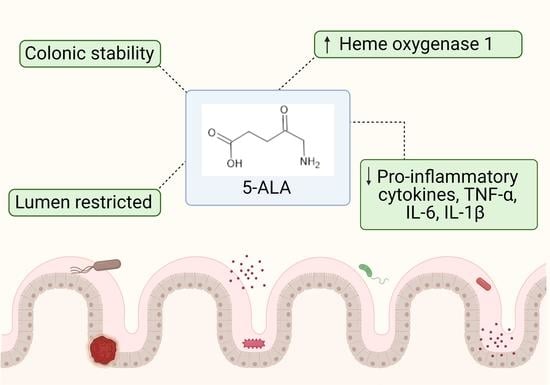
5-Aminolevulinic Acid as a Novel Therapeutic for Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
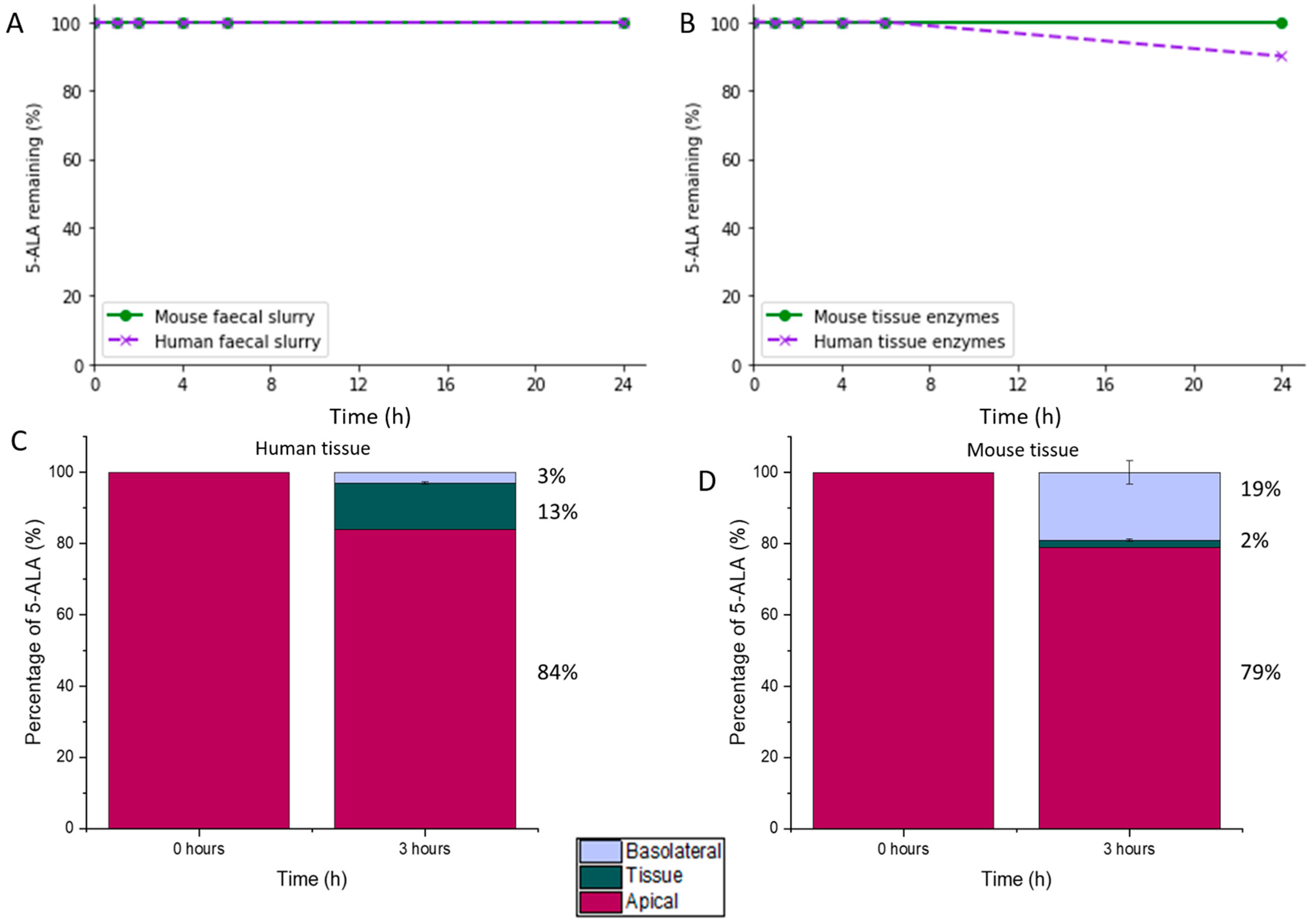
2.2.1. 5-ALA Stability and Permeability in Colonic Conditions
Preparation of Colonic Fluid
Stability of 5-ALA in Faecal Slurry (Colonic Fluid)
HPLC-FLD Quantification of 5-ALA
Preparation of Colonic Tissue Lysate for Assessment of 5-ALA Stability
Stability Assessment of 5-ALA in the Presence of Colonic Tissue Enzymes
Evaluation of 5-ALA Colonic Tissue Permeability
2.2.2. Efficacy in DSS Colitis Mouse Model
Animals and Study Design
Clinical Observations
- Weight score: 0 (maintain or increase in weight), 1 (1–5% weight loss), 2 (6–10% weight loss), 3 (11–15% weight loss), 4 (>16% weight loss).
- Stool consistency score: 0 (normal), 1 (moist/sticky), 2 (soft), 3 (diarrhoea).
- Stool blood content score: 0 (negative haemoccult test—no blood), 1 (positive haemoccult test in >30 s), 2 (positive haemoccult test in <30 s), 3 (gross observable blood).
Postmortem Analysis of Plasma and Tissue
2.2.3. Data Analysis and Statistics
3. Results and Discussion
3.1. Stability and Permeability of 5-ALA in Colonic Conditions
3.2. Efficacy in an IBD Mouse Model
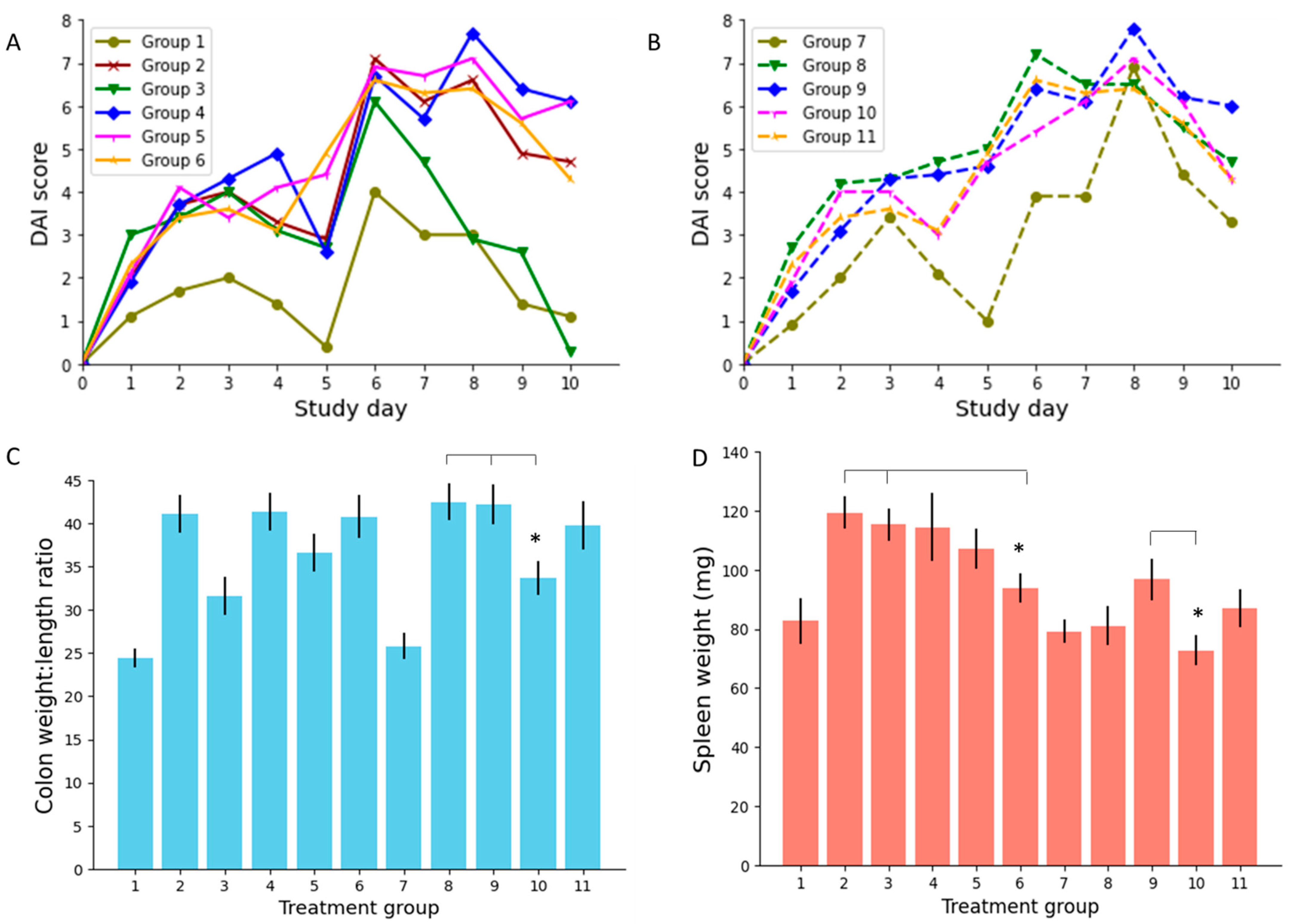
3.2.1. Disease Activity Index (DAI) Score
3.2.2. Colon Weight to Length Ratio
3.2.3. Spleen Weight
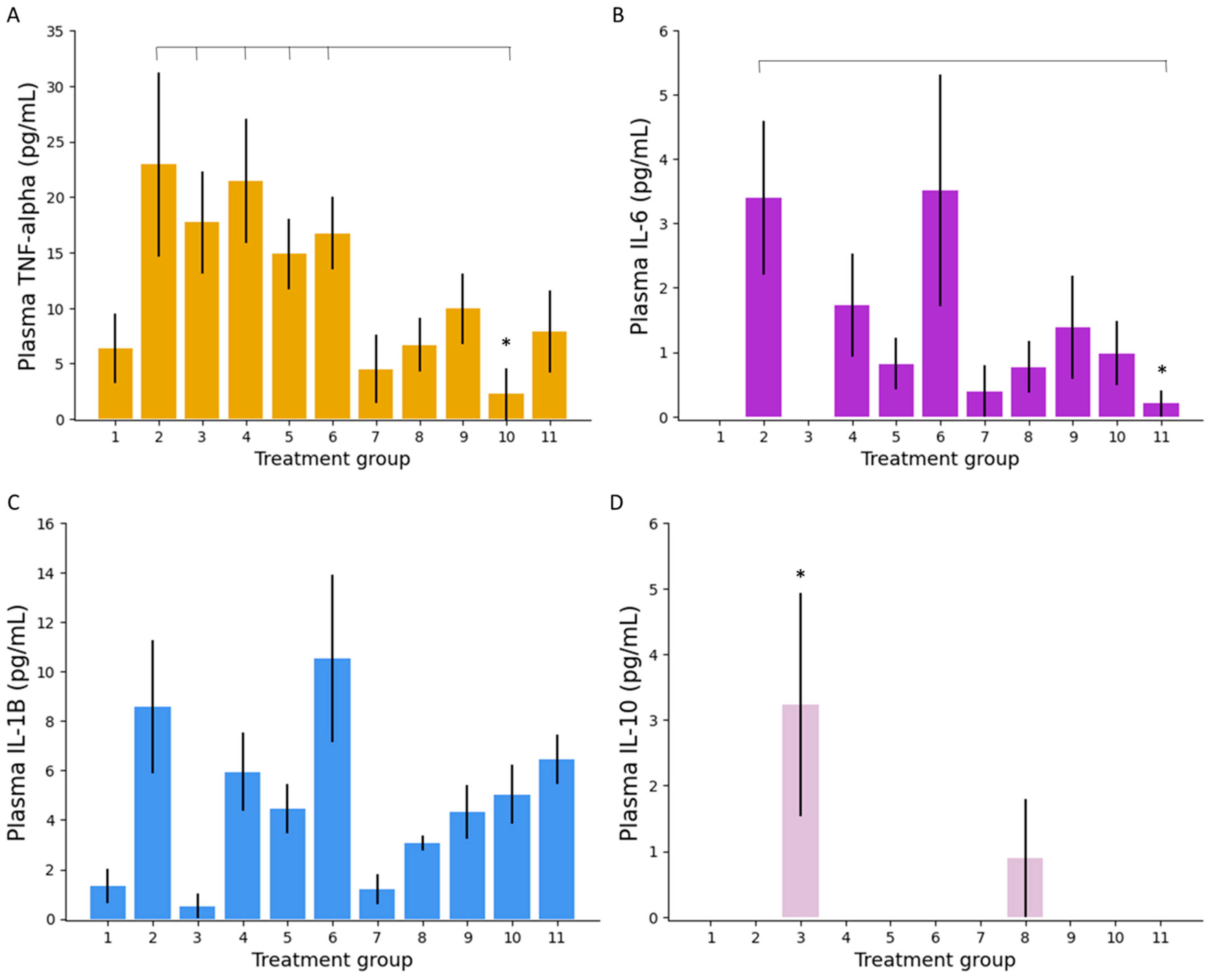
3.2.4. Colonic Tissue and Serum Analysis
Plasma Inflammatory Markers
Colonic Tissue Inflammatory Markers
Heme Oxygenase Activity
3.3. The Potential of 5-ALA as a Novel IBD Treatment
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franken, A.C.; Lokman, B.C.; Ram, A.F.; Punt, P.J.; van den Hondel, C.A.; de Weert, S. Heme biosynthesis and its regulation: Towards understanding and improvement of heme biosynthesis in filamentous fungi. Appl. Microbiol. Biotechnol. 2011, 91, 447–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujino, M.; Nishio, Y.; Ito, H.; Tanaka, T.; Li, X.K. 5-Aminolevulinic acid regulates the inflammatory response and alloimmune reaction. Int. Immunopharmacol. 2016, 37, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, D.A.; Tagarro, I.; Carmen Díez, M.; Sandborn, W.J. Prevalence of Fistulizing Crohn’s Disease in the United States: Estimate from a Systematic Literature Review Attempt and Population-Based Database Analysis. Inflamm. Bowel Dis. 2019, 25, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Shahabi, A.; Seabury, S.A.; Lakdawalla, D.N.; Espinosa, O.D.; Green, S.; Brauer, M.; Baldassano, R.N. Lifetime Economic Burden of Crohn’s Disease and Ulcerative Colitis by Age at Diagnosis. Clin. Gastroenterol. Hepatol. 2020, 18, 889–897.e10. [Google Scholar] [CrossRef]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Sandborn, W.J.; D’Haens, G.R.; Zou, G.; Stitt, L.W.; Singh, S.; Ananthakrishnan, A.N.; Dulai, P.S.; Khanna, R.; Jairath, V.; et al. Discordance Between Patient-Reported Outcomes and Mucosal Inflammation in Patients With Mild to Moderate Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 1760–1768.e1. [Google Scholar] [CrossRef]
- Neovius, M.; Arkema, E.V.; Blomqvist, P.; Ekbom, A.; Smedby, K.E. Patients with ulcerative colitis miss more days of work than the general population, even following colectomy. Gastroenterology 2013, 144, 536–543. [Google Scholar] [CrossRef]
- Ma, C.; Smith, M.K.; Guizzetti, L.; Panaccione, R.; Kaplan, G.G.; Novak, K.L.; Lu, C.; Khanna, R.; Feagan, B.G.; Singh, S.; et al. Assessing National Trends and Disparities in Ambulatory, Emergency Department, and Inpatient Visits for Inflammatory Bowel Disease in the United States (2005–2016). Clin. Gastroenterol. Hepatol. 2020, 18, 2500–2509.e1. [Google Scholar] [CrossRef]
- Turpin, W.; Lee, S.H.; Raygoza Garay, J.A.; Madsen, K.L.; Meddings, J.B.; Bedrani, L.; Power, N.; Espin-Garcia, O.; Xu, W.; Smith, M.I.; et al. Increased Intestinal Permeability Is Associated With Later Development of Crohn’s Disease. Gastroenterology 2020, 159, 2092–2100.e5. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.; Varum, F.; Bravo, R.; Furrer, E.; Bojic, D.; Basit, A.W. Inflammatory bowel disease: Exploring gut pathophysiology for novel therapeutic targets. Transl. Res. 2016, 176, 38–68. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Ulcerative Colitis. In Clinical Knowledge Summaries; 2020; Available online: https://cks.nice.org.uk/topics/ulcerative-colitis/ (accessed on 13 March 2021).
- National institute for Health and Care Excellence. Crohn’s Disease. In Clinical Knowledge Summaries; NICE: UK, 2020; Available online: https://cks.nice.org.uk/topics/crohns-disease/ (accessed on 13 March 2021).
- Ho, G.T.; Chiam, P.; Drummond, H.; Loane, J.; Arnott, I.D.; Satsangi, J. The efficacy of corticosteroid therapy in inflammatory bowel disease: Analysis of a 5-year UK inception cohort. Aliment. Pharm. 2006, 24, 319–330. [Google Scholar] [CrossRef]
- Fraser, A.G.; Orchard, T.R.; Jewell, D.P. The efficacy of azathioprine for the treatment of inflammatory bowel disease: A 30 year review. Gut 2002, 50, 485. [Google Scholar] [CrossRef] [PubMed]
- Loftus, C.G.; Loftus, E.V., Jr.; Sandborn, W.J. Cyclosporin for refractory ulcerative colitis. Gut 2003, 52, 172–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacucci, M.; de Silva, S.; Ghosh, S. Mesalazine in inflammatory bowel disease: A trendy topic once again? Can. J. Gastroenterol. 2010, 24, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Murray, A.; Nguyen, T.M.; Parker, C.E.; Feagan, B.G.; MacDonald, J.K. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Marshall, J.K.; Thabane, M.; Steinhart, A.H.; Newman, J.R.; Anand, A.; Irvine, E.J. Rectal 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2012, 11, CD004118. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Nguyen, T.M.; Parker, C.E.; Feagan, B.G.; MacDonald, J.K. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2020, 8, CD000543. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Ong, J.J.; Luzardo-Álvarez, A.; González-Barcia, M.; Basit, A.W.; Otero-Espinar, F.J.; Goyanes, A. 3D printed tacrolimus suppositories for the treatment of ulcerative colitis. Asian J. Pharm. Sci. 2021, 16, 110–119. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Gómez-Lado, N.; Lázare-Iglesias, H.; García-Otero, X.; Antúnez-López, J.R.; Ruibal, Á.; Varela-Correa, J.J.; Aguiar, P.; Basit, A.W.; Otero-Espinar, F.J.; et al. 3D Printed Tacrolimus Rectal Formulations Ameliorate Colitis in an Experimental Animal Model of Inflammatory Bowel Disease. Biomedicines 2020, 8, 563. [Google Scholar] [CrossRef]
- Hu, A.; Kotze, P.G.; Burgevin, A.; Tan, W.; Jess, A.; Li, P.S.; Kroeker, K.; Halloran, B.; Panaccione, R.; Peyrin-Biroulet, L.; et al. Combination Therapy Does Not Improve Rate of Clinical or Endoscopic Remission in Patients with Inflammatory Bowel Diseases Treated With Vedolizumab or Ustekinumab. Clin. Gastroenterol. Hepatol. 2020, 12, S1542-3565(20)30973-3. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Kotze, P.G.; Almutairdi, A.; Jairath, V.; Panaccione, R. Concomitant Use of Aminosalicylates Is Not Associated With Improved Outcomes in Patients With Ulcerative Colitis Escalated to Vedolizumab. Clin. Gastroenterol. Hepatol. 2019, 17, 2374–2376.e2. [Google Scholar] [CrossRef]
- Lukin, D.; Faleck, D.; Xu, R.; Zhang, Y.; Weiss, A.; Aniwan, S.; Kadire, S.; Tran, G.; Rahal, M.; Winters, A.; et al. Comparative Safety and Effectiveness of Vedolizumab to Tumor Necrosis Factor Antagonist Therapy for Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 10, S1542-3565(20)31388-4. [Google Scholar] [CrossRef]
- Ma, C.; Battat, R.; Dulai, P.S.; Parker, C.E.; Sandborn, W.J.; Feagan, B.G.; Jairath, V. Innovations in Oral Therapies for Inflammatory Bowel Disease. Drugs 2019, 79, 1321–1335. [Google Scholar] [CrossRef]
- Bewtra, M.; Lewis, J.D. Update on the risk of lymphoma following immunosuppressive therapy for inflammatory bowel disease. Expert Rev. Clin. Immunol. 2010, 6, 621–631. [Google Scholar] [CrossRef]
- Danese, S.; Argollo, M.; Le Berre, C.; Peyrin-Biroulet, L. JAK selectivity for inflammatory bowel disease treatment: Does it clinically matter? Gut 2019, 68, 1893. [Google Scholar] [CrossRef]
- Sands, B.E.; Armuzzi, A.; Marshall, J.K.; Lindsay, J.O.; Sandborn, W.J.; Danese, S.; Panés, J.; Bressler, B.; Colombel, J.F.; Lawendy, N.; et al. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: Results from OCTAVE Open. Aliment. Pharm. 2020, 51, 271–280. [Google Scholar] [CrossRef]
- Mowat, C.; Cole, A.; Windsor, A.; Ahmad, T.; Arnott, I.; Driscoll, R.; Mitton, S.; Orchard, T.; Rutter, M.; Younge, L.; et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011, 60, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, L.; Ma, C.; Dulai, P.S.; Prokop, L.J.; Eisenstein, S.; Ramamoorthy, S.L.; Feagan, B.G.; Jairath, V.; Sandborn, W.J.; Singh, S. Contemporary Risk of Surgery in Patients With Ulcerative Colitis and Crohn’s Disease: A Meta-Analysis of Population-Based Cohorts. Clin. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef]
- Almradi, A.; Hanzel, J.; Sedano, R.; Parker, C.E.; Feagan, B.G.; Ma, C.; Jairath, V. Clinical Trials of IL-12/IL-23 Inhibitors in Inflammatory Bowel Disease. BioDrugs 2020, 34, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Hazlewood, G.S.; Pokharel, G.; Deardon, R.; Marshall, D.A.; Bombardier, C.; Tomlinson, G.; Ma, C.; Seow, C.H.; Panaccione, R.; Kaplan, G.G. Patient preferences for maintenance therapy in Crohn’s disease: A discrete-choice experiment. PLoS ONE 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Kalainayakan, S.P.; FitzGerald, K.E.; Konduri, P.C.; Vidal, C.; Zhang, L. Essential roles of mitochondrial and heme function in lung cancer bioenergetics and tumorigenesis. Cell Biosci. 2018, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Chohan, M.O.; Berger, M.S. 5-Aminolevulinic acid fluorescence guided surgery for recurrent high-grade gliomas. J. Neurooncol. 2019, 141, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Al-Saber, F.; Aldosari, W.; Alselaiti, M.; Khalfan, H.; Kaladari, A.; Khan, G.; Harb, G.; Rehani, R.; Kudo, S.; Koda, A.; et al. The Safety and Tolerability of 5-Aminolevulinic Acid Phosphate with Sodium Ferrous Citrate in Patients with Type 2 Diabetes Mellitus in Bahrain. J. Diabetes Res. 2016, 2016, 8294805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.Z.; Van Dijk-Smith, J.P.; Van Vugt, D.A.; Kennedy, J.C.; Reid, R.L. Fluorescence and photosensitization of experimental endometriosis in the rat after systemic 5-aminolevulinic acid administration: A potential new approach to the diagnosis and treatment of endometriosis. Am. J. Obs. Gynecol. 1996, 174, 154–160. [Google Scholar] [CrossRef]
- Hijioka, M.; Kitamura, K.; Yanagisawa, D.; Nishimura, K.; Takata, K.; Inden, M.; Kitamura, Y. Neuroprotective effects of 5-aminolevulinic acid against neurodegeneration in rat models of Parkinson’s disease and stroke. J. Pharmacol. Sci. 2020, 144, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Ngwe Tun, M.M.; Kurosaki, Y.; Sakura, T.; Inaoka, D.K.; Fujine, K.; Kita, K.; Morita, K.; Yasuda, J. 5-amino levulinic acid inhibits SARS-CoV-2 infection in vitro. Biochem. Biophys. Res. Commun. 2021, 545, 203–207. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration, FDA. Aminolevulinic Acid Hydrochloride, Known as ALA HCl (Gleolan, NX Development Corp.) as an Optical Imaging Agent Indicated in Patients with Gliomas. USA; 2017. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/aminolevulinic-acid-hydrochloride-known-ala-hcl-gleolan-nx-development-corp-optical-imaging-agent (accessed on 13 March 2021).
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Darryl, L.; Shawn, L.H.-J.; Susan, C.; Annette, M.M.; Michael, W.M.; Joanna, J.P.; Mitchel, S.B. A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J. Neurosurg. JNS 2016, 124, 1300–1309. [Google Scholar] [CrossRef] [Green Version]
- Utsuki, S.; Oka, H.; Sato, S.; Shimizu, S.; Suzuki, S.; Tanizaki, Y.; Kondo, K.; Miyajima, Y.; Fujii, K. Histological Examination of False Positive Tissue Resection Using 5-Aminolevulinic Acid-Induced Fluorescence Guidance. Neurol. Med. Chir. 2007, 47, 210–214. [Google Scholar] [CrossRef] [Green Version]
- Ito, H.; Nishio, Y.; Hara, T.; Sugihara, H.; Tanaka, T.; Li, X.K. Oral administration of 5-aminolevulinic acid induces heme oxygenase-1 expression in peripheral blood mononuclear cells of healthy human subjects in combination with ferrous iron. Eur. J. Pharm. 2018, 833, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Cai, S.; Kitajima, Y.; Fujino, M.; Ito, H.; Takahashi, K.; Abe, F.; Tanaka, T.; Ding, Q.; Li, X.-K. 5-Aminolevulinic acid combined with ferrous iron induces carbon monoxide generation in mouse kidneys and protects from renal ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2013, 305, F1149–F1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.; Zhang, Q.; Fujino, M.; Cai, S.; Ito, H.; Takahashi, K.; Abe, F.; Nakajima, M.; Tanaka, T.; Xu, J.; et al. 5-Aminolevulinic acid with ferrous iron induces permanent cardiac allograft acceptance in mice via induction of regulatory cells. J. Heart Lung Transplant. 2015, 34, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Guo, H.; Chen, J.; Fujino, M.; Ito, H.; Takahashi, K.; Abe, F.; Nakajima, M.; Tanaka, T.; Wang, J.; et al. 5-Aminolevulinic acid combined with sodium ferrous citrate ameliorates H2O2-induced cardiomyocyte hypertrophy via activation of the MAPK/Nrf2/HO-1 pathway. Am. J. Physiol. Cell Physiol. 2015, 308, C665–C672. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Hida, N.; Ajioka, Y.; Hori, K.; Kamata, N.; Sogawa, M.; Yamagami, H.; Tominaga, K.; Watanabe, T.; Fujiwara, Y.; et al. Photodynamic diagnosis of endoscopically invisible flat dysplasia in patients with ulcerative colitis by visualization using local 5-aminolevulinic acid-induced photosensitization. Gastrointest. Endosc. 2010, 71, 1094–1096. [Google Scholar] [CrossRef]
- Komoike, N.; Kato, T.; Saijo, H.; Arihiro, S.; Hashimoto, H.; Okabe, M.; Ito, M.; Koido, S.; Homma, S.; Tajiri, H. Photodynamic diagnosis of colitis-associated dysplasia in a mouse model after oral administration of 5-aminolevulinic acid. In Vivo 2013, 27, 747–754. [Google Scholar] [PubMed]
- Naito, Y.; Takagi, T.; Yoshikawa, T. Heme oxygenase-1: A new therapeutic target for inflammatory bowel disease. Aliment. Pharm. 2004, 20 (Suppl. 1), 177–184. [Google Scholar] [CrossRef]
- Zhu, X.; Fan, W.G.; Li, D.P.; Kung, H.; Lin, M.C. Heme oxygenase-1 system and gastrointestinal inflammation: A short review. World J. Gastroenterol. 2011, 17, 4283–4288. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Hao, H.; Hegarty, J.P.; Lin, T.R.; Wang, Y.; Harris, L.R.; Xu, H.N.; Wu, R.; Thomas, N.J.; Floros, J. Association of the haem oxygenase-1 gene with inflammatory bowel disease. Swiss Med. Wkly. 2017, 147, w14456. [Google Scholar] [CrossRef] [Green Version]
- Namjoshi, S.; Caccetta, R.; Edwards, J.; Benson, H.A.E. Liquid chromatography assay for 5-aminolevulinic acid: Application to in vitro assessment of skin penetration via Dermaportation. J. Chromatogr. B 2007, 852, 49–55. [Google Scholar] [CrossRef]
- Axelsson, L.G.; Landström, E.; Bylund-Fellenius, A.C. Experimental colitis induced by dextran sulphate sodium in mice: Beneficial effects of sulphasalazine and olsalazine. Aliment. Pharmacol. Ther. 1998, 12, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Hatton, G.B.; Madla, C.M.; Rabbie, S.C.; Basit, A.W. All disease begins in the gut: Influence of gastrointestinal disorders and surgery on oral drug performance. Int. J. Pharm. 2018, 548, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Tubic-Grozdanis, M.; Hilfinger, J.M.; Amidon, G.L.; Kim, J.S.; Kijek, P.; Staubach, P.; Langguth, P. Pharmacokinetics of the CYP 3A substrate simvastatin following administration of delayed versus immediate release oral dosage forms. Pharm. Res. 2008, 25, 1591–1600. [Google Scholar] [CrossRef]
- Montbarbon, M.; Pichavant, M.; Langlois, A.; Erdual, E.; Maggiotto, F.; Neut, C.; Mallevaey, T.; Dharancy, S.; Dubuquoy, L.; Trottein, F.; et al. Colonic Inflammation in Mice Is Improved by Cigarette Smoke through iNKT Cells Recruitment. PLoS ONE 2013, 8, e62208. [Google Scholar] [CrossRef] [Green Version]
- Resendez, J.C.; Rehagen, D. Infusion Toxicology and Techniques. In A Comprehensive Guide to Toxicology in Nonclinical Drug Development; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 555–583. [Google Scholar]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Javdan, B.; Lopez, J.G.; Chankhamjon, P.; Lee, Y.J.; Hull, R.; Wu, Q.; Wang, X.; Chatterjee, S.; Donia, M.S. Personalized Mapping of Drug Metabolism by the Human Gut Microbiome. Cell 2020, 181, 1661–1679.e22. [Google Scholar] [CrossRef] [PubMed]
- McCoubrey, L.E.; Elbadawi, M.; Orlu, M.; Gaisford, S.; Basit, A.W. Harnessing machine learning for development of microbiome therapeutics. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. ICH Guideline M3(R2) on Non-Clinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorisation for Pharmaceuticals; The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH): London, UK, 2009.
- McConnell, E.L.; Basit, A.W.; Murdan, S. Colonic antigen administration induces significantly higher humoral levels of colonic and vaginal IgA, and serum IgG compared to oral administration. Vaccine 2008, 26, 639–646. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.L.; Liu, F.; Basit, A.W. Colonic treatments and targets: Issues and opportunities. J. Drug Target. 2009, 17, 335–363. [Google Scholar] [CrossRef]
- Ma, C.; Panaccione, R. Harnessing localised delivery of gut-selective therapy for ulcerative colitis. Lancet Gastroenterol. Hepatol. 2020, 5, 1031–1032. [Google Scholar] [CrossRef]
- Steiger, C.; Phan, N.V.; Sun, H.; Huang, H.-W.; Hess, K.; Lopes, A.; Korzenik, J.; Langer, R.; Traverso, G. Controlled Delivery of Bile Acids to the Colon. Clin. Transl. Gastroenterol. 2020, 11, e00229. [Google Scholar] [CrossRef]
- Varum, F.; Cristina Freire, A.; Fadda, H.M.; Bravo, R.; Basit, A.W. A dual pH and microbiota-triggered coating (Phloral(TM)) for fail-safe colonic drug release. Int. J. Pharm. 2020, 583, 119379. [Google Scholar] [CrossRef] [PubMed]
- Varum, F.; Cristina Freire, A.; Bravo, R.; Basit, A.W. OPTICORE, an innovative and accurate colonic targeting technology. Int. J. Pharm. 2020, 583, 119372. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, V.C.; Khela, M.K.; Evans, D.F.; Basit, A.W. A new concept in colonic drug targeting: A combined pH-responsive and bacterially-triggered drug delivery technology. Aliment. Pharmacol. Ther. 2008, 28, 911–916. [Google Scholar] [CrossRef] [PubMed]




| Component | Quantity/L (In Grams Unless Otherwise Stated) |
|---|---|
| Peptone water | 2.00 |
| Yeast extract | 2.00 |
| Sodium chloride | 0.10 |
| Monopotassium phosphate | 0.04 |
| Calcium chloride hexahydrate | 0.01 |
| Magnesium sulfate heptahydrate | 0.01 |
| Tween 80 | 2 mL |
| Bile salts | 0.50 |
| L-cysteine hydrochloride | 0.50 |
| Phytomenadione (vitamin K) | 10 µL |
| Sodium bicarbonate | 2.00 |
| Component | Quantity per 10 mL HPLC H2O |
|---|---|
| Tris-buffered saline (10X stock solution) | 2 mL |
| Phosphate buffered saline | 8 mL |
| Sodium chloride | 146 mg |
| Nonidet TM P40 substitute | 100 µL |
| Group | Number of Mice | Route | Intervention | Therapeutic Dose (mg/kg) |
|---|---|---|---|---|
| 1 | 7 | PO | PBS | NA |
| 2 | 7 | PO | 2.5% DSS + PBS | NA |
| 3 | 7 | PO | 2.5% DSS + ciclosporin | 70 |
| 4 | 7 | PO | 2.5% DSS + 5-ASA | 50 |
| 5 | 7 | PO | 2.5% DSS + 5-ALA/SFC | 10/1.5 |
| 6 | 7 | PO | 2.5% DSS + 5-ALA/SFC | 100/15.7 |
| 7 | 7 | IR | PBS | NA |
| 8 | 7 | IR | 2.5% DSS + ciclosporin | 70 |
| 9 | 7 | IR | 2.5% DSS + 5-ASA | 50 |
| 10 | 7 | IR | 2.5% DSS + 5-ALA/SFC | 10/1.5 |
| 11 | 7 | IR | 2.5% DSS + 5-ALA/SFC | 100/15.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, V.; Mai, Y.; McCoubrey, L.E.; Wada, Y.; Tomioka, M.; Kawata, S.; Charde, S.; Basit, A.W. 5-Aminolevulinic Acid as a Novel Therapeutic for Inflammatory Bowel Disease. Biomedicines 2021, 9, 578. https://doi.org/10.3390/biomedicines9050578
Yadav V, Mai Y, McCoubrey LE, Wada Y, Tomioka M, Kawata S, Charde S, Basit AW. 5-Aminolevulinic Acid as a Novel Therapeutic for Inflammatory Bowel Disease. Biomedicines. 2021; 9(5):578. https://doi.org/10.3390/biomedicines9050578
Chicago/Turabian StyleYadav, Vipul, Yang Mai, Laura E. McCoubrey, Yasufumi Wada, Motoyasu Tomioka, Satofumi Kawata, Shrikant Charde, and Abdul W. Basit. 2021. "5-Aminolevulinic Acid as a Novel Therapeutic for Inflammatory Bowel Disease" Biomedicines 9, no. 5: 578. https://doi.org/10.3390/biomedicines9050578
APA StyleYadav, V., Mai, Y., McCoubrey, L. E., Wada, Y., Tomioka, M., Kawata, S., Charde, S., & Basit, A. W. (2021). 5-Aminolevulinic Acid as a Novel Therapeutic for Inflammatory Bowel Disease. Biomedicines, 9(5), 578. https://doi.org/10.3390/biomedicines9050578