Differences in Nutritional Status and Inflammatory Biomarkers between Female and Male Patients with Bronchiectasis: A Large-Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
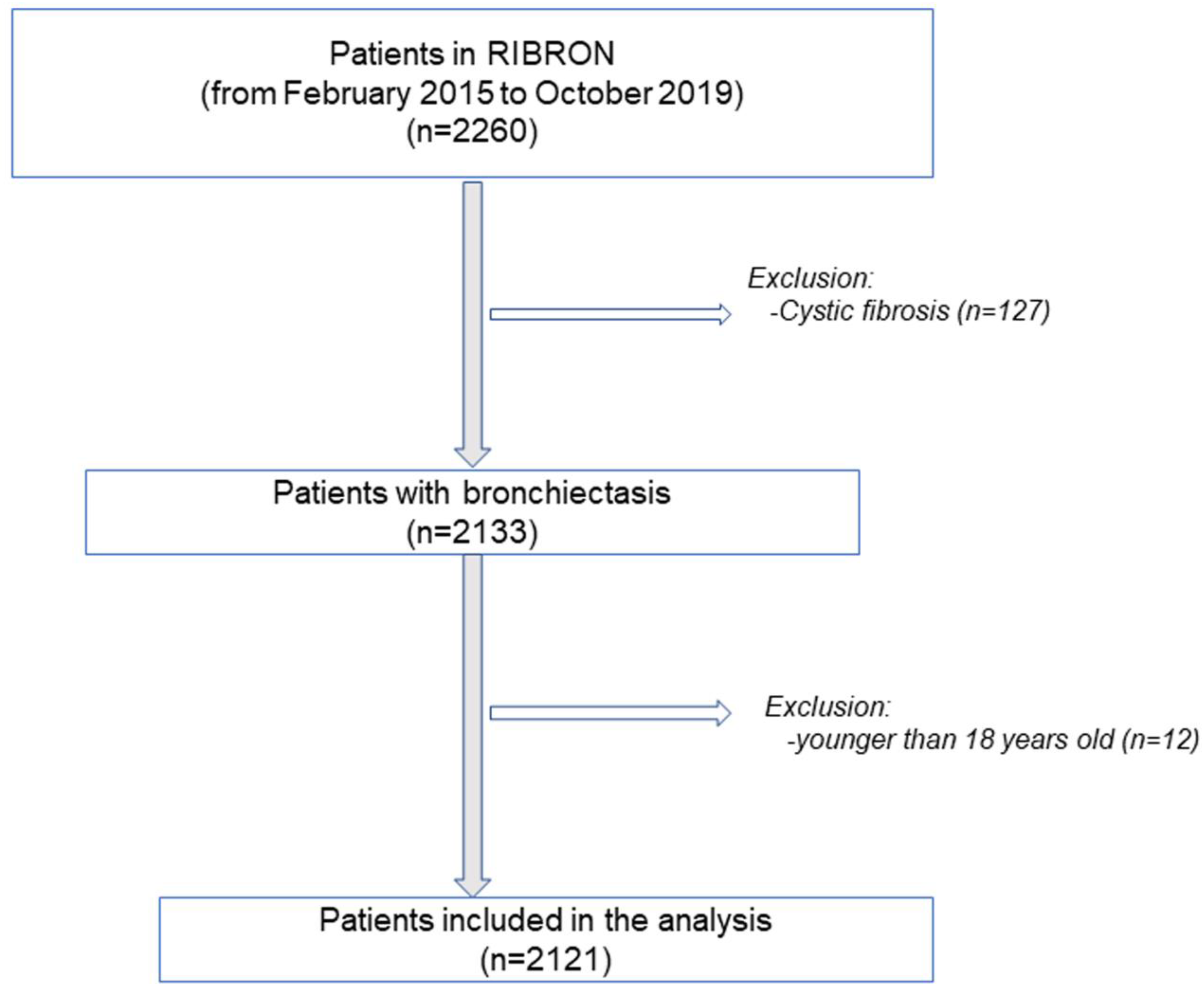
2.2. Study Population
2.3. Study Variables and Scores
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics in Male and Female Patients
3.2. Nutritional Status in Male and Female Patients
3.3. Inflammatory Parameters in Male and Female Patients
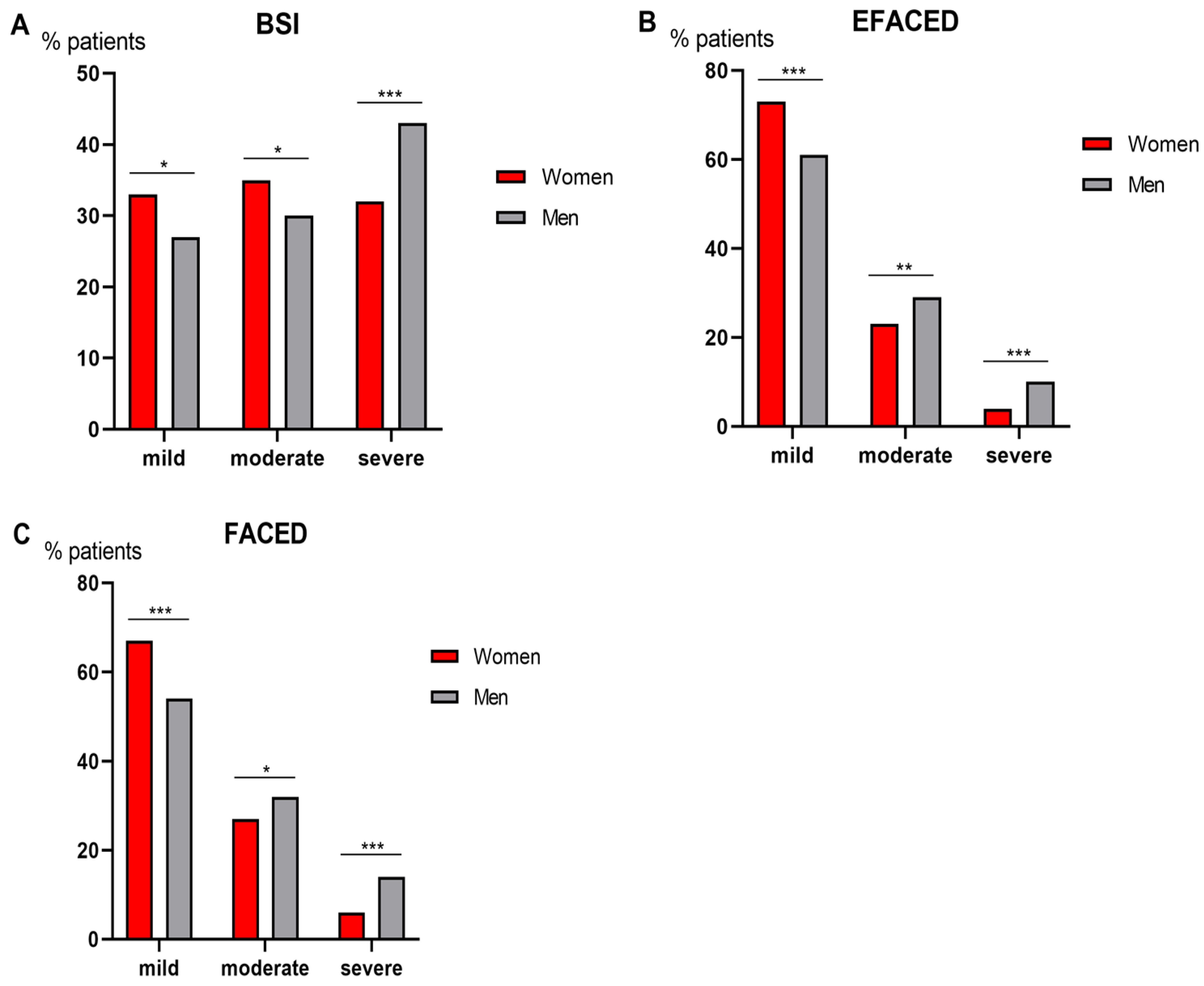
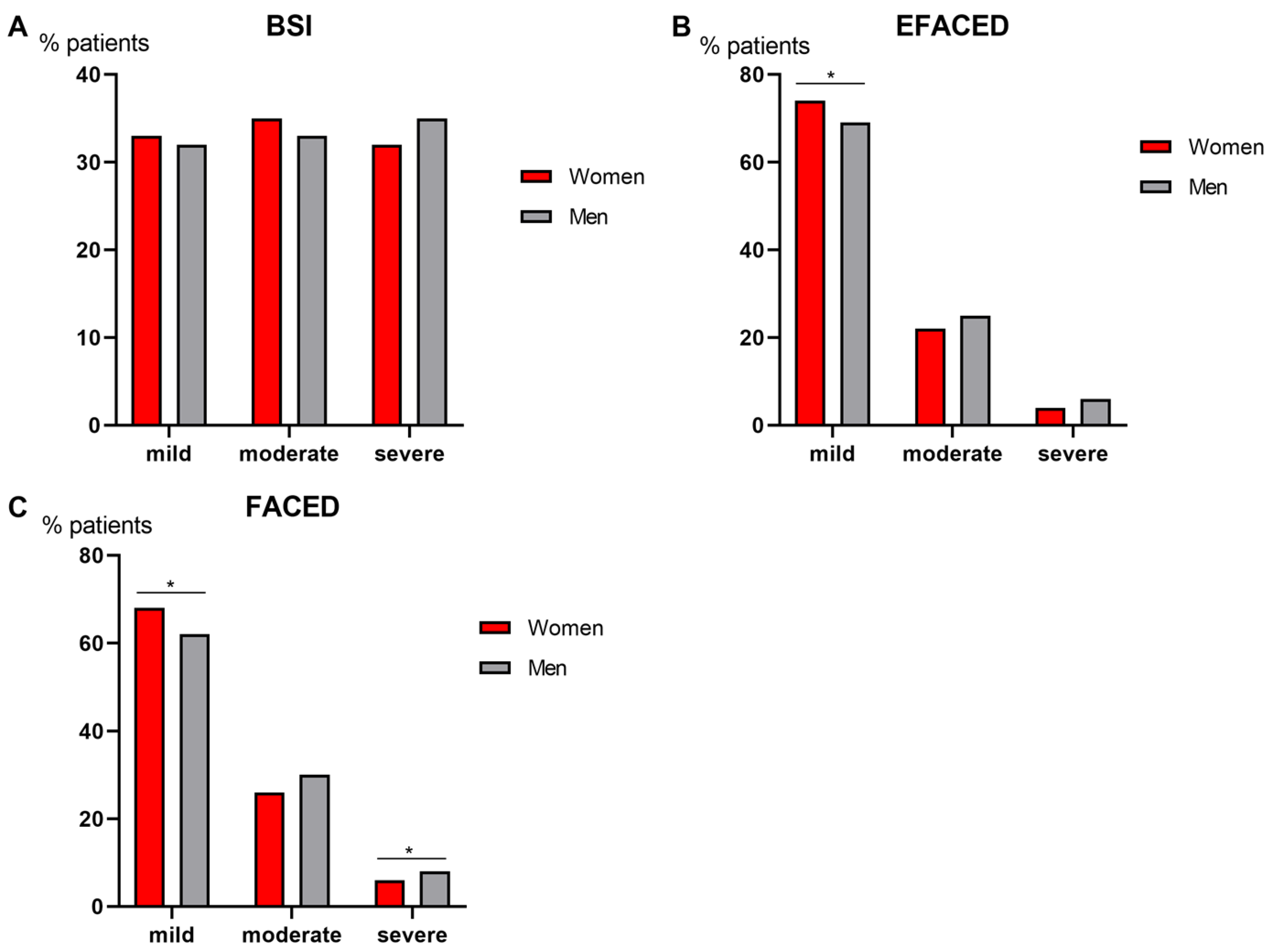
3.4. Degree of Disease Severity
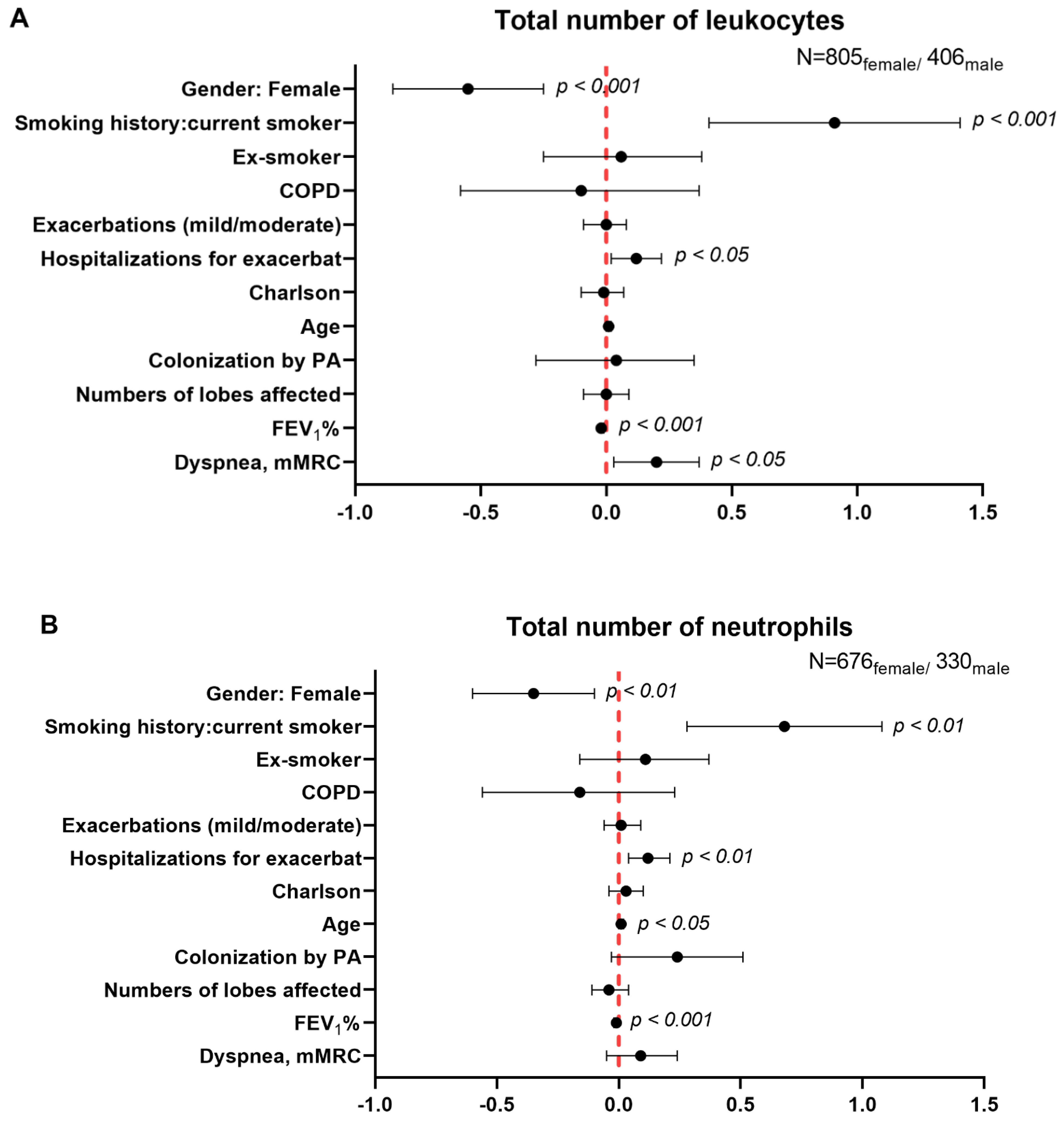
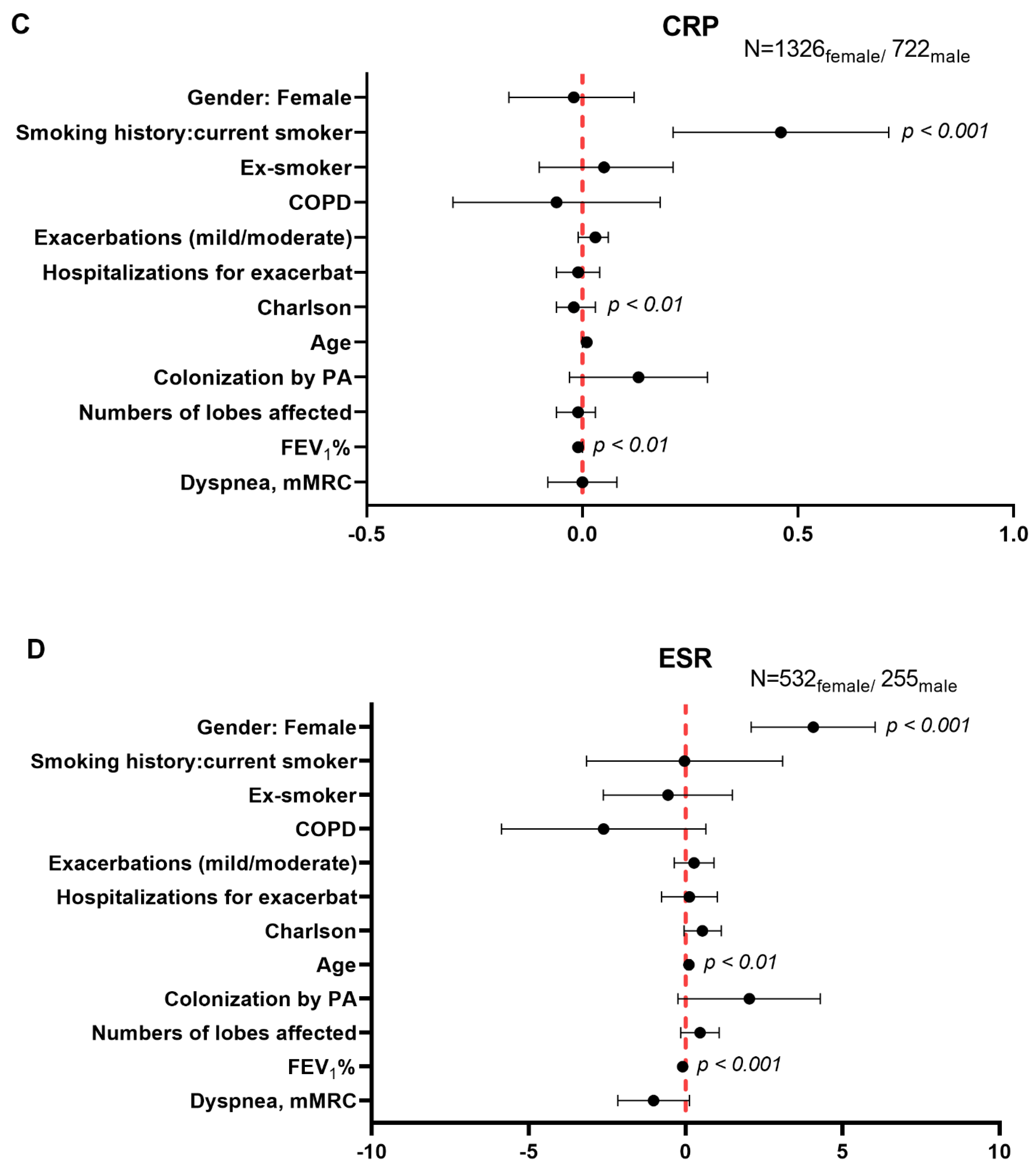
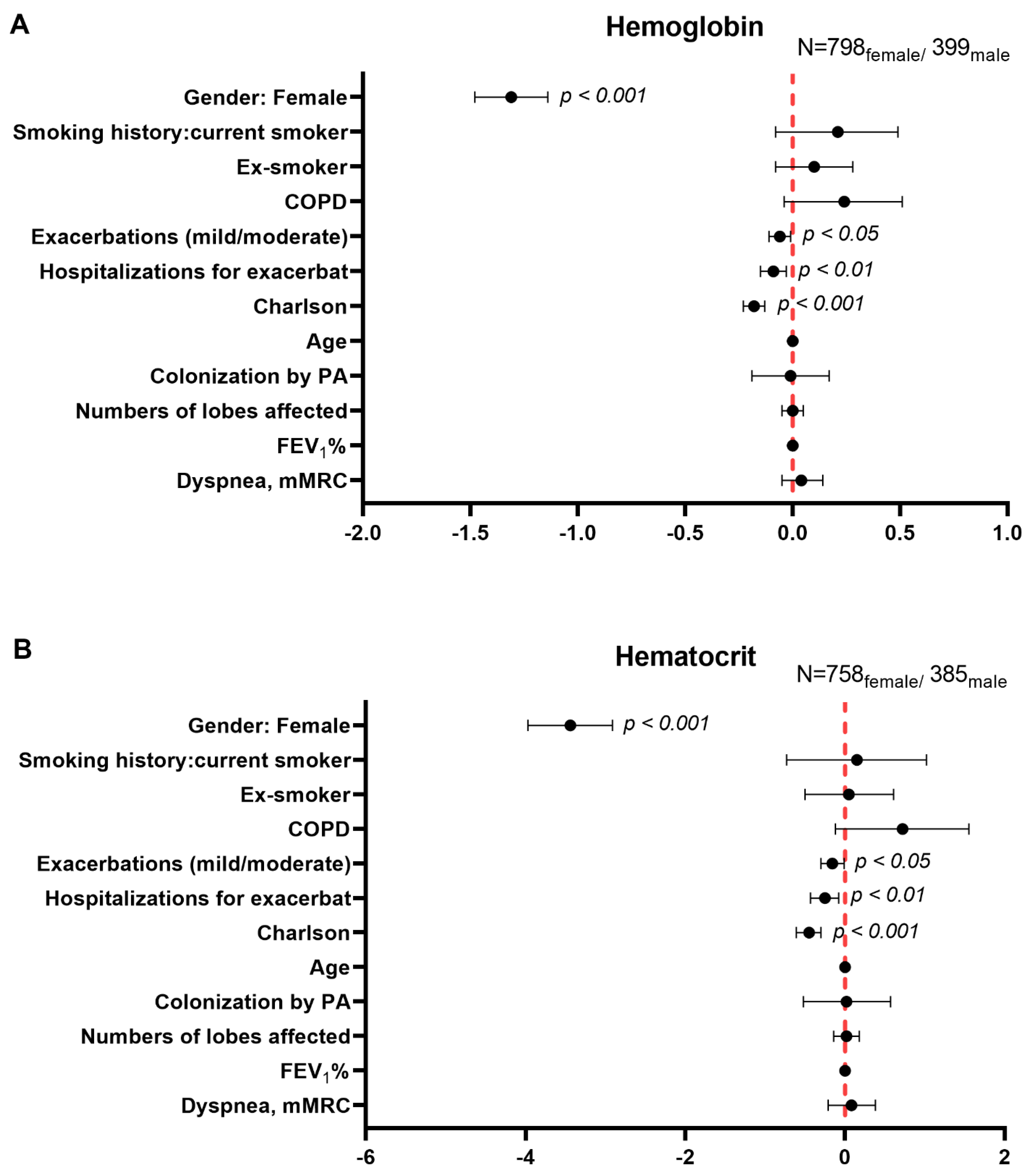
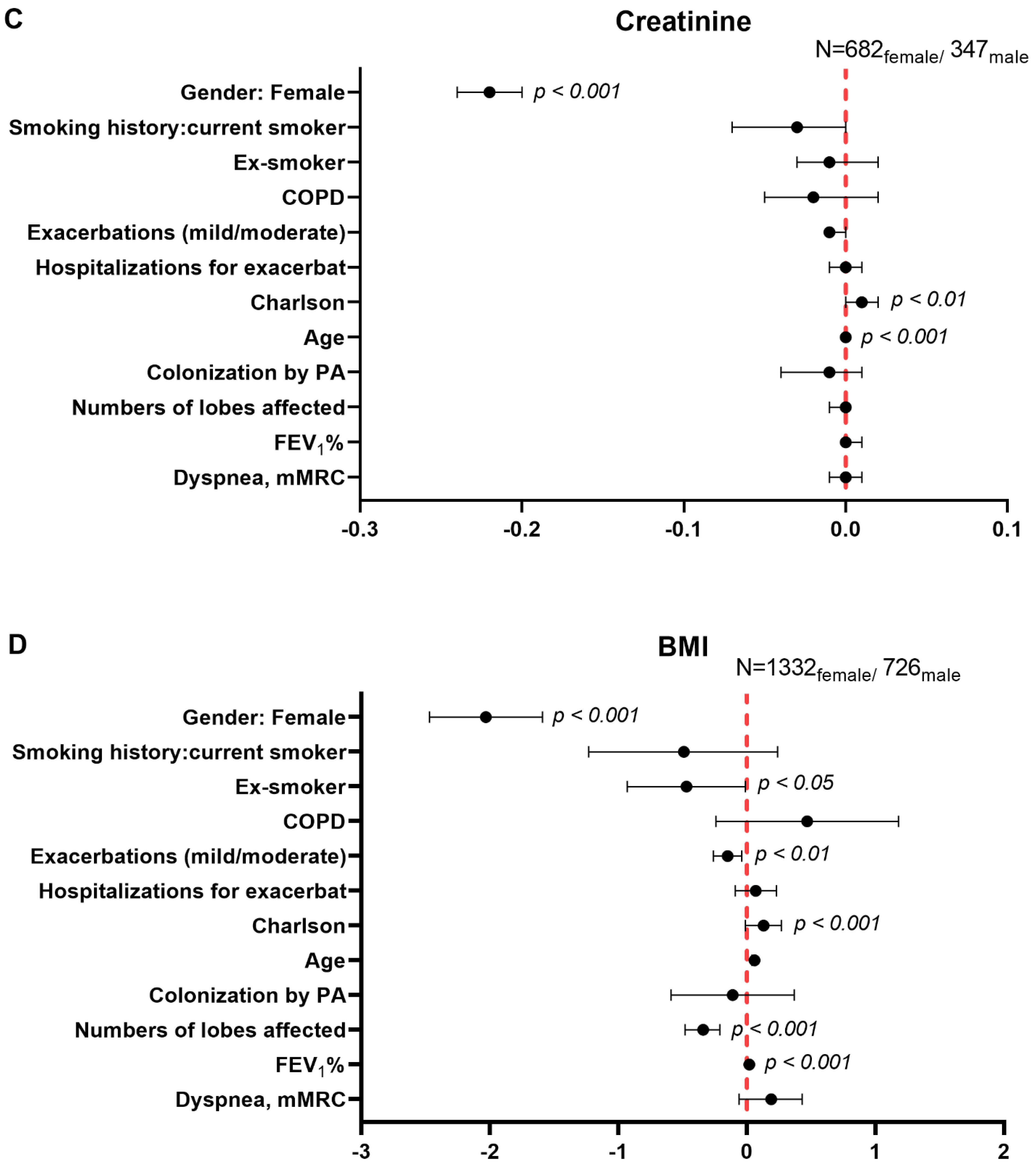
3.5. Multivariate Analysis of Systemic Inflammatory and Nutritional Parameters in Male and Female Patients
4. Discussion
5. Study Critique
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinez-Garcia, M.A.; Agustí, A. Heterogeneidad y complejidad del síndrome bronquiectasias: Un reto pendiente. Arch. Bronconeumol. 2019, 55, 187–188. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; de la Rosa, D.; Cantón, R.; Olveira, C.; Máiz-Carro, L.; Girón, R.; Prados, C.; Blanco, M. Bronquiectasias: Cuando la evidencia científica publicada no resulta suficiente. Arch. Bronconeumol. 2019, 55, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.A.; Olveira, C.; Máiz, L.; Girón, R.M.; Prados, C.; de la Rosa, D.; Blanco, M.; Agustí, A. Bronchiectasis: A Complex, Heterogeneous Disease. Arch. Bronconeumol. 2019, 55, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.Á.; Máiz, L.; Olveira, C.; Girón, R.M.; de la Rosa, D.; Blanco, M.; Cantón, R.; Vendrell, M.; Polverino, E.; de Gracia, J.; et al. Spanish Guidelines on the Evaluation and Diagnosis of Bronchiectasis in Adults. Arch. Bronconeumol. 2018, 54, 79–87. [Google Scholar] [CrossRef]
- Morrissey, B.M.; Harper, R.W. Bronchiectasis: Sex and gender considerations. Clin. Chest Med. 2004, 25, 361–372. [Google Scholar] [CrossRef]
- Vidaillac, C.; Yong, V.F.L.; Jaggi, T.K.; Soh, M.M.-M.; Chotirmall, S.H. Gender differences in bronchiectasis: A real issue? Breathe 2018, 14, 108–121. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, G.; Lopez-De-Andrés, A.; Hernández-Barrera, V.; Jiménez-García, R.; Pedraza-Serrano, F.; Puente-Maestu, L.; De Miguel-Díez, J. Bronchiectasis in patients hospitalized with acute exacerbation of COPD in Spain: Influence on mortality, hospital stay, and hospital costs (2006–2014) according to gender. PLoS ONE 2019, 14, e0211222. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, K.E.; Harbaugh, M.; Han, M.L.K.; Le Saux, C.J.; Van Winkle, L.S.; Martin, W.J.; Kosgei, R.J.; Carter, E.J.; Sitkin, N.; Smiley-Jewell, S.M.; et al. Women and lung disease: Sex differences and global health disparities. Am. J. Respir. Crit. Care Med. 2015, 192, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Nick, J.A.; Chacon, C.S.; Brayshaw, S.J.; Jones, M.C.; Barboa, C.M.; St. Clair, C.G.; Young, R.L.; Nichols, D.P.; Janssen, J.S.; Huitt, G.A.; et al. Effects of gender and age at diagnosis on disease progression in long-term survivors of cystic fibrosis. Am. J. Respir. Crit. Care Med. 2010, 182, 614–626. [Google Scholar] [CrossRef]
- Demko, C.A.; Byard, P.J.; Davis, P.B. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J. Clin. Epidemiol. 1995, 48, 1041–1049. [Google Scholar] [CrossRef]
- Menéndez, R.; Méndez, R.; Polverino, E.; Rosales-Mayor, E.; Amara-Elori, I.; Reyes, S.; Posadas, T.; Fernández-Barat, L.; Torres, A. Factors associated with hospitalization in bronchiectasis exacerbations: A one-year follow-up study. Respir. Res. 2017, 18. [Google Scholar] [CrossRef]
- He, M.; Zhu, M.; Wang, C.; Wu, Z.; Xiong, X.; Wu, H.; Cheng, D.; Ji, Y. Prognostic performance of the FACED score and bronchiectasis severity index in bronchiectasis: A systematic review and meta-analysis. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef] [PubMed]
- Martinez-García, M.A.; Villa, C.; Dobarganes, Y.; Girón, R.; Maíz, L.; García-Clemente, M.; Sibila, O.; Golpe, R.; Rodríguez, J.; Barreiro, E.; et al. RIBRON: The spanish Online Bronchiectasis Registry. Characterization of the First 1912 Patients. Arch. Bronconeumol. 2021, 57, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Chang, A.B.; Chotirmall, S.H.; Dhar, R.; McShane, P.J. Bronchiectasis. Nat. Rev. Dis. Prim. 2018, 4, 45. [Google Scholar] [CrossRef]
- Posadas, T.; Oscullo, G.; Zaldivar, E.; Villa, C.; Dobarganes, Y.; Girón, R.; Olveira, C.; Maíz, L.; García-Clemente, M.; Sibila, O.; et al. C-Reactive Protein Concentration in Steady-State Bronchiectasis: Prognostic Value of Future Severe Exacerbations. Data From the Spanish Registry of Bronchiectasis (RIBRON). Arch. Bronconeumol. 2021, 57, 21–27. [Google Scholar] [CrossRef]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Cantón, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef]
- Aliberti, S.; Masefield, S.; Polverino, E.; De Soyza, A.; Loebinger, M.R.; Menendez, R.; Ringshausen, F.C.; Vendrell, M.; Powell, P.; Chalmers, J.D. Research priorities in bronchiectasis: A consensus statement from the EMBARC Clinical Research Collaboration. Eur. Respir. J. 2016, 48, 632–647. [Google Scholar] [CrossRef]
- Shrestha, B.; Dunn, L. The Declaration of Helsinki on Medical Research involving Human Subjects: A Review of Seventh Revision. J. Nepal Health Res. Counc. 2020, 17, 548–552. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; de Gracia, J.; Vendrell Relat, M.; Giron, R.-M.; Maiz Carro, L.; de la Rosa Carrillo, D.; Olveira, C. Multidimensional approach to non-cystic fibrosis bronchiectasis: The FACED score. Eur. Respir. J. 2014, 43, 1357–1367. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Athanazio, R.A.; Girón, R.M.; Máiz-Carro, L.; de la Rosa, D.; Olveira, C.; de Gracia, J.; Vendrell, M.; Prados-Sánchez, C.; Gramblicka, G.; et al. Predicting high risk of exacerbations in bronchiectasis: The E-FACED score. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Goeminne, P.; Aliberti, S.; McDonnell, M.J.; Lonni, S.; Davidson, J.; Poppelwell, L.; Salih, W.; Pesci, A.; Dupont, L.J.; et al. The bronchiectasis severity index an international derivation and validation study. Am. J. Respir. Crit. Care Med. 2014, 189, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Lomauro, A.; Aliverti, A. Sex differences in respiratory function. Breathe 2018, 14, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Li, T.; Li, J.C.; Li, Y. Association of body mass index with disease severity and prognosis in patients with non-cystic fibrosis bronchiectasis. Braz. J. Med. Biol. Res. 2015, 48, 715–724. [Google Scholar] [CrossRef]
- Toft-Petersen, A.P.; Torp-Pedersen, C.; Weinreich, U.M.; Rasmussen, B.S. Association between hemoglobin and prognosis in patients admitted to hospital for COPD. Int. J. COPD 2016, 11, 2813–2820. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Xie, Z.; He, Q.; Chen, S.; Wang, W.; Liu, G.; Liao, Y.; Lu, C.; Hao, L.; et al. High hemoglobin is associated with increased in-hospital death in patients with chronic obstructive pulmonary disease and chronic kidney disease: A retrospective multicenter population-based study. BMC Pulm. Med. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Durmus Kocak, N.; Sasak, G.; Akturk, U.A.; Akgun, M.; Boga, S.; Sengul, A.; Gungor, S.; Arinc, S. Serum uric acid levels and uric acid/creatinine ratios in stable chronic obstructive pulmonary disease (COPD) patients: Are these parameters efficient predictors of patients at risk for exacerbation and/or severity of disease? Med. Sci. Monit. 2016, 22, 4169–4176. [Google Scholar] [CrossRef]
- Koo, H.-K.; Kang, H.K.; Song, P.; Park, H.K.; Lee, S.-S.; Jung, H. Systemic White Blood Cell Count as a Biomarker Associated with Severity of Chronic Obstructive Lung Disease. Tuberc. Respir. Dis. 2017, 80, 304. [Google Scholar] [CrossRef]
- Thorsdottir, I.; Gunnarsdottir, I.; Eriksen, B. Screening Method Evaluated by Nutritional Status Measurements can be Used to Detect Malnourishment in Chronic Obstructive Pulmonary Disease. J. Am. Diet. Assoc. 2001, 101, 648–654. [Google Scholar] [CrossRef]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef] [PubMed]
- Alende-Castro, V.; Alonso-Sampedro, M.; Vazquez-Temprano, N.; Tuñez, C.; Rey, D.; García-Iglesias, C.; Sopeña, B.; Gude, F.; Gonzalez-Quintela, A. Factors influencing erythrocyte sedimentation rate in adults. Medicine 2019, 98, e16816. [Google Scholar] [CrossRef] [PubMed]
| Women | Men | |
|---|---|---|
| N = 1368 | N = 753 | |
| Anthropometric variables, (SD) | ||
| Age (years) | 68.5 (14.4) | 69.9 (15.3) * |
| Disease severity, (SD) | ||
| FACED score | 1.9 (1.6) | 2.4 (1.8) *** |
| EFACED score | 2.4 (2) | 3.1 (2.3) *** |
| BSI score | 7.2 (4.3) | 8.1 (4.8) *** |
| # exacerbations, previous year | 1.6 (1.9) | 1.5 (1.8) |
| Hospitalizations for exacerbations in the previous year | 0.5 (1.2) | 0.7 (1.5) *** |
| Charlson Index | 1.6 (1.2) | 2.2 (1.8) *** |
| Radiological extension | 2.8 (1.4) | 2.8 (1.5) |
| Dyspnea, mMRC | 1.9 (0.9) | 2.1 (1.0) *** |
| Chronic colonization by PA, N (%) | 311 (22.7) | 189 (25.1) |
| Smoking history | ||
| Current smokers, N (%) | 109 (8) | 69 (9) |
| Ex-smokers, N (%) | 296 (22) | 409 (54) *** |
| Never smokers, N (%) | 963 (70) | 275 (37) *** |
| Packs-year, (SD) | 24 (21) | 39 (31) *** |
| Lung function testing, (SD) | ||
| FEV1, % predicted | 77 (24) | 68 (24) *** |
| FVC, % predicted | 86 (23) | 79 (19) *** |
| FEV1/FVC, % | 71 (12) | 65 (15) *** |
| DLCO, % predicted | 82 (23) | 86 (25) |
| KCO, % predicted | 78 (35) | 87 (37) ** |
| RV, % predicted | 140 (42) | 135 (56) |
| TLC, % predicted | 104 (19) | 100 (21) * |
| RV/TLC, % | 53 (13) | 47 (12) *** |
| Nutritional status | ||
| (SD) | 25 (5) | 27 (4) *** |
| BMI grade kg/m2 | ||
| <20, N (%) | 173 (13) | 45 (6) *** |
| 20–25, N (%) | 580 (42) | 231 (31) *** |
| ≥25, N (%) | 614 (45) | 476 (63) *** |
| (SD) | 13.21 (1.29) | 14.31 (1.75) *** |
| (SD) | 40.38 (3.8) | 43.4 (4.94) *** |
| (SD) | 0.75 (0.29) | 1.01 (0.55) *** |
| (SD) | 7.04 (0.61) | 6.99 (0.65) |
| (SD) | 4.19 (0.42) | 4.18 (0.49) |
| Systemic inflammatory markers | ||
| (SD) | ||
| Total number of leukocytes, cells/uL | 7.40 (3.67) × 103 | 8.43 (4.01) × 103 *** |
| Total number of neutrophils, cells/uL | 4.46 (2.41) × 103 | 5.26 (2.75) × 103 *** |
| Neutrophils, % | 58.96 (12.42) | 62.47 (12.36) *** |
| Total number of lymphocytes, cells/uL | 2.12 (2.43) × 103 | 2.08 (2.88) × 103 |
| Lymphocytes, % | 29.29 (11.18) | 25.59 (10.72) *** |
| Total number of eosinophils, cells/uL | 0.20 (0.20) × 103 | 0.21 (0.22) × 103 |
| Eosinophils, % | 2.85 (2.73) | 2.82 (2.66) |
| Platelets, cells/uL | 260 (77) × 103 | 241 (75) × 103 *** |
| (SD) | ||
| Alpha-1 antitrypsin, mg/dL | 135.85 (42.41) | 130.87 (39.79) |
| CRP, mg/dL | 5.30 (12.47) | 6.01 (13.79) * |
| Fibrinogen, mg/dL | 434.12 (136.95) | 433.64 (147.24) |
| ESR, mm/h | 21.00 (18.61) | 15.29 (14.79) *** |
| Women | Men | |
|---|---|---|
| N = 1310 | N = 575 | |
| Anthropometric variables, (SD) | ||
| Age (years) | 68.3 (14.5) | 67.4 (16) |
| Disease severity, (SD) | ||
| FACED score | 1.9 (1.6) | 2.1 (1.7) ** |
| EFACED score | 2.4 (2) | 2.7 (2.1) ** |
| BSI score | 7.1 (4.3) | 7.2 (4.4) |
| # exacerbations, previous year | 1.6 (1.9) | 1.5 (1.8) |
| Hospitalizations for exacerbations in the previous year | 0.5 (1.2) | 0.5 (1.1) |
| Charlson Index | 1.6 (1.2) | 2 (1.7) *** |
| Radiological extension | 2.8 (1.4) | 2.8 (1.5) |
| Dyspnea, mMRC | 1.8 (0.9) | 1.9 (0.9) |
| Chronic colonization by PA, N (%) | 302 (23.1) | 139 (24.2) |
| Smoking history | ||
| Current smokers, N (%) | 87 (7) | 41 (7) |
| Ex-smokers, N (%) | 267 (20) | 264 (46) *** |
| Never smokers, N (%) | 956 (73) | 270 (47) *** |
| Packs-year, (SD) | 21 (19) | 29 (24) *** |
| Lung function testing, (SD) | ||
| FEV1, % predicted | 78 (24) | 72 (24) *** |
| FVC, % predicted | 87 (23) | 81 (19) *** |
| FEV1/FVC, % | 72 (12) | 69 (14) *** |
| DLCO, % predicted | 84 (22) | 89 (25) * |
| KCO, % predicted | 78 (35) | 89 (38) ** |
| RV, % predicted | 138 (40) | 128 (54) |
| TLC, % predicted | 103 (19) | 97 (19) ** |
| RV/TLC, % | 53 (13) | 45 (13) *** |
| Nutritional status | ||
| BMI, kg/m2, (SD) | 25 (5) | 27 (4) *** |
| BMI grade kg/m2 | ||
| <20, N (%) | 165 (13) | 35 (6) *** |
| 20–25, N (%) | 556 (42) | 178 (31) *** |
| ≥25, N (%) | 588 (45) | 362 (63) *** |
| Hemoglobin, g/dL, (SD) | 13.2 (1.28) | 14.34 (1.69) *** |
| Hematocrit, %, (SD) | 40.34 (3.75) | 43.45 (4.71) *** |
| Creatinine, mg/dL, (SD) | 0.75 (0.3) | 1.03 (0.62) *** |
| Total proteins, g/dL, (SD) | 7.05 (0.6) | 7.06 (0.63) |
| Albumin, g/dL, (SD) | 4.21 (0.41) | 4.25 (0.43) |
| Systemic inflammatory markers | ||
| Systemic inflammatory cells, (SD) | ||
| Total number of leukocytes, cells/uL | 7.38 (3.72) × 103 | 8.05 (3) × 103 ** |
| Total number of neutrophils, cells/uL | 4.43 (2.39) × 103 | 5.03 (2.56) × 103 ** |
| Neutrophils, % | 58.8 (12.33) | 61.77 (11.76) *** |
| Total number of lymphocytes, cells/uL | 2.13 (2.48) × 103 | 1.93 (0.79) × 103 |
| Lymphocytes, % | 29.42 (11.15) | 26.4 (10.05) *** |
| Total number of eosinophils, cells/uL | 0.19 (0.2) × 103 | 0.21 (0.22) × 103 |
| Eosinophils, % | 2.84 (2.61) | 2.86 (2.64) |
| Platelets, cells/uL | 260 (77) × 103 | 242 (76) × 103 ** |
| Acute-phase reactants, (SD) | ||
| Alpha-1 antitrypsin, mg/dL | 135.96 (43.24) | 127.6 (40.1) * |
| CRP, mg/dL | 5.38 (12.63) | 5.38 (12.3) |
| Fibrinogen, mg/dL | 432.01 (136.49) | 420.73 (144.98) |
| ESR, mm/h | 21.15 (18.65) | 14.69 (13.56) *** |
| Women | Men | |
|---|---|---|
| N = 963 | N = 275 | |
| Anthropometric variables, (SD) | ||
| Age (years) | 69.7 (15.4) | 64.7 (18) *** |
| Lung function testing, (SD) | ||
| FEV1, % predicted | 77 (25) | 73 (23) * |
| FVC, % predicted | 86 (24) | 81 (18) *** |
| FEV1/FVC, % | 71 (12) | 69 (14) * |
| DLCO, % predicted | 85 (23) | 90 (23) |
| KCO, % predicted | 80 (35) | 94 (38) * |
| RV, % predicted | 135 (41) | 125 (54) |
| TLC, % predicted | 101 (19) | 97 (20) |
| RV/TLC, % | 53 (14) | 44 (14) *** |
| Etiology, N, % | Total | Women | Men |
|---|---|---|---|
| Post-infectious | 865, 40.8% | 603, 44.1% | 262, 34.8% *** |
| Tuberculosis | 262, 12.4% | 167, 12.2% | 95, 12.6% |
| Childhood infections | 160, 7.5% | 127, 9.3% | 33, 4.4 *** |
| Necrotizing pneumonia | 53, 2.5% | 38, 2.8% | 15, 2.0% |
| Non-tuberculous mycobacteria | 17, 0.8% | 13, 1.0% | 4, 0.5% |
| Fungal infections | 8, 0.4% | 4, 0.3% | 4, 0.5% |
| Others | 1621, 76.4% | 1019, 74.5% | 602, 79.9% ** |
| Unknown etiology | 382, 18% | 285, 20.8% | 97, 12.9% *** |
| COPD | 236, 11.1% | 58, 4.2% | 178, 23.6% *** |
| Asthma | 179, 8.4% | 128, 9.4% | 51, 6.8% * |
| Systemic disorders | 164, 7.7% | 102, 7.5% | 62, 8.2% |
| Immunodeficiencies | 85, 4.0% | 53, 3.9% | 32, 4.2% |
| Inflammatory pneumonitis | 37, 1.7% | 29, 2.1% | 8, 1.1% |
| Inflammatory bowel diseases | 17, 0.8% | 11, 0.8% | 6, 0.8% |
| Congenital malformations | 15, 0.7% | 6, 0.4% | 9, 1.2% |
| Obliterative bronchiolitis | 11, 0.5% | 8, 0.6% | 3, 0.4% |
| Hyperimmune response | 6, 0.3% | 4, 0.3% | 2, 0.3% |
| Vasculitis | 4, 0.2% | 1, 0.1% | 3, 0.4% |
| Other etiologies | 120, 5.7% | 80, 5.8% | 40, 5.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Villa, C.; Dobarganes, Y.; Olveira, C.; Girón, R.; García-Clemente, M.; Maíz, L.; Sibila, O.; Golpe, R.; Menéndez, R.; et al. Differences in Nutritional Status and Inflammatory Biomarkers between Female and Male Patients with Bronchiectasis: A Large-Cohort Study. Biomedicines 2021, 9, 905. https://doi.org/10.3390/biomedicines9080905
Wang X, Villa C, Dobarganes Y, Olveira C, Girón R, García-Clemente M, Maíz L, Sibila O, Golpe R, Menéndez R, et al. Differences in Nutritional Status and Inflammatory Biomarkers between Female and Male Patients with Bronchiectasis: A Large-Cohort Study. Biomedicines. 2021; 9(8):905. https://doi.org/10.3390/biomedicines9080905
Chicago/Turabian StyleWang, Xuejie, Carmen Villa, Yadira Dobarganes, Casilda Olveira, Rosa Girón, Marta García-Clemente, Luis Maíz, Oriol Sibila, Rafael Golpe, Rosario Menéndez, and et al. 2021. "Differences in Nutritional Status and Inflammatory Biomarkers between Female and Male Patients with Bronchiectasis: A Large-Cohort Study" Biomedicines 9, no. 8: 905. https://doi.org/10.3390/biomedicines9080905
APA StyleWang, X., Villa, C., Dobarganes, Y., Olveira, C., Girón, R., García-Clemente, M., Maíz, L., Sibila, O., Golpe, R., Menéndez, R., Rodríguez-López, J., Prados, C., Martinez-García, M. A., Rodriguez, J. L., de la Rosa, D., Duran, X., & Barreiro, E. (2021). Differences in Nutritional Status and Inflammatory Biomarkers between Female and Male Patients with Bronchiectasis: A Large-Cohort Study. Biomedicines, 9(8), 905. https://doi.org/10.3390/biomedicines9080905








