Outstanding Contributions of LAL Technology to Pharmaceutical and Medical Science: Review of Methods, Progress, Challenges, and Future Perspectives in Early Detection and Management of Bacterial Infections and Invasive Fungal Diseases
Abstract
1. Introduction
2. History of the LAL Test and Its Clinical Application
3. Gram-Negative Bacteremia and Endotoxemia
4. Factors in Blood Samples That Interfere with the Limulus Test
5. Pretreatments for Overcoming Interferents in Blood
6. Improvement of LAL Technology
7. Various Techniques Involving the LAL Assay and Other Methods
8. Fluorescence Spectroscopy and Electrochemistry-Based Rapid Determinations
9. Indirect Assay for Endotoxin
10. Relevant Analytes
11. Low-Grade Endotoxemia Related to Chronic Inflammation
12. Next-Generation LAL Technology
13. Contribution of LAL-Based β-Glucan Assay in Invasive Fungal Diseases
14. Steadily Increased Use of Fungitell in Global Diagnostic Laboratories
15. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Levin, J.; Bang, F.B. The role of endotoxin in the intracellular coagulation of Limulus blood. Bull. Johns Hopkins Hosp. 1964, 115, 265–274. [Google Scholar]
- Nakamura, T.; Morita, T.; Iwanaga, S. Lipopolysaccharide-sensitive serine-protease zymogen (factor C) found in Limulus hemocytes. Eur. J. Biochem. 1986, 154, 511–521. [Google Scholar] [CrossRef]
- Franco, E.; Garcia-Recio, V.; Jiménez, P.; Garrosa, M.; Girbés, T.; Cordoba-Diaz, M.; Cordoba-Diaz, D. Endotoxins from a pharmacopoeial point of view. Toxin 2018, 10, 331. [Google Scholar] [CrossRef]
- Iwanaga, S.; Kawabata, S.; Muta, T. New types of clotting factors and defense molecules found in horseshoe crab hemolymph: Their structures and functions. J. Biochem. 1998, 123, 1–15. [Google Scholar] [CrossRef]
- Morita, T.; Tanaka, S.; Nakamura, T.; Iwanaga, S. A new (1→3)-β-D-glucan-mediated coagulation pathway found in Limulus amebocytes. FEBS Lett. 1981, 129, 318–321. [Google Scholar] [CrossRef]
- Obayashi, T.; Tamura, H.; Tanaka, S.; Ohki, M.; Takahashi, S.; Arai, M.; Masuda, M.; Kawai, T. A new chromogenic endotoxin-specific assay using recombined Limulus coagulation enzymes and its clinical applications. Clin. Chim. Acta. 1985, 149, 55–65. [Google Scholar] [CrossRef]
- Obayashi, T.; Yoshida, M.; Tamura, H.; Aketagawa, J.; Tanaka, S.; Kawai, T. Determination of plasma (1 → 3)-β-D-glucan: A new diagnostic aid to deep mycosis. J. Med. Vet. Mycol. 1992, 30, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Obayashi, T.; Tamura, H.; Tanaka, S.; Kawai, T.; Sakamoto, S.; Miura, Y. Diagnostic and prognostic significance of plasma endotoxin determination in febrile patients with haematological malignancies. Eur. J. Cancer 1994, 30, 145–147. [Google Scholar] [CrossRef]
- Munford, R.S. Endotoxemia-menace, marker, or mistake? J. Leukoc. Biol. 2016, 100, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, T.; Kawai, T.; Yoshida, M.; Mori, T.; Goto, H.; Yasuoka, A.; Shimada, K.; Iwasaki, H.; Teshima, H.; Kohno, S.; et al. Plasma (1→3)-β-D-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet 1995, 345, 17–20. [Google Scholar] [CrossRef]
- Ostrosky-Zeichner, Z.; Alexander, B.D.; Kett, D.H.; Vazquez, J.; Pappas, P.G.; Saeki, F.; Ketchum, P.A.; Wingard, J.; Schiff, R.; Tamura, H.; et al. Multicenter clinical evaluation of the (1→3) β-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 2005, 41, 654–659. [Google Scholar] [CrossRef]
- Hurley, J.C.; Nowak, P.; Ohrmalm, L.; Gogos, C.; Armaganidis, A.; Giamarellos-Bourboulisg, E.J. Endotoxemia as a diagnostic tool for patients with suspected bacteremia caused by gram-negative organisms: A meta-analysis of 4 decades of studies. J. Clin. Microbiol. 2015, 53, 1183–1191. [Google Scholar] [CrossRef]
- Brandtzaeg, P.; Bryn, K.; Kierulf, P.; Ovstebø, R.; Namork, E.; Aase, B.; Jantzen, E. Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass-spectrometry, ultracentrifugation, and electron microscopy. J. Clin. Invest. 1992, 89, 816–823. [Google Scholar] [CrossRef][Green Version]
- Simpson, A.J.; Opal, S.M.; Angus, B.J.; Prins, J.M.; Palardy, J.E.; Parejo, N.A.; Chaowagul, W.; White, N.J. Differential antibiotic-induced endotoxin release in severe melioidosis. J. Infect. Dis. 2000, 181, 1014–1019. [Google Scholar] [CrossRef]
- Van Deventer, S.J.; de Vries, I.; Statius van Eps, L.W.; Pauw, W.; Büller, H.R.; Sturk, A.; ten Cate, J.W. Endotoxemia, bacteremia and urosepsis. Prog. Clin. Biol. Res. 1988, 272, 213–224. [Google Scholar] [PubMed]
- Tamura, H.; Obayashi, T.; Takagi, K.; Tanaka, S.; Nakahara, C.; Kawai, T. Perchloric acid treatment of human blood for quantitative endotoxin assay using synthetic chromogenic substrate for horseshoe crab clotting enzyme. Thromb. Res. 1982, 27, 51–57. [Google Scholar] [CrossRef]
- Tamura, H.; Arimoto, Y.; Tanaka, S.; Yoshida, M.; Obayashi, T.; Kawai, T. Automated kinetic assay for endotoxin and (1→3)- beta-D-glucan in human blood. Clin. Chim. Acta 1994, 226, 109–112. [Google Scholar] [CrossRef]
- Tamura, H.; Reich, J.; Nagaoka, I. Bacterial endotoxin assays relevant to host defense peptieds. Juntendo Med. J. 2016, 62, 132–140. [Google Scholar] [CrossRef]
- Reich, J.; Lang, P.; Grallert, H.; Motschmanna, H. Masking of endotoxin in surfactant samples: Effects on Limulus-based detection systems. Biologicals 2016, 44, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Reich, J.; Tamura, H.; Nagaoka, I.; Motschmann, H. Investigation of the kinetics and mechanism of low endotoxin recovery in a matrix for biopharmaceutical drug products. Biologicals 2018, 53, 1–9. [Google Scholar] [CrossRef]
- Schwarz, H.; Gornicec, J.; Neuper, T.; Parigiani, M.A.; Wallner, M.; Duschl, A.; Horejs-Hoeck, J. Biological activity of masked endotoxin. Sci. Rep. 2017, 7, 44750. [Google Scholar] [CrossRef]
- Munford, R.S.; Hall, C.L.; Dietschy, J.M. Binding of Salmonella typhimurium lipopolysaccharides to rat high-density lipoproteins. Infect. Immun. 1981, 34, 835–843. [Google Scholar] [CrossRef]
- Nachum, R.; Lipsey, A.; Siegel, S.E. Rapid detection of gram-negative bacterial meningitis by the limulus lysate test. N. Engl. J. Med. 1973, 289, 931–934. [Google Scholar] [CrossRef]
- Inagaki, M.; Nakajima, M.; Ando, Y.; Takashima, S.; Takeshita, K.; Tanaka, S.; Tamura, H.; Obayashi, T. Measurement of endotoxin in cerebrospinal fluid from neonates and infants using a new endotoxin-specific chromogenic test. Clin. Chim. Acta 1988, 175, 315–319. [Google Scholar] [CrossRef]
- Levin, J.; Poore, T.E.; Zauber, N.P.; Oser, R.S. Detection of endotoxin in the blood of patients with sepsis due to gram-negative bacteria. N. Engl. J. Med. 1970, 283, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, T. Addition of perchloric acid to blood samples for colorimetric limulus test using chromogenic substrate: Comparison with conventional procedures and clinical applications. J. Lab. Clin. Med. 1984, 104, 321–330. [Google Scholar] [PubMed]
- Novitsky, T. Limulus amebocyte lysate (LAL) detection of endotoxin in human blood. J. Endotoxin Res. 1994, 1, 253–263. [Google Scholar] [CrossRef]
- Inada, K.; Endo, S.; Takahashi, K.; Suzuki, M.; Narita, T.; Yoshida, T.; Suda, H.; Komuro, T.; Yoshida, M. Establishment of a new perchloric acid treatment method to allow determination of the total endotoxin content in human plasma by the limulus test and clinical application. Microbiol. Immunol. 1991, 35, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Gnauck, A.; Lentle, R.G.; Kruger, M.C. Chasing a ghost?--Issues with the determination of circulating levels of endotoxin in human blood. Crit. Rev. Clin. Lab. Sci. 2016, 53, 197–215. [Google Scholar] [CrossRef]
- Tamura, H.; Tanaka, S.; Obayashi, T.; Yoshida, M.; Kawai, T. A new sensitive method for determining endotoxin in whole blood. Clin. Chim. Acta 1991, 200, 35–42. [Google Scholar] [CrossRef]
- Bates, D.W.; Parsonnet, J.; Ketchum, P.A.; Miller, E.B.; Novitsky, T.J.; Sands, K.; Hibberd, P.L.; Graman, P.S.; Lanken, P.N.; Schwartz, J.S.; et al. Limulus amebocyte lysate assay for detection of endotoxin in patients with sepsis syndrome. AMCC Sepsis Project Working Group. Clin. Infect. Dis. 1998, 27, 582–591. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sezai, S.; Sakurabayashi, S.; Yoshiura, K.; Sanui, S.; Morita, T.; Shimizu, T.; Yoshino, K.; Iwase, T.; Hirano, M.; et al. The study of endotoxin levels in portal venous system in liver cirrhosis. Nihon Shokakibyo Gakkai Zasshi 1990, 87, 997–1002. (In Japanese) [Google Scholar] [PubMed]
- Kambayashi, J.; Yokota, M.; Sakon, M.; Shiba, E.; Kawasaki, T.; Mori, T.; Tsuchiya, M.; Oishi, H.; Matsuura, S. A novel endotoxin-specific assay by turbidimetry with Limulus amoebocyte lysate containing beta-glucan. J. Biochem. Biophys. Methods 1991, 22, 93–100. [Google Scholar] [CrossRef]
- Shimizu, T.; Obata, T.; Sonoda, H.; Akabori, H.; Tabata, T.; Eguchi, Y.; Endo, Y.; Tani, T. The ability of endotoxin adsorption during a longer duration of direct hemoperfusion with a polymyxin B-immobilized fiber column in patients with septic shock. Transfus. Apher. Sci. 2013, 49, 499–503. [Google Scholar] [CrossRef]
- Shimizu, T.; Obata, T.; Sonoda, H.; Akabori, H.; Miyake, T.; Yamamoto, H.; Tabata, T.; Eguchi, Y.; Tani, T. Diagnostic potential of endotoxin scattering photometry for sepsis and septic shock. Shock 2013, 40, 504–511. [Google Scholar] [CrossRef]
- Noda, K.; Goto, H.; Murakami, Y.; Ahmed, A.B.; Kuroda, A. Endotoxin assay by bioluminescence using mutant firefly luciferase. Anal. Biochem. 2010, 397, 152–155. [Google Scholar] [CrossRef]
- Suzuki, M.M.; Matsumoto, M.; Omi, H.; Kobayashi, T.; Nakamura, A.; Kishi, H.; Kobayashi, S.; Takagi, T. Interaction of peptide-bound beads with lipopolysaccharide and lipoproteins. J. Microbiol. Methods 2014, 100, 137–141. [Google Scholar] [CrossRef]
- Grallert, H.; Leopoldseder, S.; Schuett, M.; Peter Kurze, P.; Buchberger, B. EndoLISA®: A novel and reliable method for endotoxin detection. Nat. Methods 2011, 8, 3–5. [Google Scholar] [CrossRef]
- Remillard, J.F.; Gould, M.C.; Roslansky, P.F.; Novitsky, T.J. Quantitation of endotoxin in products using the LAL kinetic turbidimetric assay. Prog. Clin. Biol. Res. 1987, 231, 197–210. [Google Scholar] [PubMed]
- Maule, J.; Wainwright, N.; Steele, A.; Monaco, L.; Morris, H.; Gunter, D.; Damon, M.; Wells, M. Rapid culture-independent microbial analysis aboard the International Space Station (ISS). Astrobiology 2009, 9, 759–775. [Google Scholar] [CrossRef]
- Ding, J.L.; Ho, B. A new era in pyrogen testing. Trends Biotechnol. 2001, 19, 277–281. [Google Scholar] [CrossRef]
- Mizumura, H.; Ogura, N.; Aketagawa, J.; Aizawa, M.; Kobayashi, Y.; Kawabata, S.; Oda, T. Genetic engineering approach to develop next-generation reagents for endotoxin quantification. Innate Immun. 2017, 23, 136–146. [Google Scholar] [CrossRef]
- Maloney, T.; Phelan, R.; Simmons, N. Saving the horseshoe crab: A synthetic alternative to horseshoe crab blood for endotoxin detection. PLoS Biol. 2018, 16, e2006607. [Google Scholar] [CrossRef] [PubMed]
- Piehler, M.; Roeder, R.; Blessing, S.; Reich, J. Comparison of LAL and rFC assays-participation in a proficiency test program between 2014 and 2019. Microorganisms 2020, 8, 418. [Google Scholar] [CrossRef]
- Bolden, J.; Knutsen, C.; Levin, J.; Milne, C.; Morris, T.; Mozier, N.; Spreitzer, I.; von Wintzingerode, F. Currently available recombinant alternatives to horseshoe crab blood lysates: Are they comparable for the detection of environmental bacterial endotoxins? A review. PDA J. Pharm. Sci. Technol. 2020, 74, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.K.; Schotz, M.C.; Yoshikawa, T.T.; Guze, L.B. Determination of lipid A and endotoxin in serum by mass spectroscopy. Proc. Natl. Acad. Sci. USA 1978, 75, 3993–3997. [Google Scholar] [CrossRef]
- Tanamoto, K. Development of a new quantitative method for detection of endotoxin by fluorescence labeling of 3-hydroxy fatty acid. Adv. Exp. Med. Biol. 1990, 256, 203–213. [Google Scholar] [PubMed]
- Mohammed, A.H.; McCallus, D.E.; Norcross, N.L. Development and evaluation of an enzyme-linked immunosorbent assay for endotoxin in milk. Vet. Microbiol. 1988, 18, 27–39. [Google Scholar] [CrossRef]
- Inoue, K.Y. Research of electrochemical sensor for highly sensitive and quantitative analysis with simple operation. Rev. Polarogr. 2019, 65, 3–10. (In Japanese) [Google Scholar] [CrossRef]
- Munford, R.S.; Hall, C.L. Radioimmunoassay for Gram-negative bacterial lipopolysaccharide O antigens: Influence of antigen solubility. Infect Immun. 1979, 26, 42–48. [Google Scholar] [CrossRef]
- Sloyer, J.; Novitsky, T.J. Use of rENP to quantitate endotoxin by fluorescence polarization. J. Endotoxin Res. 2000, 6, 101. [Google Scholar]
- Takano, S.; Inoue, K.Y.; Takahashi, S.; Ino, K.; Hitoshi Shiku, H.; Matsue, T. Electrochemical sensor with substitutional stripping voltammetry for highly sensitive endotoxin assay. Analyst 2014, 139, 5001–5006. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Chai, Y.; Pu, X.; Ruo Yuan, R. A signal-on electrochemical aptasensor for ultrasensitive detection of endotoxin using three-way DNA junction-aided enzymatic recycling and graphene nanohybrid for amplification. Nanoscale 2014, 6, 2902–2908. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Peterbauer, A.; Schindler, S.; Fennrich, S.; Poole, S.; Mistry, Y.; Montag-Lessing, T.; Spreitzer, I.; Löschner, B.; van Aalderen, M.; et al. International validation of novel pyrogen tests based on human monocytoid cells. J. Immunol. Methods. 2005, 298, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ghosh, S. Molecular mechanisms of NF-kappaB activation induced by bacterial lipopolysaccharide through Toll-like receptors. J. Endotoxin Res. 2000, 6, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Hatao, T.; Hiki, N.; Shioiri, T.; Ogawa, T.; Mimura, Y.; Kaminishi, N.; Muroi, M.; Tanamoto, K. Lipopolysaccharide biological activity assay (LBA). Endotoxin Innate Immun. 2007, 10, 126–132. (In Japanese) [Google Scholar]
- Hartung, T. Pyrogen testing revisited on occasion of the 25th anniversary of the whole blood monocyte activation test. ALTEX 2021, 38, 3–19. [Google Scholar] [CrossRef]
- Marshall, J.C.; Walker, P.M.; Foster, D.M.; Harris, D.; Ribeiro, M.; Paice, J.; Alexander, D.; Romaschin, A.D.; Derzko, A.N. Measurement of endotoxin activity in critically ill patients using whole blood neutrophil dependent chemiluminescence. Crit. Care 2002, 6, 342–348. [Google Scholar] [CrossRef]
- Klein, D.J.; Foster, D.; Walker, P.M.; Bagshaw, S.M.; Mekonnen, H.; Antonelli, M. Polymyxin B hemoperfusion in endotoxemic septic shock patients without extreme endotoxemia: A post hoc analysis of the EUPHRATES trial. Intensive Care Med. 2018, 44, 2205–2212. [Google Scholar] [CrossRef]
- Ishihata, K.; Kakihana, Y.; Yasuda, T.; Imabayashi, T.; Nakamura, N. Newly developed endotoxin measurement method (the Endotoxin Activity Assay) may reflect the severity of sepsis. Open J. Pathol. 2013, 3, 1–6. [Google Scholar] [CrossRef]
- Levels, J.H.; Lemaire, L.C.; van den Ende, A.E.; van Deventer, S.J.; van Lanschot, J.J. Lipid composition and lipopolysaccharide binding capacity of lipoproteins in plasma and lymph of patients with systemic inflammatory response syndrome and multiple organ failure. Crit. Care Med. 2003, 31, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Kitchens, R.L.; Thompson, P.A.; Munford, R.S.; O’Keefe, G.E. Acute inflammation and infection maintain circulating phospholipid levels and enhance lipopolysaccharide binding to plasma lipoproteins. J. Lipid Res. 2003, 44, 2339–2348. [Google Scholar] [CrossRef]
- Allan, E.; Poxton, I.R.; Barclay, G.R. Anti-bacteroides lipopolysaccharide IgG levels in healthy adults and sepsis patients. FEMS Immunol. Med. Microbiol. 1995, 11, 5–12. [Google Scholar] [CrossRef]
- Opal, S.M.; Scannon, P.J.; Vincent, J.L.; White, M.; Carroll, S.F.; Palardy, J.E.; Parejo, N.A.; Pribble, J.P.; Lemke, J.H. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 1999, 180, 1584–1589. [Google Scholar] [CrossRef]
- Herrmann, W.; Ecker, D.; Quast, S.; Klieden, M.; Rose, S.; Marzi, I. Comparison of procalcitonin, sCD14 and interleukin-6 values in septic patients. Clin. Chem. Lab. Med. 2000, 38, 41–46. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Mitaka, C. Clinical laboratory differentiation of infectious versus non-infectious systemic inflammatory response syndrome. Clin. Chim. Acta 2005, 351, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H. Increased intestinal permeability and decreased barrier function: Does it really influence the risk of inflammation? Inflamm. Intest. Dis. 2016, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Sciarretta, S.; Valenti, V.; di Nonno, F.; Calvieri, C.; Nocella, C.; Frati, G.; Forte, M.; d’Amati, G.; Maria, G.; et al. Low-grade endotoxaemia enhances artery thrombus growth via Toll-like receptor 4: Implication for myocardial infarction. Eur. Heart J. 2020, 41, 3156–3165. [Google Scholar] [CrossRef] [PubMed]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef]
- Boroni Moreira, A.P.; de Cássia Gonçalves Alfenas, R. The influence of endotoxemia on the molecular mechanisms of insulin resistance. Nutr. Hosp. 2012, 27, 382–390. [Google Scholar] [PubMed]
- Finkelman, M. Specificity influences in (1→3)-β-D-glucan-supported diagnosis of invasive fungal disease. J. Fungi 2020, 7, 14. [Google Scholar] [CrossRef]
- Cummins, B.M.; Ligler, F.S.; Walker, G.M. Point-of-Care diagnostics for niche applications. Biotechnol. Adv. 2016, 34, 161–176. [Google Scholar] [CrossRef]
- Perlroth, J.; Choi, B.; Spellberg, B. Nosocomial fungal infections: Epidemiology, diagnosis, and treatment. Med. Mycol. 2007, 45, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.; Verhaegen, J.; Lagrou, K.; Eldere, J.V.; Boogaerts, M. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: A prospective validation. Blood 2001, 97, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, Y.Q.; Guo, Y.L.; Qin, S.M.; Wu, C.; Ke Wang, K. Diagnosis of invasive fungal disease using serum (1→3)-β-D-glucan: A bivariate meta-analysis. Intern. Med. 2011, 50, 2783–2791. [Google Scholar] [CrossRef]
- Karageorgopoulos, D.E.; Vouloumanou, E.K.; Ntziora, F.; Michalopoulos, A.; Rafailidis, P.I.; Matthew, E.; Falagas, M.E. β-D-glucan assay for the diagnosis of invasive fungal infections: A meta-analysis. Clin. Infect. Dis. 2011, 52, 750–770. [Google Scholar] [CrossRef]
- Onishi, A.; Sugiyama, D.; Kogata, Y.; Saegusa, J.; Sugimoto, T.; Kawano, S.; Morinobu, A.; Nishimura, K.; Kumagai, S. Diagnostic accuracy of serum 1,3-β-D-glucan for pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: Systematic review and meta-analysis. J. Clin. Microbiol. 2012, 50, 7–15. [Google Scholar] [CrossRef]
- White, S.K.; Walker, B.S.; Hanson, K.E.; Robert, L.; Schmidt, R.L. Diagnostic accuracy of β-D-glucan (fungitell) testing among patients with hematologic malignancies or solid organ tumors: A systematic review and meta-analysis. Am. J. Clin. Pathol. 2019, 151, 275–285. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar]
- Sunamura, E.; Manami Iwasaki, M.; Shiina, S.; Shin-Ichiro Kitahara, S.; Takuya Yotani, T.; Manabe, M.; Miyazaki, O. A novel enzyme immunoassay for the measurement of plasma (1→3)-β-D-glucan levels. J. Immunol. Methods 2020, 487, 112872. [Google Scholar] [CrossRef] [PubMed]
- Desmet, S.; van Wijngaerden, E.; Maertens, J.; Verhaegen, J.; Verbeken, E.; De Munter, P.; Meersseman, W.; Van Meensel, B.; Van Eldere, J.; Lagrou, K. Serum (1-3)-β-D-glucan as a tool for diagnosis of pneumocystis jirovecii pneumonia in patients with human immunodeficiency virus infection or hematological malignancy. J. Clin. Microbiol. 2009, 47, 3871–3874. [Google Scholar] [CrossRef]
- Muroi, M.; Ogura, N.; Mizumura, H.; Aketagawa, J.; Oda, T.; Tanamoto, K.-I. Application of a recombinant three-factor chromogenic reagent, pyrosmart, for bacterial endotoxins test filed in the pharmacopeias. Biol. Pharm. Bull. 2019, 42, 2024–2037. [Google Scholar] [CrossRef]
- Nagaoka, I.; Tamura, H.; Reich, J. Therapeutic potential of cathelicidin peptide LL-37, an antimicrobial agent, in a murine sepsis model. Int. J. Mol. Sci. 2020, 21, 5973. [Google Scholar] [CrossRef] [PubMed]
- Prüller, F.; Wagner, J.; Raggam, R.B.; Hoenigl, M.; Kessler, H.H.; Martie Truschnig-Wilders, M.; Robert Krause, R. Automation of serum (1→3)-beta-D-glucan testing allows reliable and rapid discrimination of patients with and without candidemia. Med. Mycol. 2014, 52, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P.; Najvar, L.K.; Bocanegra, R.; Kirkpatrick, W.R.; Patterson, T.F.; Thornton, C.R. Interlaboratory and interstudy reproducibility of a novel lateral-flow device and influence of antifungal therapy on detection of invasive pulmonary aspergillosis. J. Clin. Microbiol. 2013, 51, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Tanaka, S.; Oda, T.; Uemura, Y.; Aketagawa, J.; Hashimoto, Y. Purification and characterization of a (1→3)-beta-D-glucan-binding protein from horseshoe crab (Tachypleus tridentatus) amoebocytes. Carbohydr. Res. 1996, 295, 103–116. [Google Scholar]
- Adachi, Y.; Ishii, M.; Kanno, T.; Tetsui, J.; Ishibashi, K.; Yamanaka, D.; Miura, N.; Ohno, N. N-Terminal (1→3)-β-D-glucan recognition proteins from insects recognize the difference in ultra-structures of (1→3)-β-D-glucan. Int. J. Mol. Sci. 2019, 20, 3498. [Google Scholar] [CrossRef]
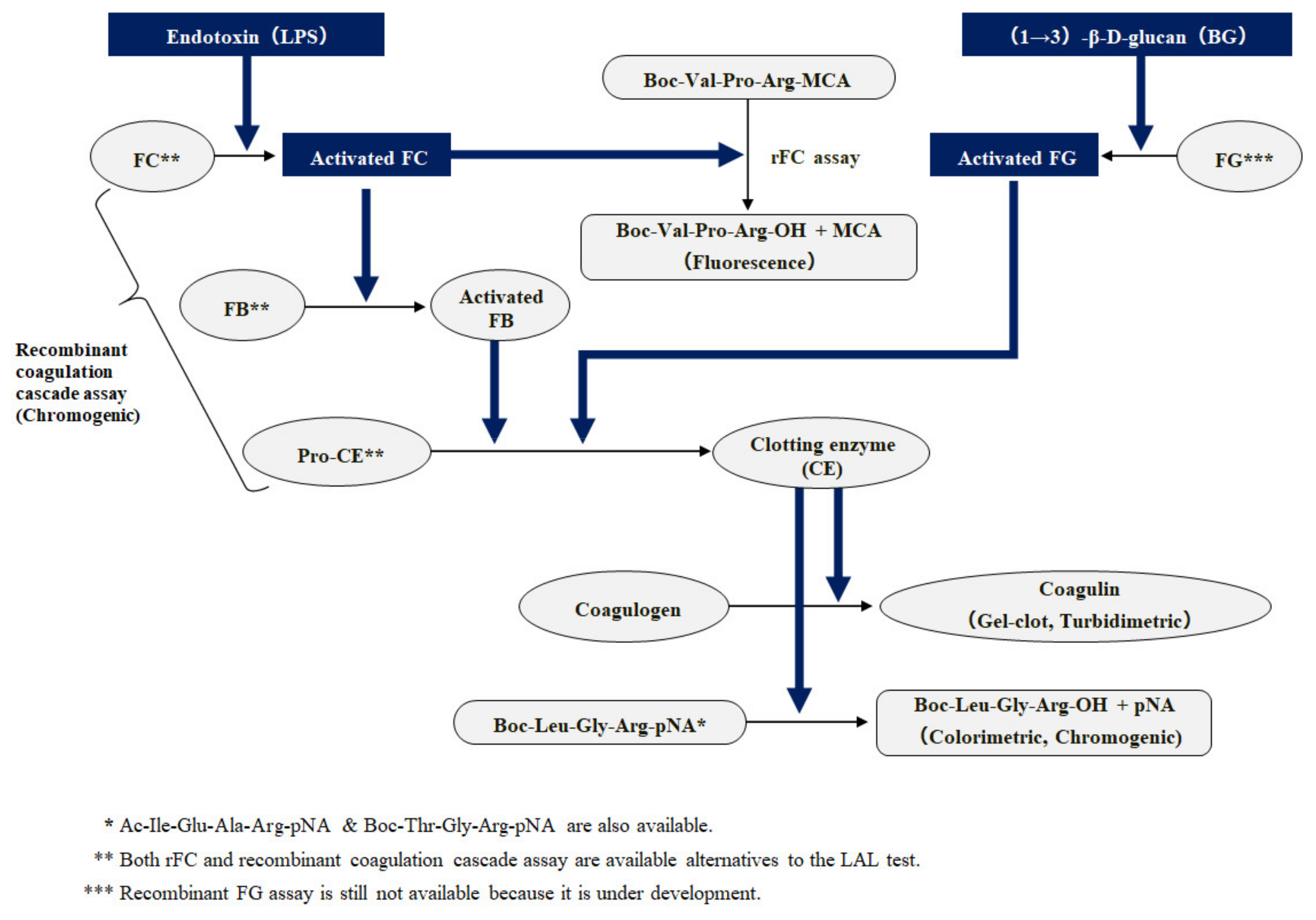
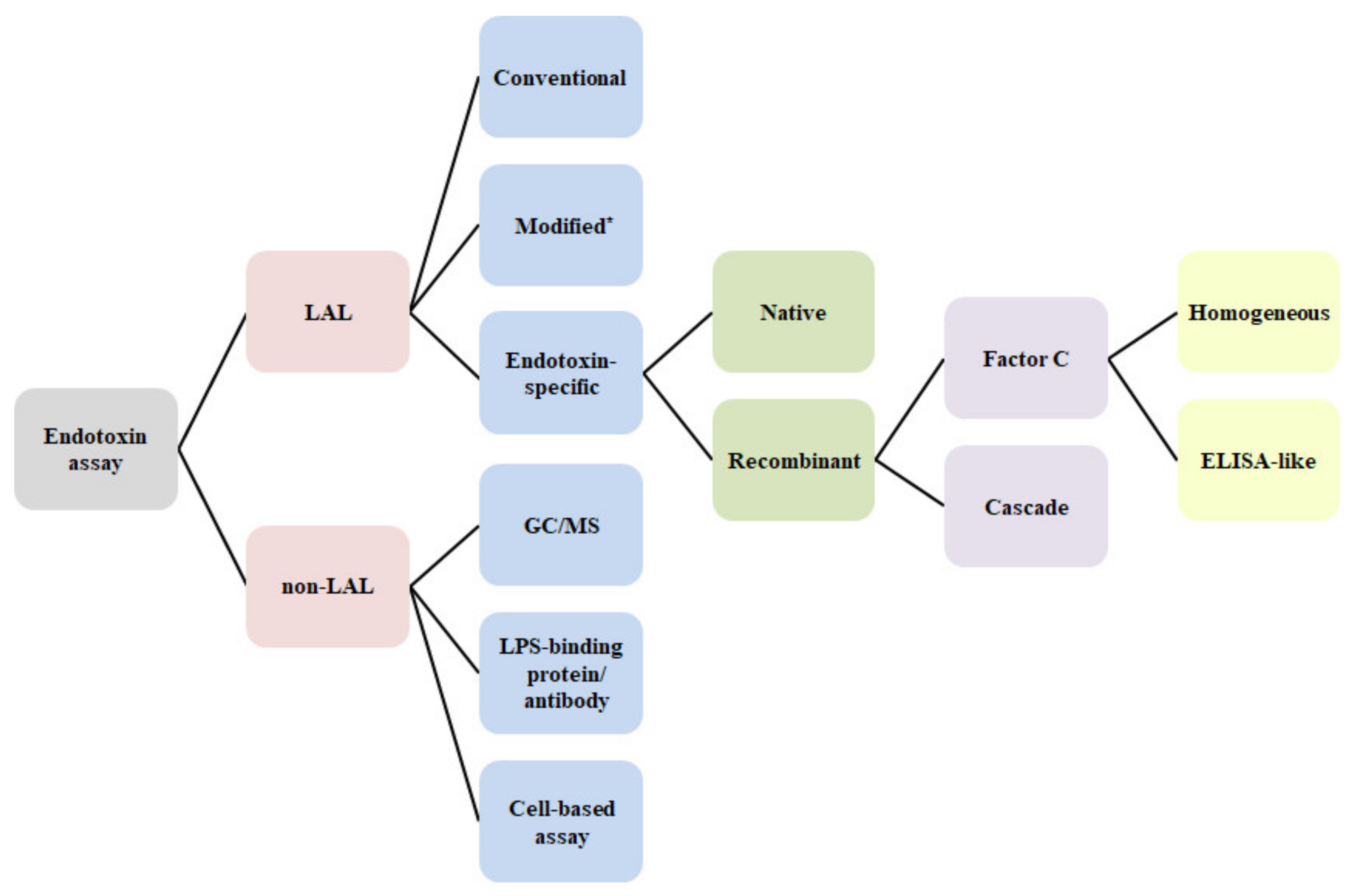
| LAL/ | Analyte | Technique | Principle/Key Elements | Method | Sample | Reference |
|---|---|---|---|---|---|---|
| Non-LAL | ||||||
| LAL | Endotoxin | Conventional/ Endotoxin-specific | Activation of pro-clotting enzyme in Limulus amebocyte lysate | Gel-clot | Plasma | Levin, J. [25] |
| Chromogenic/Turbidimetric | Plasma | van Deventer, S.J. [15] Tamura, H. [16,17] Obayashi, T. [26] Inada, K. [28] Kambayashi, J. [33] | ||||
| LAL alternatives (Endotoxin-specific) | Recombinant Factor C | Fluorogenic | Non-clinical | Ding, J.L. [41] | ||
| Recombinant coagulation enzymes | Chromogenic | Pharmaceutical Non-clinical | Mizumura, T. [42] | |||
| Modified LAL | Endotoxin scattering photometry (ESP) | Light scattering by small particles | Plasma | Shimizu, T. [35] | ||
| Engineered firefly luciferases with improved sensitivity | Chromogenic | Dialysate | Noda, K. [36] | |||
| Target LPS capture with LPS binding peptide produced by phage-display | Chromogenic | Pharmaceutical Non-clinical | Suzuki, M.M. [37] | |||
| Target LPS capture with phage-derived protein | Chromogenic/ Fluorogenic | Pharmaceutical Non-clinical | Grallert, H. [38] | |||
| Endosafe PTS device with a disposable cartridge | Chromogenic | Nuclear medicine, Pharmaceutical | Maule, J. [40] | |||
| Electrochemical LAL assay | Chromogenic | Dialysate | Takano, S. [52] | |||
| Non-LAL (Direct) | Endotoxin/ | GC/MS | β-hydroxymyristic acid content of Lipid A | Gas chromatograph-Mass spectrometer | Serum (Rabbit) | Maitra, S.K. [46] |
| Lipid A | Immunological techniques | Antiserum to J5 mutant of E.coli 0111:B4 | ELISA | Milk | Mohammed, A.H. [48] | |
| Endotoxin | Target LPS capture with polymyxin probe | ELISA | Non-clinical | Inoue, K.Y. [49] | ||
| LPS O-antigen | Antiserum to O-polysaccharides | Radioimmuno-assay | Non-clinical | Munford, R.S. [50] | ||
| Endotoxin | Fluorescence spectroscopy | Fluorescence-labelled Endotoxin Neutralizing Protein (ENP) | Fluorescence polarization | Non-clinical | Sloyer, J. [51] | |
| Electro-chemistry-based | Electrochemical aptasensor | Signal amplification by enzymatic recycling | Non-clinical | Bai, L.J. [53] | ||
| Non-LAL (Indirect, Cell-based) | Endotoxin | NF-κB | Monocyte activation test | Cytokines production | Pharmaceutical | Hoffman, S. [54] |
| Pyrogen | MAT | Toll-like receptor 4/MD-2/CD14 | NFkB- reporter assay | Plasma (Rat) | Nishida, M. [56] | |
| Endotoxin activity | EAA | Human neutrophil-complement opsonized LPS-IgM complexes | Chemilumi-nescent emission | Whole blood | Marshall, J.C. [58] | |
| Relevant analytes | Anti-endotoxin antibody | Immunological techniques | Anti-bacteroides lipopolysaccharide IgG | Inhibition ELISA | Serum | Allan, E. [63] |
| LBP | Endotoxin-LBP-sCD14 complexes | ELISA | Serum | Opal, S.M. [64] |
| Clinical Papers | Sensitivity (%) | Specificity (%) | Evidence | β-Glucan Assay Kits | |
|---|---|---|---|---|---|
| Obayashi, T., et al. [10] | 90 | 100 | Multi-center clinical studies, 9 sites | Japan | Fungitec G-test |
| Ostrosky-Zeichner, L., et al. [11] | 70 | 87 | Multi-center clinical evaluation, 6 sites | USA | Fungitell |
| Lu, Y., et al. [76] | 76 | 85 | 13 ** | Fungitell (9/13) | |
| WAKO (2/13) | |||||
| Fungitec G-test (1/13) | |||||
| Karageorgopoulos, D.E., et al. [77] | 77 | 85 | 23 ** | Fungitell (10/23) | |
| Fungitec G-test (7/23) | |||||
| WAKO (5/23) | |||||
| Meta-analysis * | Gold Mountain River (1/23) | ||||
| Onishi, A., et al. [78] | 80 | 82 | 36 ** | Fungitell (17/36) | |
| Fungitec G-test (9/36) WAKO (6/36) | |||||
| Gold Mountain River (3/36) Others (1/36) | |||||
| White, S.K., et al. [79] | 83 | 79 | 19 ** | Fungitell (19/19) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamura, H.; Reich, J.; Nagaoka, I. Outstanding Contributions of LAL Technology to Pharmaceutical and Medical Science: Review of Methods, Progress, Challenges, and Future Perspectives in Early Detection and Management of Bacterial Infections and Invasive Fungal Diseases. Biomedicines 2021, 9, 536. https://doi.org/10.3390/biomedicines9050536
Tamura H, Reich J, Nagaoka I. Outstanding Contributions of LAL Technology to Pharmaceutical and Medical Science: Review of Methods, Progress, Challenges, and Future Perspectives in Early Detection and Management of Bacterial Infections and Invasive Fungal Diseases. Biomedicines. 2021; 9(5):536. https://doi.org/10.3390/biomedicines9050536
Chicago/Turabian StyleTamura, Hiroshi, Johannes Reich, and Isao Nagaoka. 2021. "Outstanding Contributions of LAL Technology to Pharmaceutical and Medical Science: Review of Methods, Progress, Challenges, and Future Perspectives in Early Detection and Management of Bacterial Infections and Invasive Fungal Diseases" Biomedicines 9, no. 5: 536. https://doi.org/10.3390/biomedicines9050536
APA StyleTamura, H., Reich, J., & Nagaoka, I. (2021). Outstanding Contributions of LAL Technology to Pharmaceutical and Medical Science: Review of Methods, Progress, Challenges, and Future Perspectives in Early Detection and Management of Bacterial Infections and Invasive Fungal Diseases. Biomedicines, 9(5), 536. https://doi.org/10.3390/biomedicines9050536






