Multimodal Tracking of Hematopoietic Stem Cells from Young and Old Mice Labeled with Magnetic–Fluorescent Nanoparticles and Their Grafting by Bioluminescence in a Bone Marrow Transplant Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Aspects in the Use of Animals
2.2. In Vitro Study
2.2.1. Extraction and Isolation of Bone Marrow Mononuclear Cells (BM-MNC)
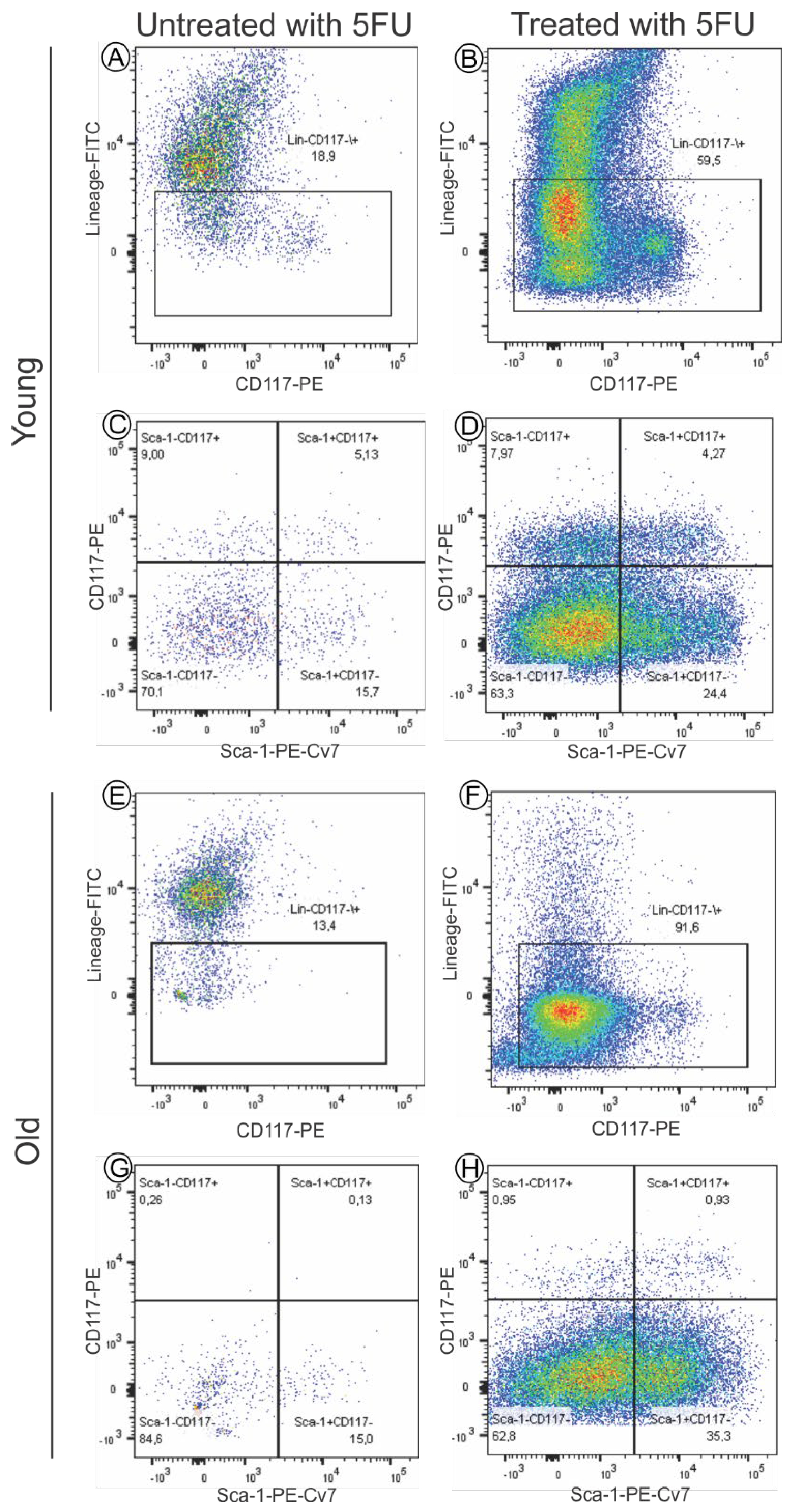
2.2.2. Immunophenotypic Characteristics of BM-MNC
2.2.3. BM-MNC Lentiviral Transduction for Luciferase Expressing
2.2.4. Efficacy Evaluation of Luciferase Transduction in BM-MNC by Bioluminescent Imaging (BLI)
2.2.5. Multimodal Superparamagnetic Iron Oxide Nanoparticles
2.2.6. Polydispersion, Stability, Optical Caracterization and Zeta Potention Analysis of SPIONNIRF-Rh
2.2.7. BM-MNC Labeling with SPIONNIRF-Rh
2.2.8. Internalization of SPIONNIRF-Rh into BM-MNC
2.2.9. BM-MNC Viability after Labeling with SPIONNIRF-Rh
2.2.10. Signal and Quantification Analysis of the SPIONNIRF-Rh Loud Internalized into BM-MNC Using NIRF, ICP-MS, and MRI Techniques
2.3. In Vivo Study
2.3.1. BMT Model
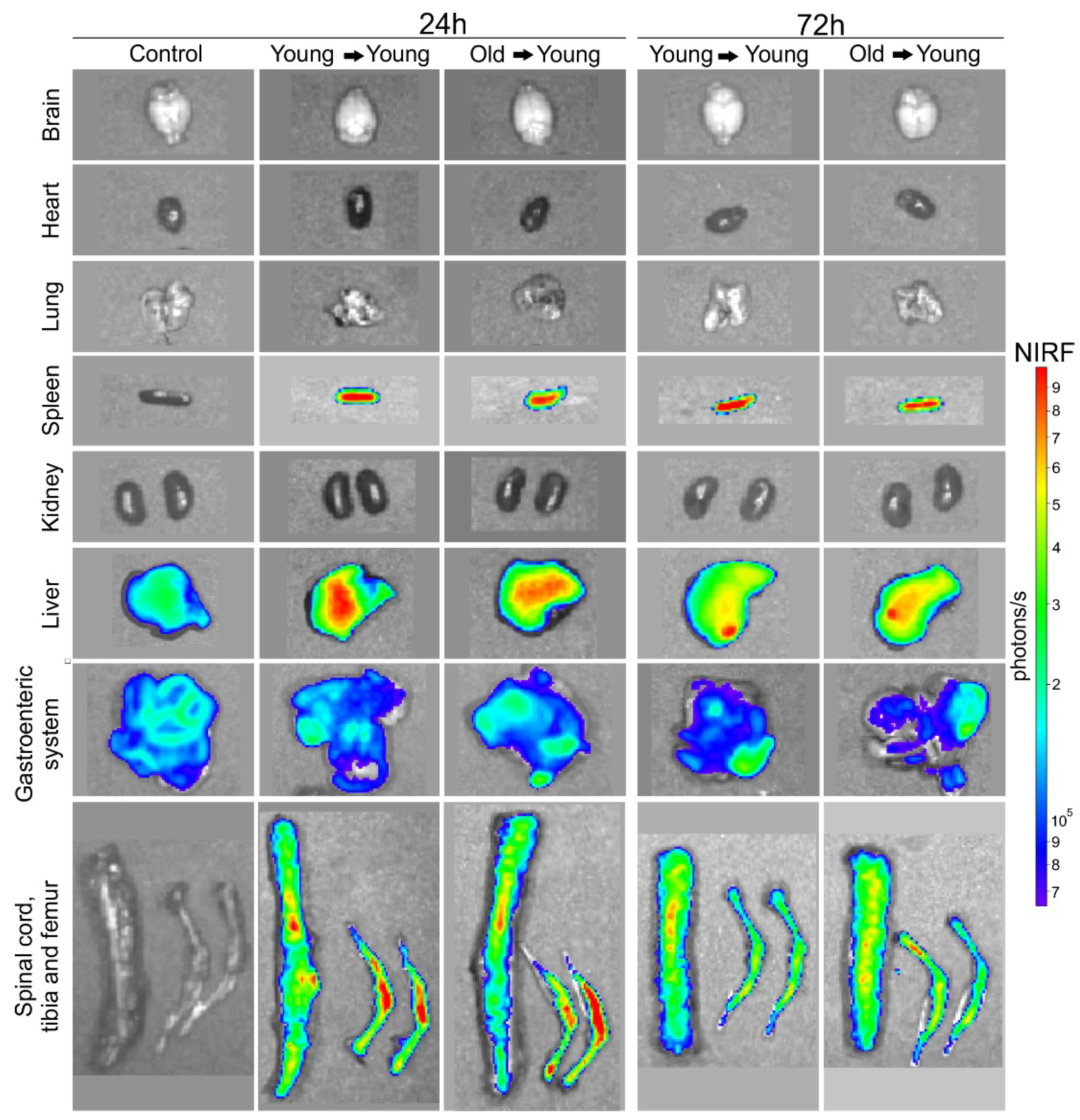
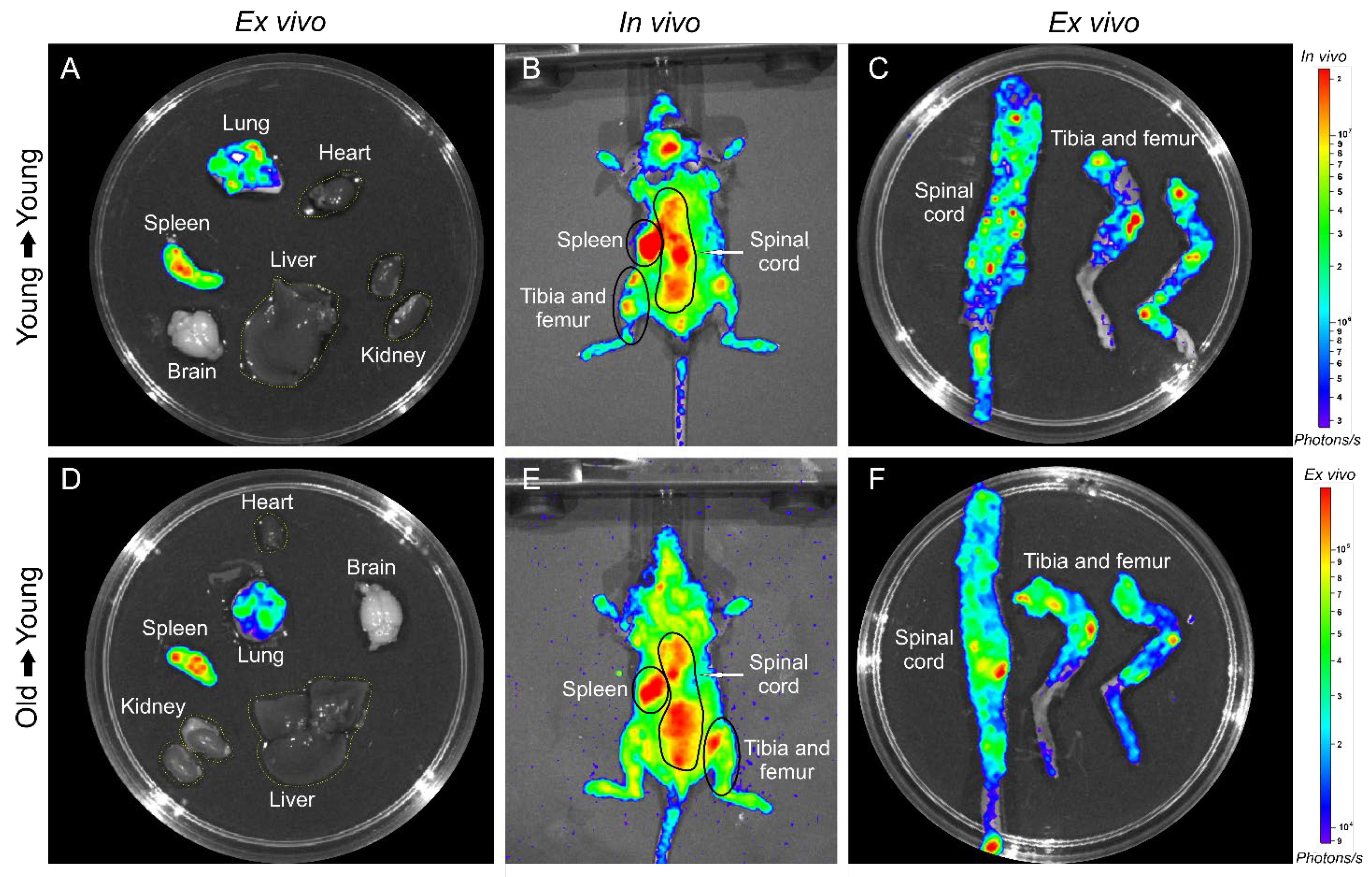
2.3.2. BM-MNC Labeled with SPIONNIRF-Rh Homing Evaluation by NIRF
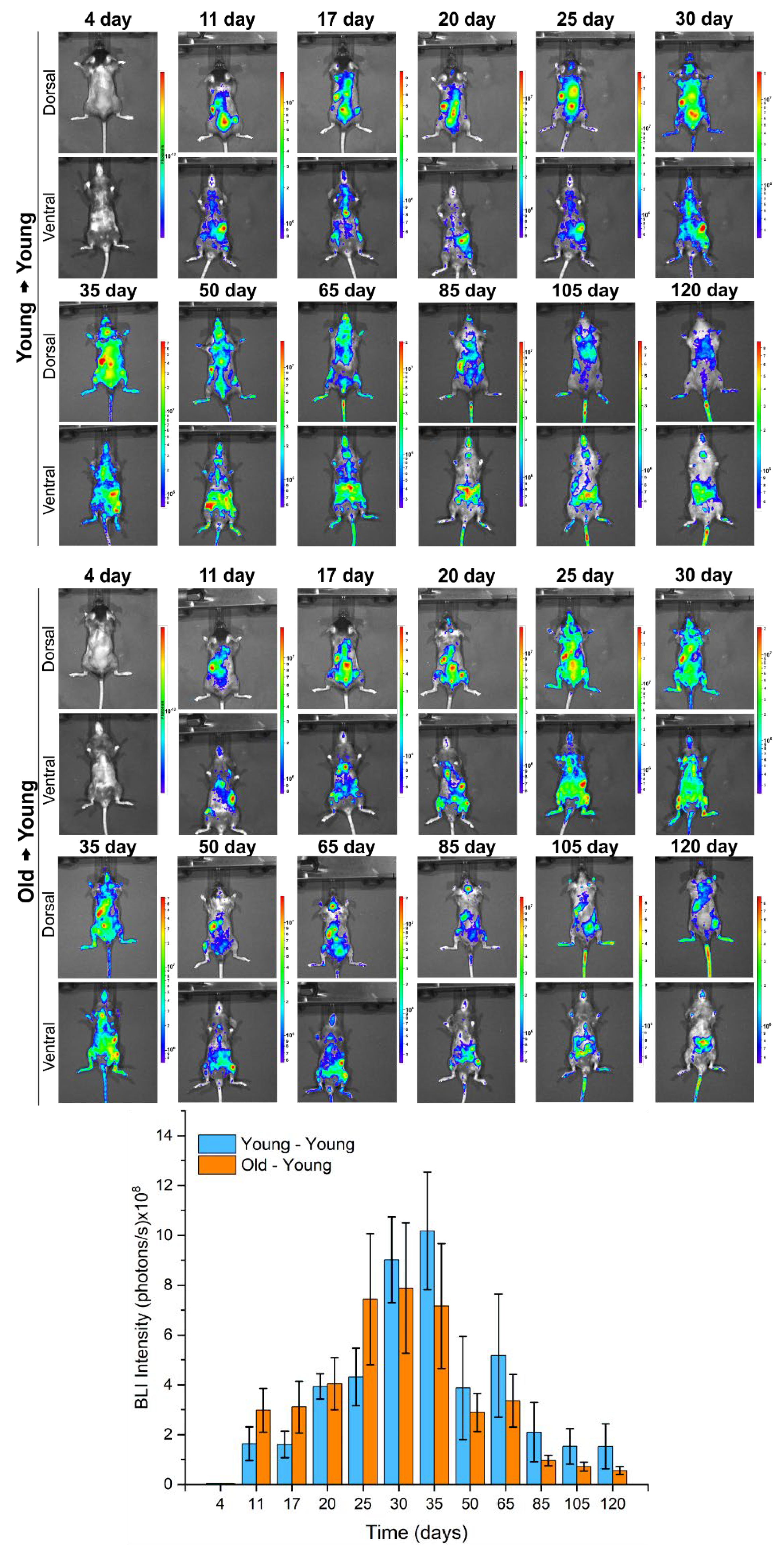
2.3.3. BM-MNC Labeled with SPIONNIRF-Rh Tracking and Their Grafting by BLI
2.3.4. Histological Analysis of Iron Present in Tissues
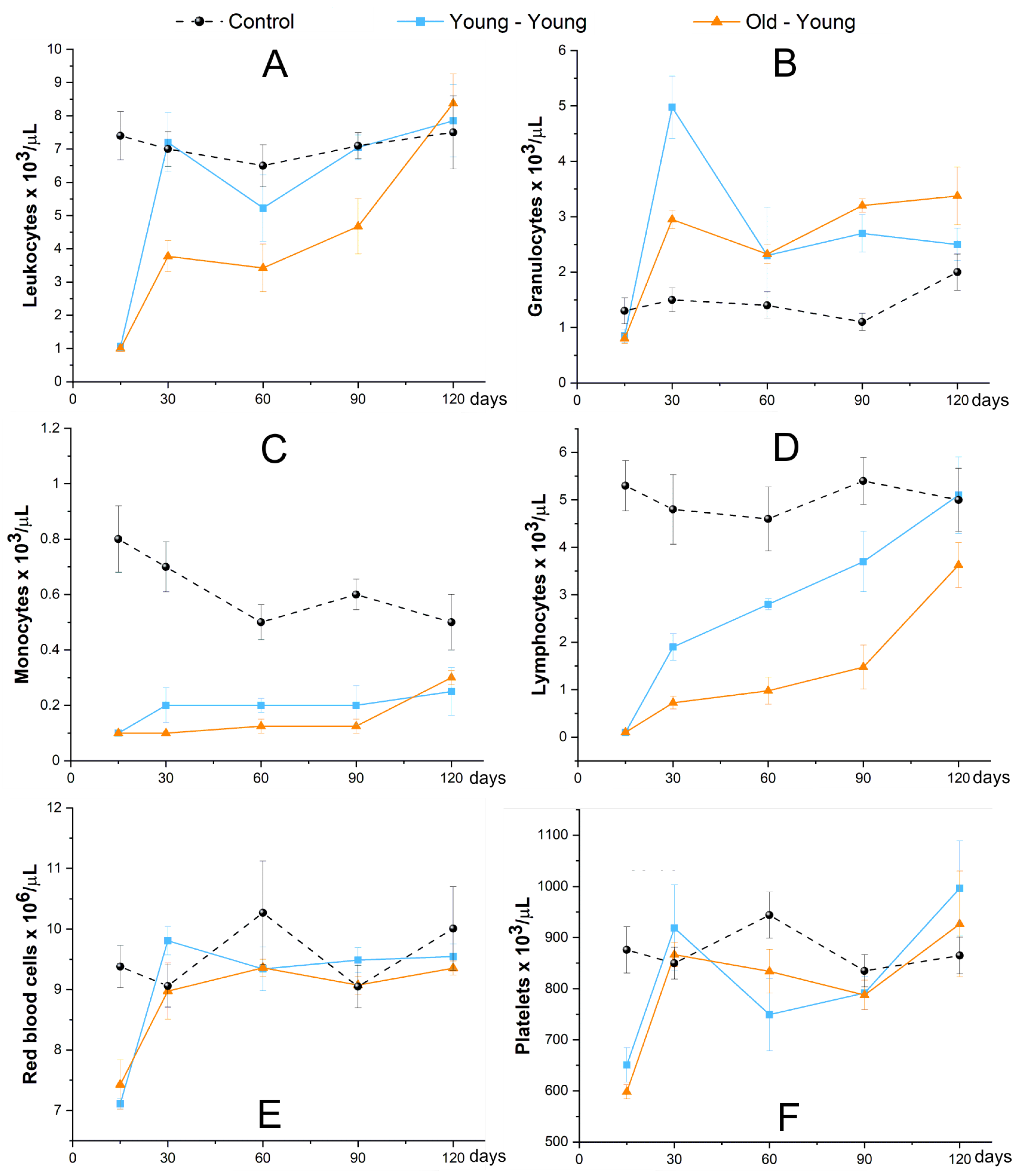
2.3.5. Evaluation of Hematological Reconstitution (Blood Count and LKS Quantification)
2.4. Statistical Analysis
3. Results
3.1. In Vitro Study
3.1.1. Immunofenotypic Characteristics of Bone Marrow Mononuclear Cells (BM-MNC)
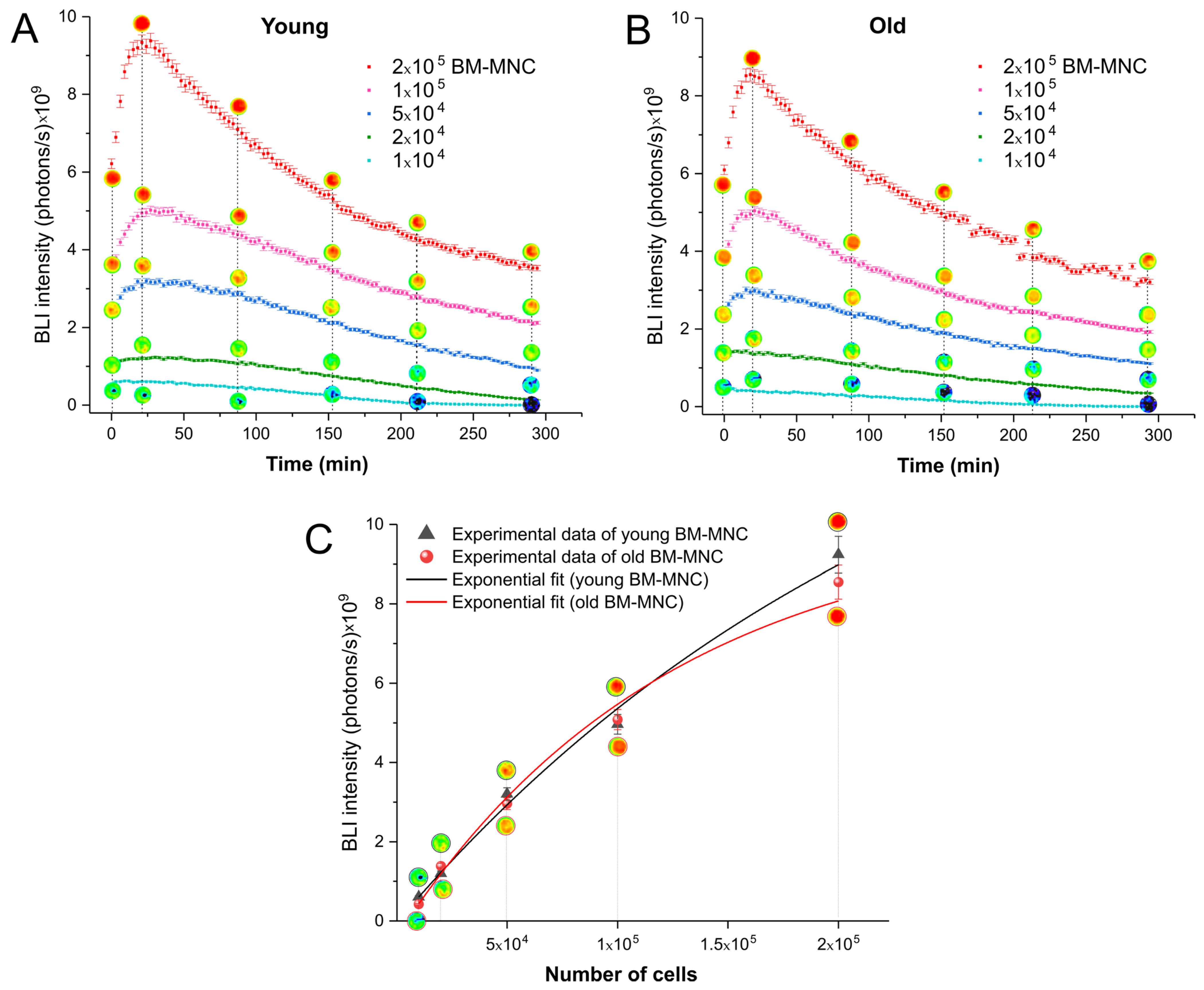
3.1.2. Time Course of Activity of Luciferase between Young and Old Bone Marrow Mononuclear Cells
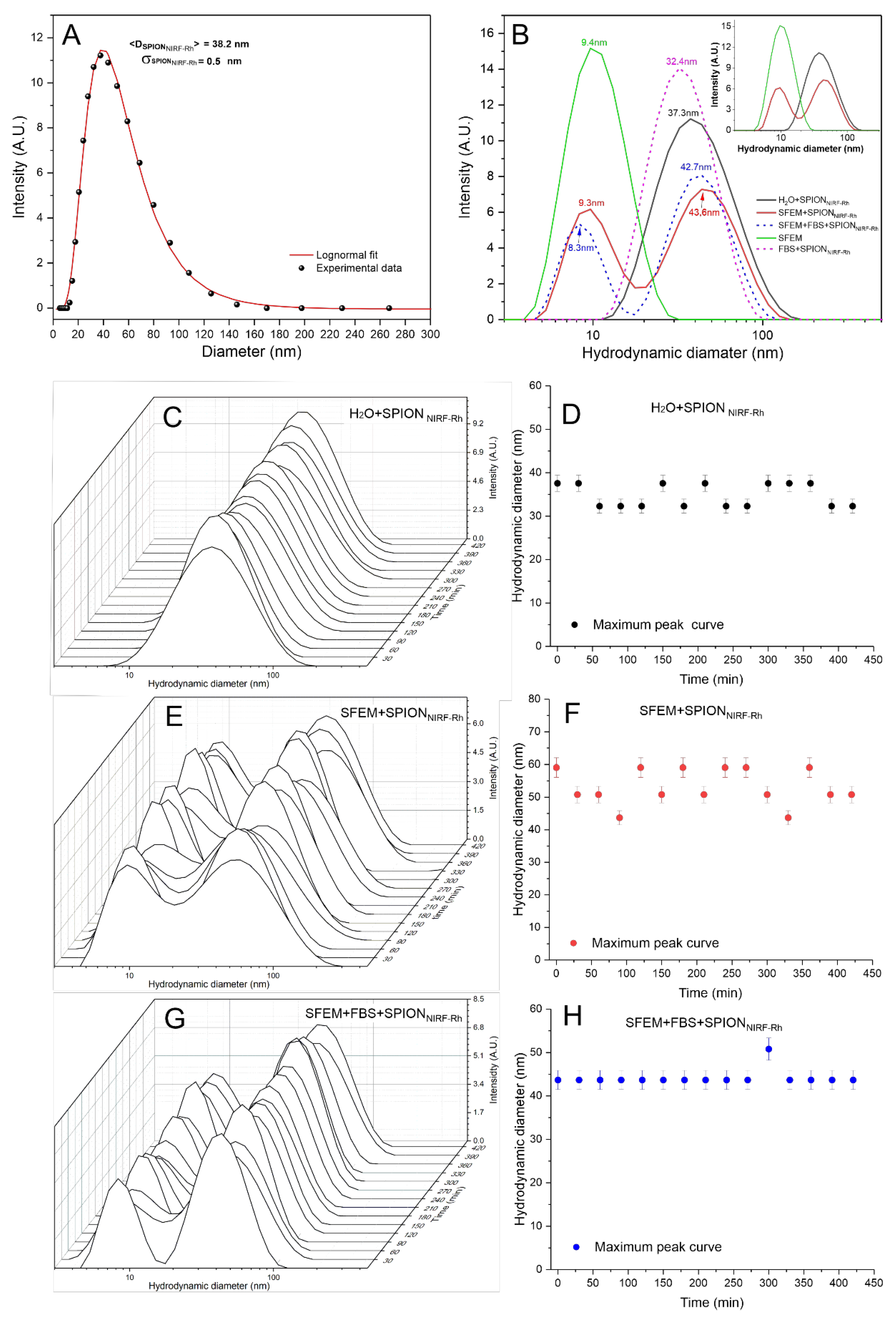
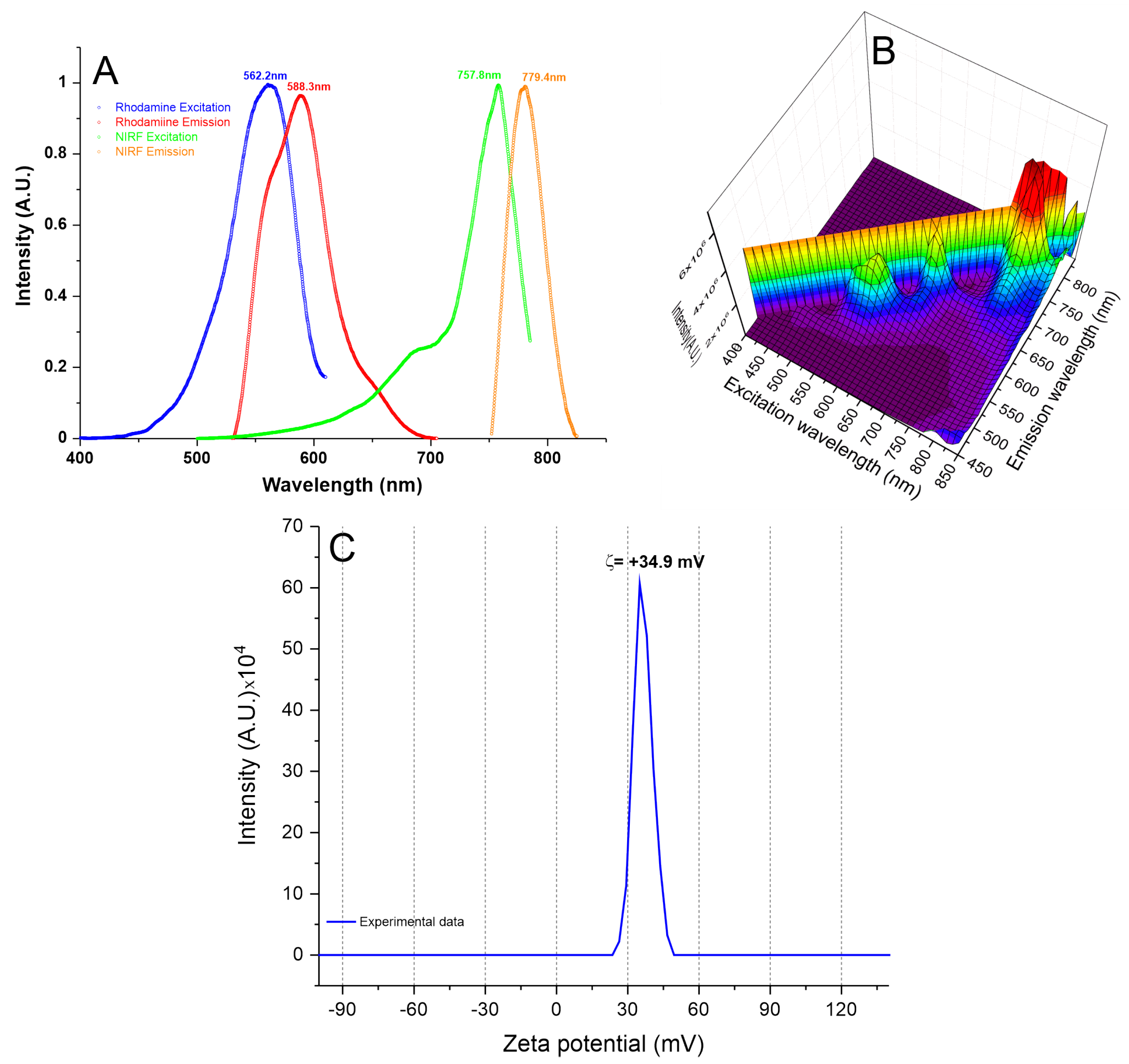
3.1.3. Analysis of SPIONNIRF-Rh Polydispersion, Stability, Optical Characterization, and Zeta Potential
3.1.4. A SPIONNIRF-Rh Internalization into BM-MNC Evaluation by Brightfield and Fluorescence Microscopy
3.1.5. Quantification of SPIONNIRF-Rh Loud Internalized into BM-MNC by NIRF, ICP-MS, and MRI
3.2. In Vivo Study
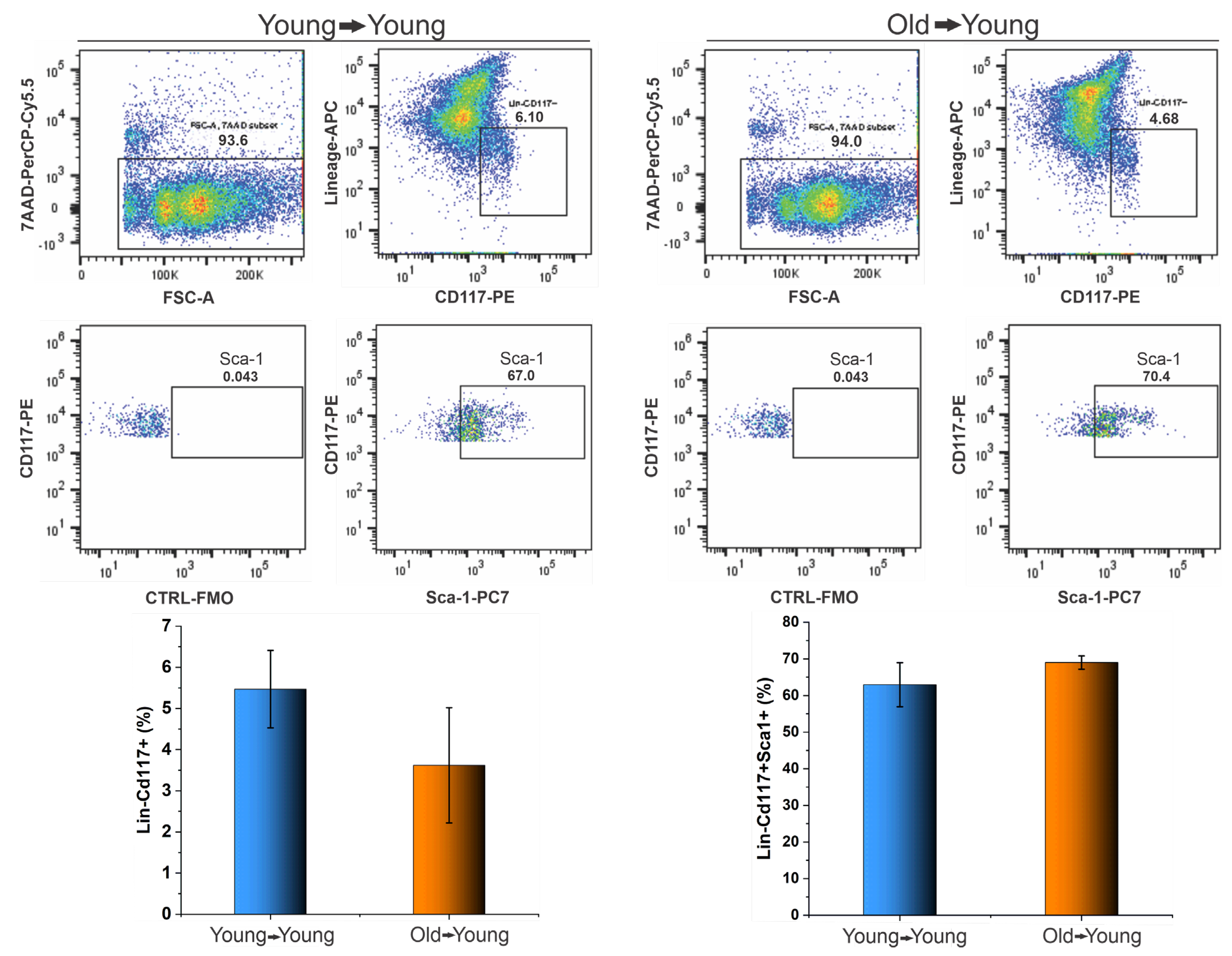
3.2.1. BMT of Young to Young and Old to Young Mice
3.2.2. The Iron Presence in Tissues Evaluated by Histological
3.2.3. Evaluation of Hematological Reconstitution (Blood Count and LKS Quantification)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gratwohl, A.; Baldomero, H. Trends of hematopoietic stem cell transplantation in the third millennium. Curr. Opin. Hematol. 2009, 16, 420–426. [Google Scholar] [CrossRef]
- Bregni, M.; Badoglio, M.; Pedrazzoli, P.; Lanza, F. Is allogeneic transplant for solid tumors still alive? Bone Marrow Transplant. 2016, 51, 751–752. [Google Scholar] [CrossRef]
- Armitage, J.O. Bone Marrow Transplantation. N. Engl. J. Med. 1994, 330, 827–838. [Google Scholar] [CrossRef]
- Smith, A.R.; Wagner, J.E. Alternative haematopoietic stem cell sources for transplantation: Place of umbilical cord blood. Br. J. Haematol. 2009, 147, 246–261. [Google Scholar] [CrossRef]
- Jethava, Y.S.; Sica, S.; Savani, B.; Socola, F.; Jagasia, M.; Mohty, M.; Nagler, A.; Bacigalupo, A. Conditioning regimens for allogeneic hematopoietic stem cell transplants in acute myeloid leukemia. Bone Marrow Transplant. 2017, 52, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Liesveld, J.L.; Sharma, N.; Aljitawi, O.S. Stem cell homing: From physiology to therapeutics. Stem Cells 2020, 38, 1241–1253. [Google Scholar] [CrossRef]
- Lapidot, T.; Dar, A.; Kollet, O. How do stem cells find their way home? Blood 2005, 106, 1901–1910. [Google Scholar] [CrossRef] [PubMed]
- Celso, C.L.; Fleming, H.E.; Wu, J.W.; Zhao, C.X.; Miake-Lye, S.; Fujisaki, J.; Côté, D.; Rowe, D.W.; Lin, C.P.; Scadden, D.T. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 2008, 457, 92–96. [Google Scholar] [CrossRef]
- Xie, Y.; Yin, T.; Wiegraebe, W.; He, X.C.; Miller, D.; Stark, D.; Perko, K.; Alexander, R.B.; Schwartz, J.; Grindley, J.C.; et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature 2008, 457, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Shaw, C.; Gatza, C.; Fisk, C.J.; Donehower, L.; Goodell, M. Aging Hematopoietic Stem Cells Decline in Function and Exhibit Epigenetic Dysregulation. PLoS Biol. 2007, 5, e201. [Google Scholar] [CrossRef]
- Xing, Z.; Ryan, M.A.; Daria, D.; Nattamai, K.J.; Van Zant, G.; Wang, L.; Zheng, Y.; Geiger, H. Increased hematopoietic stem cell mobilization in aged mice. Blood 2006, 108, 2190–2197. [Google Scholar] [CrossRef]
- Khurana, S. The effects of proliferation and DNA damage on hematopoietic stem cell function determine aging. Dev. Dyn. 2016, 245, 739–750. [Google Scholar] [CrossRef]
- Sudo, K.; Ema, H.; Morita, Y.; Nakauchi, H. Age-Associated Characteristics of Murine Hematopoietic Stem Cells. J. Exp. Med. 2000, 192, 1273–1280. [Google Scholar] [CrossRef]
- Lee, H.W.; Gangadaran, P.; Kalimuthu, S.; Ahn, B.-C. Advances in Molecular Imaging Strategies forIn VivoTracking of Immune Cells. BioMed Res. Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Colvin, G.A.; Lambert, J.-F.; Dooner, M.S.; Cerny, J.; Quesenberry, P.J. Murine allogeneic in vivo stem cell homing. J. Cell. Physiol. 2007, 211, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Papa, E.F.; Dooner, M.S.; Machan, J.T.; Johnson, K.W.; Goldberg, L.R.; Quesenberry, P.J.; Colvin, G.A. Homing and Long-Term Engraftment of Long- and Short-Term Renewal Hematopoietic Stem Cells. PLoS ONE 2012, 7, e31300. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.A.; Nucci, M.P.; Filgueiras, I.S.; Ferreira, J.M.; Nucci, L.P.; Mamani, J.B.; Alvieri, F.; Souza, L.E.B.; Rego, G.N.A.; Kondo, A.T.; et al. Noninvasive Tracking of Hematopoietic Stem Cells in a Bone Marrow Transplant Model. Cells 2020, 9, 939. [Google Scholar] [CrossRef]
- Mankoff, D.A. A definition of molecular imaging. J. Nucl. Med. 2007, 48, 18–21. [Google Scholar]
- Xie, B.-W.; Mol, I.M.; Keereweer, S.; Van Beek, E.R.; Que, I.; Snoeks, T.J.A.; Chan, A.; Kaijzel, E.L.; Löwik, C.W.G.M. Dual-Wavelength Imaging of Tumor Progression by Activatable and Targeting Near-Infrared Fluorescent Probes in a Bioluminescent Breast Cancer Model. PLoS ONE 2012, 7, e31875. [Google Scholar] [CrossRef][Green Version]
- Nucci, M.P.; Filgueiras, I.; Ferreira, J.M.; De Oliveira, F.A.; Nucci, L.P.; Mamani, J.B.; Rego, G.N.A.; Gamarra, L.F. Stem cell homing, tracking and therapeutic efficiency evaluation for stroke treatment using nanoparticles: A systematic review. World J. Stem Cells 2020, 12, 381–405. [Google Scholar] [CrossRef]
- Liang, Y.; Van Zant, G.; Szilvassy, S.J. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood 2005, 106, 1479–1487. [Google Scholar] [CrossRef]
- Morrison, S.; Wandycz, A.M.; Akashi, K.; Globerson, A.; Weissman, I.L. The aging of hematopoietic stem cells. Nat. Med. 1996, 2, 1011–1016. [Google Scholar] [CrossRef]
- Accomasso, L.; Gallina, C.; Turinetto, V.; Giachino, C. Stem Cell Tracking with Nanoparticles for Regenerative Medicine Purposes: An Overview. Stem Cells Int. 2016, 2016, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef]
- Bengtsson, N.; Kim, S.; Lin, L.; Walter, G.; Scott, E.W. Ultra-high-field MRI real-time imaging of HSC engraftment of the bone marrow niche. Leukemia 2011, 25, 1223–1231. [Google Scholar] [CrossRef][Green Version]
- Niemeyer, M.; Oostendorp, R.A.J.; Kremer, M.; Hippauf, S.; Jacobs, V.R.; Baurecht, H.; Ludwig, G.; Piontek, G.; Bekker-Ruz, V.; Timmer, S.; et al. Non-invasive tracking of human haemopoietic CD34+ stem cells in vivo in immunodeficient mice by using magnetic resonance imaging. Eur. Radiol. 2010, 20, 2184–2193. [Google Scholar] [CrossRef]
- Sweeney, S.K.; Manzar, G.S.; Zavazava, N.; Assouline, J.G. Tracking embryonic hematopoietic stem cells to the bone marrow: Nanoparticle options to evaluate transplantation efficiency. Stem Cell Res. Ther. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Walczyk, D.; Bombelli, F.B.; Monopoli, M.P.; Lynch, I.; Dawson, K.A. What the Cell “Sees” in Bionanoscience. J. Am. Chem. Soc. 2010, 132, 5761–5768. [Google Scholar] [CrossRef] [PubMed]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, M.S.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef] [PubMed]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Chen, D.; Monteiro-Riviere, N.A.; Zhang, L.W. Intracellular imaging of quantum dots, gold, and iron oxide nanoparticles with associated endocytic pathways. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 9, e1419. [Google Scholar] [CrossRef]
- Mamani, J.B.; Pavon, L.F.; Miyaki, L.A.M.; Sibov, T.T.; Rossan, F.; Silveira, P.H.; Cárdenas, W.H.Z.; Junior, E.A.; Gamarra, L.F. Intracellular labeling and quantification process by magnetic resonance imaging using iron oxide magnetic nanoparticles in rat C6 glioma cell line. Einstein 2012, 10, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef]
- Barrera, C.; Herrera, A.P.; Bezares, N.; Fachini, E.; Olayo-Valles, R.; Hinestroza, J.; Rinaldi, C. Effect of poly(ethylene oxide)-silane graft molecular weight on the colloidal properties of iron oxide nanoparticles for biomedical applications. J. Colloid Interface Sci. 2012, 377, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Alvieri, F.; Mamani, J.B.; Nucci, M.P.; Oliveira, F.A.; Filgueiras, I.; Rego, G.N.A.; De Barboza, M.F.; Da Silva, H.R.; Gamarra, L.F. Methods of Granulocyte Isolation from Human Blood and Labeling with Multimodal Superparamagnetic Iron Oxide Nanoparticles. Molecules 2020, 25, 765. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, M.Z.H.; Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Yucel, D.; Kocabas, F. Developments in Hematopoietic Stem Cell Expansion and Gene Editing Technologies. In Cell Biology and Translational Medicine, Volume 1: Stem Cells in Regenerative Medicine: Advances and Challenges; Turksen, K., Ed.; Springer: Cham, Switzerland, 2018; pp. 103–125. [Google Scholar]
- Shaikh, A.; Bhartiya, D.; Kapoor, S.; Nimkar, H. Delineating the effects of 5-fluorouracil and follicle-stimulating hormone on mouse bone marrow stem/progenitor cells. Stem Cell Res. Ther. 2016, 7, 59. [Google Scholar] [CrossRef]
- Troy, T.; Jekic-McMullen, D.; Sambucetti, L.; Rice, B. Quantitative Comparison of the Sensitivity of Detection of Fluorescent and Bioluminescent Reporters in Animal Models. Mol. Imaging 2004, 3, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Wu, J.C. Comparison of Imaging Techniques for Tracking Cardiac Stem Cell Therapy. J. Nucl. Med. 2007, 48, 1916–1919. [Google Scholar] [CrossRef] [PubMed]
- Säwen, P.; Eldeeb, M.; Erlandsson, E.; Kristiansen, T.A.; Laterza, C.; Kokaia, Z.; Karlsson, G.; Yuan, J.; Soneji, S.; Mandal, P.K.; et al. Murine HSCs contribute actively to native hematopoiesis but with reduced differentiation capacity upon aging. eLife 2018, 7. [Google Scholar] [CrossRef]
- Wierzbiński, K.R.; Szymanski, T.; Rozwadowska, N.; Rybka, J.D.; Zimna, A.; Zalewski, T.; Nowicka-Bauer, K.; Malcher, A.; Nowaczyk, M.; Krupinski, M.; et al. Potential use of superparamagnetic iron oxide nanoparticles for in vitro and in vivo bioimaging of human myoblasts. Sci. Rep. 2018, 8, 1–17. [Google Scholar] [CrossRef]
- Mamani, J.B.; Malheiros, J.M.; Cardoso, E.F.; Tannús, A.; Silveira, P.H.; Gamarra, L.F. In vivo magnetic resonance imaging tracking of C6 glioma cells labeled with superparamagnetic iron oxide nanoparticles. Einstein 2012, 10, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Arbab, A.S.; Yocum, G.T.; Rad, A.M.; Khakoo, A.Y.; Fellowes, V.; Read, E.J.; Frank, J.A. Labeling of cells with ferumoxides-protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cells. NMR Biomed. 2005, 18, 553–559. [Google Scholar] [CrossRef]
- Miyashita, S.-I.; Groombridge, A.S.; Fujii, S.; Takatsu, A.; Chiba, K.; Inagaki, K. Time-resolved ICP-MS Measurement: A New Method for Elemental and Multiparametric Analysis of Single Cells. Anal. Sci. 2014, 30, 219–224. [Google Scholar] [CrossRef]
- Kim, S.J.; Lewis, B.K.; Steiner, M.-S.; Bissa, U.V.; Dose, C.; Frank, J.A. Superparamagnetic iron oxide nanoparticles for direct labeling of stem cells andin vivoMRI tracking. Contrast Media Mol. Imaging 2016, 11, 55–64. [Google Scholar] [CrossRef]
- Thu, M.S.; Bryant, L.H.; Coppola, T.; Jordan, E.K.; Budde, M.D.; Lewis, B.K.; Chaudhry, A.; Ren, J.; Varma, N.R.S.; Arbab, A.S.; et al. Self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine for cell tracking by magnetic resonance imaging. Nat. Med. 2012, 18, 463–467. [Google Scholar] [CrossRef]
- Arbab, A.S.; Yocum, G.T.; Kalish, H.; Jordan, E.K.; Anderson, S.A.; Khakoo, A.Y.; Read, E.J.; Frank, J.A. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood 2004, 104, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Ushiki, T.; Kizaka-Kondoh, S.; Ashihara, E.; Tanaka, S.; Masuko, M.; Hirai, H.; Kimura, S.; Aizawa, Y.; Maekawa, T.; Hiraoka, M. Noninvasive Tracking of Donor Cell Homing by Near-Infrared Fluorescence Imaging Shortly after Bone Marrow Transplantation. PLoS ONE 2010, 5, e11114. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, D.J.; Bonde, J.; Hess, D.A.; Hohm, S.A.; Lahey, R.; Zhou, P.; Creer, M.H.; Piwnica-Worms, D.; Nolta, J.A. Fluorophore-Conjugated Iron Oxide Nanoparticle Labeling and Analysis of Engrafting Human Hematopoietic Stem Cells. Stem Cells 2008, 26, 517–524. [Google Scholar] [CrossRef]
- Asiedu, K.O.; Koyasu, S.; Szajek, L.P.; Choyke, P.L.; Sato, N. Bone Marrow Cell Trafficking Analyzed by 89Zr-oxine Positron Emission Tomography in a Murine Transplantation Model. Clin. Cancer Res. 2017, 23, 2759–2768. [Google Scholar] [CrossRef]
- Short, C.; Lim, H.K.; Tan, J.; O’Neill, H.C. Targeting the Spleen as an Alternative Site for Hematopoiesis. BioEssays 2019, 41. [Google Scholar] [CrossRef]
- Miller, S.C. Hematopoietic Reconstitution of Irradiated, Stem Cell-Injected Mice: Early Dynamics of Restoration of the Cell Lineages of the Spleen and Bone Marrow. J. Hematotherapy 2002, 11, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Montfort, M.J.; Olivares, C.R.; Mulcahy, J.M.; Fleming, W.H. Adult blood vessels restore host hematopoiesis following lethal irradiation. Exp. Hematol. 2002, 30, 950–956. [Google Scholar] [CrossRef]
- Cao, Y.-A.; Wagers, A.J.; Beilhack, A.; Dusich, J.; Bachmann, M.H.; Negrin, R.S.; Weissman, I.L.; Contag, C.H. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc. Natl. Acad. Sci. USA 2004, 101, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.; Ge, S.; Symbatyan, G.; Rosol, M.S.; Olch, A.J.; Crooks, G.M. Effects of Sublethal Irradiation on Patterns of Engraftment after Murine Bone Marrow Transplantation. Biol. Blood Marrow Transplant. 2011, 17, 608–619. [Google Scholar] [CrossRef]
- O’Connell, K.; Mikkola, A.M.; Stepanek, A.M.; Vernet, A.; Hall, C.D.; Sun, C.C.; Yildirim, E.; Staropoli, J.F.; Lee, J.T.; Brown, D. Practical Murine Hematopathology: A Comparative Review and Implications for Research. Comp. Med. 2015, 65, 96–113. [Google Scholar]
- Rossi, D.J.; Bryder, D.; Zahn, J.M.; Ahlenius, H.; Sonu, R.; Wagers, A.J.; Weissman, I.L. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl. Acad. Sci. USA 2005, 102, 9194–9199. [Google Scholar] [CrossRef]
- Mejia-Ramirez, E.; Florian, M.C. Understanding intrinsic hematopoietic stem cell aging. Haematologica 2019, 105, 22–37. [Google Scholar] [CrossRef]
- Steiner, D.; Gelovani, J.; Savoldo, B.; Robinson, S.N.; Decker, W.K.; Brouard, N.; Najjar, A.; Xing, D.; Yang, H.; Li, S.; et al. Noninvasive Bioluminescent Imaging Demonstrates Long-Term Multilineage Engraftment of Ex Vivo-Expanded CD34-Selected Umbilical Cord Blood Cells. Stem Cells 2009, 27, 1932–1940. [Google Scholar] [CrossRef]
- Astuti, Y.; Kramer, A.C.; Blake, A.L.; Blazar, B.R.; Tolar, J.; Taisto, M.E.; Lund, T.C. A Functional Bioluminescent Zebrafish Screen for Enhancing Hematopoietic Cell Homing. Stem Cell Rep. 2017, 8, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cheung, P.; Roth, J.C.; Wilson, D.L.; Gerson, S.L. Imaging Stem Cell-derived Persistent Foci After In Vivo Selection of Lentiviral MGMT-P140K Transduced Murine Bone Marrow Cells. Mol. Ther. 2011, 19, 1342–1352. [Google Scholar] [CrossRef]
- Saia, M.; Termanini, A.; Rizzi, N.; Mazza, M.; Barbieri, E.; Valli, D.; Ciana, P.; Gruszka, A.M.; Alcalay, M. AML1/ETO accelerates cell migration and impairs cell-to-cell adhesion and homing of hematopoietic stem/progenitor cells. Sci. Rep. 2016, 6, 34957. [Google Scholar] [CrossRef]
- Wang, X.; Rosol, M.; Ge, S.; Peterson, D.; McNamara, G.; Pollack, H.; Kohn, D.B.; Nelson, M.D.; Crooks, G.M. Dynamic tracking of human hematopoietic stem cell engraftment using in vivo bioluminescence imaging. Blood 2003, 102, 3478–3482. [Google Scholar] [CrossRef]
- Ohmori, T.; Kashiwakura, Y.; Ishiwata, A.; Madoiwa, S.; Mimuro, J.; Furukawa, Y.; Sakata, Y. Vinculin Is Indispensable for Repopulation by Hematopoietic Stem Cells, Independent of Integrin Function. J. Biol. Chem. 2010, 285, 31763–31773. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Tian, Y.; Feng, S.; Wu, Y.; Shen, X.; Chen, K.; He, Y.; Sun, Q.; Li, X.; Xu, J.; et al. In vivo single-cell lineage tracing in zebrafish using high-resolution infrared laser-mediated gene induction microscopy. eLife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Thin, M.Z.; Allan, H.; Bofinger, R.; Kostelec, T.D.; Guillaume, S.; Connell, J.J.; Patrick, P.S.; Hailes, H.C.; Tabor, A.B.; Lythgoe, M.F.; et al. Multi-modal imaging probe for assessing the efficiency of stem cell delivery to orthotopic breast tumours. Nanoscale 2020, 12, 16570–16585. [Google Scholar] [CrossRef]
- Belderbos, S.; González-Gómez, M.A.; Cleeren, F.; Wouters, J.; Piñeiro, Y.; Deroose, C.M.; Coosemans, A.; Gsell, W.; Bormans, G.; Rivas, J.; et al. Simultaneous in vivo PET/MRI using fluorine-18 labeled Fe3O4@Al(OH)3 nanoparticles: Comparison of nanoparticle and nanoparticle-labeled stem cell distribution. EJNMMI Res. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Hamilton, N.; Sabroe, I.; Renshaw, S.A. A method for transplantation of human HSCs into zebrafish, to replace humanised murine transplantation models. F1000 Res. 2018, 7. [Google Scholar] [CrossRef]
- Lopez, D.; Lin, L.; Monaghan, J.R.; Cogle, C.R.; Bova, F.J.; Maden, M.; Scott, E.W. Mapping hematopoiesis in a fully regenerative vertebrate: The axolotl. Blood 2014, 124, 1232–1241. [Google Scholar] [CrossRef]
- Parada-Kusz, M.; Penaranda, C.; Hagedorn, E.J.; Clatworthy, A.; Nair, A.V.; Henninger, J.E.; Ernst, C.; Li, B.; Riquelme, R.; Jijon, H.; et al. Generation of mouse-zebrafish hematopoietic tissue chimeric embryos for hematopoiesis and host-pathogen interaction studies. Dis. Model. Mech. 2018, 11, dmm034876. [Google Scholar] [CrossRef]
- Staal, F.J.; Spaink, H.P.; Fibbe, W.E. Visualizing Human Hematopoietic Stem Cell Trafficking In Vivo Using a Zebrafish Xenograft Model. Stem Cells Dev. 2016, 25, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Faivre, L.; Chaussard, M.; Vercellino, L.; Vanneaux, V.; Hosten, B.; Teixera, K.; Parietti, V.; Merlet, P.; Sarda-Mantel, L.; Rizzo-Padoin, N.; et al. 18F-FDG labelling of hematopoietic stem cells: Dynamic study of bone marrow homing by PET-CT imaging and impact on cell functionality. Curr. Res. Transl. Med. 2016, 64, 141–148. [Google Scholar] [CrossRef]
- Pantin, J.M.; Hoyt, R.F.; Aras, O.; Sato, N.; Chen, M.Y.; Hunt, T.; Clevenger, R.; Eclarinal, P.; Adler, S.; Choyke, P.; et al. Optimization of Intrabone Delivery of Hematopoietic Progenitor Cells in a Swine Model Using Cell Radiolabeling with [89] zirconium. Arab. Archaeol. Epigr. 2015, 15, 606–617. [Google Scholar] [CrossRef]
- Lange, S.; Steder, A.; Killian, D.; Knuebel, G.; Sekora, A.; Vogel, H.; Lindner, I.; Dunkelmann, S.; Prall, F.; Escobar, H.M.; et al. Engraftment Efficiency after Intra–Bone Marrow versus Intravenous Transplantation of Bone Marrow Cells in a Canine Nonmyeloablative Dog Leukocyte Antigen-Identical Transplantation Model. Biol. Blood Marrow Transplant. 2017, 23, 247–254. [Google Scholar] [CrossRef]
- Massollo, M.; Podestà, M.; Marini, C.; Morbelli, S.; Cassanelli, C.; Pinto, V.; Ubezio, G.; Curti, G.; Uccelli, A.; Frassoni, F.; et al. Contact with the bone marrow microenvironment readdresses the fate of transplanted hematopoietic stem cells. Exp. Hematol. 2010, 38, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Sambuceti, G.; Massollo, M.; Marini, C.; Podestà, M.; Cassanelli, C.; Morbelli, S.; Fiz, F.; Buschiazzo, A.; Capitanio, S.; Augeri, C.; et al. Trafficking and homing of systemically administered stem cells: The need for appropriate analysis tools of radionuclide images. Q. J. Nucl. Med. Mol. Imaging 2013, 57, 207–215. [Google Scholar] [PubMed]




| SPIONNIRF-Rh Concentrations (μg Fe/mL) | Mean ± SD × 108 (Photons/s) of BLI Intensity Signal | N | |
|---|---|---|---|
| Young BM-MNC | Old BM-MNC | ||
| 0 | 1.378 ± 0.009 | 1.369 ± 0.001 | 10 |
| 10 | 1.360 ± 0.001 | 1.355 ± 0.007 | 10 |
| 30 | 1.343 ± 0.001 | 1.336 ± 0.009 | 10 |
| 50 | 1.325 ± 0.003 | 1.326 ± 0.003 | 10 |
| [Fe] (µg/mL) | BM-MNC of Animal | NIRF | ICP-MS | MRI | |||
|---|---|---|---|---|---|---|---|
| Mass (pg Fe/Cell) | N° of SPION × 104/Cell | Mass (pg Fe/Cell) | N° of SPION × 104/Cell | Mass (pg Fe/Cell) | N° of SPION × 104/Cell | ||
| 10 | Young | 2.27 ± 0.09 | 2.85 ± 0.11 | 1.90 ± 0.10 | 2.39 ± 0.12 | - | - |
| Old | 2.07 ± 0.07 | 2.61 ± 0.08 | 1.67 ± 0.06 | 2.10 ± 0.74 | - | - | |
| 30 | Young | 3.06 ± 0.12 | 3.85 ± 0.15 | 3.21 ± 0.07 | 4.03 ± 0.93 | - | - |
| Old | 3.02 ± 0.09 | 3.79 ± 0.11 | 3.01 ± 0.10 | 3.78 ± 0.13 | - | - | |
| 50 | Young | 3.98 ± 0.16 | 5.00 ± 0.20 | 4.23 ± 0.09 | 5.32 ± 0.11 | 3.13 ± 0.24 | 3.93 ± 0.30 |
| Old | 3.98 ± 0.14 | 5.00 ± 0.18 | 4.00 ± 0.07 | 5.03 ± 0.81 | 3.08 ± 0.17 | 3.87 ± 0.21 | |
| Time Evaluation (Days) | Mean ± Standard Deviation of BLI Intensity (Photons/s) × 108 | |
|---|---|---|
| Young → Young | Old → Young | |
| 4 | 0 | 0 |
| 11 | 1.64 ± 0.68 | 2.98 ± 0.88 |
| 17 | 1.61 ± 0.53 | 3.11 ± 1.04 |
| 20 | 3.93 ± 0.50 | 4.04 ± 1.05 |
| 25 | 4.32 ± 1.15 | 7.44 ± 2.64 |
| 30 | 9.02 ± 1.72 | 7.88 ± 2.61 |
| 35 | 10.18 ± 2.35 | 7.16 ± 2.51 |
| 50 | 3.87 ± 2.07 | 2.89 ± 0.76 |
| 65 | 5.17 ± 2.47 | 3.36 ± 1.05 |
| 85 | 2.10 ± 1.19 | 0.96 ± 0.21 |
| 105 | 1.53 ± 0.71 | 0.71 ± 0.18 |
| 120 | 1.53 ± 0.90 | 0.55 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, F.A.; Nucci, M.P.; Mamani, J.B.; Alves, A.H.; Rego, G.N.A.; Kondo, A.T.; Hamerschlak, N.; Junqueira, M.S.; de Souza, L.E.B.; Gamarra, L.F. Multimodal Tracking of Hematopoietic Stem Cells from Young and Old Mice Labeled with Magnetic–Fluorescent Nanoparticles and Their Grafting by Bioluminescence in a Bone Marrow Transplant Model. Biomedicines 2021, 9, 752. https://doi.org/10.3390/biomedicines9070752
Oliveira FA, Nucci MP, Mamani JB, Alves AH, Rego GNA, Kondo AT, Hamerschlak N, Junqueira MS, de Souza LEB, Gamarra LF. Multimodal Tracking of Hematopoietic Stem Cells from Young and Old Mice Labeled with Magnetic–Fluorescent Nanoparticles and Their Grafting by Bioluminescence in a Bone Marrow Transplant Model. Biomedicines. 2021; 9(7):752. https://doi.org/10.3390/biomedicines9070752
Chicago/Turabian StyleOliveira, Fernando A., Mariana P. Nucci, Javier B. Mamani, Arielly H. Alves, Gabriel N. A. Rego, Andrea T. Kondo, Nelson Hamerschlak, Mara S. Junqueira, Lucas E. B. de Souza, and Lionel F. Gamarra. 2021. "Multimodal Tracking of Hematopoietic Stem Cells from Young and Old Mice Labeled with Magnetic–Fluorescent Nanoparticles and Their Grafting by Bioluminescence in a Bone Marrow Transplant Model" Biomedicines 9, no. 7: 752. https://doi.org/10.3390/biomedicines9070752
APA StyleOliveira, F. A., Nucci, M. P., Mamani, J. B., Alves, A. H., Rego, G. N. A., Kondo, A. T., Hamerschlak, N., Junqueira, M. S., de Souza, L. E. B., & Gamarra, L. F. (2021). Multimodal Tracking of Hematopoietic Stem Cells from Young and Old Mice Labeled with Magnetic–Fluorescent Nanoparticles and Their Grafting by Bioluminescence in a Bone Marrow Transplant Model. Biomedicines, 9(7), 752. https://doi.org/10.3390/biomedicines9070752









