Hydrolysable Tannins Exhibit Acetylcholinesterase Inhibitory and Anti-Glycation Activities In Vitro and Learning and Memory Function Improvements in Scopolamine-Induced Amnesiac Mice
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemical and Reagents
2.2. Crude Extract Preparation
2.3. Isolation of Purified cCmpounds
2.4. AChE Inhibitory Activities In Vitro
2.5. Effects of Extracts and Purified Compounds of TT-Hull on Oxygen Radical Absorbance Capacity
2.6. Effects of TT-Hull Extracts on Anti-Glycation in Non-Enzymatic BSA/Galactose Models
2.7. Effects of Purified Compounds of TT-Hull on Inhibition of Aβ (1–42) Aggregation In Vitro
2.8. The Oral Administration of TT-Hull Extracts or Purified Compounds in Scopolamine-Induced Amnesiac ICR Mice
2.9. Analyses of Learning and Memory Functions in Scopolamine-Induced Amnesiac ICR Mice
2.10. AChE Activities and Reduced GSH Levels in Brain Tissue Extracts
2.11. Statistical Analyses
3. Results
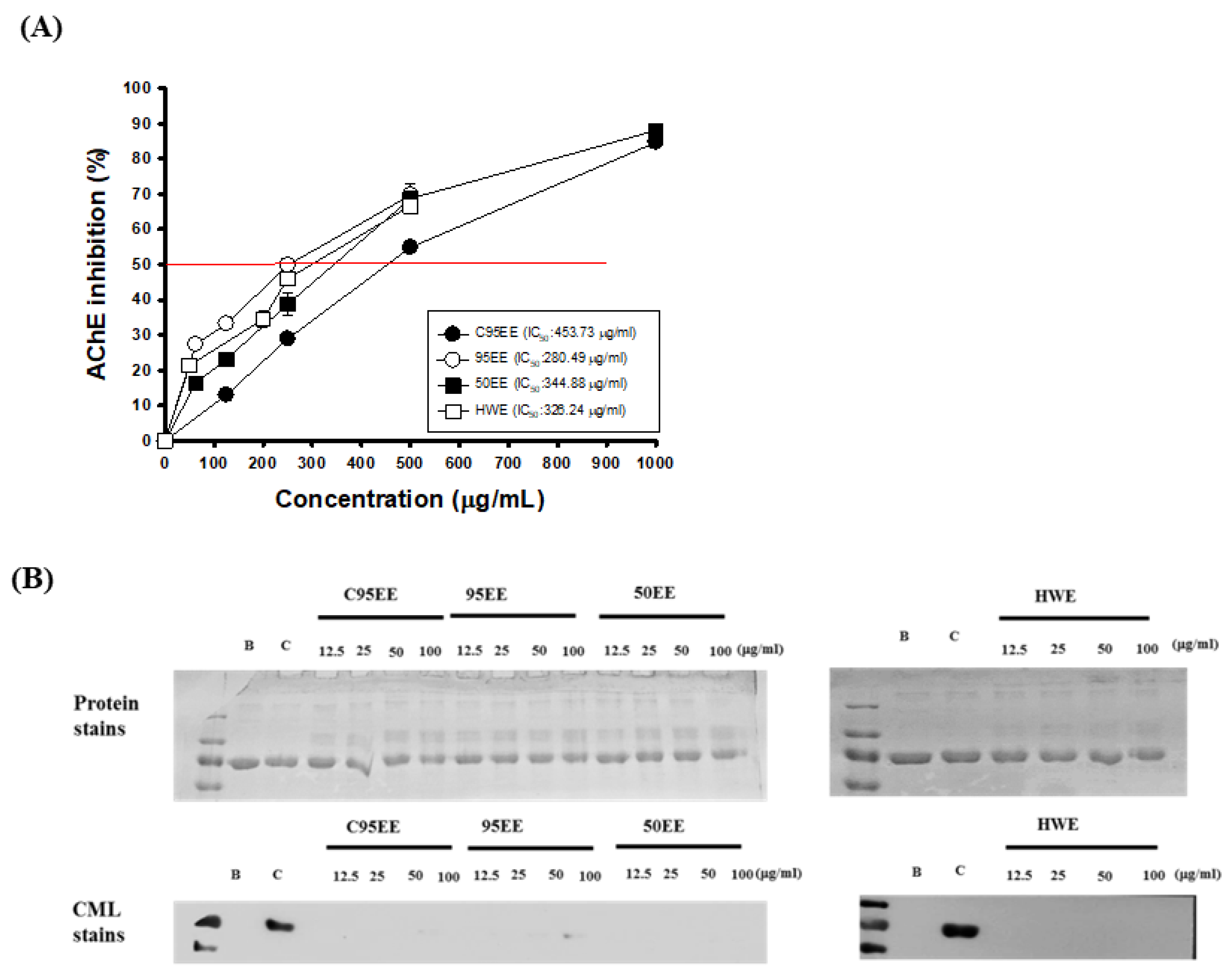
3.1. Effects of TT-Hull Extracts on AChE Inhibitory Activities, ORAC, and Anti-Non-Enzymatic Glycation Activities
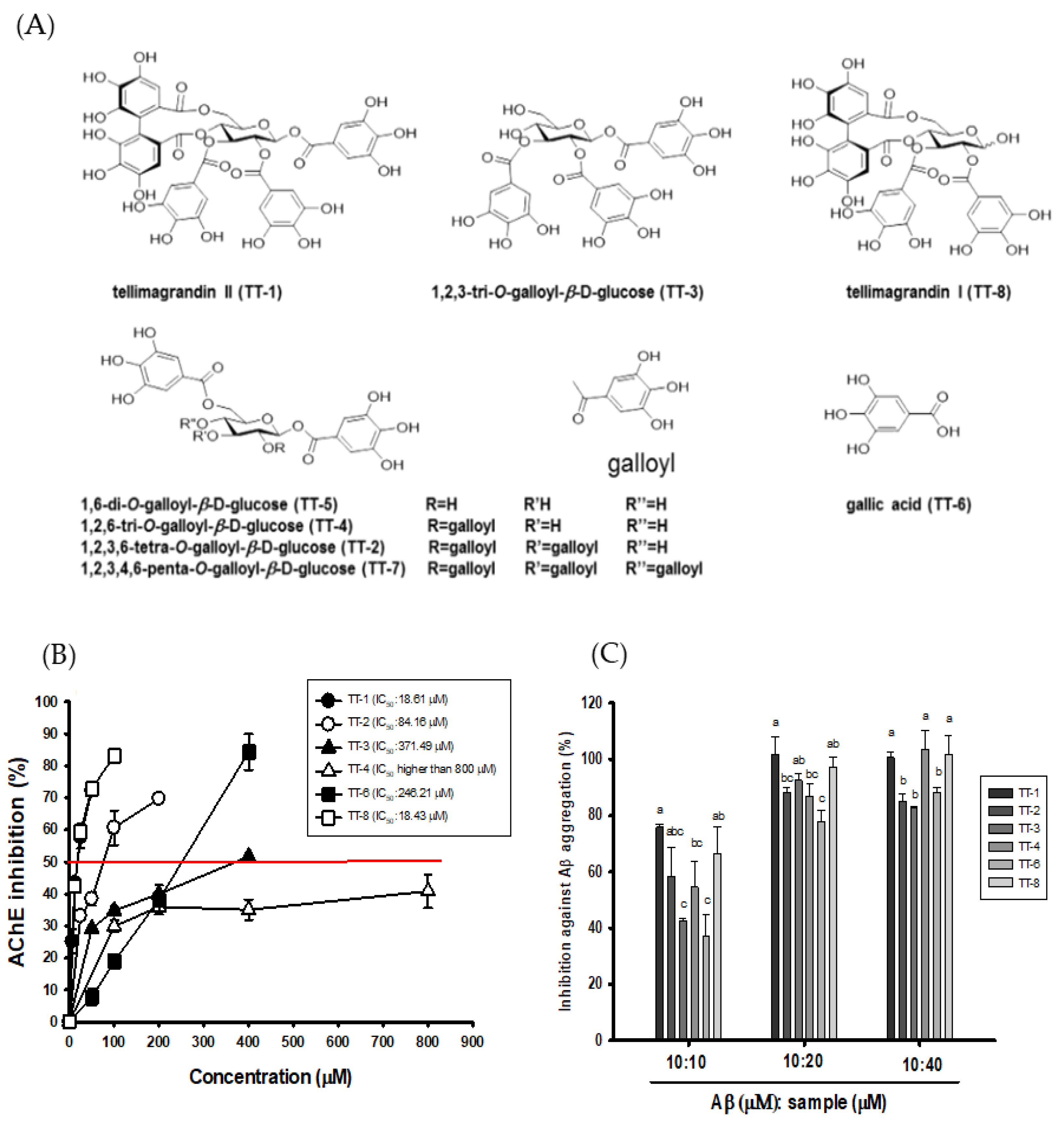
3.2. Effects of the Isolated Compounds from 50EE on AChE Inhibitory Activities, ORAC, and Anti-Aβ (1-42) Aggregations
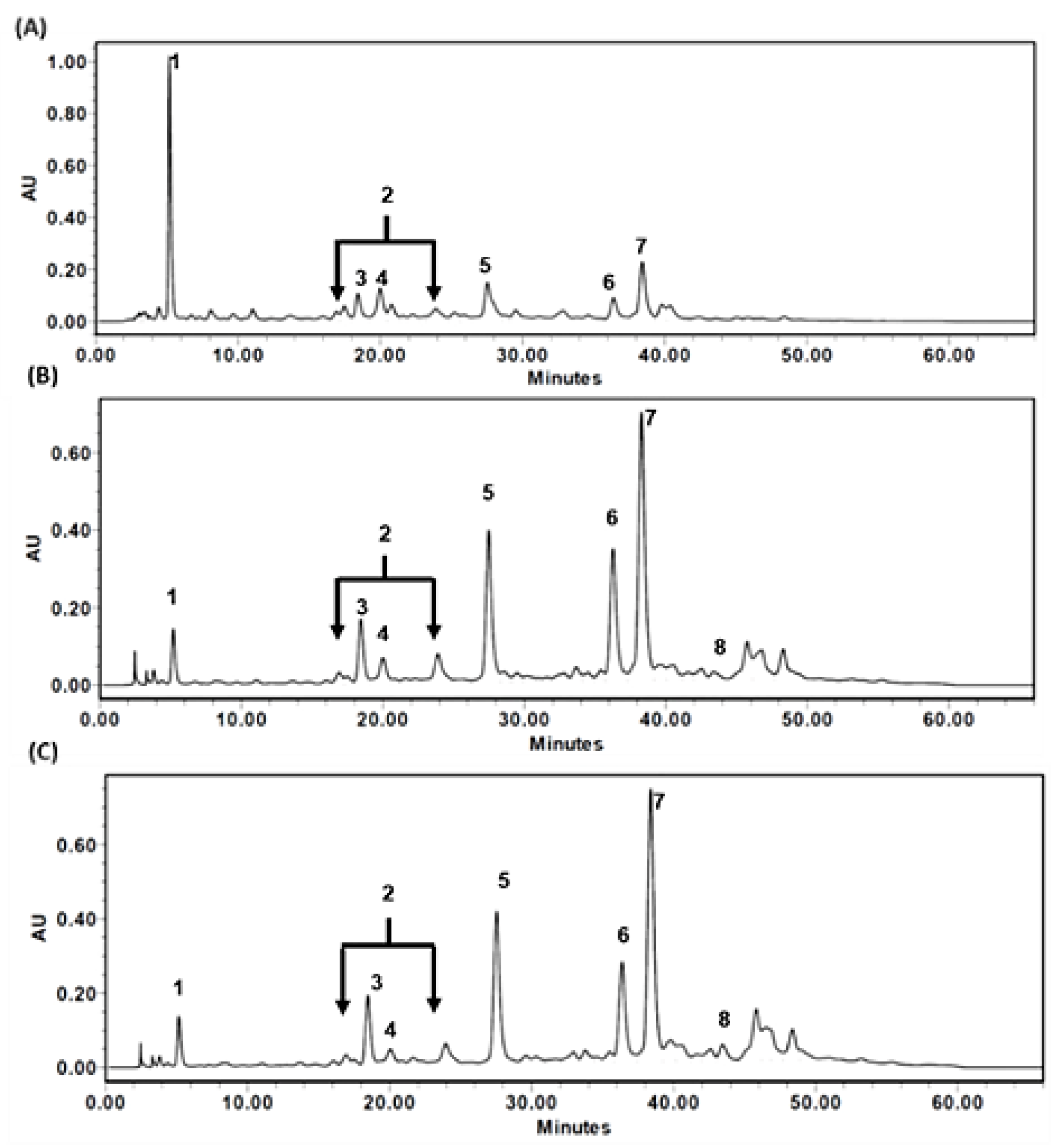
3.3. HPLC Chromatograms of TT-Hull Extracts
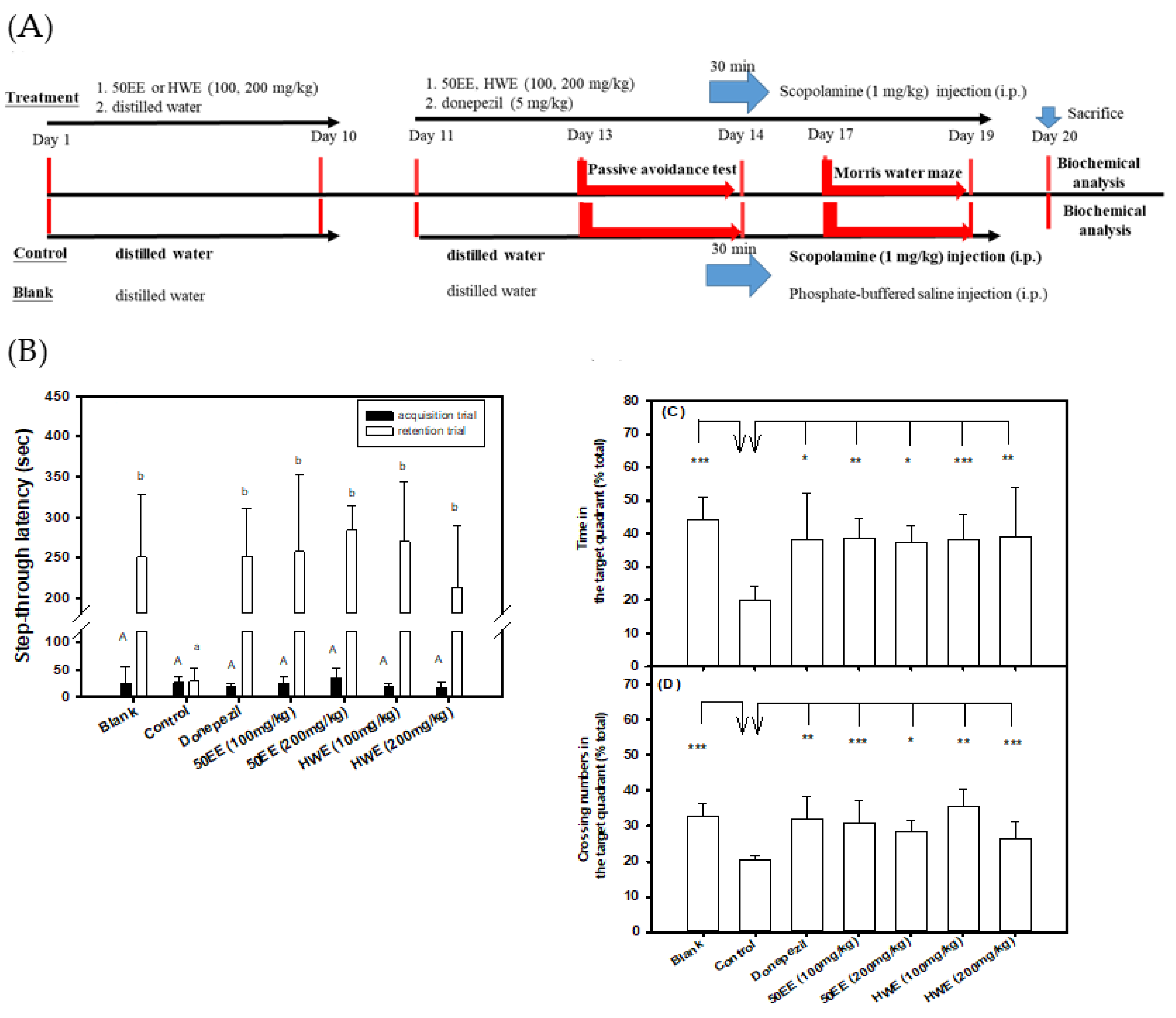
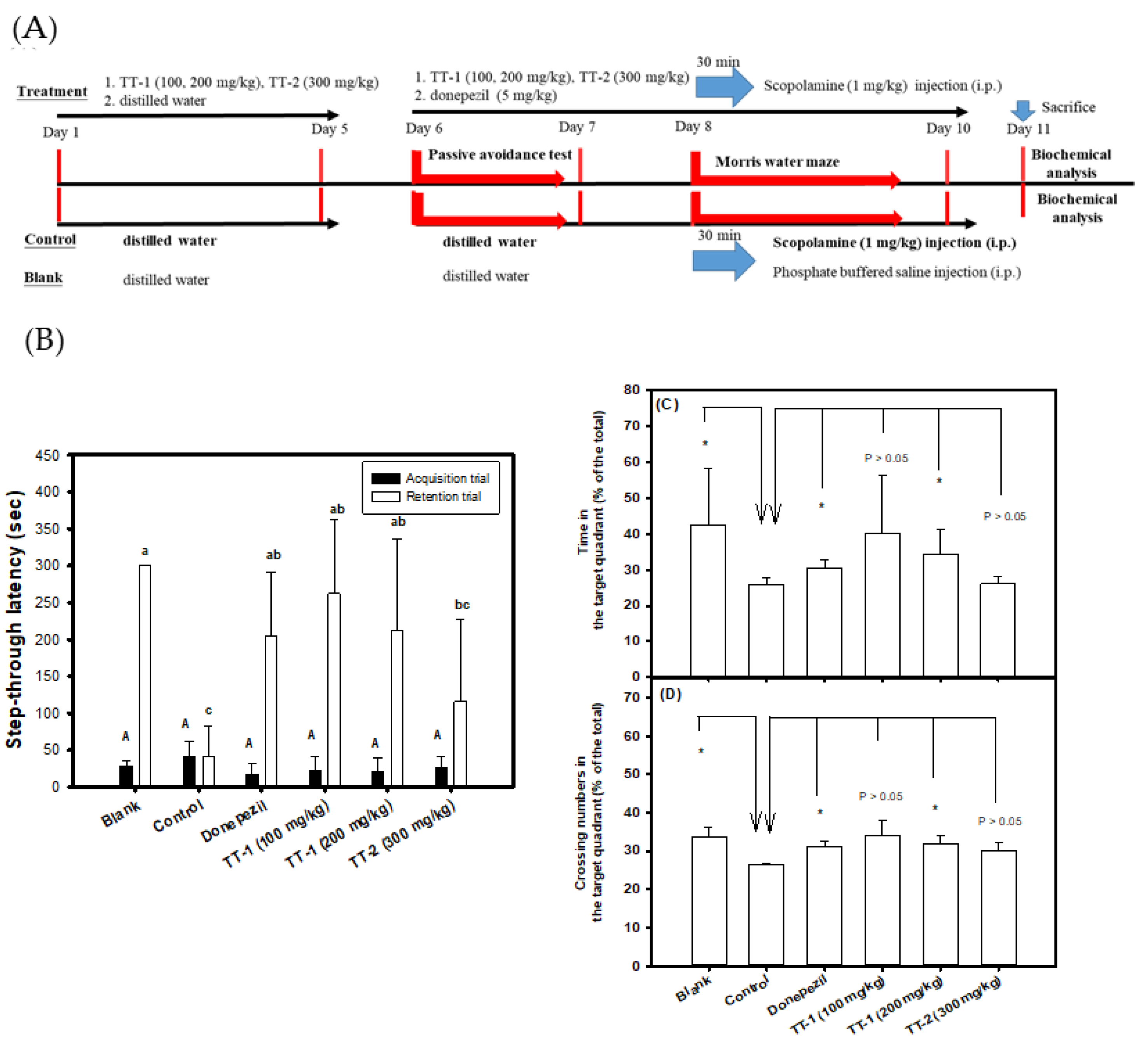
3.4. Effects of the Oral Administration of TT-Hull Extracts on Learning and Memory Functions in Scopolamine-Induced Amnesiac ICR Mice
3.5. Effects of the Oral Administration of Purified Compounds of TT-1 and TT-2 on Learning and Memory Functions in Scopolamine-Induced Amnesiac ICR Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Dementia Observatory (GDO). 2021. Available online: https://www.who.int/data/gho/data/themes/theme-details/GHO/global-dementia-observatory-(gdo) (accessed on 19 August 2021).
- WHO. Dementia. 2019. Available online: https://www.who.int/en/news-room/fact-sheets/detail/dementia (accessed on 27 June 2021).
- Plassman, B.L.; Lang, K.M.; Fisher, G.G.; Heeringa, S.G.; Weir, D.R.; Ofstedal, M.B.; Burke, J.R.; Hurd, M.D.; Potter, G.G.; Rodgers, W.L.; et al. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology 2007, 29, 125–132. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Eng. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.K.; Chao, S.P.; Hu, C.J. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 2020, 27, 18. [Google Scholar] [CrossRef]
- McGleenon, B.M.; Dynan, K.B.; Passmore, A.P. Acetylcholinesterase inhibitors in Alzheimer’s disease. Br. J. Clin. Pharmacol. 1999, 8, 471–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, L.A.; Hong, N.S.; McDonald, R.J. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2011, 35, 1397–1409. [Google Scholar] [CrossRef] [Green Version]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef]
- Čolović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [Green Version]
- Herholz, K. Acetylcholine esterase activity in mild cognitive impairment and Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2008, 35 (Suppl. S1), S25–S29. [Google Scholar] [CrossRef]
- Davies, P.; Maloney, A.J.F. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976, 308, 1403. [Google Scholar] [CrossRef]
- Squire, L.R. The neuropsychology of human memory. Annu. Rev. Neurosci. 1982, 5, 241–273. [Google Scholar] [CrossRef]
- Chen, X.Q.; Mobley, W.C. Exploring the pathogenesis of Alzheimer disease in basal forebrain cholinergic neurons: Onverging insights from alternative hypotheses. Front. Neurosci. 2019, 3, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, L.; Mazzitelli, S.; Arciello, M.; Capo, C.R.; Rotilio, G. Benefits from dietary polyphenols for brain aging and Alzheimer’s disease. Neurochem. Res. 2008, 33, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011, 141, 989S–1009S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plents and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [Green Version]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A mini review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Gey, K.F. The antioxidant hypothesis of cardiovascular disease: Epidemiology and mechanisms. Biochem. Soc. Trans. 1990, 18, 1041–1045. [Google Scholar] [CrossRef] [Green Version]
- González-Burgos, E.; Liaudanskas, M.; Viškelis, J.; Zvikas, V.; Janulis, V.; Gómez-Serranillos, M.P. Antioxidant activity, neuroprotective properties and bioactive constituents analysis of varying polarity extracts from Eucalyptus globulus leaves. J. Food Drug Anal. 2018, 26, 1293–1302. [Google Scholar] [CrossRef]
- Kikuchia, S.; Shinpoa, K.; Takeuchi, M.; Yamagishi, S.; Makita, Z.; Sasaki, N.; Tashiroa, K. Glycation-a sweet tempter for neuronal death. Brain Res. Rev. 2003, 41, 306–323. [Google Scholar] [CrossRef]
- Delgado-Andrade, C. Carboxymethyl-lysine: Thirty years of investigation in the field of AGE formation. Food Funct. 2016, 7, 46–57. [Google Scholar] [CrossRef]
- Müch, G.; Westcott, B.; Menini, T.; Gugliucci, A. Advanced glycation endproducts and their pathogenic roles in neurological disorders. Amino Acids 2012, 42, 1221–1236. [Google Scholar]
- Gella, A.; Durany, N. Oxidative stress in Alzheimer disease. Cell Adh. Migr. 2009, 3, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.H.; Pan, Z.B.; Chen, C.R.; Wei, M.X.; Chen, C.A.; Lin, H.P.; Hsu, C.H. Synthesis of multiporous carbons from the water caltrop shell for high-performance supercapacitors. ACS Omega 2020, 5, 10626–10632. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Lee, K.T.; Chih, Y.K.; Eng, C.F.; Lin, H.P.; Chiou, Y.B.; Cheng, C.L.; Lin, Y.X.; Chang, J.S. Novel renewable double-energy system for activated biochar production and thermoelectric generation from waste heat. Energy Fuels 2020, 34, 3383–3393. [Google Scholar] [CrossRef]
- Wang, C.C.R.; Ciou, J.Y.; Chiang, P.Y. Effect of micronization on functional properties of the water caltrop (Trapa taiwanensis Nakai) pericarp. Food Chem. 2009, 113, 970–974. [Google Scholar] [CrossRef]
- Yasuda, M.; Yasutake, K.; Hino, M.; Ohwatari, H.; Ohmagari, N.; Takedomi, K.; Tanaka, T.; Nonaka, G.-I. Inhibitory effects of polyphenols from water chestnut (Trapa japonica) husk on glycolytic enzymes and postprandial blood glucose elevation in mice. Food Chem. 2014, 165, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.H.; Lee, C.J.; Chen, L.C.; Lee, T.L.; Hsieh, Y.Y.; Han, C.H.; Yang, C.H.; Huang, W.J.; Hou, W.C. Acetylcholinesterase inhibitory activity and neuroprotection in vitro, molecular docking, and improved learning and memory functions of demethylcurcumin in scopolamine-induced amnesia ICR mice. Food Funct. 2020, 11, 2328–2338. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lee, T.L.; Han, C.H.; Lee, Y.S.; Hou, W.C. Anti-glycation, anti-hemolysis, and ORAC activities of demethylcurcumin and tetrahydroxycurcumin in vitro and reductions of oxidative stress in D-galactose-induced BALB/c mice in vivo. Bot. Stud. 2019, 60, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.H.; Lu, Y.L.; Liu, D.Z.; Hou, W.C. Anti-glycation, radical scavenging, and semicarbazide-sensitive amine oxidase inhibitory activities of acetohydroxamic acid in vitro. Drug Des. Dev. Ther. 2017, 11, 2139–2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.C.; Chen, H.F.; Wu, J.Y.; Chen, L.G. Stability of principal hydrolysable tannins from Trapa taiwanensis hulls. Molecules 2019, 24, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuominen, A.; Sundman, T. Stability and oxidation products of hydrolysable tannins in basic conditions detected by HPLC/DAD-ESI/QTOF/MS. Phytochem. Anal. 2013, 24, 424–435. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Nanoparticle mediated brain targeted delivery of gallic acid: In vivo behavioral and biochemical studies for protection against scopolamine-induced amnesia. Drug Deliv. 2013, 20, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Skalicka-Wozniak, K.; Budzynska, B.; Biala, G.; Boguszewska-Czubara, A. Scopolamine-induced memory impairment is alleviated by xanthotoxin: Role of acetylcholinesterase and oxidative stress processes. ACS Chem. Neurosci. 2018, 9, 1184–1194. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Y.; Xu, T.; Li, Q.; Wang, D.; Zhang, L.; Fan, B.; Wang, F.; Liu, X. Genistein ameliorates scopolamine-induced amnesia in mice through the regulation of the cholinergic neurotransmission, antioxidant system and ERK/CREB/BDNF signaling. Front. Pharmacol. 2018, 9, 1153. [Google Scholar] [CrossRef]
- Bryan, K.J.; Lee, H.G.; Perry, G.; Smith, M.A.; Casadesus, G. Transgenic mouse models of Alzheimer’s disease: Behavioral testing and considerations. In Methods of Behavior Analysis in Neuroscience, 2nd ed.; Buccafusco, J.J., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009; Chapter 1. [Google Scholar]
- Thapa, A.; Carroll, N.J. Dietary modulation of oxidative stress in Alzheimer’s disease. Int. J. Mol. Sci. 2017, 18, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vries, H.E.; Witte, M.; Hondius, D.; Rozemuller, A.J.M.; Drukarch, B.; Hoozemans, J.; Horssen, J. Nrf2-induced antioxidant protection: A promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Rad. Biol. Med. 2008, 45, 1375–1383. [Google Scholar] [CrossRef]
- Han, C.H.; Lin, Y.S.; Lee, T.L.; Liang, H.J.; Hou, W.C. Asn-Trp dipeptides improve the oxidative stress and learning dysfunctions in D-galactose-induced BALB/c mice. Food Funct. 2014, 5, 2228–2236. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Value of water mazes for assessing spatial and egocentric learning and memory in rodent basic research and regulatory studies. Neurotoxicol. Teratol. 2014, 45, 75–90. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-G.; Lin, S.-Y.; Lee, Y.-S.; Wang, C.-C.; Hou, W.-C. Hydrolysable Tannins Exhibit Acetylcholinesterase Inhibitory and Anti-Glycation Activities In Vitro and Learning and Memory Function Improvements in Scopolamine-Induced Amnesiac Mice. Biomedicines 2021, 9, 1066. https://doi.org/10.3390/biomedicines9081066
Chen L-G, Lin S-Y, Lee Y-S, Wang C-C, Hou W-C. Hydrolysable Tannins Exhibit Acetylcholinesterase Inhibitory and Anti-Glycation Activities In Vitro and Learning and Memory Function Improvements in Scopolamine-Induced Amnesiac Mice. Biomedicines. 2021; 9(8):1066. https://doi.org/10.3390/biomedicines9081066
Chicago/Turabian StyleChen, Lih-Geeng, Shyr-Yi Lin, Yi-Shan Lee, Ching-Chiung Wang, and Wen-Chi Hou. 2021. "Hydrolysable Tannins Exhibit Acetylcholinesterase Inhibitory and Anti-Glycation Activities In Vitro and Learning and Memory Function Improvements in Scopolamine-Induced Amnesiac Mice" Biomedicines 9, no. 8: 1066. https://doi.org/10.3390/biomedicines9081066
APA StyleChen, L.-G., Lin, S.-Y., Lee, Y.-S., Wang, C.-C., & Hou, W.-C. (2021). Hydrolysable Tannins Exhibit Acetylcholinesterase Inhibitory and Anti-Glycation Activities In Vitro and Learning and Memory Function Improvements in Scopolamine-Induced Amnesiac Mice. Biomedicines, 9(8), 1066. https://doi.org/10.3390/biomedicines9081066





