Enhanced Effect of Polyethyleneimine-Modified Graphene Oxide and Simvastatin on Osteogenic Differentiation of Murine Bone Marrow-Derived Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
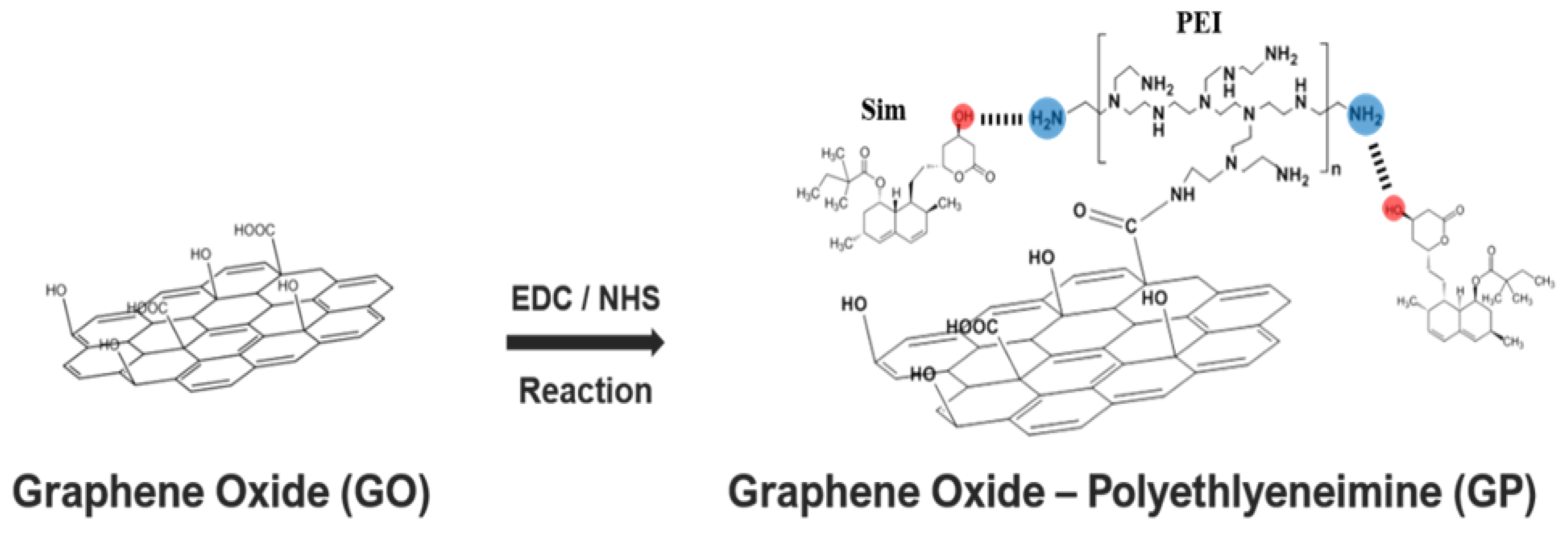
2.2. Synthesis of Go-Polyethylenimine (GP)/Simvastatin (Sim) Complexes
2.3. Property Investigations
2.4. Tests for Cell Viability
2.5. Alkaline Phosphatase (ALP) Activity Assay
2.6. Alizarin Red S (ARS) Staining
2.7. Quantitative Real Time Polymerase Chain Reaction
2.8. Statistical Analysis
3. Results
3.1. Simvastatin Immobilization by PEI Modified-GO (GP)
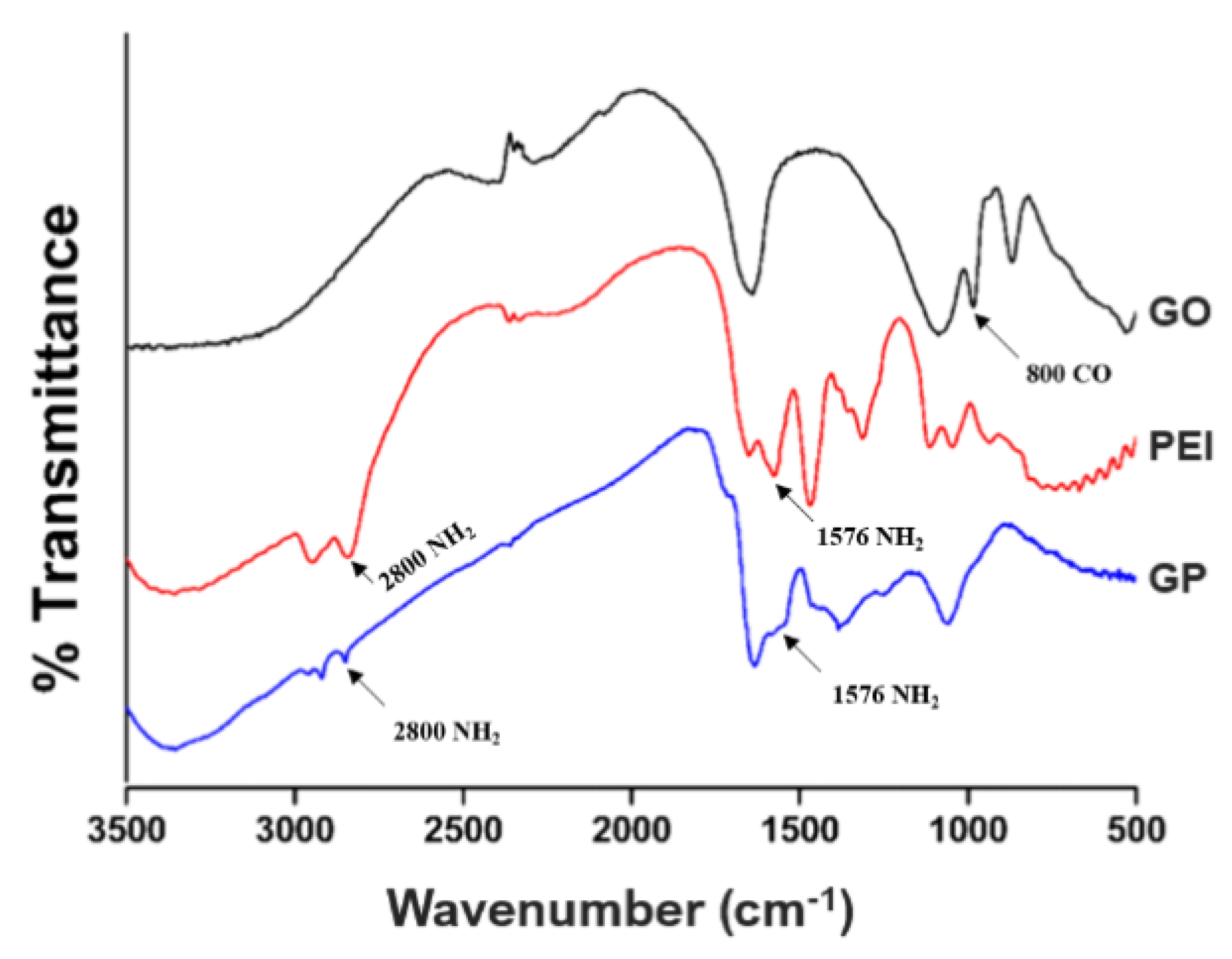
3.2. Chemical Bond Identification
3.3. Zeta Potential Determination
3.4. Cytotoxicity and Proliferation
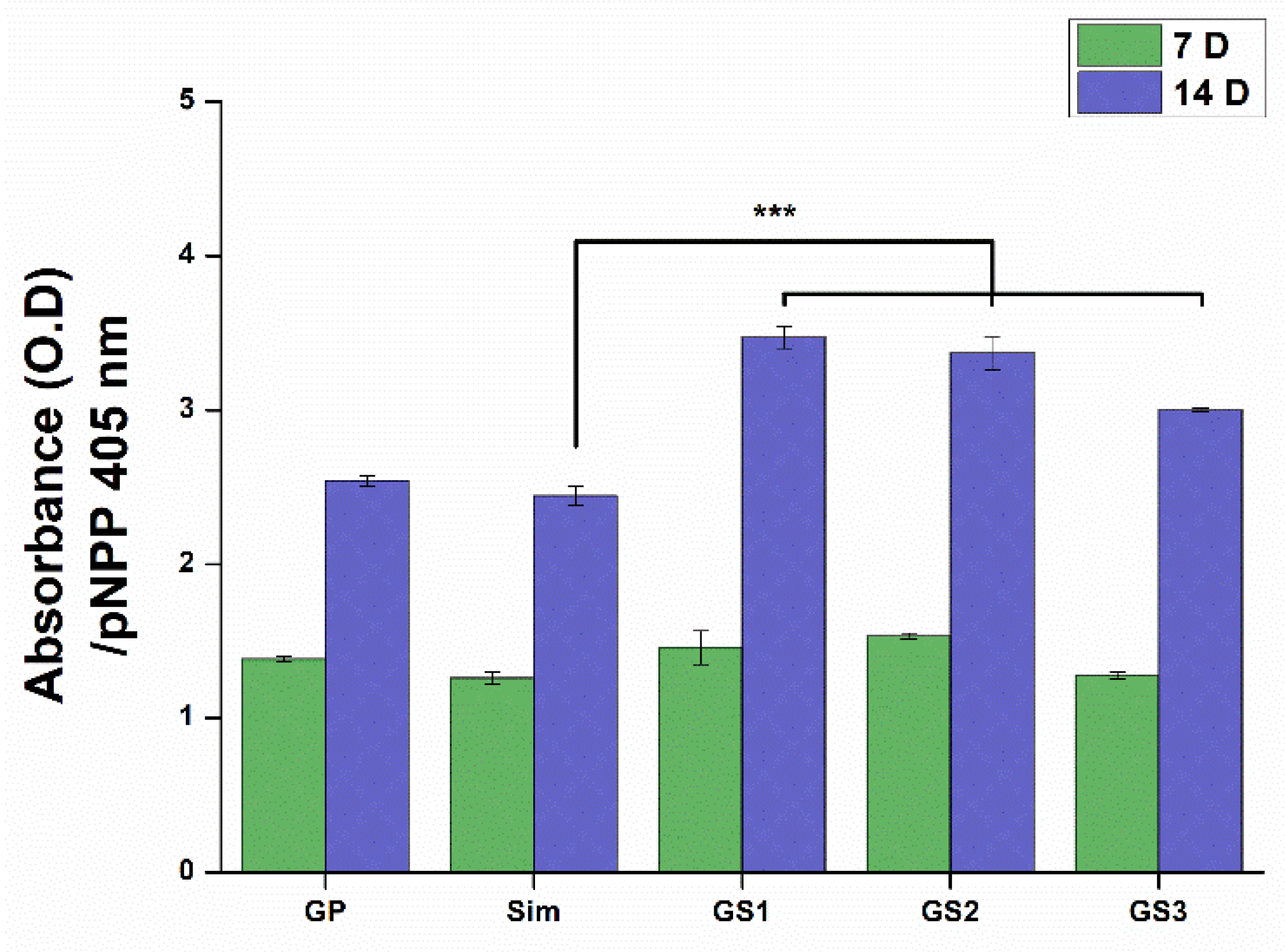
3.5. ALP Activity
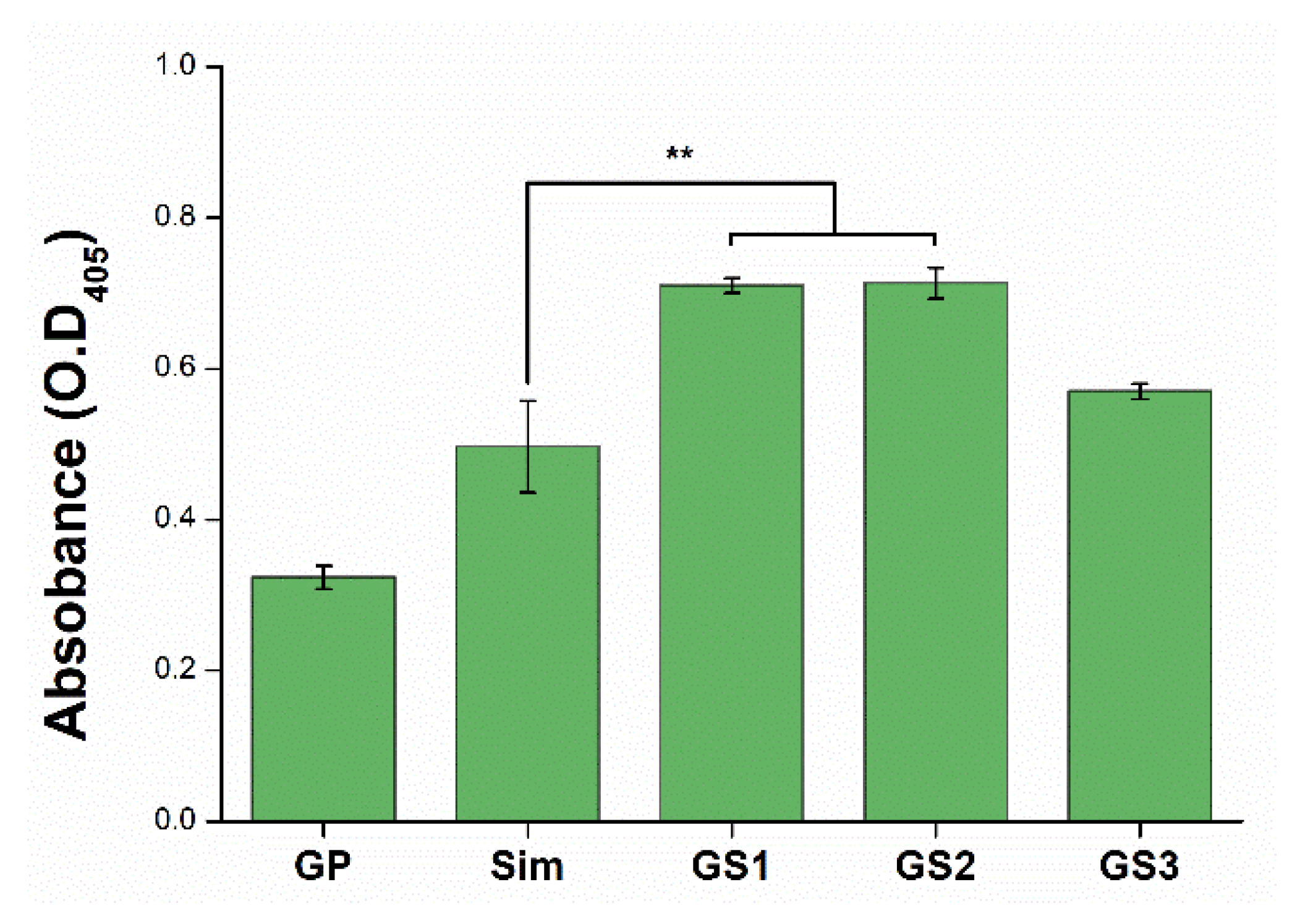
3.6. Mineralization of Stem Cells
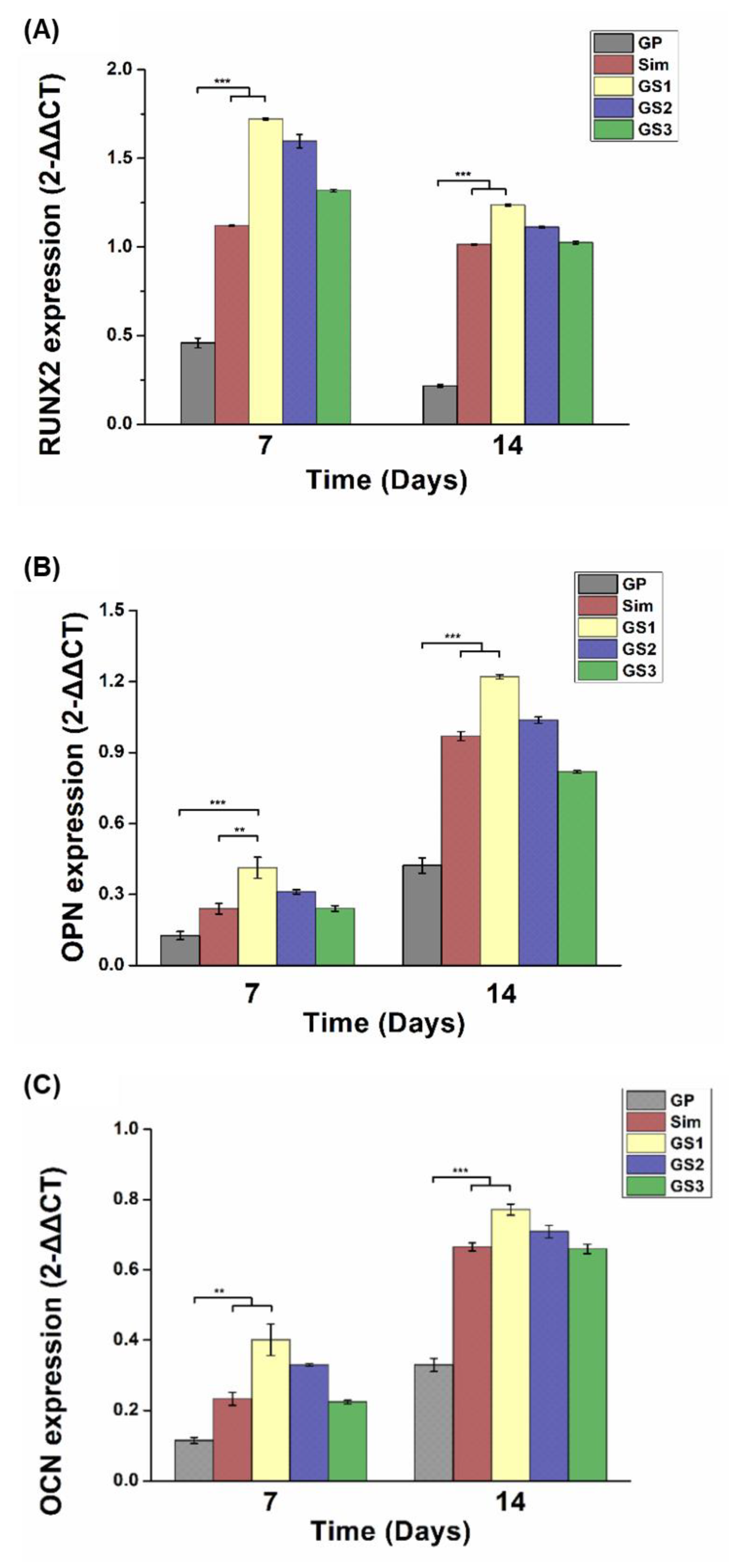
3.7. Osteogenic Gene Expression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jain, K.K. Drug Delivery Systems—An Overview. Methods in Mol. Biol. 2008, 437, 1–50. [Google Scholar] [PubMed]
- Witharana, C.; Wanigasekara, J. Drug delivery systems: A new frontier in nano-technology. IJMRHS 2017, 2017, 11–14. [Google Scholar]
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of Nanoparticle-Based Carriers for Targeted Drug Delivery. J. Nanomater. 2016, 2016, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Poste, G.; Kirsh, R. Site–Specific (Targeted) Drug Delivery in Cancer Therapy. Bio/Technology 1983, 1, 869–878. [Google Scholar] [CrossRef]
- Levy-Nissenbaum, E.; Radovic-Moreno, A.F.; Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanotechnology and aptamers: Applications in drug delivery. Trends Biotechnol. 2008, 26, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Henna, T.K.; Nivitha, K.P.; Raphey, V.R.; Sabu, C.; Pramod, K. Functionalized Graphene for Drug Delivery Applications. In Carbon Nanoparticles and Nanostructures; Springer: Berlin/Heidelberg, Germany, 2019; pp. 247–278. [Google Scholar]
- McCallion, C.; Burthem, J.; Rees-Unwin, K.; Golovanov, A.; Pluen, A. Graphene in therapeutics delivery: Problems, solutions and future opportunities. Eur. J. Pharm. Biopharm. 2016, 104, 235–250. [Google Scholar] [CrossRef]
- Pan, Y.; Sahoo, N.G.; Li, L. The application of graphene oxide in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 1365–1376. [Google Scholar] [CrossRef]
- Liu, B.; Wang, W. Comprehensive Review on Graphene Oxide for Use in Drug Delivery System. Curr. Med. Chem. 2020, 27, 3665–3685. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Amatore, C.; Chen, Y.; Jiang, H.; Wang, X.-M. Gold Nanoclusters and Graphene Nanocomposites for Drug Delivery and Imaging of Cancer Cells. Angew. Chem. Int. Ed. 2011, 50, 11644–11648. [Google Scholar] [CrossRef]
- Huang, X.-M.; Liu, L.-Z.; Zhou, S.; Zhao, J.-J. Physical properties and device applications of graphene oxide. Front. Phys. 2020, 15, 33301. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, D.-E.; Zhao, Y.; Nagase, S.; Zhang, S.; Chen, Z. Theoretical Insights into the Structures of Graphene Oxide and Its Chemical Conversions Between Graphene. J. Comput. Theor. Nanosci. 2011, 8, 2406–2422. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, L.; Liu, M.; Zhang, Z. Biomedical Applications of Graphene. Theranostics 2012, 2, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Weng, F.; Yin, J.; Bao, F.; Gao, J.; Ma, R.; Yan, S.; Liu, Y.; Ding, H. Preparation and the controlled release effect study of graphene oxide-modified poly(ε-caprolactone). Int. J. Polym. Mater. 2018, 67, 307–312. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2010, 6, 8. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W. Cytotoxicity of carbon nanotubes. Sci. China Ser. B Chem. 2008, 51, 1021–1029. [Google Scholar] [CrossRef]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.-L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G.; et al. Graphene for Controlled and Accelerated Osteogenic Differentiation of Human Mesenchymal Stem Cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef]
- Kalbacova, M.; Broz, A.; Kong, J.; Kalbac, M. Graphene substrates promote adherence of human osteoblasts and mesenchymal stromal cells. Carbon 2010, 48, 4323–4329. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, M.; Chen, L.; Fang, H.; Liu, S.; Zhou, C.; Sun, J.; Wang, Z. Alendronate loaded graphene oxide functionalized collagen sponge for the dual effects of osteogenesis and anti-osteoclastogenesis in osteoporotic rats. Bioact. Mater. 2020, 5, 859–870. [Google Scholar] [CrossRef]
- Fu, C.; Yang, X.; Tan, S.; Song, L. Enhancing Cell Proliferation and Osteogenic Differentiation of MC3T3-E1 Pre-osteoblasts by BMP-2 Delivery in Graphene Oxide-Incorporated PLGA/HA Biodegradable Microcarriers. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Ouyang, L.; Qi, M.; Wang, S.; Tu, S.; Li, B.; Deng, Y.; Yang, W. Osteogenesis and Antibacterial Activity of Graphene Oxide and Dexamethasone Coatings on Porous Polyetheretherketone via Polydopamine-Assisted Chemistry. Coatings 2018, 8, 203. [Google Scholar] [CrossRef]
- Niu, J.; Ding, G.; Zhang, L. Effects of simvastatin on the osteogenic differentiation and immunomodulation of bone marrow mesenchymal stem cells. Mol. Med. Rep. 2012, 12, 8237–8240. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.-C.; Chen, C.-H.; Fu, Y.-C.; Tai, I.-C.; Li, C.-J.; Chang, L.-F.; Ho, M.-L.; Chang, J.-K. Estrogen receptor mediates simvastatin-stimulated osteogenic effects in bone marrow mesenchymal stem cells. Biochem. Pharmacol. 2015, 98, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Pagkalos, J.; Cha, J.M.; Kang, Y.; Heliotis, M.; Tsiridis, E.; Mantalaris, A. Simvastatin induces osteogenic differentiation of murine embryonic stem cells. J. Bone Miner. Res. 2010, 25, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.J.; Kang, K.L.; Heo, J.S. Simvastatin Promotes Osteogenic Differentiation of Mouse Embryonic Stem Cells Via Canonical Wnt/β-Catenin Signaling. Mol. Cells 2011, 32, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Chen, S.-Q.; Zhou, W.-T.; Yu, F.-Y.; Bao, L.; Qiu, G.-X.; Qiao, Q.; Hu, F.-Q.; Wang, J.-W.; Yuan, H. A novel biocompatible, simvastatin-loaded, bone-targeting lipid nanocarrier for treating osteoporosis more effectively. RSC Adv. 2020, 10, 20445–20459. [Google Scholar] [CrossRef]
- Chen, B.; Liu, M.; Zhang, L.; Huang, J.; Yao, J.; Zhang, Z. Polyethylenimine-functionalized graphene oxide as an efficient gene delivery vector. J. Mater. Chem. 2011, 21, 7736–7741. [Google Scholar] [CrossRef]
- Bahulekar, R.; Ayyangar, N.; Ponrathnam, S. Polyethyleneimine in immobilization of biocatalysts. Enzym. Microb. Technol. 1991, 13, 858–868. [Google Scholar] [CrossRef]
- Park, J.-S. Carbon-Based Graphene as a Drug Carrier for Use in Tissue Engineering. Master’s Thesis, Dankook University, Cheonan, Korea, 8 January 2018. (In Korean). [Google Scholar]
- Papavlu, A.P.; Dinca, V.; Filipescu, M.; Dinescu, M.F.A.M. Matrix-Assisted Pulsed Laser Evaporation of Organic Thin Films: Applications in Biology and Chemical Sensors. In Laser Ablation—From Fundamentals to Applications; IntechOpen: London, UK, 2017. [Google Scholar]
- Cai, X.; Lin, M.; Tan, S.; Mai, W.; Zhang, Y.; Liang, Z.; Lin, Z.; Zhang, X. The use of polyethyleneimine-modified reduced graphene oxide as a substrate for silver nanoparticles to produce a material with lower cytotoxicity and long-term antibacterial activity. Carbon 2012, 50, 3407–3415. [Google Scholar] [CrossRef]
- Liu, H.; Kuila, T.; Kim, N.H.; Ku, B.-C.; Lee, J.H. In situ synthesis of the reduced graphene oxide–polyethyleneimine composite and its gas barrier properties. J. Mater. Chem. A 2013, 1, 3739–3746. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Wang, M.-H. Preparation, characterization and anti-cancer activity of graphene oxide-‑silver nanocomposite. J. Photochem. Photobiol. B Biol. 2020, 210, 111984. [Google Scholar] [CrossRef]
- Yueyi, C.; Xiaoguang, H.; JingYing, W.; Quansheng, S.; Jie, T.; Xin, F.; Yingsheng, X.; Chunli, S. Calvarial defect healing by recruitment of autogenous osteogenic stem cells using locally applied simvastatin. Biomaterials 2013, 34, 9373–9380. [Google Scholar] [CrossRef]
- Ho, M.-L.; Tai, I.-C.; Wang, Y.-H.; Chen, C.-H.; Chuang, S.-C.; Chang, J.-K. Simvastatin enhances Rho/actin/cell rigidity pathway contributing to mesenchymal stem cells’ osteogenic differentiation. Int. J. Nanomed. 2015, 10, 5881–5894. [Google Scholar] [CrossRef]
- Sun, S.-H.; Lee, I.-K.; Lee, J.-W.; Shim, I.-S.; Kim, S.-H.; Kim, K.-S. Simvastatin Induces Osteogenic Differentiation and Suppresses Adipogenic Differentiation in Primarily Cultured Human Adipose-Derived Stem Cells. Biomol. Ther. 2009, 17, 353–361. [Google Scholar] [CrossRef]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.-I.; Kim, K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016, 2016, 1–19. [Google Scholar] [CrossRef]
- Stein, G.S.; Lian, J.B. Molecular Mechanisms Mediating Proliferation/Differentiation Interrelationships during Progressive Development of the Osteoblast Phenotype. Endocr. Rev. 1993, 14, 424–442. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, Y.C.; Jin, O.S.; Kang, S.H.; Hwang, Y.-S.; Park, J.-C.; Hong, S.W.; Han, D.-W. Reduced graphene oxide-coated hydroxyapatite composites stimulate spontaneous osteogenic differentiation of human mesenchymal stem cells. Nanoscale 2015, 7, 11642–11651. [Google Scholar] [CrossRef]
- Yang, J.-W.; Hsieh, K.Y.; Kumar, P.V.; Cheng, S.-J.; Lin, Y.-R.; Shen, Y.-C.; Chen, G.-Y. Enhanced Osteogenic Differentiation of Stem Cells on Phase-Engineered Graphene Oxide. ACS Appl. Mater. Interfaces 2018, 10, 12497–12503. [Google Scholar] [CrossRef]
- Xue, D.; Chen, E.; Zhong, H.; Zhang, W.; Wang, S.; Joomun, M.U.; Yao, T.; Tan, Y.; Lin, S.; Zheng, Q.; et al. Immunomodulatory properties of graphene oxide for osteogenesis and angiogenesis. Int. J. Nanomed. 2018, ume 13, 5799–5810. [Google Scholar] [CrossRef]
- Králík, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cell Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef]
- Huang, W.; Yang, S.; Shao, J.; Li, Y.-P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. 2007, 12, 3068–3092. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.-S.; Park, J.-S.; Lee, E.-J. Enhanced Effect of Polyethyleneimine-Modified Graphene Oxide and Simvastatin on Osteogenic Differentiation of Murine Bone Marrow-Derived Mesenchymal Stem Cells. Biomedicines 2021, 9, 501. https://doi.org/10.3390/biomedicines9050501
Oh J-S, Park J-S, Lee E-J. Enhanced Effect of Polyethyleneimine-Modified Graphene Oxide and Simvastatin on Osteogenic Differentiation of Murine Bone Marrow-Derived Mesenchymal Stem Cells. Biomedicines. 2021; 9(5):501. https://doi.org/10.3390/biomedicines9050501
Chicago/Turabian StyleOh, Jun-Sung, Jeong-Sun Park, and Eun-Jung Lee. 2021. "Enhanced Effect of Polyethyleneimine-Modified Graphene Oxide and Simvastatin on Osteogenic Differentiation of Murine Bone Marrow-Derived Mesenchymal Stem Cells" Biomedicines 9, no. 5: 501. https://doi.org/10.3390/biomedicines9050501
APA StyleOh, J.-S., Park, J.-S., & Lee, E.-J. (2021). Enhanced Effect of Polyethyleneimine-Modified Graphene Oxide and Simvastatin on Osteogenic Differentiation of Murine Bone Marrow-Derived Mesenchymal Stem Cells. Biomedicines, 9(5), 501. https://doi.org/10.3390/biomedicines9050501





