miR-31-5p Promotes Oxidative Stress and Vascular Smooth Muscle Cell Migration in Spontaneously Hypertensive Rats via Inhibiting FNDC5 Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Determination of VSMC Migration
2.3. Transfection of miR-31-5p Mimic and Inhibitor
2.4. Luciferase Reporter Assay
2.5. Western Blot Analysis
2.6. RT-PCR
2.7. DHE Fluorescence Staining
2.8. Evaluation of Cell Viability
2.9. Statistical Analysis
3. Results
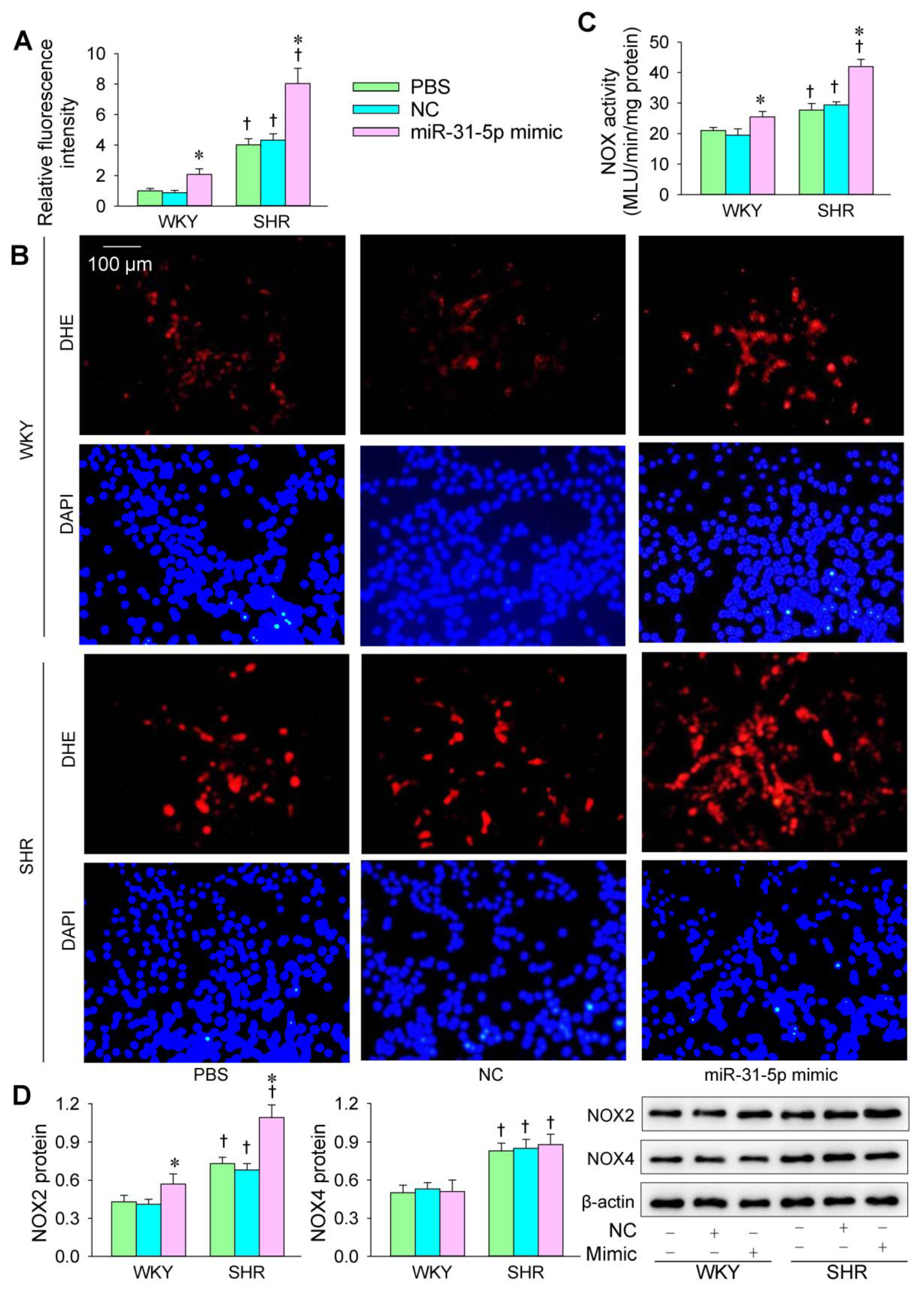
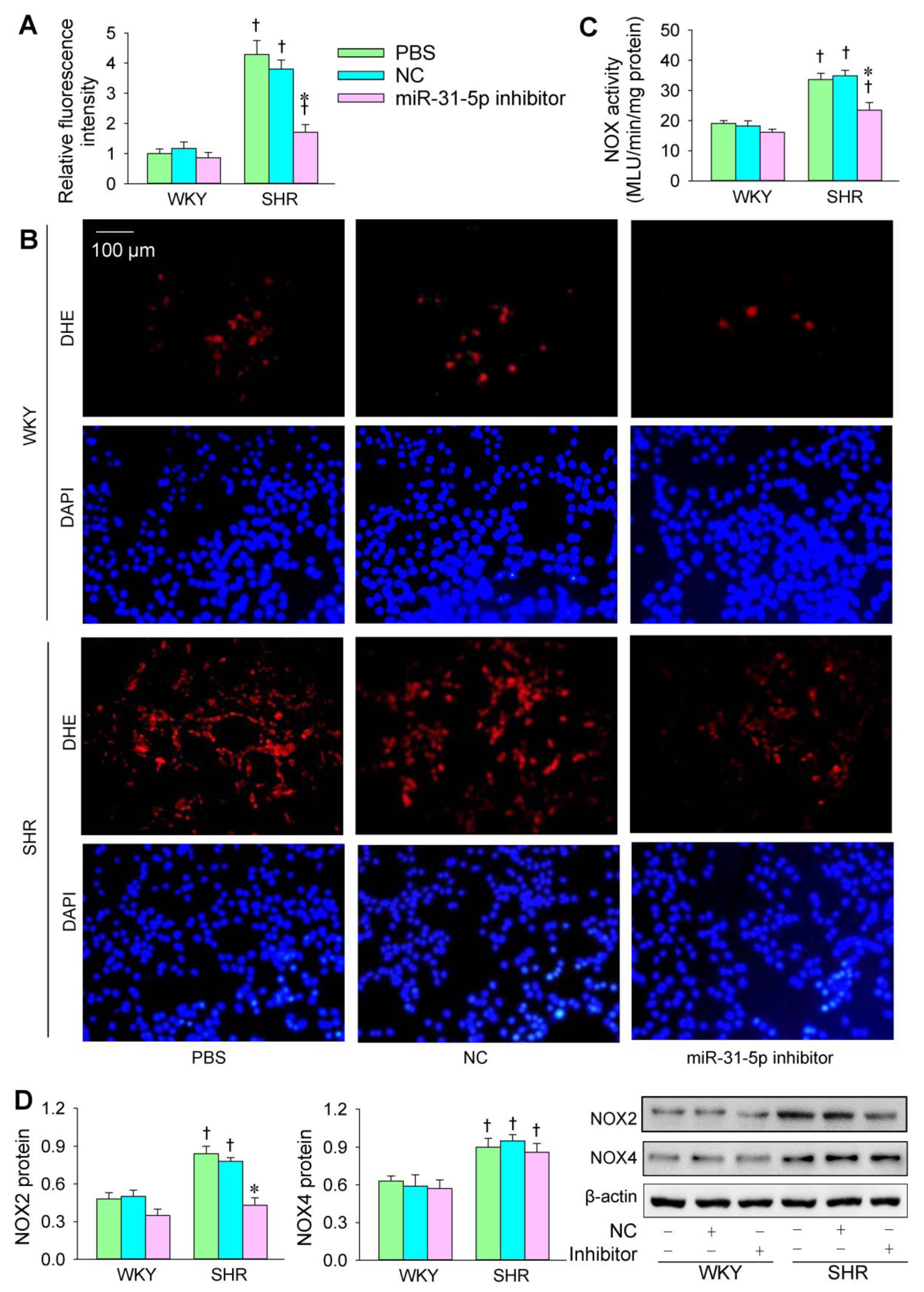
3.1. Roles of miR-31-5p in Oxidative Stress
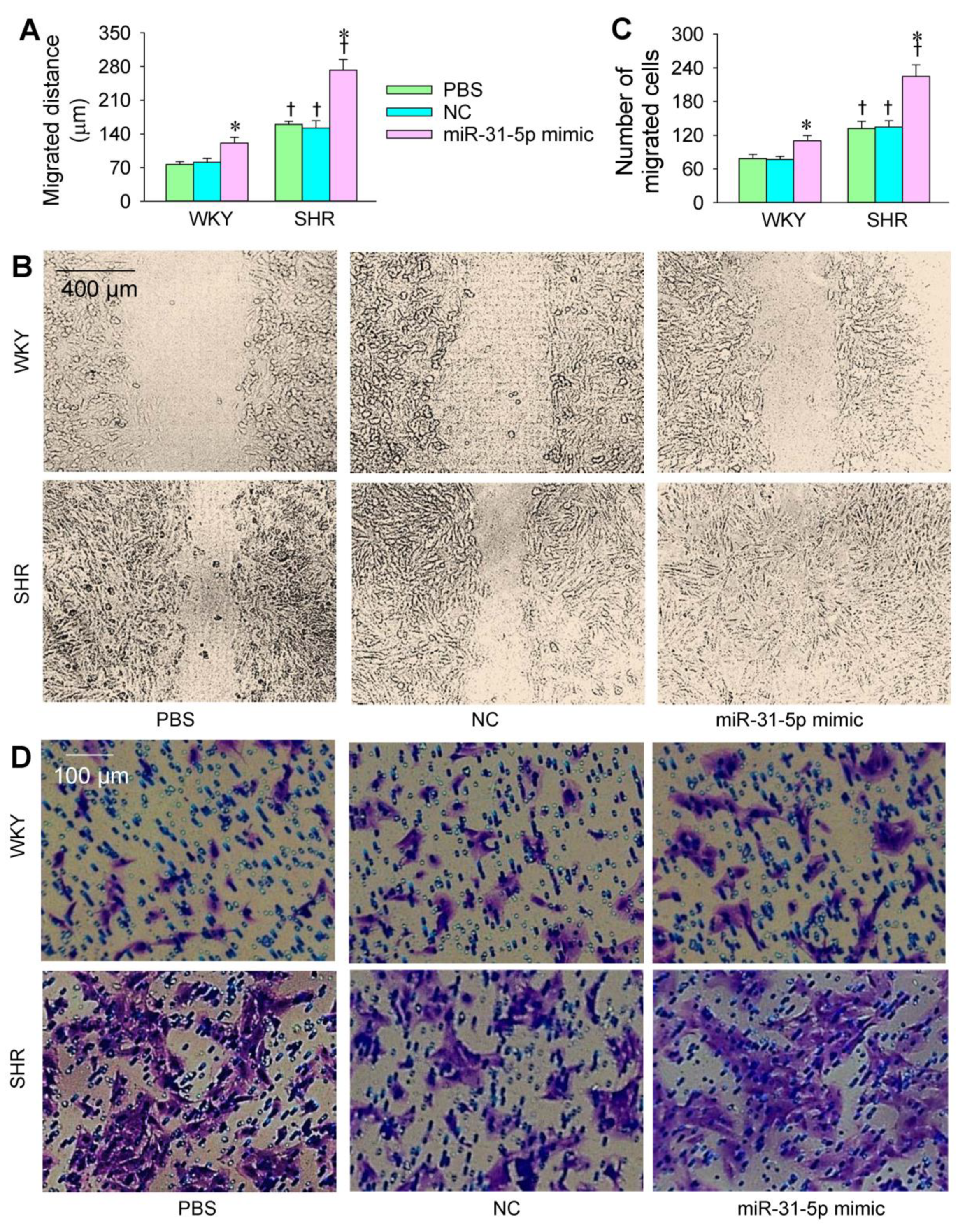
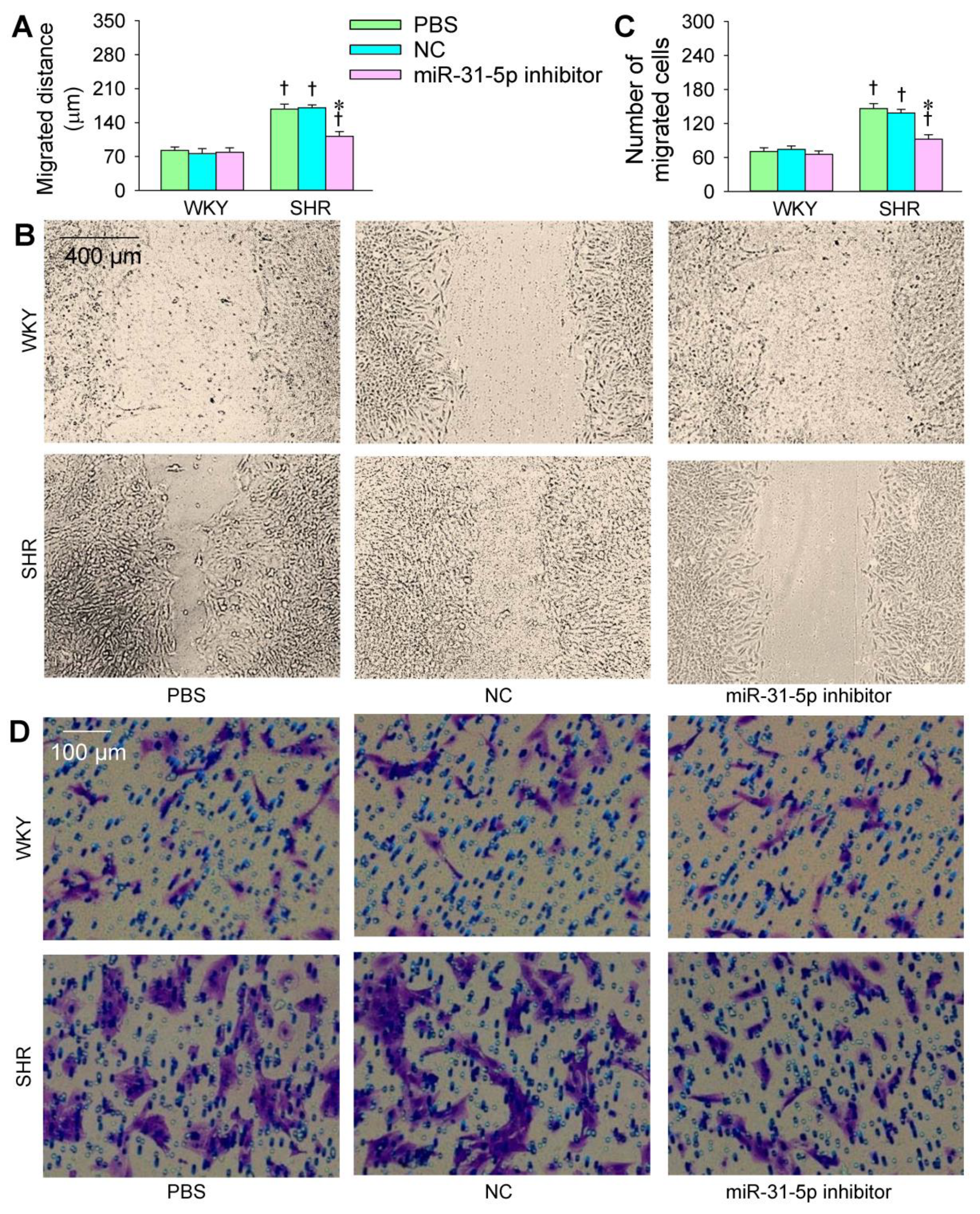
3.2. Roles of miR-31-5p in VSMC Migration
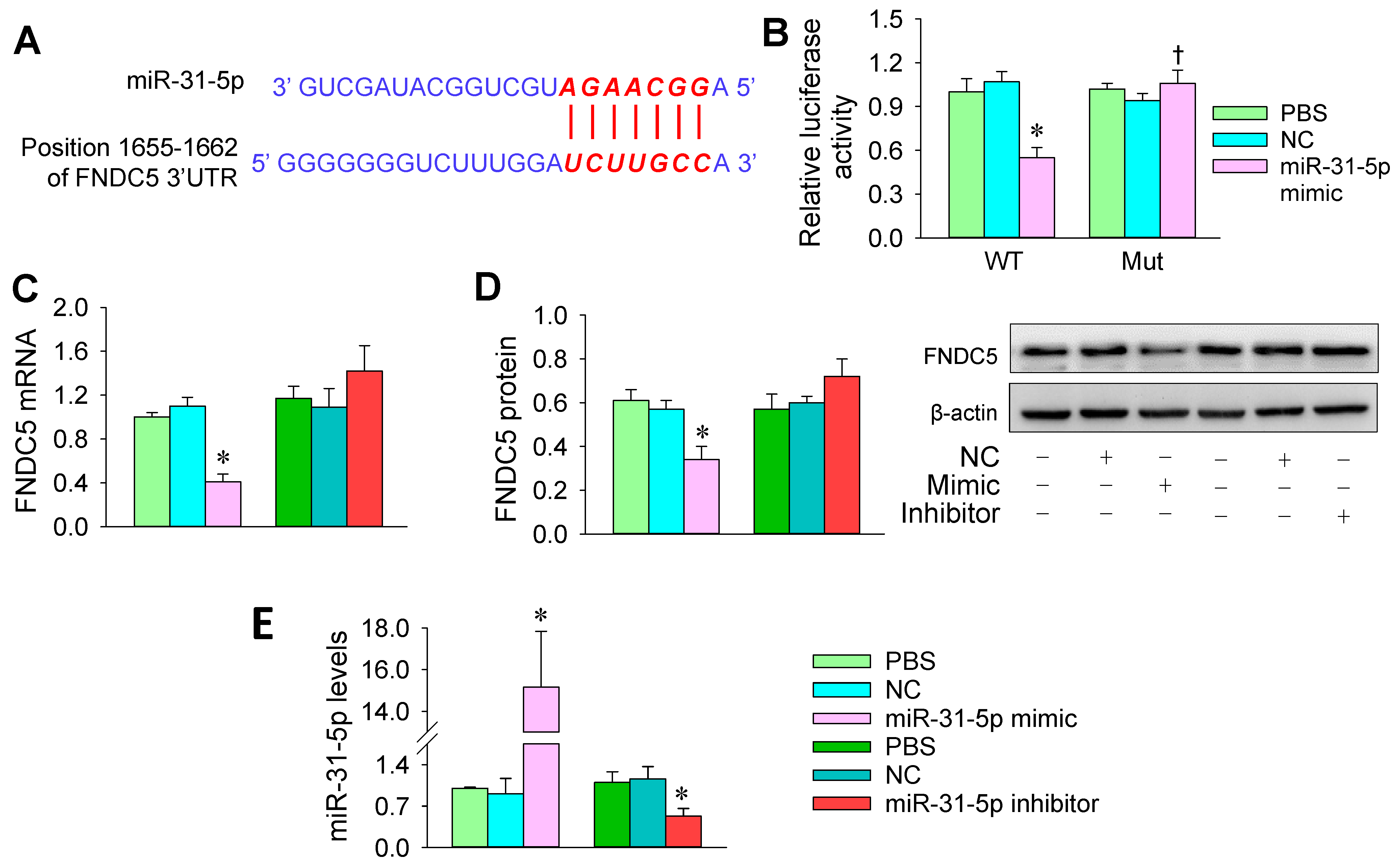
3.3. FNDC5 Is a Target of miR-31-5p
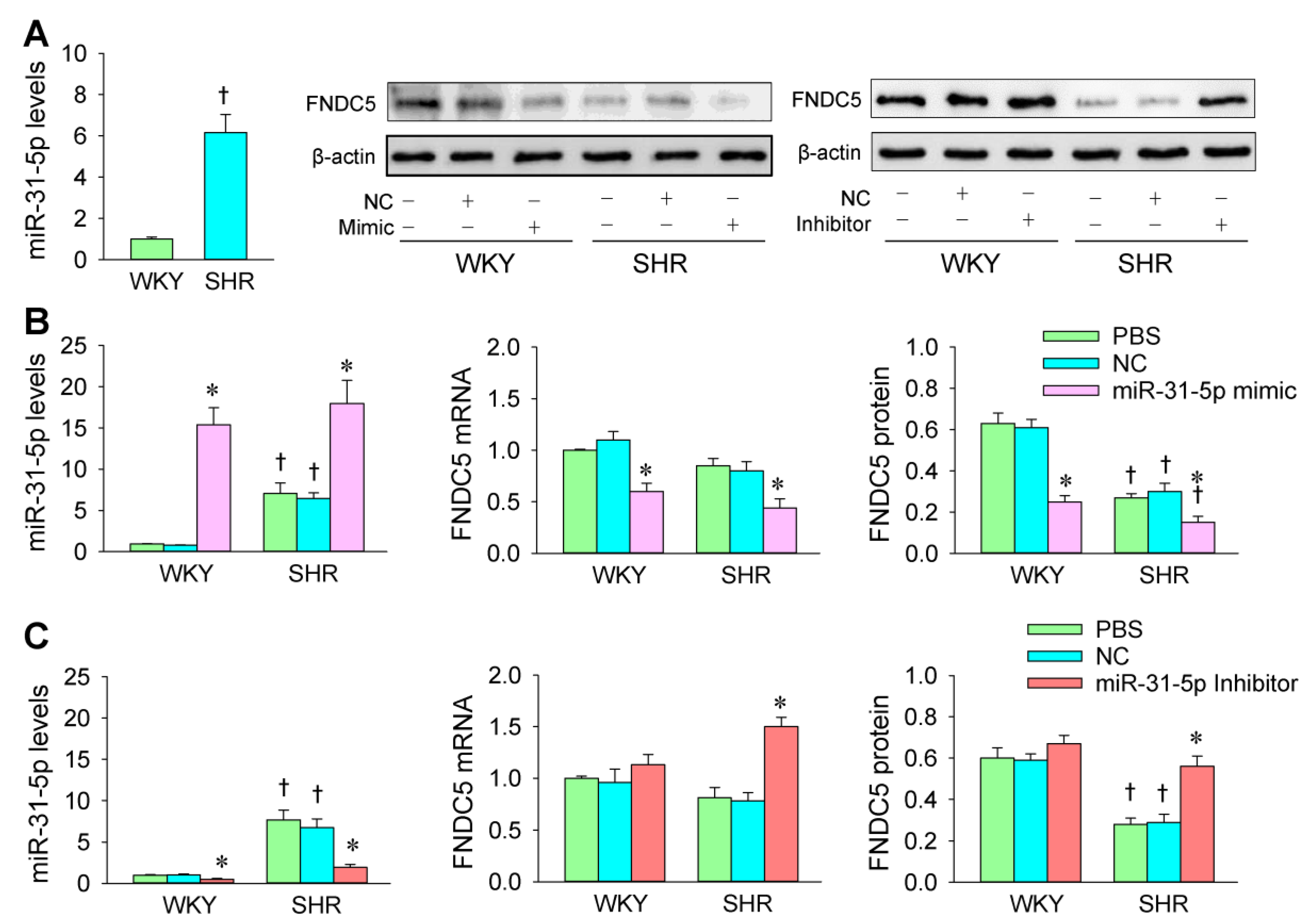
3.4. miR-31-5p Levels and the Roles of miR-31-5p in Regulating FNDC5 Expression
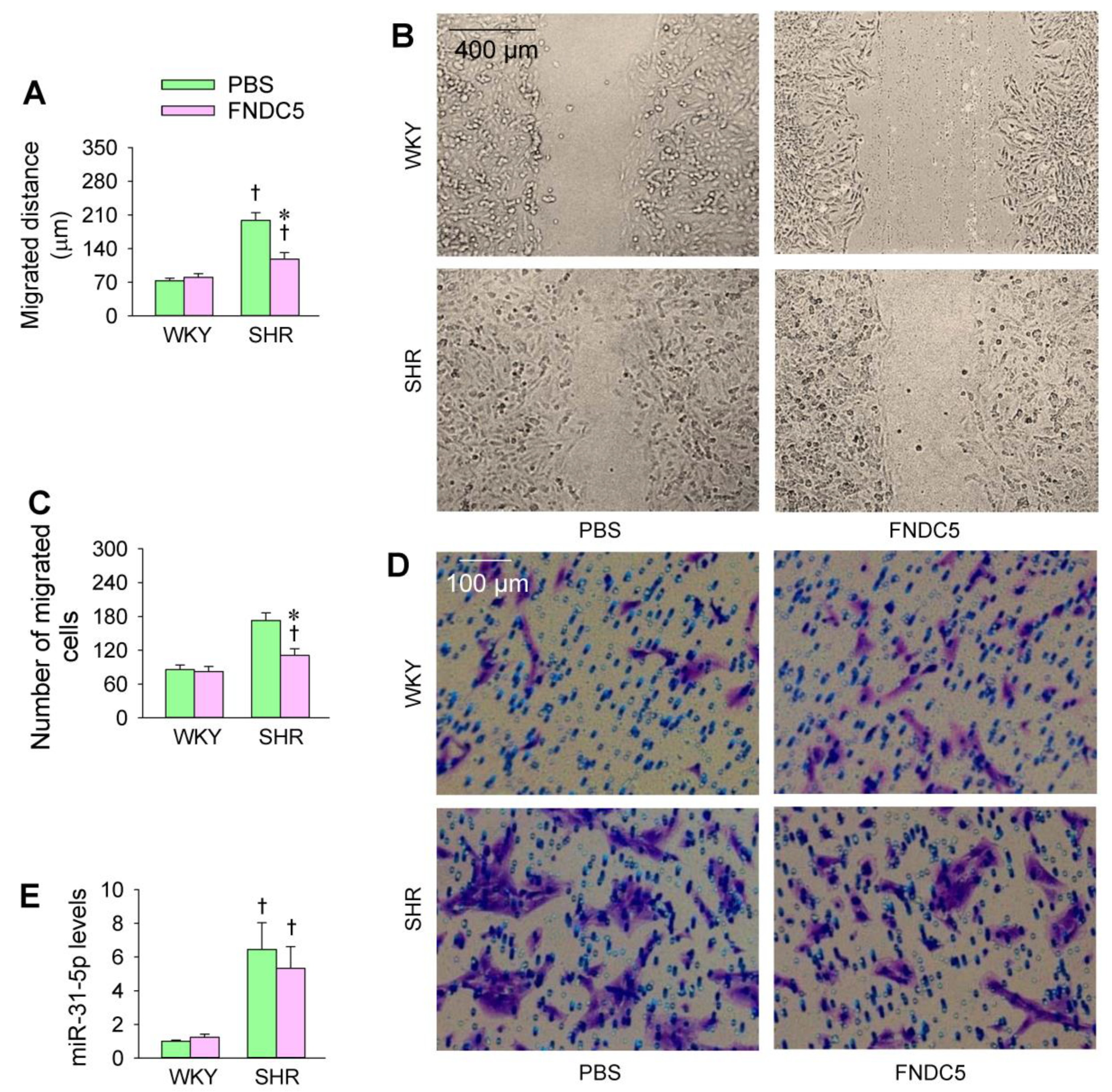
3.5. Effects of Exogenous FNDC5 on Oxidative Stress and VSMC Migration
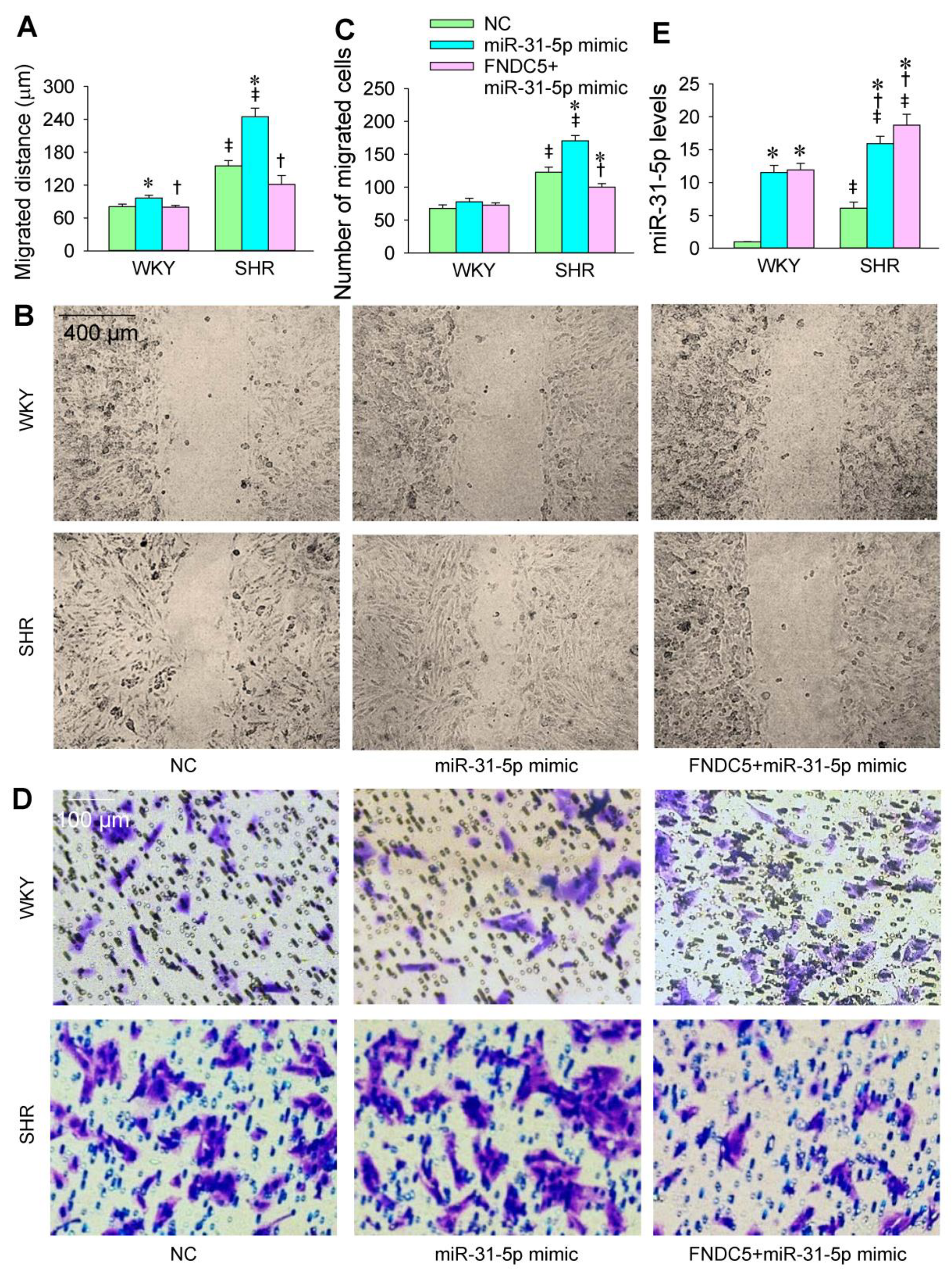
3.6. FNDC5 Prevents miR-31-5p Mimic-Induced Oxidative Stress and VSMC Migration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, X. Regulation of smooth muscle cells in development and vascular disease: Current therapeutic strategies. Expert. Rev. Cardiovasc. Ther. 2006, 4, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, J.; Chobanian, A.V.; Haudenschild, C.C. Smooth muscle cell migration and proliferation: Atherogenic mechanisms in hypertension. Atherosclerosis 1987, 67, 215–221. [Google Scholar] [CrossRef]
- Wu, N.; Ye, C.; Zheng, F.; Wan, G.W.; Wu, L.L.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Zhu, G.Q. MiR155-5p inhibits cell migration and oxidative stress in vascular smooth muscle cells of spontaneously hypertensive rats. Antioxidants 2020, 9, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Zang, Y.H.; Qiu, Y.; Zhang, F.; Chen, A.D.; Wang, J.J.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Zhu, G.Q. BCL6 attenuates proliferation and oxidative stress of vascular smooth muscle cells in hypertension. Oxid. Med. Cell. Longev. 2019, 2019, 5018410. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Redondo, A.B.; Aguado, A.; Briones, A.M.; Salaices, M. NADPH oxidases and vascular remodeling in cardiovascular diseases. Pharmacol. Res. 2016, 114, 110–120. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstadter, J.; Kroller-Schon, S.; Munzel, T.; et al. Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [Green Version]
- Poznyak, A.V.; Grechko, A.V.; Orekhova, V.A.; Khotina, V.; Ivanova, E.A.; Orekhov, A.N. NADPH oxidases and their role in atherosclerosis. Biomedicines 2020, 8, 206. [Google Scholar] [CrossRef]
- Ismaeel, A.; Brumberg, R.S.; Kirk, J.S.; Papoutsi, E.; Farmer, P.J.; Bohannon, W.T.; Smith, R.S.; Eidson, J.L.; Sawicki, I.; Koutakis, P. Oxidative stress and arterial dysfunction in peripheral artery disease. Antioxidants 2018, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Bordoni, L.; Fedeli, D.; Piangerelli, M.; Pelikant-Malecka, I.; Radulska, A.; Samulak, J.J.; Sawicka, A.K.; Lewicki, L.; Kalinowski, L.; Olek, R.A.; et al. Gender-related differences in trimethylamine and oxidative blood biomarkers in cardiovascular disease patients. Biomedicines 2020, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.; Castillo, G.; Gonzalez, J.; Valls, N.; Rodrigo, R. Oxidative stress in hypertension: Mechanisms and therapeutic opportunities. Exp. Clin. Endocrinol. Diabetes 2015, 123, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Novelle, M.G.; Contreras, C.; Romero-Pico, A.; Lopez, M.; Dieguez, C. Irisin, two years later. Int. J. Endocrinol. 2013, 2013, 746281. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, M.A.; Chinnam, N.; Ohashi, T.; Shah, R.S.; Erickson, H.P. The structure of irisin reveals a novel intersubunit beta-sheet fibronectin type III (FNIII) dimer: Implications for receptor activation. J. Biol. Chem. 2013, 288, 33738–33744. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.Y.; Xiong, X.Q.; Ren, X.S.; Zhao, M.X.; Shi, C.X.; Wang, J.J.; Zhou, Y.B.; Zhang, F.; Han, Y.; Gao, X.Y.; et al. FNDC5 alleviates hepatosteatosis by restoring AMPK/mTOR-mediated autophagy, fatty acid oxidation, and lipogenesis in mice. Diabetes 2016, 65, 3262–3275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.Y.; Shi, C.X.; Gao, R.; Sun, H.J.; Xiong, X.Q.; Ding, L.; Chen, Q.; Li, Y.H.; Wang, J.J.; Kang, Y.M.; et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Chen, D.; Sun, H.J.; Ding, L.; Wang, J.J.; Chen, Q.; Li, Y.H.; Zhou, Y.B.; Han, Y.; Zhang, F.; et al. FNDC5 overexpression and irisin ameliorates glucose/lipid metabolic derangements and enhances lipolysis in obesity. Biochim. Biophys. Acta 2015, 1852, 1867–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, X.Q.; Geng, Z.; Zhou, B.; Zhang, F.; Han, Y.; Zhou, Y.B.; Wang, J.J.; Gao, X.Y.; Chen, Q.; Li, Y.H.; et al. FNDC5 attenuates adipose tissue inflammation and insulin resistance via AMPK-mediated macrophage polarization in obesity. Metabolism 2018, 83, 31–41. [Google Scholar] [CrossRef]
- Zang, Y.H.; Chen, D.; Zhou, B.; Chen, A.D.; Wang, J.J.; Gao, X.Y.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Zhu, G.Q. FNDC5 inhibits foam cell formation and monocyte adhesion in vascular smooth muscle cells via suppressing NFkB-mediated NLRP3 upregulation. Vascul. Pharmacol. 2019, 121, 106579. [Google Scholar] [CrossRef]
- Zhou, B.; Qiu, Y.; Wu, N.; Chen, A.D.; Zhou, H.; Chen, Q.; Kang, Y.M.; Li, Y.H.; Zhu, G.Q. FNDC5 attenuates oxidative stress and NLRP3 inflammasome activation in vascular smooth muscle cells via activating the AMPK-SIRT1 signal pathway. Oxid. Med. Cell. Longev. 2020, 2020, 6384803. [Google Scholar] [CrossRef] [PubMed]
- Bowen, T.; Jenkins, R.H.; Fraser, D.J. MicroRNAs, transforming growth factor beta-1, and tissue fibrosis. J. Pathol. 2013, 229, 274–285. [Google Scholar] [CrossRef]
- Engedal, N.; Zerovnik, E.; Rudov, A.; Galli, F.; Olivieri, F.; Procopio, A.D.; Rippo, M.R.; Monsurro, V.; Betti, M.; Albertini, M.C. From Oxidative Stress Damage to Pathways, Networks, and Autophagy via MicroRNAs. Oxid. Med. Cell. Longev. 2018, 2018, 4968321. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, Q.; Cao, Q.; Wang, S.; Liu, H.; Li, Q. Integrated Analysis of miRNA-mRNA Interaction Network in Porcine Granulosa Cells Undergoing Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 2019, 1041583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanathan, S.; Shenoda, B.B.; Lin, Z.; Alexander, G.M.; Huppert, A.; Sacan, A.; Ajit, S.K. Inflammation potentiates miR-939 expression and packaging into small extracellular vesicles. J. Extracell. Vesicles 2019, 8, 1650595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.; Lei, J.; Du, L. miR-31-5p may enhance the efficacy of chemotherapy with Taxol and cisplatin in TNBC. Exp. Ther. Med. 2020, 19, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.S.; Tong, Y.; Qiu, Y.; Ye, C.; Wu, N.; Xiong, X.Q.; Wang, J.J.; Han, Y.; Zhou, Y.B.; Zhang, F.; et al. MiR155-5p in adventitial fibroblasts-derived extracellular vesicles inhibits vascular smooth muscle cell proliferation via suppressing angiotensin-converting enzyme expression. J. Extracell. Vesicles 2020, 9, 1698795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, C.; Tong, Y.; Wu, N.; Wan, G.W.; Zheng, F.; Chen, J.Y.; Lei, J.Z.; Zhou, H.; Chen, A.D.; Wang, J.J.; et al. Inhibition of miR-135a-5p attenuates vascular smooth muscle cell proliferation and vascular remodeling in hypertensive rats. Acta Pharmacol. Sin. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Quan, J.; Chen, F.; Pan, X.; Zhuang, C.; Xiong, T.; Zhuang, C.; Li, J.; Huang, X.; Ye, J.; et al. MiR-31-5p acts as a tumor suppressor in renal cell carcinoma by targeting cyclin-dependent kinase 1 (CDK1). Biomed. Pharmacother. 2019, 111, 517–526. [Google Scholar] [CrossRef]
- Zhao, G.; Han, C.; Zhang, Z.; Wang, L.; Xu, J. Increased expression of microRNA-31-5p inhibits cell proliferation, migration, and invasion via regulating Sp1 transcription factor in HepG2 hepatocellular carcinoma cell line. Biochem. Biophys. Res. Commun. 2017, 490, 371–377. [Google Scholar] [CrossRef]
- Peng, H.; Wang, L.; Su, Q.; Yi, K.; Du, J.; Wang, Z. MiR-31-5p promotes the cell growth, migration and invasion of colorectal cancer cells by targeting NUMB. Biomed. Pharmacother. 2019, 109, 208–216. [Google Scholar] [CrossRef]
- Huang, S.; Chen, Z.; Wu, W.; Wang, M.; Wang, R.; Cui, J.; Li, W.; Wang, S. MicroRNA-31 promotes arterial smooth muscle cell proliferation and migration by targeting mitofusin-2 in arteriosclerosis obliterans of the lower extremitie. Exp. Ther. Med. 2018, 15, 633–640. [Google Scholar] [CrossRef]
- Sun, H.J.; Ren, X.S.; Xiong, X.Q.; Chen, Y.Z.; Zhao, M.X.; Wang, J.J.; Zhou, Y.B.; Han, Y.; Chen, Q.; Li, Y.H.; et al. NLRP3 inflammasome activation contributes to VSMC phenotypic transformation and proliferation in hypertension. Cell Death. Dis. 2017, 8, e3074. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.J.; Zhao, M.X.; Ren, X.S.; Liu, T.Y.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Wang, J.J.; Zhu, G.Q. Salusin-b promotes vascular smooth muscle cell migration and intimal hyperplasia after vascular injury via ROS/NFkB/MMP-9 pathway. Antioxid. Redox Signal. 2016, 24, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Azimi, M.; Gharakhanlou, R.; Naghdi, N.; Khodadadi, D.; Heysieattalab, S. Moderate treadmill exercise ameliorates amyloid-b-induced learning and memory impairment, possibly via increasing AMPK activity and up-regulation of the PGC-1a/FNDC5/BDNF pathway. Peptides 2018, 102, 78–88. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Lee, K.S.; Choi, S.; Kim, J.; Lee, D.K.; Park, M.; Park, W.; Kim, T.H.; Hwang, J.Y.; Won, M.H.; et al. NF-kB-responsive miRNA-31-5p elicits endothelial dysfunction associated with preeclampsia via down-regulation of endothelial nitric-oxide synthase. J. Biol. Chem. 2018, 293, 18989–19000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Li, L.; Hu, D.; Zhang, X. LINC00612/miR-31-5p/Notch1 axis regulates apoptosis, inflammation, and oxidative stress in human pulmonary microvascular endothelial cells induced by cigarette smoke extract. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 2049–2060. [Google Scholar] [CrossRef]
- Li, W.; Yu, N.; Fan, L.; Chen, S.H.; Wu, J.L. Circ_0063517 acts as ceRNA, targeting the miR-31-5p-ETBR axis to regulate angiogenesis of vascular endothelial cells in preeclampsia. Life Sci. 2020, 244, 117306. [Google Scholar] [CrossRef]
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef]
- Cui, C.; Wang, X.; Shang, X.M.; Li, L.; Ma, Y.; Zhao, G.Y.; Song, Y.X.; Geng, X.B.; Zhao, B.Q.; Tian, M.R.; et al. lncRNA 430945 promotes the proliferation and migration of vascular smooth muscle cells via the ROR2/RhoA signaling pathway in atherosclerosis. Mol. Med. Rep. 2019, 19, 4663–4672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Tian, C.; Sun, L.; Cao, F.; Meng, Z. The lncRNA TUG1/miR-145-5p/FGF10 regulates proliferation and migration in VSMCs of hypertension. Biochem. Biophys. Res. Commun. 2018, 501, 688–695. [Google Scholar] [CrossRef]
- Tong, Y.; Ye, C.; Ren, X.S.; Qiu, Y.; Zang, Y.H.; Xiong, X.Q.; Wang, J.J.; Chen, Q.; Li, Y.H.; Kang, Y.M.; et al. Exosome-mediated transfer of ACE (angiotensin-converting enzyme) from adventitial fibroblasts of spontaneously hypertensive rats promotes vascular smooth muscle cell migration. Hypertension 2018, 72, 881–888. [Google Scholar] [CrossRef]
- Mironov, A.A.; Sesorova, I.S.; Dimov, I.D.; Karelina, N.R.; Beznoussenko, G.V. Intracellular transports and atherogenesis. Front. Biosci. 2020, 25, 1230–1258. [Google Scholar] [CrossRef]
- Belo, V.A.; Guimaraes, D.A.; Castro, M.M. Matrix metalloproteinase 2 as a potential mediator of vascular smooth muscle cell migration and chronic vascular remodeling in hypertension. J. Vasc. Res. 2015, 52, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Nawata, J.; Wang, H.; Onoue, N.; Zhulanqiqige, D.; Ito, K.; Sugimura, K.; Fukumoto, Y.; Shimokawa, H. Enhanced pulsatile pressure accelerates vascular smooth muscle migration: Implications for atherogenesis of hypertension. Cardiovasc. Res. 2008, 80, 346–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capaci, V.; Bascetta, L.; Fantuz, M.; Beznoussenko, G.V.; Sommaggio, R.; Cancila, V.; Bisso, A.; Campaner, E.; Mironov, A.A.; Wisniewski, J.R.; et al. Mutant p53 induces Golgi tubulo-vesiculation driving a prometastatic secretome. Nat. Commun. 2020, 11, 3945. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Durgin, B.G.; Straub, A.C. Redox control of vascular smooth muscle cell function and plasticity. Lab. Investig. 2018, 98, 1254–1262. [Google Scholar] [CrossRef]
- Huetsch, J.C.; Suresh, K.; Shimoda, L.A. Regulation of smooth muscle cell proliferation by NADPH oxidases in pulmonary hypertension. Antioxidants 2019, 8, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowala, M.C.; Cuenoud, H.F.; Joris, I.; Majno, G. Cellular changes during hypertension: A quantitative study of the rat aorta. Exp. Mol. Pathol. 1986, 45, 323–335. [Google Scholar] [CrossRef]


| Gene | Primer | Sequence (5′ → 3′) |
|---|---|---|
| FNDC5 | Forward | AGAGAGCAAGCACCAAGACT |
| Reverse | GATGGAGTCGGAACCCTGAA | |
| β-actin | Forward | GGACCTGACAGACTACCTCA |
| Reverse | GTTGCCAATAGTGATGACCT | |
| miR-31-5p | Forward | GCGCGTGAGATGGCTCCCTG |
| U6 | Forward | CTCGCTTCGGCAGCACA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Wu, L.-L.; Zheng, F.; Wu, N.; Chen, A.-D.; Zhou, H.; Chen, J.-Y.; Chen, Q.; Li, Y.-H.; Kang, Y.-M.; et al. miR-31-5p Promotes Oxidative Stress and Vascular Smooth Muscle Cell Migration in Spontaneously Hypertensive Rats via Inhibiting FNDC5 Expression. Biomedicines 2021, 9, 1009. https://doi.org/10.3390/biomedicines9081009
Zhou B, Wu L-L, Zheng F, Wu N, Chen A-D, Zhou H, Chen J-Y, Chen Q, Li Y-H, Kang Y-M, et al. miR-31-5p Promotes Oxidative Stress and Vascular Smooth Muscle Cell Migration in Spontaneously Hypertensive Rats via Inhibiting FNDC5 Expression. Biomedicines. 2021; 9(8):1009. https://doi.org/10.3390/biomedicines9081009
Chicago/Turabian StyleZhou, Bing, Lu-Lu Wu, Fen Zheng, Nan Wu, Ai-Dong Chen, Hong Zhou, Jing-Yu Chen, Qi Chen, Yue-Hua Li, Yu-Ming Kang, and et al. 2021. "miR-31-5p Promotes Oxidative Stress and Vascular Smooth Muscle Cell Migration in Spontaneously Hypertensive Rats via Inhibiting FNDC5 Expression" Biomedicines 9, no. 8: 1009. https://doi.org/10.3390/biomedicines9081009
APA StyleZhou, B., Wu, L.-L., Zheng, F., Wu, N., Chen, A.-D., Zhou, H., Chen, J.-Y., Chen, Q., Li, Y.-H., Kang, Y.-M., & Zhu, G.-Q. (2021). miR-31-5p Promotes Oxidative Stress and Vascular Smooth Muscle Cell Migration in Spontaneously Hypertensive Rats via Inhibiting FNDC5 Expression. Biomedicines, 9(8), 1009. https://doi.org/10.3390/biomedicines9081009








