Tumor-Infiltrating Lymphocytes in the Tumor Microenvironment of Laryngeal Squamous Cell Carcinoma: Systematic Review and Meta-Analysis †
Abstract
1. Introduction
2. Materials and Methods
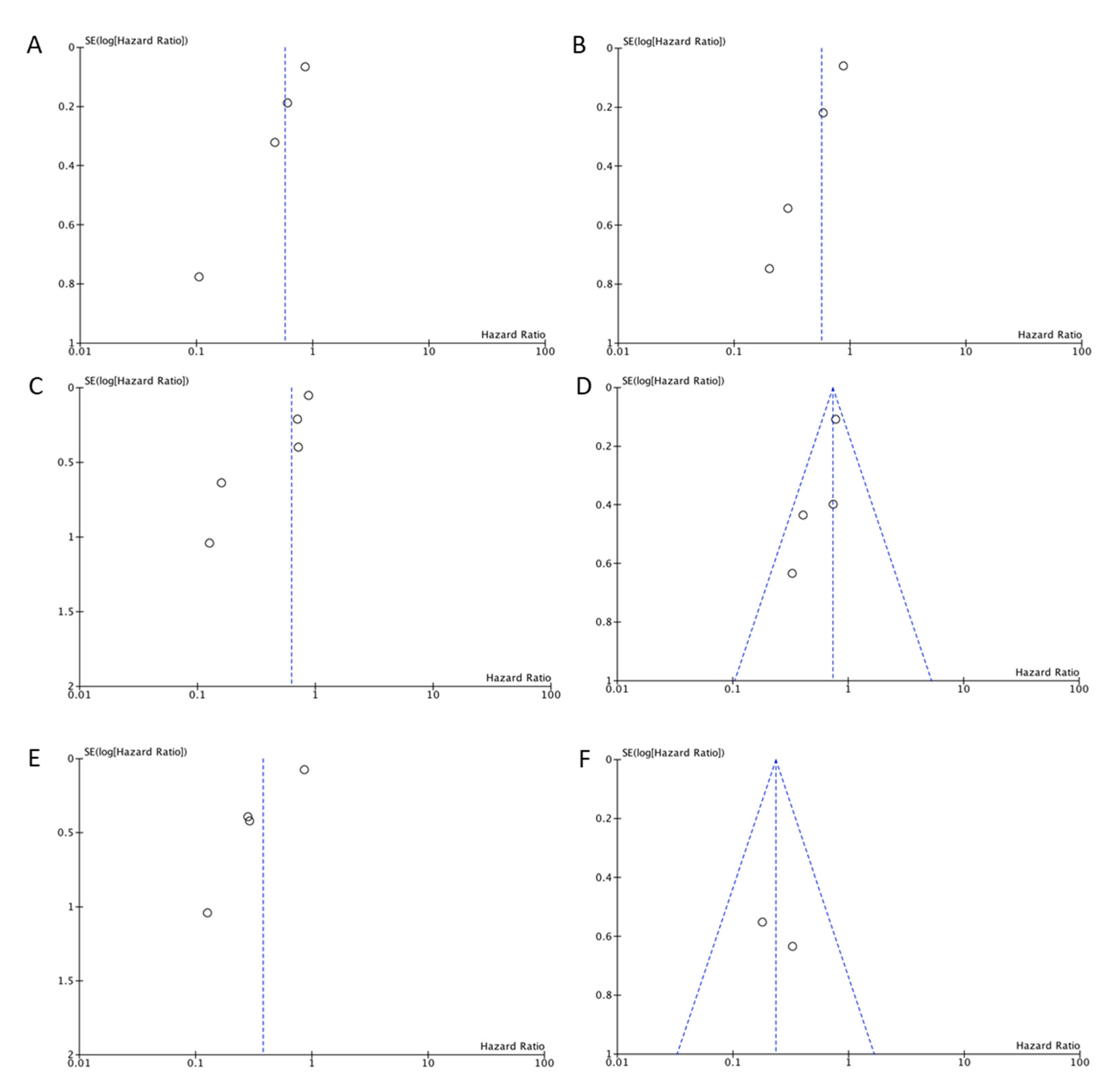
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef]
- Angell, H.K.; Bruni, D.; Barrett, J.C.; Herbst, R.; Galon, J. The Immunoscore: Colon Cancer and Beyond. Clin. Cancer Res. 2020, 26, 332–339. [Google Scholar] [CrossRef]
- Ferris, R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. 2015, 33, 3293–3304. [Google Scholar] [CrossRef]
- Jones, T.M. Tumour-infiltrating lymphocytes in the risk stratification of squamous cell carcinoma of the head and neck. Br. J. Cancer 2014, 110, 269–270. [Google Scholar] [CrossRef]
- Galon, J.; Mlecnik, B.; Bindea, G.; Angell, H.K.; Berger, A.; Lagorce, C.; Lugli, A.; Zlobec, I.; Hartmann, A.; Bifulco, C.; et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J. Pathol. 2014, 232, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Gooden, M.J.M.; De Bock, G.H.; Leffers, N.; Daemen, T.; Nijman, H.W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br. J. Cancer 2011, 105, 93–103. [Google Scholar] [CrossRef]
- Galon, J.; Pagès, F.; Marincola, F.M.; Thurin, M.; Trinchieri, G.; Fox, B.; Gajewski, T.F.; Ascierto, P. The immune score as a new possible approach for the classification of cancer. J. Transl. Med. 2012, 10, 1. [Google Scholar] [CrossRef]
- Spector, M.E.; Bellile, E.; Amlani, L.; Zarins, K.; Smith, J.; Brenner, J.C.; Rozek, L.; Nguyen, A.; Thomas, D.; McHugh, J.B.; et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol. Neck Surg. 2019, 145, 1012–1019. [Google Scholar] [CrossRef]
- De Ruiter, E.J.; Ooft, M.L.; Devriese, L.A.; Willems, S.M. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. OncoImmunology 2017, 6, e1356148. [Google Scholar] [CrossRef] [PubMed]
- Borsetto, D.; Tomasoni, M.; Payne, K.; Polesel, J.; Deganello, A.; Bossi, P.; Tysome, J.; Masterson, L.; Tirelli, G.; Tofanelli, M.; et al. Prognostic Significance of CD4+ and CD8+ Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Cancers 2021, 13, 781. [Google Scholar] [CrossRef]
- Nguyen, N.; Bellile, E.; Thomas, D.; McHugh, J.; Rozek, L.; Virani, S.; Peterson, L.; Carey, T.E.; Walline, H.; Moyer, J.; et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck 2016, 38, 1074–1084. [Google Scholar] [CrossRef]
- De Meulenaere, A.; Vermassen, T.; Aspeslagh, S.; Vandecasteele, K.; Rottey, S.; Ferdinande, L. TILs in Head and Neck Cancer: Ready for Clinical Implementation and Why (Not)? Head Neck Pathol. 2016, 11, 354–363. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ Br. Med. J. 2015, 349, g7647. [Google Scholar] [CrossRef]
- Karpathiou, G.; Casteillo, F.; Giroult, J.-B.; Forest, F.; Fournel, P.; Monaya, A.; Froudarakis, M.; Dumollard, J.M.; Prades, J.M.; Peoc’H, M. Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: Immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget 2016, 8, 19310–19322. [Google Scholar] [CrossRef] [PubMed]
- Badoual, C.; Hans, S.; Rodriguez, J.; Peyrard, S.; Klein, C.; Agueznay, N.E.H.; Mosseri, V.; Laccourreye, O.; Bruneval, P.; Fridman, W.H.; et al. Prognostic Value of Tumor-Infiltrating CD4+ T-Cell Subpopulations in Head and Neck Cancers. Clin. Cancer Res. 2006, 12, 465–472. [Google Scholar] [CrossRef]
- Balermpas, P.; Michel, Y.; Wagenblast, J.; Seitz, O.; Weiss, C.; Rodel, F.; Rodel, C.; Fokas, E. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br. J. Cancer 2013, 110, 501–509. [Google Scholar] [CrossRef]
- Yang, F.; Zeng, Z.; Li, J.; Zheng, Y.; Wei, F.; Ren, X. PD-1/PD-L1 Axis, Rather Than High-Mobility Group Alarmins or CD8+ Tumor-Infiltrating Lymphocytes, Is Associated With Survival in Head and Neck Squamous Cell Carcinoma Patients Who Received Surgical Resection. Front. Oncol. 2018, 8, 604. [Google Scholar] [CrossRef]
- Ngamphaiboon, N.; Chureemas, T.; Siripoon, T.; Arsa, L.; Trachu, N.; Jiarpinitnun, C.; Pattaranutaporn, P.; Sirachainan, E.; Larbcharoensub, N. Characteristics and impact of programmed death-ligand 1 expression, CD8+ tumor-infiltrating lymphocytes, and p16 status in head and neck squamous cell carcinoma. Med. Oncol. 2019, 36, 21. [Google Scholar] [CrossRef]
- De Ruiter, E.J.; De Roest, R.H.; Brakenhoff, R.H.; Leemans, C.R.; De Bree, R.; Terhaard, C.H.J.; Willems, S.M. Digital pathology-aided assessment of tumor-infiltrating T lymphocytes in advanced stage, HPV-negative head and neck tumors. Cancer Immunol. Immunother. 2020, 69, 581–591. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Song, L.-J.; Shen, J.; Yue, H.; Han, Y.-Q.; Yang, C.-L.; Liu, S.-Y.; Deng, J.-W.; Jiang, Y.; Fu, G.-H.; et al. Prognostic and predictive values of immune infiltrate in patients with head and neck squamous cell carcinoma. Hum. Pathol. 2018, 82, 104–112. [Google Scholar] [CrossRef]
- Alessandrini, L.; Franz, L.; Ottaviano, G.; Ghi, M.G.; Lanza, C.; Blandamura, S.; Marioni, G. Prognostic role of programmed death ligand 1 (PD-L1) and the immune microenvironment in laryngeal carcinoma. Oral Oncol. 2020, 108, 104836. [Google Scholar] [CrossRef]
- Yu, D.; Cheng, J.; Xue, K.; Zhao, X.; Wen, L.; Xu, C. Expression of Programmed Death-Ligand 1 in Laryngeal Carcinoma and its Effects on Immune Cell Subgroup Infiltration. Pathol. Oncol. Res. 2018, 25, 1437–1443. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, W.-J.; Tang, D.; Zhou, J.; Chou, L.; Tao, L.; Lu, L.-M. The ratio of CD4/CD8 T-cells in human papillomavirus-positive laryngeal squamous cell carcinoma accounts for improved outcome. Acta Oto-Laryngol. 2016, 136, 826–833. [Google Scholar] [CrossRef]
- Tao, Y.; Shen, H.; Liu, Y.; Li, G.; Huang, Z.; Liu, Y. IL-23R in laryngeal cancer: A cancer immunoediting process that facilitates tumor cell proliferation and results in cisplatin resistance. Carcinogenesis 2021, 42, 118–126. [Google Scholar] [CrossRef]
- Franz, L.; Alessandrini, L.; Ottaviano, G.; Di Carlo, R.; Fasanaro, E.; Ramacciotti, G.; Contro, G.; Marioni, G. Postoperative radiotherapy for laryngeal cancer. The prognostic role of programmed death-ligand 1: An immune microenvironment-based cluster analysis. Pathol. Res. Pract. 2020, 216, 153120. [Google Scholar] [CrossRef]
- Gabriel, A.; Namysłowski, G.; Ziółkowski, A.; Morawski, K.; Stęplewska-Mazur, K.; Urbaniec, P. Immunohistochemical analysis of lymphocytic infiltration in the tumor microenvironment in patients operated on for laryngeal cancer. Eur. Arch. Oto-Rhino-Laryngol. 1999, 256, 384–387. [Google Scholar] [CrossRef]
- Birtalan, E.; Danos, K.; Gurbi, B.; Brauswetter, D.; Halasz, J.; Piurko, V.K.; Acs, B.; Antal, B.; Mihalyi, R.; Pato, A.; et al. Expression of PD-L1 on Immune Cells Shows Better Prognosis in Laryngeal, Oropharygeal, and Hypopharyngeal Cancer. Appl. Immunohistochem. Mol. Morphol. 2018, 26, e79–e85. [Google Scholar] [CrossRef]
- Schneider, K.; Marbaix, E.; Bouzin, C.; Hamoir, M.; Mahy, P.; Bol, V.; Grégoire, V. Immune cell infiltration in head and neck squamous cell carcinoma and patient outcome: A retrospective study. Acta Oncol. 2018, 57, 1165–1172. [Google Scholar] [CrossRef]
- Ono, T.; Azuma, K.; Kawahara, A.; Kakuma, T.; Sato, F.; Akiba, J.; Tanaka, N.; Abe, T.; Chitose, S.; Umeno, H. Predictive value of CD8 / FOXP3 ratio combined with PD-L1 expression for radiosensitivity in patients with squamous cell carcinoma of the larynx receiving definitive radiation therapy. Head Neck 2020, 42, 3518–3530. [Google Scholar] [CrossRef]
- Tao, Y.; Gross, N.; Liu, Y.; Zhang, L.; Li, G.; Huang, Z.; Yang, J. A high ratio of IL-12Rβ2-positive tumor-infiltrating lymphocytes indicates favorable prognosis in laryngeal cancer. Oral Oncol. 2017, 74, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, W.-J.; Fu, Q.-L.; Wu, C.-Y.; Lin, J.-Z.; Zhu, X.-L.; Hou, W.-J.; Wei, Y.; Wen, Y.-H.; Wang, Y.-J.; et al. Functionally distinct subsets of CD4+ regulatory T cells in patients with laryngeal squamous cell carcinoma are indicative of immune deregulation and disease progression. Oncol. Rep. 2014, 33, 354–362. [Google Scholar] [CrossRef]
- Vassilakopoulou, M.; Avgeris, M.; Velcheti, V.; Kotoula, V.; Rampias, T.; Chatzopoulos, K.; Perisanidis, C.; Kontos, C.K.; Giotakis, A.I.; Scorilas, A.; et al. Evaluation of PD-L1 Expression and Associated Tumor-Infiltrating Lymphocytes in Laryngeal Squamous Cell Carcinoma. Clin. Cancer Res. 2016, 22, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S.; Song, X.; Zeng, W.; Wang, S.; Chen, F.; Ding, H. The prognostic value of systemic and local inflammation in patients with laryngeal squamous cell carcinoma. OncoTargets Ther. 2016, 9, 7177–7185. [Google Scholar] [CrossRef]
- Hoesli, R.; Birkeland, A.C.; Rosko, A.J.; Issa, M.; Chow, K.L.; Michmerhuizen, N.L.; Mann, J.E.; Chinn, S.B.; Shuman, A.G.; Prince, M.E.; et al. Proportion of CD4 and CD8 tumor infiltrating lymphocytes predicts survival in persistent/recurrent laryngeal squamous cell carcinoma. Oral Oncol. 2018, 77, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.E.; Smith, J.D.; Birkeland, A.C.; Bellile, E.; Swiecicki, P.; Mierzwa, M.; Chinn, S.B.; Shuman, A.G.; Malloy, K.M.; Casper, K.A.; et al. Analysis of tumor-infiltrating CD103 resident memory T-cell content in recurrent laryngeal squamous cell carcinoma. Cancer Immunol. Immunother. 2019, 68, 213–220. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Gao, W.; Huangfu, H.; Wen, S.; Zhang, C.; Zhao, Q.; Dong, Z.; Qu, C.; Li, G.; et al. Assessment of tumor-associated immune cells in laryngeal squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2019, 145, 1761–1772. [Google Scholar] [CrossRef]
- Chatzopoulos, K.; Kotoula, V.; Manoussou, K.; Markou, K.; Vlachtsis, K.; Angouridakis, N.; Nikolaou, A.; Vassilakopoulou, M.; Psyrri, A.; Fountzilas, G. Tumor Infiltrating Lymphocytes and CD8+ T Cell Subsets as Prognostic Markers in Patients with Surgically Treated Laryngeal Squamous Cell Carcinoma. Head Neck Pathol. 2020, 14, 689–700. [Google Scholar] [CrossRef]
- Sanchez-Canteli, M.; Granda-Díaz, R.; Del Rio-Ibisate, N.; Allonca, E.; López-Alvarez, F.; Agorreta, J.; Garmendia, I.; Montuenga, L.M.; García-Pedrero, J.M.; Rodrigo, J.P. PD-L1 expression correlates with tumor-infiltrating lymphocytes and better prognosis in patients with HPV-negative head and neck squamous cell carcinomas. Cancer Immunol. Immunother. 2020, 69, 2089–2100. [Google Scholar] [CrossRef]
- Tagliabue, M.; Maffini, F.; Fumagalli, C.; Gandini, S.; Lepanto, D.; Corso, F.; Cacciola, S.; Ranghiero, A.; Rappa, A.; Vacirca, D.; et al. A role for the immune system in advanced laryngeal cancer. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, D.; Heng, Y.; Zhu, X.; Zhou, L.; Tao, L.; Lu, L. Prognostic Impact of Tumor-Infiltrating Lymphocytes in Laryngeal Squamous Cell Carcinoma Patients. Laryngoscope 2020. [Google Scholar] [CrossRef]
- Franz, L.; Alessandrini, L.; Fasanaro, E.; Gaudioso, P.; Carli, A.; Nicolai, P.; Marioni, G. Prognostic impact of neutrophils-to-lymphocytes ratio (NLR), PD-L1 expression, and tumor immune microenvironment in laryngeal cancer. Ann. Diagn. Pathol. 2021, 50, 151657. [Google Scholar] [CrossRef]
- Sala, O.; Ferlito, A. Morphological Observations of Immunobiology of Laryngeal Cancer: Evaluation of the Defensive Activity of Immunocompetent Cells present In Tumour Stroma. Acta Oto-Laryngol. 1976, 81, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Hartman, D.J.; Ahmad, F.; Ferris, R.L.; Rimm, D.L.; Pantanowitz, L. Utility of CD8 score by automated quantitative image analysis in head and neck squamous cell carcinoma. Oral Oncol. 2018, 86, 278–287. [Google Scholar] [CrossRef]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.-S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non–Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv. Anat. Pathol. 2017, 24, 311–335. [Google Scholar] [CrossRef]
- Almangush, A.; Leivo, I.; Mäkitie, A.A. Overall assessment of tumor-infiltrating lymphocytes in head and neck squamous cell carcinoma: Time to take notice. Acta Oto-Laryngol. 2020, 140, 246–248. [Google Scholar] [CrossRef]
- Eynde, M.V.D.; Mlecnik, B.; Bindea, G.; Fredriksen, T.; Church, S.E.; Lafontaine, L.; Haicheur, N.; Marliot, F.; Angelova, M.; Vasaturo, A.; et al. The Link between the Multiverse of Immune Microenvironments in Metastases and the Survival of Colorectal Cancer Patients. Cancer Cell 2018, 34, 1012–1026.e3. [Google Scholar] [CrossRef]




| References | Analytic Cohort | Covariant | Sample | Stage | Treatment | Technique | Other Biomarkers |
|---|---|---|---|---|---|---|---|
| Vassilakopoulou et al., 2016 [33] | 260 | High stromal TIL (≥30%) | Postoperative sample | All | S, S+RT | HE in WS | PD-L1 (IHC and mRNA) |
| Wang et al., 2016 [34] | 120 | High stromal TIL (≥30%) | Postoperative sample | All | S+RT, S+CRT | HE in WS | No |
| Hoesli et al., 2018 [35] | 183 | High tumor-infiltrating CD8+ (dichotomized at the median) | Postoperative sample | All | S (post RT, CRT) | IHC in TMAs | PD-L1 CPS (IHC) |
| Mann et al., 2019 [36] | 183 | High tumor-infiltrating CD103+ and CD4+ | Postoperative sample | All | S (post RT, CRT) | IHC in TMAs | No |
| Spector et al., 2019 [9] | 74 | High tumor-infiltrating CD8+ (dichotomized at the median) and high tumoral TILws (0.35 × CD8+ 0.35 × CD4+ 0.3 × FOXP3+) | Incisional biopsies | All | S, S+(C)RT, RT, CRT | IHC in TMAs | No |
| Zhou et al., 2019 [37] | 71 | High tumor- and stromal-infiltrating CD8+ and high stromal-infiltrating CD3+ (dichotomized at the median) | Postoperative sample | All | S | IHC in WS | CD163 (IHC) |
| Chatzopoulos et al., 2020 [38] | 283 | Stromal TIL (10% increments) and high tumor-infiltrating CD8+ (dichotomized at the median) | Postoperative sample | All | S, S+RT | HE in WS and IHC in TMAs | No |
| Sánchez-Canteli et al., 2020 [39] | 67 | High tumor-infiltrating CD8+ (dichotomized at the median) | Postoperative sample | All | S, S+RT | IHC in TMAs | PD-L1 CPS and TPS (IHC) |
| Tagliabue et al., 2020 [40] | 56 | High stromal TIL (≥5%) | Postoperative sample | III/IV | S, S+(C)RT, RT, CRT | HE in WS | No |
| Zhang et al., 2020 [41] | 41 | Immunoscore (2–4) vs (0–1) (CD3+/CD8+) | Postoperative sample | All | S | IHC in WS | CD66b+ (IHC) |
| Franz et al., 2021 [42] | 60 | High stromal TIL (≥30%) | Postoperative sample | All | S, S+RT | HE in WS | PD-L1 CPS (IHC) NLR |
| References | Key Findings |
|---|---|
| Vassilakopoulou et al., 2016 [33] | Increased TIL density in tumor stroma was associated with better outcome in laryngeal squamous cell carcinoma. |
| Wang et al., 2016 [34] | High TIL density in tumor stroma, which represents the local inflammation, was predictive of longer OS and RFS. |
| Hoesli et al., 2018 [35] | CD8+ TIL status was associated with a significant improvement in DFS and DSS in patients with recurrent/persistent laryngeal squamous cell carcinoma surgically treated after RT/CRT. |
| Mann et al., 2019 [36] | An immune profile driven by CD103+ TIL content, alone and in combination with CD4+ TIL content, was a prognostic biomarker of survival in patients with recurrent/persistent LSCC. |
| Spector et al., 2019 [9] | The levels of TIL were an independent prognostic factor in patients with head and neck squamous cell carcinoma, including laryngeal squamous cell carcinoma, treated with different modalities. Subsets of TILs and combined TILs scores may be clinically useful predictive and prognostic factors. |
| Zhou et al., 2019 [37] | High density of peritumoral CD3+ and CD8+ immune cells in both tumor stroma and tumor nests was significantly associated with a favorable OS. |
| Chatzopoulos et al., 2020 [38] | Favorable prognostic impact of higher TIL density in laryngeal squamous cell carcinoma patients. The assessment of CD8+ infiltrates does not seem to offer additional prognostic information over the morphologically assessed TIL density. |
| Sánchez-Canteli et al., 2020 [39] | High tumoral infiltration by CD8+ TIL was associated with better survival outcomes. |
| Tagliabue et al., 2020 [40] | Low TIL and altered expression of specific genes associated with tumor–immune systems interactions emerged as independent risk factors, associated with poor prognosis and relapse within 2 years in advanced laryngeal squamous cell carcinoma. |
| Zhang et al., 2020 [41] | High density of CD3+ TIL were associated with better OS. Patients with an Immunoscore of 0–1 experienced the worst OS and DFS, compared with Immunoscore 2–4. |
| Franz et al., 2021 [42] | TIL count rate ≥30% was associated with higher DFS and reduced recurrence risk. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigo, J.P.; Sánchez-Canteli, M.; López, F.; Wolf, G.T.; Hernández-Prera, J.C.; Williams, M.D.; Willems, S.M.; Franchi, A.; Coca-Pelaz, A.; Ferlito, A. Tumor-Infiltrating Lymphocytes in the Tumor Microenvironment of Laryngeal Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Biomedicines 2021, 9, 486. https://doi.org/10.3390/biomedicines9050486
Rodrigo JP, Sánchez-Canteli M, López F, Wolf GT, Hernández-Prera JC, Williams MD, Willems SM, Franchi A, Coca-Pelaz A, Ferlito A. Tumor-Infiltrating Lymphocytes in the Tumor Microenvironment of Laryngeal Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Biomedicines. 2021; 9(5):486. https://doi.org/10.3390/biomedicines9050486
Chicago/Turabian StyleRodrigo, Juan P., Mario Sánchez-Canteli, Fernando López, Gregory T. Wolf, Juan C. Hernández-Prera, Michelle D. Williams, Stefan M. Willems, Alessandro Franchi, Andrés Coca-Pelaz, and Alfio Ferlito. 2021. "Tumor-Infiltrating Lymphocytes in the Tumor Microenvironment of Laryngeal Squamous Cell Carcinoma: Systematic Review and Meta-Analysis" Biomedicines 9, no. 5: 486. https://doi.org/10.3390/biomedicines9050486
APA StyleRodrigo, J. P., Sánchez-Canteli, M., López, F., Wolf, G. T., Hernández-Prera, J. C., Williams, M. D., Willems, S. M., Franchi, A., Coca-Pelaz, A., & Ferlito, A. (2021). Tumor-Infiltrating Lymphocytes in the Tumor Microenvironment of Laryngeal Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Biomedicines, 9(5), 486. https://doi.org/10.3390/biomedicines9050486









