The Role of the Bisphenol A in Diabetes and Obesity
Abstract
1. Introduction
1.1. Epidemiology of Obesity and Diabetes
1.2. Bisphenol A
1.2.1. Oestrogenic Characteristics of BPA
1.2.2. Sources and Levels of Exposure to BPA in Humans
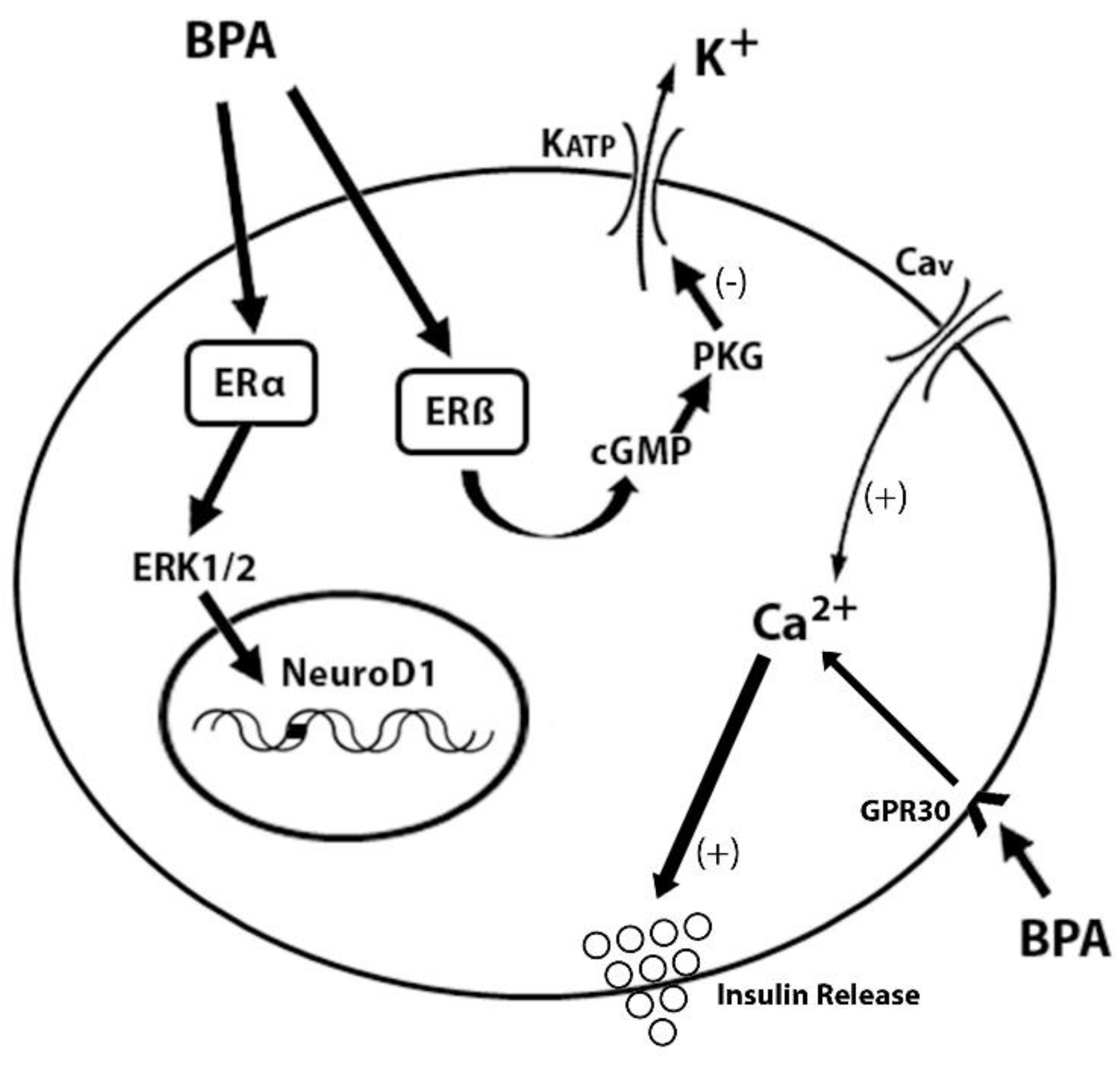
1.2.3. The Role of BPA in the Disruption of Glucose Metabolism and Insulin Resistance
2. Search Methodology
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Nutrition. Challenges. Available online: https://www.who.int/nutrition/challenges/en (accessed on 6 December 2020).
- Dhurandhar, N.V.; Schoeller, D.; Brown, A.W.; Heymsfield, S.B.; Thomas, D.; Sørensen, T.I.; Speakman, J.R.; Jeansonne, M.; Allison, D.B.; Energy Balance Measurement Working Group. Response to “Energy balance measurement: When something is not better than nothing”. Int. J. Obes. 2015, 39, 1175–1176. [Google Scholar] [CrossRef]
- Dhurandhar, N.V.; Schoeller, D.; Brown, A.W.; Heymsfield, S.B.; Thomas, D.; Sørensen, T.I.; Speakman, J.R.; Jeansonne, M.; Allison, D.B.; Energy Balance Measurement Working Group. Energy balance measurement: When something is not better than nothing. Int. J. Obes. 2015, 39, 1109–1113. [Google Scholar] [CrossRef]
- Zulet, M.A.; Puchau, B.; Navarro, C.; Martí, A.; Martínez, J.A. Biomarcadores del estado inflamatorio: Nexo de unión con la obesidad y complicaciones asociadas. Nutr. Hosp. 2007, 22, 511–527. [Google Scholar]
- Freedman, L.S.; Commins, J.M.; Moler, J.E.; Arab, L.; Baer, D.J.; Kipnis, V.; Midthune, D.; Moshfegh, A.J.; Neuhouser, M.L.; Prentice, R.L.; et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am. J. Epidemiol. 2014, 180, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Baillie-Hamilton, P.F. Chemical toxins: A hypothesis to explain the global obesity epidemic. J. Altern. Complement. Med. 2002, 8, 185–192. [Google Scholar] [CrossRef]
- Kelishadi, R.; Poursafa, P.; Jamshidi, F. Role of environmental chemicals in obesity: A systematic review on the current evidence. J. Environ. Public Health 2013, 896789. [Google Scholar] [CrossRef]
- Dodds, E.C.; Lawson, W. Synthetic strogenic Agents without the Phenanthrene Nucleus. Nature 1936, 137, 996. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-disrupting chemicals and public health protection: A statement of principles from The Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef]
- Bergman, A.; Brandt, I.; Brouwer, A.; Harrison, P.; Holmes, P.; Humfrey, C.; Keiding, N.; Randall, G.; Sharpe, R.; Skakkebaek, N. In Proceedings of the European Workshop on the Impact of Endocrine Disrupters on Human Health and Wildlife, Weybridge, UK, 2–4 December 1996.
- Cunha, S.C.; Almeida, C.; Mendes, E.; Fernandes, J.O. Simultaneous determination of bisphenol A and bisphenol B in beverages and powdered infant formula by dispersive liquid-liquid micro-extraction and heart-cutting multidimensional gas chromatography-mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2011, 28, 513–526. [Google Scholar] [CrossRef]
- La Merrill, M.; Birnbaum, L.S. Childhood obesity and environmental chemicals. Mt. Sinai J. Med. 2011, 78, 22–48. [Google Scholar] [CrossRef]
- Agay-Shay, K.; Martinez, D.; Valvi, D.; Garcia-Esteban, R.; Basagaña, X.; Robinson, O.; Casas, M.; Sunyer, J.; Vrijheid, M. Exposure to Endocrine-Disrupting Chemicals during Pregnancy and Weight at 7 Years of Age: A Multi-pollutant Approach. Environ. Health Perspect. 2015, 123, 1030–1037. [Google Scholar] [CrossRef]
- Scinicariello, F.; Buser, M.C. Urinary polycyclic aromatic hydrocarbons and childhood obesity: NHANES (2001–2006). Environ. Health Perspect. 2014, 122, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, M.; Chen, B.; Xu, M.; Xu, Y.; Huang, Y.; Lu, J.; Chen, Y.; Wang, W.; Li, X.; et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2012, 97, E223–E227. [Google Scholar] [CrossRef]
- Carwile, J.L.; Michels, K.B. Urinary bisphenol A and obesity: NHANES 2003-2006. Environ. Res. 2011, 111, 825–830. [Google Scholar] [CrossRef]
- Bhandari, R.; Xiao, J.; Shankar, A. Urinary bisphenol A and obesity in U.S. children. Am. J. Epidemiol. 2013, 177, 1263–1270. [Google Scholar] [CrossRef]
- Trasande, L.; Attina, T.M.; Blustein, J. Association between urinary bisphenol a concentration and obesity prevalence in children and adolescents. JAMA 2012, 308, 1113–1121. [Google Scholar] [CrossRef]
- Thayer, K.A.; Doerge, D.R.; Hunt, D.; Schurman, S.H.; Twaddle, N.C.; Churchwell, M.I.; Garantziotis, S.; Kissling, G.E.; Easterling, M.R.; Bucher, J.R.; et al. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ. Int. 2015, 83, 107–115. [Google Scholar] [CrossRef]
- Deckelbaum, R.J.; Williams, C.L. Childhood obesity: The health issue. Obes. Res. 2001, 9 (Suppl. S4), 239S–243S. [Google Scholar] [CrossRef]
- Caballero, B. The global epidemic of obesity: An overview. Epidemiol. Rev. 2007, 29, 1–5. [Google Scholar] [CrossRef]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Pérez-Rodrigo, C. Current mapping of obesity. Nutr. Hosp. 2013, 28 (Suppl. S5), 21–31. [Google Scholar] [CrossRef]
- National Health Survey Spain 2017. Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/encuestaNacional/encuestaNac2017/ENSE17_pres_web.pdf (accessed on 10 May 2021).
- Martos-Moreno, G.A.; Gil-Campos, M.; Bueno, G.; Bahillo, P.; Bernal, S.; Feliu, A.; Lechuga-Sancho, A.M.; Palomo, E.; Ruiz, R.; Vela, A.; et al. Obesity associated metabolic impairment is evident at early ages: Spanish collaborative study. Nutr. Hosp. 2014, 30, 787–793. [Google Scholar] [CrossRef]
- Must, A. Does overweight in childhood have an impact on adult health? Nutr. Rev. 2003, 61, 139–142. [Google Scholar] [CrossRef]
- World Health Organization. Diabetes. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 2 June 2021).
- World Health Organization. Diabetes. Available online: https://www.who.int/en/news-room/fact-sheets/detail/diabetes (accessed on 2 June 2021).
- World Health Organization. Global Report on Diabetes. Available online: https://www.who.int/publications/i/item/9789241565257 (accessed on 2 June 2021).
- American Diabetes Association Classification and Diagnosis of Diabetes. 2016 Standards of Medical Care in Diabetes. Diabetes Care 2016, 39, S13–S22. [Google Scholar] [CrossRef]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF diabetes atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. [Google Scholar] [CrossRef]
- Yang, C.; Lee, H.K.; Kong, A.P.S.; Lim, L.L.; Cai, Z.; Chung, A.C.K. Early-life exposure to endocrine disrupting chemicals associates with childhood obesity. Ann. Pediatr. Endocrinol. Metab. 2018, 23, 182–195. [Google Scholar] [CrossRef]
- Birnbaum, L.S.; Bucher, J.R.; Collman, G.W.; Zeldin, D.C.; Johnson, A.F.; Schug, T.T.; Heindel, J.J. Consortium-based science: The NIEHS’s multipronged, collaborative approach to assessing the health effects of bisphenol A. Environ. Health Perspect. 2012, 120, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.T.; Vogel, S.A.; Vandenberg, L.N.; Braun, J.M.; Hauser, R.; Taylor, J.A.; vom Saal, F.S.; Heindel, J.J. Bisphenol, A. In Dioxins and Health: Including Other Persistent Organic Pollutants and Endocrine Disruptors, 3rd ed.; Schecter, A., Ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 381–414. [Google Scholar]
- Pence, H.E.; Williams, A. ChemSpider: An Online Chemical Information Resource. J. Chem. Educ. 2010, 87, 1123–1124. [Google Scholar] [CrossRef]
- Welshons, W.V.; Thayer, K.A.; Judy, B.M.; Taylor, J.A.; Curran, E.M.; vom Saal, F.S. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ. Health Perspect. 2003, 111, 994–1006. [Google Scholar] [CrossRef]
- Welshons, W.V.; Nagel, S.C.; vom Saal, F.S. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 2006, 147 (Suppl. S6), S56–S69. [Google Scholar] [CrossRef]
- Routledge, E.J.; White, R.; Parker, M.G.; Sumpter, J.P. Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) alpha and ERbeta. J. Biol. Chem. 2000, 275, 35986–35993. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Ropero, A.B.; Soriano, S.; García-Arévalo, M.; Ripoll, C.; Fuentes, E.; Quesada, I.; Nadal, A. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol. Cell Endocrinol. 2012, 355, 201–207. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Ropero, A.B.; Carrera, M.P.; Cederroth, C.R.; Baquié, M.; Gauthier, B.R.; Nef, S.; Stefani, E.; Nadal, A. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS ONE 2008, 3, e2069. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N. Non-monotonic dose responses in studies of endocrine disrupting chemicals: Bisphenol a as a case study. Dose Response 2013, 12, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Kannan, K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J. Agric Food Chem. 2013, 61, 4655–4662. [Google Scholar] [CrossRef]
- Rosenmai, A.K.; Dybdahl, M.; Pedersen, M.; Alice van Vugt-Lussenburg, B.M.; Wedebye, E.B.; Taxvig, C.; Vinggaard, A.M. Are structural analogues to bisphenol a safe alternatives? Toxicol. Sci. 2014, 139, 35–47. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Wu, Y.; Zhao, Y.; Luo, F.; Li, S.; Yang, L.; Moez, E.K.; Dinu, I.; Martin, J.W. Bisphenol A Metabolites and Bisphenol S in Paired Maternal and Cord Serum. Environ. Sci. Technol. 2017, 51, 2456–2463. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L.; Corriveau, J.; Popovic, S. Sources of low concentrations of bisphenol A in canned beverage products. J. Food Prot. 2010, 73, 1548–1551. [Google Scholar] [CrossRef] [PubMed]
- Talsness, C.E.; Andrade, A.J.; Kuriyama, S.N.; Taylor, J.A.; vom Saal, F.S. Components of plastic: Experimental studies in animals and relevance for human health. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2079–2096. [Google Scholar] [CrossRef]
- Konieczna, A.; Rutkowska, A.; Rachoń, D. Health risk of exposure to Bisphenol A (BPA). Rocz. Panstw. Zakl. Hig. 2015, 66, 5–11. [Google Scholar] [PubMed]
- Abraham, A.; Chakraborty, P. A review on sources and health impacts of bisphenol A. Rev. Environ. Health 2020, 35, 201–210. [Google Scholar] [CrossRef]
- Rahman, M.S.; Adegoke, E.O.; Pang, M.G. Drivers of owning more BPA. J. Hazard. Mater. 2021, 417, 126076. [Google Scholar] [CrossRef]
- Goodson, A.; Robin, H.; Summerfield, W.; Cooper, I. Migration of bisphenol A from can coatings--effects of damage, storage conditions and heating. Food Addit. Contam. 2004, 21, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Repossi, A.; Farabegoli, F.; Gazzotti, T.; Zironi, E.; Pagliuca, G. Bisphenol A in Edible Part of Seafood. Ital. J. Food Saf. 2016, 5, 5666. [Google Scholar] [CrossRef]
- Biedermann, S.; Tschudin, P.; Grob, K. Transfer of bisphenol A from thermal printer paper to the skin. Anal. Bioanal. Chem. 2010, 398, 571–576. [Google Scholar] [CrossRef]
- Ehrlich, S.; Calafat, A.M.; Humblet, O.; Smith, T.; Hauser, R. Handling of thermal receipts as a source of exposure to bisphenol A. JAMA 2014, 311, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Wong, C.K.; Zheng, J.S.; Bouwman, H.; Barra, R.; Wahlström, B.; Neretin, L.; Wong, M.H. Bisphenol A (BPA) in China: A review of sources, environmental levels, and potential human health impacts. Environ. Int. 2012, 42, 91–99. [Google Scholar] [CrossRef]
- Schönfelder, G.; Wittfoht, W.; Hopp, H.; Talsness, C.E.; Paul, M.; Chahoud, I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002, 110, A703–A707. [Google Scholar] [CrossRef]
- Todaka, E.; Mori, C. Necessity to establish new risk assessment and risk communication for human fetal exposure to multiple endocrine disruptors in Japan. Congenit. Anom. 2002, 42, 87–93. [Google Scholar] [CrossRef]
- Ikezuki, Y.; Tsutsumi, O.; Takai, Y.; Kamei, Y.; Taketani, Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 2002, 17, 2839–2841. [Google Scholar] [CrossRef]
- Ye, X.; Kuklenyik, Z.; Needham, L.L.; Calafat, A.M. Measuring environmental phenols and chlorinated organic chemicals in breast milk using automated on-line column-switching-high performance liquid chromatography-isotope dilution tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 831, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Brede, C.; Fjeldal, P.; Skjevrak, I.; Herikstad, H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit. Contam. 2003, 20, 684–689. [Google Scholar] [CrossRef] [PubMed]
- LOI n° 2018-938 du 30 Octobre 2018 Pour L’équilibre des Relations Commerciales Dans le Secteur Agricole et al. Imentaire et une Alimentation Saine, Durable et Accessible à Tous. Available online: https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000037547946?r=540rP0j2hP (accessed on 12 May 2021).
- EFSA Explains the Safety of Bisphenol A. Available online: https://www.efsa.europa.eu/en/corporate/pub/factsheetbpa150121 (accessed on 5 December 2020).
- Oldring, P.K.; Castle, L.; O’Mahony, C.; Dixon, J. Estimates of dietary exposure to bisphenol A (BPA) from light metal packaging using food consumption and packaging usage data: A refined deterministic approach and a fully probabilistic (FACET) approach. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2014, 31, 466–489. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Toxicological and Health Aspects of Bisphenol A: Report of Joint FAO/WHO Expert Meeting 2–5 November 2010 and Report of Stakeholder Meeting on Bisphenol A; WHO: Ottawa, ON, Canada, 2011. [Google Scholar]
- Pjanic, M. The role of polycarbonate monomer bisphenol-A in insulin resistance. PeerJ 2017, 5, e3809. [Google Scholar] [CrossRef]
- Mitra, S.W.; Hoskin, E.; Yudkovitz, J.; Pear, L.; Wilkinson, H.A.; Hayashi, S.; Pfaff, D.W.; Ogawa, S.; Rohrer, S.P.; Schaeffer, J.M.; et al. Immunolocalization of estrogen receptor beta in the mouse brain: Comparison with estrogen receptor alpha. Endocrinology 2003, 144, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.S.; Bulayeva, N.N.; Wozniak, A.L.; Alyea, R.A. Xenoestrogens are potent activators of nongenomic estrogenic responses. Steroids 2007, 72, 124–134. [Google Scholar] [CrossRef]
- Thomas, P.; Dong, J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: A potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006, 102, 175–179. [Google Scholar] [CrossRef]
- Viñas, R.; Watson, C.S. Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: Effects on cell functions. Environ. Health Perspect. 2013, 121, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenschein, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In vitro molecular mechanisms of bisphenol a action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kuzuya, H.; Nakao, K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.P.; Tiano, J.P.; Liu, S.; Hewitt, S.C.; Le May, C.; Dalle, S.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S.; Korach, K.S.; Mauvais-Jarvis, F. Extranuclear estrogen receptor-alpha stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 13057–13062. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Morimoto, S.; Ripoll, C.; Fuentes, E.; Nadal, A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ. Health Perspect. 2006, 114, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Nadal, A.; Alonso-Magdalena, P.; Soriano, S.; Quesada, I.; Ropero, A.B. The pancreatic beta-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Mol. Cell Endocrinol. 2009, 304, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Mack, L.R.; Tomich, P.G. Gestational Diabetes: Diagnosis, Classification, and Clinical Care. Obstet. Gynecol. Clin. N. Am. 2017, 44, 207–217. [Google Scholar] [CrossRef]
- Catalano, P.M.; Kirwan, J.P.; Haugel-de Mouzon, S.; King, J. Gestational diabetes and insulin resistance: Role in short- and long-term implications for mother and fetus. J. Nutr. 2003, 133, 1674S–1683S. [Google Scholar] [CrossRef] [PubMed]
- Baz, B.; Riveline, J.P.; Gautier, J.F. Endocrinology of pregnancy: Gestational diabetes mellitus: Definition, aetiological and clinical aspects. Eur. J. Endocrinol. 2016, 174, R43–R51. [Google Scholar] [CrossRef]
- Lopez, L.M.; Grimes, D.A.; Schulz, K.F. Steroidal contraceptives: Effect on carbohydrate metabolism in women without diabetes mellitus. Cochrane Database Syst. Rev. 2019, 11, CD006133. [Google Scholar] [CrossRef]
- Godsland, I.F.; Crook, D.; Simpson, R.; Proudler, T.; Felton, C.; Lees, B.; Anyaoku, V.; Devenport, M.; Wynn, V. The effects of different formulations of oral contraceptive agents on lipid and carbohydrate metabolism. N. Engl. J. Med. 1990, 323, 1375–1381. [Google Scholar] [CrossRef]
- Batista, T.M.; Alonso-Magdalena, P.; Vieira, E.; Amaral, M.E.; Cederroth, C.R.; Nef, S.; Quesada, I.; Carneiro, E.M.; Nadal, A. Short-term treatment with bisphenol-A leads to metabolic abnormalities in adult male mice. PLoS ONE 2012, 7, e33814. [Google Scholar] [CrossRef]
- García-Arevalo, M.; Alonso-Magdalena, P.; Rebelo Dos Santos, J.; Quesada, I.; Carneiro, E.M.; Nadal, A. Exposure to bisphenol-A during pregnancy partially mimics the effects of a high-fat diet altering glucose homeostasis and gene expression in adult male mice. PLoS ONE 2014, 9, e100214. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Magdalena, P.; Vieira, E.; Soriano, S.; Menes, L.; Burks, D.; Quesada, I.; Nadal, A. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ. Health Perspect. 2010, 118, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Magdalena, P.; García-Arévalo, M.; Quesada, I.; Nadal, A. Bisphenol-A treatment during pregnancy in mice: A new window of susceptibility for the development of diabetes in mothers later in life. Endocrinology 2015, 156, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Ohlstein, J.F.; Strong, A.L.; McLachlan, J.A.; Gimble, J.M.; Burow, M.E.; Bunnell, B.A. Bisphenol A enhances adipogenic differentiation of human adipose stromal/stem cells. J. Mol. Endocrinol. 2014, 53, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.G.; Boudreau, A.; Ahmed, S.; Atlas, E. In Vitro Effects of Bisphenol a β-D-Glucuronide (BPA-G) on Adipogenesis in Human and Murine Preadipocytes. Environ. Health Perspect. 2015, 123, 1287–1293. [Google Scholar] [CrossRef]
- Valentino, R.; D’Esposito, V.; Passaretti, F.; Liotti, A.; Cabaro, S.; Longo, M.; Perruolo, G.; Oriente, F.; Beguinot, F.; Formisano, P. Bisphenol-A impairs insulin action and up-regulates inflammatory pathways in human subcutaneous adipocytes and 3T3-L1 cells. PLoS ONE 2013, 8, e82099. [Google Scholar] [CrossRef]
- Dai, Y.E.; Chen, W.; Qi, H.; Liu, Q.Q. Effect of bisphenol a on SOCS-3 and insulin signaling transduction in 3T3-L1 adipocytes. Mol. Med. Rep. 2016, 14, 331–336. [Google Scholar] [CrossRef]
- Angle, B.M.; Do, R.P.; Ponzi, D.; Stahlhut, R.W.; Drury, B.E.; Nagel, S.C.; Welshons, W.V.; Besch-Williford, C.L.; Palanza, P.; Parmigiani, S.; et al. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): Evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod. Toxicol. 2013, 42, 256–268. [Google Scholar] [CrossRef]
- Susiarjo, M.; Xin, F.; Bansal, A.; Stefaniak, M.; Li, C.; Simmons, R.A.; Bartolomei, M.S. Bisphenol a exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology 2015, 156, 2049–2058. [Google Scholar] [CrossRef]
- Bansal, A.; Li, C.; Xin, F.; Duemler, A.; Li, W.; Rashid, C.; Bartolomei, M.S.; Simmons, R.A. Transgenerational effects of maternal bisphenol: A exposure on offspring metabolic health. J. Dev. Orig. Health Dis. 2019, 10, 164–175. [Google Scholar] [CrossRef] [PubMed]
- García-Arévalo, M.; Alonso-Magdalena, P.; Servitja, J.M.; Boronat-Belda, T.; Merino, B.; Villar-Pazos, S.; Medina-Gómez, G.; Novials, A.; Quesada, I.; Nadal, A. Maternal Exposure to Bisphenol-A during Pregnancy Increases Pancreatic β-Cell Growth During Early Life in Male Mice Offspring. Endocrinology 2016, 157, 4158–4171. [Google Scholar] [CrossRef]
- Whitehead, R.; Guan, H.; Arany, E.; Cernea, M.; Yang, K. Prenatal exposure to bisphenol A alters mouse fetal pancreatic morphology and islet composition. Horm. Mol. Biol. Clin. Investig. 2016, 25, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Lin, Y.; Li, Y.; Ying, C.; Chen, J.; Song, L.; Zhou, Z.; Lv, Z.; Xia, W.; Chen, X.; et al. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology 2011, 152, 3049–3061. [Google Scholar] [CrossRef]
- Veiga-Lopez, A.; Moeller, J.; Sreedharan, R.; Singer, K.; Lumeng, C.; Ye, W.; Pease, A.; Padmanabhan, V. Developmental programming: Interaction between prenatal BPA exposure and postnatal adiposity on metabolic variables in female sheep. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E238–E247. [Google Scholar] [CrossRef]
- Soriano, S.; Alonso-Magdalena, P.; García-Arévalo, M.; Novials, A.; Muhammed, S.J.; Salehi, A.; Gustafsson, J.A.; Quesada, I.; Nadal, A. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: Role of estrogen receptor β. PLoS ONE 2012, 7, e31109. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, P.; Qian, W.; Li, Y.; Zhao, J.; Huan, F.; Wang, J.; Xiao, H. Perinatal bisphenol A exposure and adult glucose homeostasis: Identifying critical windows of exposure. PLoS ONE 2013, 8, e64143. [Google Scholar] [CrossRef]
- Rubin, B.S.; Paranjpe, M.; DaFonte, T.; Schaeberle, C.; Soto, A.M.; Obin, M.; Greenberg, A.S. Perinatal BPA exposure alters body weight and composition in a dose specific and sex specific manner: The addition of peripubertal exposure exacerbates adverse effects in female mice. Reprod. Toxicol. 2017, 68, 130–144. [Google Scholar] [CrossRef]
- Lee, H.A.; Kim, Y.J.; Lee, H.; Gwak, H.S.; Park, E.A.; Cho, S.J.; Kim, H.S.; Ha, E.H.; Park, H. Effect of urinary bisphenolA on androgenic hormones and insulin resistance in preadolescent girls: A pilot study from the Ewha Birth & Growth Cohort. Int. J. Environ. Res. Public Health 2013, 10, 5737–5749. [Google Scholar] [CrossRef]
- Wang, B.; Li, M.; Zhao, Z.; Lu, J.; Chen, Y.; Xu, Y.; Xu, M.; Wang, W.; Wang, T.; Bi, Y.; et al. Urinary bisphenol A concentration and glucose homeostasis in non-diabetic adults: A repeated-measures, longitudinal study. Diabetologia 2019, 62, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Savastano, S.; Tarantino, G.; D’Esposito, V.; Passaretti, F.; Cabaro, S.; Liotti, A.; Liguoro, D.; Perruolo, G.; Ariemma, F.; Finelli, C.; et al. Bisphenol-A plasma levels are related to inflammatory markers, visceral obesity and insulin-resistance: A cross-sectional study on adult male population. J. Transl. Med. 2015, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Stahlhut, R.W.; Myers, J.P.; Taylor, J.A.; Nadal, A.; Dyer, J.A.; Vom Saal, F.S. Experimental BPA Exposure and Glucose-Stimulated Insulin Response in Adult Men and Women. J. Endocr. Soc. 2018, 2, 1173–1187. [Google Scholar] [CrossRef] [PubMed]

| Ref. | Year | No. of Subjects | Exposure Dose | Stage | Conclusions |
|---|---|---|---|---|---|
| [81] | 2012 | 2 groups 6–9 mice/group | 100 µg/kg body weight/day | 3-month-old male OF-1 mice | The mice exposed to BPA developed insulin resistance and hyperinsulinaemia and increased secretion in response to glucose stimulation. They also presented a lower body temperature and were less active than those in the control group. |
| [83] | 2010 | 3 groups 8–13 mice/group | 10 or 100 μg/kg body weight/day | 3-month pregnant OF-1 mice | BPA produces metabolic disorders that disrupt glucose homoeostasis, which is considered a risk factor for diabetes. The offspring has lower glucose tolerance, as well as higher insulin resistance and higher plasma levels of insulin, leptin, triglycerides, and glycerol. |
| [90] | 2013 | 7 groups 13–17 mice/group | 5, 50, 500, 5000, and 50,000 μg/kg body weight/day | 3-month-old nulliparous female CD-1 | The mice exposed to BPA at four different doses significantly lose the ability to maintain normal glucose levels and show greater insulin insensitivity. |
| [91] | 2015 | 3 groups 9–13 mice/group | lower dose: 10 μg/kg body weight/day; upper dose: 10 mg/kg body weight/day | Six-week-old virgin C57BL/6 females | Early life exposure to environmental EDs can disrupt the metabolism of the developing foetus, as well as that of its offspring. Weight gain was observed in male offspring compared to controls, as well as glucose intolerance during adulthood. |
| [92] | 2019 | 3 groups 11 mice/group | lower dose: 10 μg/kg body weight/day; upper dose: 10 mg/kg body weight/day | C57BL/6J virgin female mice (F0) crossed with 8–10 weeks old C57BL/6J males | This study suggests that BPA exposure can be passed down through generations by epigenetic modifications. The third generation has a lower number of pancreatic ß cells and an increase in insulin secretion. |
| [93] | 2016 | Control group: 73 mice; BPA10 group: 63; BPA100 group: 56. | 10 or 100 μg/kg body weight/day | 3-month pregnant OF-1 mice | The offspring of pregnant females exposed to BPA at doses of either 10 μg/kg/day or higher doses of 100 μg/kg/day presented higher levels of insulin in the blood compared to the control group. |
| [94] | 2016 | Not Available | 5 mg/kg body weight/day | 6-8-week-old breeding pairs of adult C57BL/6 mice | Exposure to BPA increases glucagon expression and the number of ß-cell islets in the pancreas that express it, suggesting that BPA may disrupt pancreatic cell differentiation. |
| [95] | 2011 | 64 mice (32 female and 32 male) | 50, 250, or 1250 μg/kg body weight/day. Dosages were adjusted daily for body weight changes of pregnant rats (2.0 mL/kg body weight) | Virgin female (270–300 g) and male (350–400 g) genitor Wistar rats. Pups measured on postnatal days 1, 5 10, 15, and 21 | Perinatal exposure to BPA is implicated in the development of obesity and compromises the proper metabolism functioning, particularly when exposure is at small doses (50 ug/kg). The males of the offspring present hyperinsulinaemia and metabolic disturbances that increase with age. |
| [96] | 2016 | Groups of 6–9 female sheep | 0.05, 0.5, or 5 mg/kg body weight/day from days 30 through 90 of gestation | Adult Suffolk breed sheep (2–5 y old) | Exposure to BPA during foetal life at levels equivalent to those found in humans can disrupt metabolism, leading to insulin resistance and adipocyte hypertrophy. The defects produced in the metabolism of glucose and insulin are similar to those produced by a high-fat diet. |
| [97] | 2012 | Not Available | 1 nM | Adult C57 female mice | Environmentally significant doses of BPA have an insulinotropic action on the islets of Langerhans. |
| [98] | 2013 | 15–30 mice per group | 100 µg/kg body weight/day | 8 weeks old male and female C57BL6 mice (F0) | The results suggest that exposure to BPA could contribute to the appearance of metabolic disorders that lead to significant disruptions in glucose homoeostasis. Furthermore, the effects of BPA seem to be dose-, sex-, and time-dependent and are greater if exposure occurs during the foetal development period. |
| [99] | 2017 | 15–30 mice per group | 0, 0.25, 2.5, 25, or 250 μg BPA/kg body weight/day from GD 8 to lactational day 16 | Breeding pairs of adult C57BL/6 mice | The effects of BPA are dose- and sex-dependent. The second exposure exacerbated the adverse effects of BPA exposure in females, who presented signs of obesity and metabolic disturbances such as increased triglyceride levels, hyperinsulinaemia, and insulin resistance. |
| Ref. | Year | Subjects | Conclusions |
|---|---|---|---|
| [15] | 2012 | 3390 aged 40 y or older | Levels of BPA in urine are positively associated with obesity, increased abdominal fat, and insulin resistance in Chinese adults and the elderly. |
| [100] | 2013 | 48 children aged 7 to 8 years | Exposure to BPA in pre-adolescents can disrupt endocrine metabolism due to its ability to act as a natural oestrogen. In the group exposed to BPA, the levels of base oestradiol and androstenedione were significantly higher than in the control group. A year later, the girls who had been exposed to BPA showed elevated levels of these hormones as well as insulin resistance. |
| [101] | 2019 | 2336 aged 40 y or older followed for 4 years | Exposure to BPA was independently associated with impaired glucose homoeostasis prior to development of diabetes in middle-aged and elderly women, but not in men. In women, increased urinary concentrations of BPA were associated with an increased risk of developing hyperglycaemia and dysfunction of pancreatic ß cells. |
| [102] | 2015 | 76 Caucasian male 53.5 ± 5.7 mean y age | In subjects with higher levels of BPA in the blood, higher levels of inflammation markers were found, and they had higher percentages of visceral fat and higher metabolic syndrome and insulin resistance prevalence. |
| [103] | 2018 | 8 healthy adult males and 8 adult females (postmenopausal), with obesity and prediabetes by HbA1c | This study suggests that exposure to BPA at a dose of 50 ug/kg may disrupt the glucose-stimulated insulin response in humans. A strong positive relationship is found between HbA1c and the percentage changes in the insulin index. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Bermejo, M.; Mas-Pérez, I.; Murillo-Llorente, M.T. The Role of the Bisphenol A in Diabetes and Obesity. Biomedicines 2021, 9, 666. https://doi.org/10.3390/biomedicines9060666
Pérez-Bermejo M, Mas-Pérez I, Murillo-Llorente MT. The Role of the Bisphenol A in Diabetes and Obesity. Biomedicines. 2021; 9(6):666. https://doi.org/10.3390/biomedicines9060666
Chicago/Turabian StylePérez-Bermejo, Marcelino, Irene Mas-Pérez, and Maria Teresa Murillo-Llorente. 2021. "The Role of the Bisphenol A in Diabetes and Obesity" Biomedicines 9, no. 6: 666. https://doi.org/10.3390/biomedicines9060666
APA StylePérez-Bermejo, M., Mas-Pérez, I., & Murillo-Llorente, M. T. (2021). The Role of the Bisphenol A in Diabetes and Obesity. Biomedicines, 9(6), 666. https://doi.org/10.3390/biomedicines9060666







