Prognostic Relevance of CD4+, CD8+ and FOXP3+ TILs in Oral Squamous Cell Carcinoma and Correlations with PD-L1 and Cancer Stem Cell Markers
Abstract
1. Introduction
2. Materials and methods
2.1. Patients and Tissue Specimens
2.2. Immunohistochemistry (IHC)
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
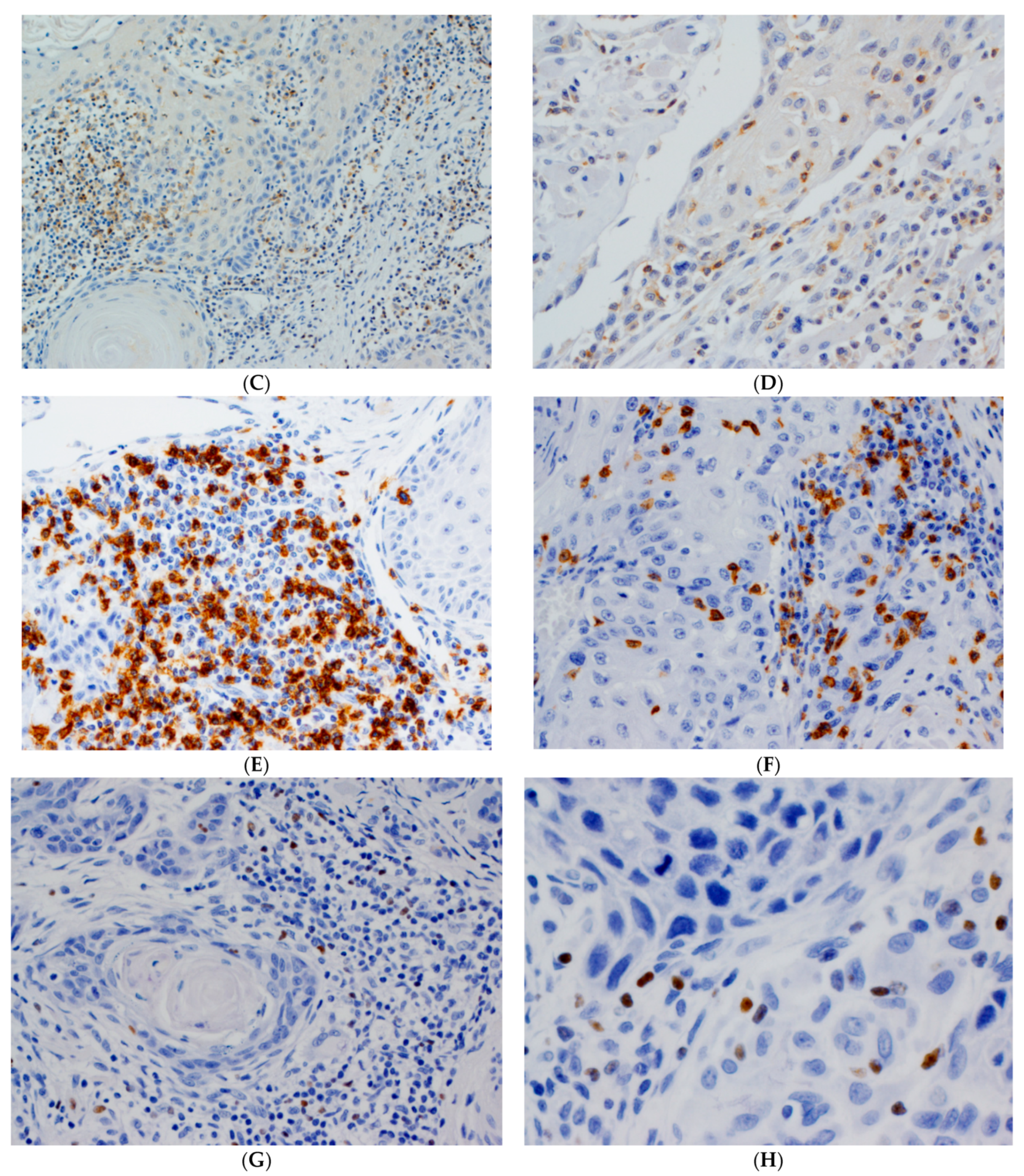
3.2. Immunohistochemical Evaluation of CD4+, CD8+ and FOXP3+ TILs in OSCC Tissue Specimens
3.3. Associations between CD4+, CD8+ and FOXP3+ TILs Density and Clinicopathological Variables
3.4. Associations between CD4+, CD8+, FOXP3+ TILs Density and Expression of CSC Markers
3.5. Associations between CD4+, CD8+, FOXP3+ TILs Density and PD-L1 Expression
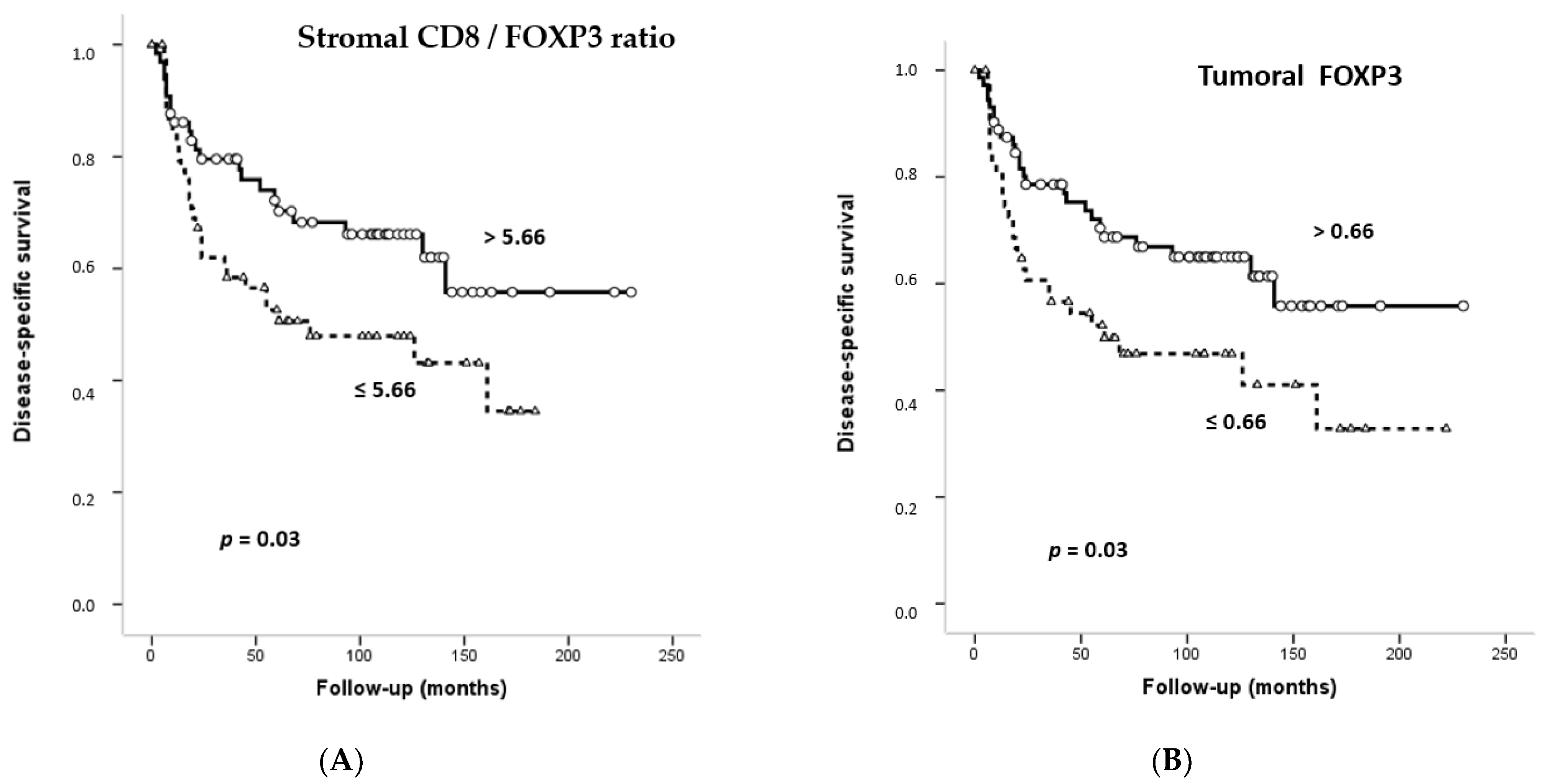
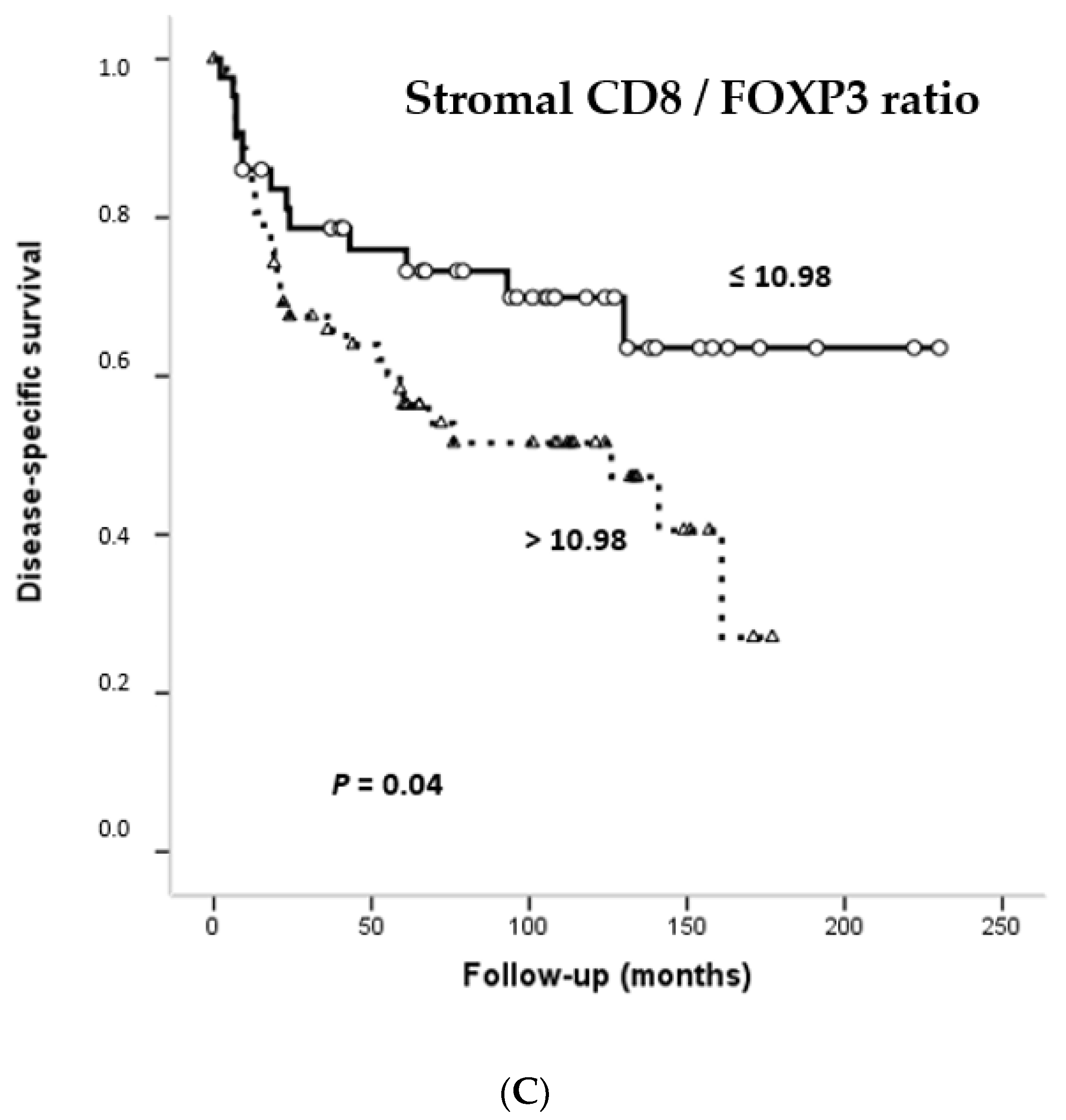
3.6. Impact of CD4+, CD8+ and FOXP3+ Infiltrating TILs on the Survival of OSCC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferris, R.L. Immunology and immunotherapy of head and neck cancer. J. Clin. Oncol. 2015, 33, 3293–3304. [Google Scholar] [CrossRef]
- Arneth, B. Tumor microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Nguyen, N.; Bellile, E.; Thomas, D.; McHugh, J.; Rozek, L.; Virani, S.; Peterson, L.; Carey, T.E.; Walline, H.; Moyer, J.; et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck 2016, 38, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Luen, S.; Virassamy, B.; Savas, P.; Salgado, R.; Loi, S. The genomic landscape of breast cancer and its interaction with host immunity. Breast 2016, 29, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef]
- Kythreotou, A.; Siddique, A.; Mauri, F.A.; Bower, M.; Pinato, D.J. PD-L1. J. Clin. Pathol. 2018, 71, 189–194. [Google Scholar] [CrossRef]
- Valle, S.; Martin-Hijano, L.; Alcalá, S.; Alonso-Nocelo, M.; Sainz, B., Jr. The ever-evolving concept of the cancer stem cell in pancreatic cancer. Cancers 2018, 10, 33. [Google Scholar] [CrossRef]
- Martin-Hijano, L.; Sainz, B., Jr. The interactions between cancer stem cells and the innate interferon signaling pathway. Front. Immunol. 2020, 11, 526. [Google Scholar] [CrossRef]
- Davis, S.J.; Divi, V.; Owen, J.H.; Bradford, C.R.; Carey, T.E.; Papagerakis, S.; Prince, M.E.P. Metastatic potential of cancer stem cells in head and neck squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- de Vicente, J.C.; Rodríguez-Santamarta, T.; Rodrigo, J.P.; Allonca, E.; Vallina, A.; Singhania, A.; Donate-Pérez Del Molino, P.; García-Pedrero, J.M. The emerging role of NANOG as an early cancer risk biomarker in patients with oral potentially malignant disorders. J. Clin. Med. 2019, 8, 1376. [Google Scholar] [CrossRef]
- de Vicente, J.C.; Donate-Pérez Del Molino, P.; Rodrigo, J.P.; Allonca, E.; Hermida-Prado, F.; Granda-Díaz, R.; Rodríguez Santamarta, T.; García-Pedrero, J.M. SOX2 expression is an independent predictor of oral cancer progression. J. Clin. Med. 2019, 8, 1744. [Google Scholar] [CrossRef] [PubMed]
- Major, A.G.; Pitty, L.P.; Farah, C.S. Cancer stem cell markers in head and neck squamous cell carcinoma. Stem Cells Int. 2013, 2013, 319489. [Google Scholar] [CrossRef] [PubMed]
- Grubelnik, G.; Boštjančič, E.; Grošelj, A.; Zidar, N. Expression of NANOG and its regulation in oral squamous cell carcinoma. Biomed. Res. Int. 2020, 2020, 8573793. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.L.; Yu, C.C.; Chang, Y.C.; Yu, C.H.; Chou, M.Y. Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J. Oral. Pathol. Med. 2011, 40, 621–628. [Google Scholar] [CrossRef]
- Chiou, S.H.; Yu, C.C.; Huang, C.Y.; Lin, S.C.; Liu, C.J.; Tsai, T.H.; Chou, S.H.; Chien, C.S.; Ku, H.H.; Lo, J.F. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin. Cancer Res. 2008, 14, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Curtarelli, R.B.; Gonçalves, J.M.; Dos Santos, L.G.P.; Savi, M.G.; Nör, J.E.; Mezzomo, L.A.M.; Rodríguez Cordeiro, M.M. Expression of cancer stem cell biomarkers in human head and neck carcinomas: A systematic review. Stem. Cell Rev. Rep. 2018, 14, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Oh, S.Y.; Do, S.I.; Lee, H.J.; Kang, H.J.; Rho, Y.S.; Bae, W.J.; Lim, Y.C. SOX2 regulates self-renewal and tumorigenicity of stem-like cells of head and neck squamous cell carcinoma. Br. J. Cancer 2014, 111, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Li, S.; Peng, B.; Ye, Y.; Deng, X.; Yao, K. Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma. PLoS ONE 2013, 8, e56324. [Google Scholar]
- Lothian, C.; Lendahl, U. An evolutionarily conserved region in the second intron of the human nestin gene directs gene expression to CNS progenitor cells and to early neural crest cells. Eur. J. Neurosci. 1997, 9, 452–462. [Google Scholar] [CrossRef]
- Ravindran, G.; Devaraj, H. Prognostic significance of neural stem cell markers, Nestin and Musashi-1, in oral squamous cell carcinoma: Expression pattern of Nestin in the precancerous stages of oral squamous epithelium. Clin. Oral Investig. 2015, 19, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, N.; Ishii, G.; Kojima, M.; Sanada, M.; Fujii, S.; Ochiai, A. Podoplanin, a novel marker of tumor-initiating cells in human squamous cell carcinoma A431. Biochem. Biophys. Res. Commun. 2008, 373, 36–41. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, P.; Mei, Q.; Sun, W.; Zhou, L.; Yin, T. Podoplanin is a useful prognostic marker and indicates better differentiation in lung squamous cell cancer patients? A systematic review and meta-analysis. BMC Cancer 2020, 20, 424. [Google Scholar] [CrossRef] [PubMed]
- de Vicente, J.C.; Rodrigo, J.P.; Rodriguez-Santamarta, T.; Lequerica-Fernández, P.; Allonca, E.; García-Pedrero, J.M. Podoplanin expression in oral leukoplakia: Tumorigenic role. Oral Oncol. 2013, 49, 598–603. [Google Scholar] [CrossRef]
- Kindt, N.; Descamps, G.; Seminerio, I.; Bellier, J.; Lechien, J.R.; Mat, Q.; Pottier, C.; Delvenne, P.; Journé, F.; Saussez, S. High stromal Foxp3-positive T cell number combined to tumor stage improved prognosis in head and neck squamous cell carcinoma. Oral Oncol. 2017, 67, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Kuang, H.; Fan, W.; Chen, X.; Yu, T.; Tang, Q.; Zhou, Z.; Liang, F. Downregulation of FOXP3 in neutrophils by IL-8 promotes the progression of oral squamous cell carcinoma. Oncol. Lett. 2019, 18, 4771–4777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, C.; Zhang, Z.; Yan, K.; Li, C.; Li, Y.; Li, L. CXCL12 is associated with FoxP3+ tumor-infiltrating lymphocytes and affects the survival of patients with oral squamous cell carcinoma. Oncol. Lett. 2019, 18, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Zhao, S.J.; Fang, J.; Ma, D.; Liu, X.Q.; Chen, X.B.; Wang, Y.; Cheng, B.; Wang, Z. Foxp3 overexpression in tumor cells predicts poor survival in oral squamous cell carcinoma. BMC Cancer 2016, 16, 530. [Google Scholar] [CrossRef]
- Tao, H.; Mimura, Y.; Aoe, K.; Kobayashi, S.; Yamamoto, H.; Matsuda, E.; Okabe, K.; Matsumoto, T.; Sugi, K.; Ueoka, H. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer 2012, 75, 95–101. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, X.; Guo, S.; Zhang, C.; Tang, Y.; Tian, Y.; Ni, B.; Lu, B.; Wang, H. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS ONE 2014, 9, e91551. [Google Scholar] [CrossRef]
- Sayour, E.J.; McLendon, P.; McLendon, R.; De Leon, G.; Reynolds, R.; Kresak, J.; Sampson, J.H.; Mitchell, D.A. Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol. Immunother. 2015, 64, 419–427. [Google Scholar] [CrossRef]
- Vlad, C.; Kubelac, P.; Fetica, B.; Vlad, D.; Irimie, A.; Achimas-Cadariu, P. The prognostic value of FOXP3+ T regulatory cells in colorectal cancer. J. Buon. 2015, 20, 114–119. [Google Scholar]
- Haas, M.; Dimmler, A.; Hohenberger, W.; Grabenbauer, G.G.; Niedobitek, G.; Distel, L.V. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol. 2009, 9, 65. [Google Scholar] [CrossRef]
- Badoual, C.; Hans, S.; Rodriguez, J.; Peyrard, S.; Klein, C.; Agueznay, N.E.H.; Mosseri, V.; Laccourreye, O.; Bruneval, P.; Fridman, W.H.; et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin. Cancer Res. 2006, 12, 465–472. [Google Scholar] [CrossRef]
- Shimizu, S.; Hiratsuka, H.; Koike, K.; Tsuchihashi, K.; Sonoda, T.; Ogi, K.; Miyakawa, A.; Kobayashi, J.; Kaneko, T.; Igarashi, T.; et al. Tumor-infiltrating CD8+ T-cell density is an independent prognostic marker for oral squamous cell carcinoma. Cancer Med. 2019, 8, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xie, N.; Liu, H.; Wan, Y.; Zhu, Y.; Zhang, M.; Tao, Y.; Zhou, H.; Liu, X.; Hou, J.; et al. The prognostic role of tumour-infiltrating lymphocytes in oral squamous cell carcinoma: A meta-analysis. J. Oral Pathol. Med. 2019, 48, 788–798. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, E.J.; Ooft, M.L.; Devriese, L.A.; Willems, S.M. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology 2017, 6, e1356148. [Google Scholar] [CrossRef]
- Borsetto, D.; Tomasoni, M.; Payne, K.; Polesel, J.; Deganello, A.; Bossi, P.; Tysome, J.R.; Masterson, L.; Tirelli, G.; Tofanelli, M.; et al. Prognostic Significance of CD4+ and CD8+ tumor-infiltrating lymphocytes in head and neck squamous cell carcinoma: A meta-analysis. Cancers 2021, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Liu, Y.; Jiang, S.J.; Liu, Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef]
- Park, K.; Cho, K.J.; Lee, M.; Yoon, D.H.; Kim, S.B. Importance of FOXP3 in prognosis and its relationship with p16 in tonsillar squamous cell carcinoma. Anticancer Res. 2013, 33, 5667–5673. [Google Scholar]
- de Leeuw, R.J.; Kost, S.E.; Kakal, J.A.; Nelson, B.H. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: A critical review of the literature. Clin. Cancer Res. 2012, 18, 3022–3029. [Google Scholar] [CrossRef]
- Müller, S. Update from the 4th Edition of the World Health Organization of Head and Neck Tumours: Tumours of the Oral Cavity and Mobile Tongue. Head Neck Pathol. 2017, 11, 33–40. [Google Scholar] [CrossRef]
- Amin, M.B. AJCC Cancer Staging Manual, 8th ed.; Springer: Chicago, IL, USA, 2017; pp. 79–94. [Google Scholar]
- de Vicente, J.C.; Rodríguez-Santamarta, T.; Rodrigo, J.P.; Blanco-Lorenzo, V.; Allonca, E.; García-Pedrero, J.M. PD-L1 expression in tumor cells is an independent unfavorable prognostic factor in oral squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2019, 28, 546–554. [Google Scholar] [CrossRef]
- de Vicente, J.C.; Santamarta, T.R.; Rodrigo, J.P.; García-Pedrero, J.M.; Allonca, E.; Blanco-Lorenzo, V. Expression of podoplanin in the invasion front of oral squamous cell carcinoma is not prognostic for survival. Virchows Arch. 2015, 466, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Schicketanz, K.H. Immunohistochemical determination of estrogen and progesterone receptor content in human breast cancer. Computer-assisted image analysis (QIC score) vs. Subjective grading (IRS). Pathol. Res. Pract. 1993, 189, 862–866. [Google Scholar] [CrossRef]
- Suárez-Sánchez, F.J.; Lequerica-Fernández, P.; Rodrigo, J.P.; Hermida-Prado, F.; Suárez-Canto, J.; Rodríguez-Santamarta, T.; Domínguez-Iglesias, F.; García-Pedrero, J.M.; de Vicente, J.C. Tumor-infiltrating CD20+ B lymphocytes: Significance and prognostic implications in oral cancer microenvironment. Cancers 2021, 13, 395. [Google Scholar] [CrossRef] [PubMed]
- DiPaolo, R.J.; Glass, D.D.; Bijwaard, K.E.; Shevach, E.M. CD4+CD25+ T cells prevent the development of organ-specific autoimmune disease by inhibiting the differentiation of autoreactive effector T cells. J. Immunol. 2005, 175, 7135–7142. [Google Scholar] [CrossRef]
- Swann, J.B.; Smyth, M.J. Immune surveillance of tumors. J. Clin. Investig. 2007, 117, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Shan, Z.; Liu, Z.; Liu, S.; Yang, L.; Fang, X.; Li, K.; Wang, B.; Deng, Z.; Hu, Y.; et al. The repertoire of tumor-infiltrating lymphocytes within the microenvironment of oral squamous cell carcinoma reveals immune dysfunction. Cancer Immunol. Immunother. 2020, 69, 465–476. [Google Scholar] [CrossRef]
- Wolf, G.T.; Chepeha, D.B.; Bellile, E.; Nguyen, A.; Thomas, D.; McHugh, J. University of Michigan Head and Neck SPORE Program. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: A preliminary study. Oral Oncol. 2015, 51, 90–95. [Google Scholar] [CrossRef]
- Ono, T.; Azuma, K.; Kawahara, A.; Sasada, T.; Hattori, S.; Sato, F.; Shin, B.; Chitose, S.I.; Akiba, J.; Hirohito, U. Association between PD-L1 expression combined with tumor-infiltrating lymphocytes and the prognosis of patients with advanced hypopharyngeal squamous cell carcinoma. Oncotarget 2017, 8, 92699–92714. [Google Scholar] [CrossRef][Green Version]
- Hu, C.; Tian, S.; Lin, L.; Zhang, J.; Ding, H. Prognostic and clinicopathological significance of PD-L1 and tumor infiltrating lymphocytes in hypopharyngeal squamous cell carcinoma. Oral Oncol. 2020, 102, 104560. [Google Scholar] [CrossRef] [PubMed]
- Peske, J.D.; Woods, A.B.; Engelhard, V.H. Control of CD8 T-cell infiltration into tumors by vasculature and microenvironment. Adv. Cancer Res. 2015, 128, 263–307. [Google Scholar]
- Balermpas, P.; Rödel, F.; Weiss, C.; Rödel, C.; Fokas, E. Tumor-infiltrating lymphocytes favor the response to chemoradiotherapy of head and neck cancer. Oncoimmunology 2014, 3, e27403. [Google Scholar] [CrossRef]
- Balermpas, P.; Michel, Y.; Wagenblast, J.; Seitz, O.; Weiss, C.; Rödel, F.; Rödel, C.; Fokas, E. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br. J. Cancer 2014, 110, 501–509. [Google Scholar] [CrossRef]
- Drennan, S.; Stafford, N.D.; Greenman, J.; Green, V.L. Increased frequency and suppressive activity of CD127(low/-) regulatory T cells in the peripheral circulation of patients with head and neck squamous cell carcinoma are associated with advanced stage and nodal involvement. Immunology 2013, 140, 335–343. [Google Scholar]
- Schott, A.K.; Pries, R.; Wollenberg, B. Permanent up-regulation of regulatory T-lymphocytes in patients with head and neck cancer. Int. J. Mol. Med. 2010, 26, 67–75. [Google Scholar] [PubMed]
- Troiano, G.; Rubini, C.; Togni, L.; Caponio, V.C.A.; Zhurakivska, K.; Santarelli, A.; Cirillo, N.; Lo Muzio, L.; Mascitti, M. The immune phenotype of tongue squamous cell carcinoma predicts early relapse and poor prognosis. Cancer Med. 2020, 9, 8333–8344. [Google Scholar] [CrossRef]
- De Meulenaere, A.; Vermassen, T.; Aspeslagh, S.; Zwaenepoel, K.; Deron, P.; Duprez, F.; Rottey, S.; Ferdinande, L. Prognostic markers in oropharyngeal squamous cell carcinoma: Focus on CD70 and tumour infiltrating lymphocytes. Pathology 2017, 49, 397–404. [Google Scholar] [CrossRef]
- Lei, Y.; Xie, Y.; Tan, Y.S.; Prince, M.E.; Moyer, J.S.; Nör, J.; Wolf, G.T. Telltale tumor infiltrating lymphocytes (TIL) in oral, head & neck cancer. Oral Oncol. 2016, 61, 159–165. [Google Scholar]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Spranger, S.; Gajewski, T.F. Tumor-intrinsic oncogene pathways mediating immune avoidance. Oncoimmunology 2015, 5, e1086862. [Google Scholar] [CrossRef]
- Schipmann, S.; Wermker, K.; Schulze, H.J.; Kleinheinz, J.; Brunner, G. Cutaneous and oral squamous cell carcinoma-dual immunosuppression via recruitment of FOXP3+ regulatory T cells and endogenous tumour FOXP3 expression? J. Craniomaxillofac. Surg. 2014, 42, 1827–1833. [Google Scholar] [CrossRef]
- Salama, P.; Phillips, M.; Grieu, F.; Morris, M.; Zeps, N.; Joseph, D.; Platell, C.; Iacopetta, B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J. Clin. Oncol. 2009, 27, 186–192. [Google Scholar] [CrossRef]
- Boxberg, M.; Leising, L.; Steiger, K.; Jesinghaus, M.; Alkhamas, A.; Mielke, M.; Pfarr, N.; Götz, C.; Wolff, C.D.; Weichert, W.; et al. Composition and clinical impact of the immunologic tumor microenvironment in oral squamous cell carcinoma. J. Immunol. 2019, 202, 278–291. [Google Scholar] [CrossRef]
- Miyara, M.; Sakaguchi, S. Human FoxP3(+)CD4(+) regulatory T cells: Their knowns and unknowns. Immunol. Cell Biol. 2011, 89, 346–351. [Google Scholar] [CrossRef]
- Teng, F.; Mu, D.; Meng, X.; Kong, L.; Zhu, H.; Liu, S.; Zhang, J.; Yu, J. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am. J. Cancer Res. 2015, 5, 2064–2074. [Google Scholar]
- Homma, Y.; Taniguchi, K.; Murakami, T.; Nakagawa, K.; Nakazawa, M.; Matsuyama, R.; Mori, R.; Takeda, K.; Ueda, M.; Ichikawa, Y.; et al. Immunological impact of neoadjuvant chemoradiotherapy in patients with borderline resectable pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2014, 21, 670–676. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, E.J.; de Roest, R.H.; Brakenhoff, R.H.; Leemans, C.R.; de Bree, R.; Terhaard, C.H.J.; Willems, S.M. Digital pathology-aided assessment of tumor-infiltrating T lymphocytes in advanced stage, HPV-negative head and neck tumors. Cancer Immunol. Immunother. 2020, 69, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.H.; Zhang, X.X.; Lu, Z.Y.; Huang, X.F.; Wang, Z.Y.; Yang, Y.; Dong, Y.C.; Jing, Y.; Song, Y.; Hou, Y.Y.; et al. Tumor-infiltrating CD1a+ DCs and CD8+/FoxP3+ ratios served as predictors for clinical outcomes in tongue squamous cell carcinoma patients. Pathol. Oncol. Res. 2019, 26, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Kashima, Y.; Nishii, N.; Tachinami, H.; Furusawa, E.; Nagai, S.; Harada, H.; Azuma, M. Orthotopic tongue squamous cell carcinoma (SCC) model exhibiting a different tumor-infiltrating T-cell status with margin-restricted CD8+ T cells and regulatory T cell-dominance, compared to skin SCC. Biochem. Biophys. Res. Commun. 2020, 526, 218–224. [Google Scholar] [CrossRef]
- Naito, Y.; Saito, K.; Shiiba, K.; Ohuchi, A.; Saigenji, K.; Nagura, H.; Ohtani, H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998, 58, 3491–3494. [Google Scholar] [PubMed]
- Menon, A.G.; Janssen-van Rhijn, C.M.; Morreau, H.; Putter, H.; Tollenaar, R.A.; van de Velde, C.J.; Fleuren, G.J.; Kuppen, P.J. Immune system and prognosis in colorectal cancer: A detailed immunohistochemical analysis. Lab. Investig. 2004, 84, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Herbst, R.S.; Chen, L. Defining and understanding adaptive resistance in cancer immunotherapy. Trends Immunol. 2018, 39, 624–631. [Google Scholar] [CrossRef]
- Zhu, Q.; Cai, M.Y.; Weng, D.S.; Zhao, J.J.; Pan, Q.Z.; Wang, Q.J.; Tang, Y.; He, J.; Li, M.; Xia, J.C. PD-L1 expression patterns in tumour cells and their association with CD8(+) tumour infiltrating lymphocytes in clear cell renal cell carcinoma. J. Cancer 2019, 10, 154–1161. [Google Scholar] [CrossRef]
- Piazzolla, D.; Palla, A.R.; Pantoja, C.; Cañamero, M.; Perez de Castro, I.; Ortega, S.; Gómez-López, G.; Dominguez, O.; Megías, D.; Roncador, G.; et al. Lineage-restricted function of the pluripotency factor NANOG in stratified epithelia. Nat. Comm. 2015, 5, 4226. [Google Scholar] [CrossRef]
- Stasikowska-Kanicka, O.; Wągrowska-Danilewicz, M.; Danilewicz, M. CD8+ and CD163+ infiltrating cells and PD-L1 immunoexpression in oral leukoplakia and oral carcinoma. APMIS 2018, 126, 732–738. [Google Scholar] [CrossRef]
- Thompson, E.D.; Zahurak, M.; Murphy, A.; Cornish, T.; Cuka, N.; Abdelfatah, E.; Yang, S.; Duncan, M.; Ahuja, N.; Taube, J.M.; et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 2017, 66, 794–801. [Google Scholar] [CrossRef]
- Sharma, A.; Rudra, D. Emerging functions of regulatory T cells in tissue homeostasis. Front. Immunol. 2018, 9, 883. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Puig, P.E.; Roux, S.; Parcellier, A.; Schmitt, E.; Solary, E.; Kroemer, G.; Martin, F.; Chauffert, B.; Zitvogel, L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 2005, 202, 919–929. [Google Scholar] [CrossRef]
- Pedregal-Mallo, D.; Hermida-Prado, F.; Granda-Díaz, R.; Montoro-Jiménez, I.; Allonca, E.; Pozo-Agundo, E.; Álvarez-Fernández, M.; Álvarez-Marcos, C.; García-Pedrero, J.M.; Rodrigo, J.P. Prognostic significance of the pluripotency factors NANOG, SOX2, and OCT4 in head and neck squamous cell carcinomas. Cancers 2020, 12, 1794. [Google Scholar] [CrossRef]
- Ghazi, N.; Aali, N.; Shahrokhi, V.R.; Mohajertehran, F.; Saghravanian, N. Relative expression of SOX2 and OCT4 in oral squamous cell carcinoma and oral epithelial dysplasia. Rep. Biochem. Mol. Biol. 2020, 9, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, G.; Narwal, A.; Kamboj, M.; Sen, R. Association of SOX2, OCT4 and WNT5A expression in oral epithelial dysplasia and oral squamous cell carcinoma: An immunohistochemical study. Head Neck Pathol. 2020, 14, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Sánchez, F.J.; Lequerica-Fernández, P.; Suárez-Canto, J.; Rodrigo, J.P.; Rodriguez-Santamarta, T.; Domínguez-Iglesias, F.; García-Pedrero, J.M.; de Vicente, J.C. Macrophages in oral carcinomas: Relationship with cancer stem cell markers and PD-L1 expression. Cancers 2020, 12, 1764. [Google Scholar] [CrossRef]

| Variable | Number | Stromal CD4 Mean (SD) | p | Tumoral CD4 Mean (SD) | p | Stromal CD8 Mean (SD) | p | Tumoral CD8 Mean (SD) | p | Stromal FOXP3 Mean (SD) | p | Tumoral FOXP3 Mean (SD) | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | |||||||||||||
| <65 | 77 | 43.87 (42.35) | 0.05 | 3.51 (5.43) | 0.01 | 158.24 (160.38) | 0.16 | 43.47 (58.60) | 0.28 | 18.32 (27.33) | 0.13 | 3.79 (7.70) | 0.09 |
| ≥65 | 48 | 71.82 (94.14) | 10.01 (17.94) | 210.88 (256.15) | 54.85 (54.68) | 11.46 (18.92) | 2.05 (3.67) | ||||||
| Gender | |||||||||||||
| Female | 43 | 66.37 (96.18) | 0.25 | 7.92 (13.85) | 0.03 * | 205.31 (276.68) | 0.37 | 51.88 (67.82) | 0.57 | 16.19 (22.44) | 0.85 | 3.40 (6.00) | 0.71 |
| Male | 82 | 48.43 (46.92) | 5.02 (11.34) | 164.37 (151.46) | 45.75 (50.94) | 15.35 (25.73) | 2.95 (6.74) | ||||||
| Tobacco | |||||||||||||
| No | 41 | 76.18 (96.80) | 0.03 * | 10.26 (18.58) | 0.01 * | 215.76 (226.61) | 0.18 | 67.25 (69.83) | 0.02 | 14.65 (22.50) | 0.75 | 2.76 (4.95) | 0.67 |
| Yes | 84 | 44.07 (45.62) | 3.93 (6.70) | 160.25 (189.54) | 47.34 (5.19) | 16.15 (25.62) | 3.28 (7.13) | ||||||
| Alcohol consumption | |||||||||||||
| No | 56 | 67.10 (86.31) | 0.08 | 8.54 (16.18) | 0.004 * | 208.14 (222.55) | 0.14 | 58.69 (64.46) | 0.04 * | 13.84 (20.70) | 0.45 | 2.38 (4.40) | 0.25 |
| Yes | 69 | 44.46 (47.02) | 3.95 (7.27) | 154.36 (184.20) | 38.98 (49.08) | 17.15 (27.40) | 3.72 (7.78) | ||||||
| T classification | |||||||||||||
| T1 + 2 | 81 | 55.93 (54.83) | 0.76 | 6.28 (12.52) | 0.75 | 192.29 (206.21) | 0.3 | 45.69 (58.84) | 0.56 | 17.96 (26.50) | 0.02 * | 3.55 (7.14) | 0.3 |
| T3 + 4 | 44 | 52.15 (88.27) | 5.56 (11.99) | 152.99 (197.33) | 51.85 (54.40) | 11.34 (19.97) | 2.30 (4.96) | ||||||
| N classification | |||||||||||||
| N0 | 76 | 52.74 (50.56) | 0.7 | 5.22 (8.31) | 0.43 | 176.77 (196.70) | 0.9 | 50.73 (59.57) | 0.49 | 14.24 (21.01) | 0.42 | 2.78 (4.83) | 0.48 |
| N+ | 49 | 57.48 (89.39) | 7.25 (16.68) | 181.07 (214.97) | 43.52 (53.56) | 17.84 (29.32) | 3.62 (8.46) | ||||||
| Stage | |||||||||||||
| I + II | 52 | 57.77 (55.79) | 0.66 | 5.93 (9.88) | 0.94 | 197.14 (224.40) | 0.38 | 47.67 (63.87) | 0.97 | 16.93 (24.08) | 0.62 | 3.43 (5.61) | 0.64 |
| III + IV | 73 | 52.34 (76.04) | 6.09 (13.80) | 165.14 (187.14) | 48.02 (52.42) | 14.74 (24.98) | 2.88 (7.05) | ||||||
| Grade | |||||||||||||
| Well | 80 | 63.23 (80.43) | 0.02 | 6.09 (11.39) | 0.94 | 204.61 (235.63) | 0.02 | 48.23 (59.11) | 0.92 | 14.70 (23.95) | 0.57 | 3.15 (6.98) | 0.92 |
| Moderate + Poor | 45 | 39.26 (33.17) | 5.91 (13.86) | 131.96 (115.05) | 47.25 (54.20) | 17.28 (25.72) | 3.04 (5.56) | ||||||
| Site | |||||||||||||
| Tongue | 51 | 73.87 (90.52) | 0.01 * | 9.52 (17.06) | 0.02 | 200.96 (229.44) | 0.3 | 43.11 (50.32) | 0.44 | 21.67 (32.63) | 0.04 | 4.26 (8.85) | 0.15 |
| Others | 74 | 41.32 (42.89) | 3.58 (6.43) | 162.95 (183.01) | 51.21 (61.61) | 11.52 (15.92) | 2.33 (4.01) | ||||||
| Recurrence | |||||||||||||
| No | 71 | 47.50 (49.79) | 0.25 | 4.78 (8.86) | 0.23 | 171.85 (173.57) | 0.67 | 45.65 (55.24) | 0.62 | 18.85 (27.78) | 0.06 | 3.61 (7.26) | 0.05 |
| Yes | 54 | 62.63 (86.47) | 7.64 (15.61) | 187.13 (238.10) | 50.76 (59.96) | 11.55 (19.12) | 2.47 (5.30) | ||||||
| Second primary tumor | |||||||||||||
| No | 106 | 53.13 (70.30) | 0.57 | 5.72 (12.24) | 0.52 | 170.75 (205.11) | 0.31 | 44.95 (51.89) | 0.18 | 15.99 (25.91) | 0.71 | 3.09 (6.64) | 0.92 |
| Yes | 19 | 62.79 (55.56) | 7.70 (12.80) | 221.45 (191.76) | 64.07 (80.24) | 13.76 (15.36) | 3.24 (5.65) |
| Factor | SOX2 | p | NANOG | p | Nestin | p | PDPN (IRS) | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Low | High | Low | High | |||||
| Stromal CD4 (mean, SD) | 63.66 (81.02) | 43.61 (43.92) | 0.11 | 63.00 78.30) | 36.71 (35.64) | 0.01 | 58.28 (53.67) | 49.61 (52.18) | 0.30 | 61.45 (88.09) | 47.78 (36.98) | 0.61 |
| Tumoral CD4 (mean, SD) | 6.89 (14.65) | 5.06 (8.13) | 0.43 | 7.51 (14.32) | 3.29 (6.12) | 0.03 | 6.26 (6.83) | 5.15 (11.36) | 0.21 | 8.02 (16.48) | 4.63 (7.09) | 0.96 |
| Stromal CD8 (mean, SD) | 215.23 (234.37) | 128.21 (142.87) | 0.01 | 200.83 (229.44) | 134.07 (131.46) | 0.04 | 229.90 (172.05) | 163.05 (187.08) | 0.13 | 189.45 (239.22) | 186.60 (161.68) | 0.45 |
| Tumoral CD8 (mean, SD) | 53.44 (58.72) | 41.95 (55.66) | 0.04 | 53.95 (61.19) | 36.11 (48.35) | 0.11 | 39.28 (67.17) | 45.52 (58.46) | 0.55 | 51.36 (63.00) | 45.38 (42.81) | 0.90 |
| Stromal FOXP3 (mean, SD) | 15.57 (22.17) | 16.48 (28.32) | 0.84 | 14.81 (24.46) | 17.05 (24.92) | 0.64 | 32.85 (37.47) | 13.48 (20.90) | 0.01 | 9.75 (17.19) | 20.02 (21.76) | 0.003 |
| Tumoral FOXP3 (mean, SD) | 2.54 (4.60) | 4.12 (8.57) | 0.24 | 3.22 (7.37) | 2.90 (4.36) | 0.80 | 7.54 (9.36) | 2.25 (4.39) | 0.05 | 2.10 (4.20) | 3.29 (5.53) | 0.03 |
| Stromal CD8/FOXP3 ratio (mean, SD) | 56.48 (124.57) | 38.03 (78.71) | 0.15 | 62.08 (129.78) | 25.05 (36.24) | 0.64 | 14.15 (10.93) | 52.28 (127.31) | 0.54 | 71.66 (137.10) | 48.51 (113.58) | 0.08 |
| Tumoral CD8/FOXP3 ratio (mean, SD) | 35.32 (56.90) | 22.39 (57.74) | 0.008 | 39.29 (67.86) | 12.58 (15.63) | 0.02 | 7.42 (6.04) | 35.28 (68.76) | 0.35 | 46.52 (84.19) | 29.58 (35.36) | 0.96 |
| Factor | Tumor PD-L1 | p | |
|---|---|---|---|
| ≤10% | >10% | ||
| Stromal CD4 (mean, SD) | 50.09 (48.50) | 86.40 (134.59) | 0.27 |
| Tumoral CD4 (mean, SD) | 4.57 (7.89) | 15.16 (24.77) | 0.01 |
| Stromal CD8 (mean, SD) | 161.87 (158.97) | 285.01 (365.01) | 0.17 |
| Tumoral CD8 (mean, SD) | 40.10 (49.76) | 98.49 (73.77) | 0.004 |
| Stromal FOXP3 (mean, SD) | 16.18 (25.41) | 14.57 (21.23) | 0.80 |
| Tumoral FOXP3 (mean, SD) | 3.22 (6.86) | 2.98 (4.43) | 0.88 |
| Stromal CD8/FOXP3 ratio (mean, SD) | 50.03 (116.61) | 43.89 (49.56) | 0.08 |
| Tumoral CD8/FOXP3 ratio (mean, SD) | 26.63 (48.44) | 49.02 (92.22) | 0.01 |
| Parameter | Number | Censored Patients (%) | Cancer-Free Survival Time (95% CI) | p | Hazard Ratio | 95% CI |
|---|---|---|---|---|---|---|
| pT classification | ||||||
| 81 | 53 (65) | 151.82 (129.03–174.99) | 0.001 | 2.49 | 1.44–4.30 |
| 44 | 19 (43) | 77.62 (54.19–101.04) | |||
| pN classification | ||||||
| 76 | 49 (65) | 127.96 (109.43–146.49) | 0.01 | 1.92 | 1.12–3.31 |
| 49 | 23 (4) | 108.58 (77.88–139.28) | |||
| Stage | ||||||
| 52 | 36 (69) | 140.09 (120.15–160.04) | 0.002 | 2.4 | 1.33–4.32 |
| 73 | 36 (49) | 113.33 (87.73–138.93) | |||
| Grade | ||||||
| 80 | 44 (55) | 127.85 (103.73–151.98) | 0.59 | 0.85 | 0.48–1.52 |
| 45 | 28 (62) | 121.63 (96.25–147.01) | |||
| Stromal CD4+ | ||||||
| 62 | 34 (55) | 127.86 (102.28–153.45) | 0.6 | 0.86 | 0.50–1.49 |
| 63 | 38 (60) | 137.49 (110.54–164.44) | |||
| Tumoral CD4+ | ||||||
| 59 | 31 (53) | 120.39 (93.85–146.93) | 0.42 | 0.8 | 0.47–1.38 |
| 66 | 41 (62) | 142.01 (115.42–168.60) | |||
| Stromal CD8+ | ||||||
| 63 | 32 (51) | 109.62 (81.85–137.39) | 0.05 | 0.58 | 0.33–1.01 |
| 62 | 40 (65) | 152.28 (126.67–177.90) | |||
| Tumoral CD8+ | ||||||
| 61 | 35 (57) | 133.16 (105.84–160.47) | 0.95 | 1.01 | 0.59–1.74 |
| 64 | 37 (58) | 111.28 (90.91–131.66) | |||
| Stromal FOXP3+ | ||||||
| 60 | 29 (48) | 96.14 (75.24–117.05) | 0.03 | 0.56 | 0.32–0.98 |
| 65 | 43 (66) | 152.79 (126.93–178.66) | |||
| Tumoral FOXP3+ | ||||||
| 53 | 25 (47) | 105.47 (77.32–133.62) | 0.03 | 0.54 | 0.31–0.93 |
| 72 | 47 (65) | 151.97 (127.55–176.38) | |||
| Stromal CD8/FOXP3+ ratio | ||||||
| 43 | 30 (70) | 162.89 (132.67–193.11) | 0.04 | 1.9 | 0.99–3.64 |
| 63 | 32 (51) | 97.82 (78.78–116.87) | |||
| Tumoral CD8/FOXP3+ ratio | ||||||
| 39 | 28 (72) | 163.64 (131.68–195.59) | 0.06 | 2.01 | 0.95–4.27 |
| 41 | 22 (54) | 100.67 (77.71–123.63) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lequerica-Fernández, P.; Suárez-Canto, J.; Rodriguez-Santamarta, T.; Rodrigo, J.P.; Suárez-Sánchez, F.J.; Blanco-Lorenzo, V.; Domínguez-Iglesias, F.; García-Pedrero, J.M.; de Vicente, J.C. Prognostic Relevance of CD4+, CD8+ and FOXP3+ TILs in Oral Squamous Cell Carcinoma and Correlations with PD-L1 and Cancer Stem Cell Markers. Biomedicines 2021, 9, 653. https://doi.org/10.3390/biomedicines9060653
Lequerica-Fernández P, Suárez-Canto J, Rodriguez-Santamarta T, Rodrigo JP, Suárez-Sánchez FJ, Blanco-Lorenzo V, Domínguez-Iglesias F, García-Pedrero JM, de Vicente JC. Prognostic Relevance of CD4+, CD8+ and FOXP3+ TILs in Oral Squamous Cell Carcinoma and Correlations with PD-L1 and Cancer Stem Cell Markers. Biomedicines. 2021; 9(6):653. https://doi.org/10.3390/biomedicines9060653
Chicago/Turabian StyleLequerica-Fernández, Paloma, Julián Suárez-Canto, Tania Rodriguez-Santamarta, Juan Pablo Rodrigo, Faustino Julián Suárez-Sánchez, Verónica Blanco-Lorenzo, Francisco Domínguez-Iglesias, Juana María García-Pedrero, and Juan Carlos de Vicente. 2021. "Prognostic Relevance of CD4+, CD8+ and FOXP3+ TILs in Oral Squamous Cell Carcinoma and Correlations with PD-L1 and Cancer Stem Cell Markers" Biomedicines 9, no. 6: 653. https://doi.org/10.3390/biomedicines9060653
APA StyleLequerica-Fernández, P., Suárez-Canto, J., Rodriguez-Santamarta, T., Rodrigo, J. P., Suárez-Sánchez, F. J., Blanco-Lorenzo, V., Domínguez-Iglesias, F., García-Pedrero, J. M., & de Vicente, J. C. (2021). Prognostic Relevance of CD4+, CD8+ and FOXP3+ TILs in Oral Squamous Cell Carcinoma and Correlations with PD-L1 and Cancer Stem Cell Markers. Biomedicines, 9(6), 653. https://doi.org/10.3390/biomedicines9060653






