | 46 (18–81) | 57 (65) 31 (35) | 0A 71, (81) LA 17, (19) | - | 36.5 | |
| Leboulleux et al., 2010 [53] | 2003–2009 | Retrospective | 17 | 64 | 54 (23–79) | 36 (56) 28 (44) | 35 (30) | 0A 58, (90) LA 6, (10) | - | 35 |
| Brix et al., 2010 [42] | 1996–2009 | Retrospective | 19 | 152 | 51 | 10844 | 63:34 | 0A 117, (77) LA 35, (23) | 11 (34) | 39.3 |
| Study | Tumor Stage (ENSAT) | Tumor Size (OA: MIS) cm, Median | R0 Resection (OA: MIS) n, (%) | LND (OA: MIS) n | Local Recurrence (OA: MIS) n, (%) | Overall Recurrence (OA: MIS) n, (%) | Disease Free Survival (OA: MIS) Median, Months, (%) | Overall Survival (OA: MIS) Months (%) |
|---|---|---|---|---|---|---|---|---|
| Kastelan et al., (2020) [41] | I–III | 12:7.5 | 23:23 (100:100) | - | 2:1 (9:4) | 5:3 (22:13) | - p:0.55 | - p:0.76 |
| Zheng et al., (2018) [46] | I–III | 10.1:6.3 | 22:20 (100:100) | - | 5:8 (23:40) | 13:11 p:0.08 | 45:17 p:0.02 | - |
| Wu et al., (2018) [47] | I–II | 6.8:5.8 | - | 3:0 | 5:9 (22:43) | 12:11 (52:52) | 22:25 (36:39) p:0.8 | 42:63 (43:47) p:0.63 |
| Calcatera et al., (2018) [38] | I–IV | 12.4:8.9 | 289:141 (74:70) | - | - | - | - | - |
| Maurice et al., 2017 [39] | I–IV | 11.7:7.5 | 266:129 (83:80) | 42:2 p:0.01 | - | - | - | (62:58) p:0.42 |
| Lee et al., 2017 [40] | I–IV | 10.9:5.5 | 114:36 (74:77) | 63 | - | 82:22 (64:48) p:0.07 | 10:14 (3.8:9.1) p:0.2 | 53.8:90.9 (49:68) p:0.23 |
| Vanbrugghe et al., 2016 [48] | I–III | 11.6:6.2 | 9:12 (100:75) | - | 0:2 (0:12) | 4:6 (44:37) | (62:56) p:1.0 | (89:69) p:0.36 |
| Donatini et al., 2014 [43] | I–II | 6.8:5.5 | 21:13 (100:100) | - | - | 5:4 (24:31) | 47:46 | (81:85) p:0.63 |
| Mir et al., 2013 [49] | I–IV | 13:7 | 16:11 (61:61) | 14:6 | 12:10 (46:55) | (27:22) | 13.8:9.7 (60:39) | (54:58) p:0.6 |
| Fossa et al., 2013 [44] | I–III | 13:8 | 12:12 (80:70) | - | 1:1 (7:6) | 5:3 (33:17) | 8.1:15.2 | 36:103 p.0.22 |
| Cooper et al., 2013 [50] | I–IV | 12:8 | 134:25 (52:71) | - | - | 73:76.1 | 16.7:10.9 | 110:54 p:0.07 |
| Miller et al., 2012 [52] | I–III | 12:7.4 | 72:26 (65:56) | - | - | (40:86) | - | Stage II 103/51 p:0.002 Stage III 44/28 p:0.77 |
| Lombardi et al., 2012 [45] | I–II | 9:7.7 | 126:30 (100:100) | 23:1 | 14:4 (11:13) | 48:8 (38:26) | 48:72 (38:58) | - (47:66) p:0.2 |
| Porpiglia et al., 2010 [54] | I–II | 10.5:9 | 25:18 (100:100) | - | 6:6 (24:33) | 16:9 (64:50) | 18:23 | (72:95) |
| Miller et al., 2010 [51] | I–III | 12.3:7 | (82:50) | - | (20:25) | (65:63) | 19:10 | - |
| Leboulleux et al., 2010 [53] | I–IV | 14:7 | 37:5 (63:83) | - | (72:34) | - | 20 | 38:5 - |
| Brix et al., 2010 [42] | I–III | 8:6.2 | 64:24 (55:69) | - | (38:50) | 81:27 (69:77) | 21.5–24.2 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallaro, G.; Tarallo, M.; Chiappini, A.; Crocetti, D.; Polistena, A.; Petramala, L.; Sibio, S.; De Toma, G.; Fiori, E.; Letizia, C. Surgical Management of Adrenocortical Carcinoma: Current Highlights. Biomedicines 2021, 9, 909. https://doi.org/10.3390/biomedicines9080909
Cavallaro G, Tarallo M, Chiappini A, Crocetti D, Polistena A, Petramala L, Sibio S, De Toma G, Fiori E, Letizia C. Surgical Management of Adrenocortical Carcinoma: Current Highlights. Biomedicines. 2021; 9(8):909. https://doi.org/10.3390/biomedicines9080909
Chicago/Turabian StyleCavallaro, Giuseppe, Mariarita Tarallo, Ambra Chiappini, Daniele Crocetti, Andrea Polistena, Luigi Petramala, Simone Sibio, Giorgio De Toma, Enrico Fiori, and Claudio Letizia. 2021. "Surgical Management of Adrenocortical Carcinoma: Current Highlights" Biomedicines 9, no. 8: 909. https://doi.org/10.3390/biomedicines9080909
APA StyleCavallaro, G., Tarallo, M., Chiappini, A., Crocetti, D., Polistena, A., Petramala, L., Sibio, S., De Toma, G., Fiori, E., & Letizia, C. (2021). Surgical Management of Adrenocortical Carcinoma: Current Highlights. Biomedicines, 9(8), 909. https://doi.org/10.3390/biomedicines9080909






