Role of MicroRNAs in Human Osteosarcoma: Future Perspectives
Abstract
1. Introduction
2. Human Osteosarcoma
3. MicroRNAs: Biogenesis and Biological Functions
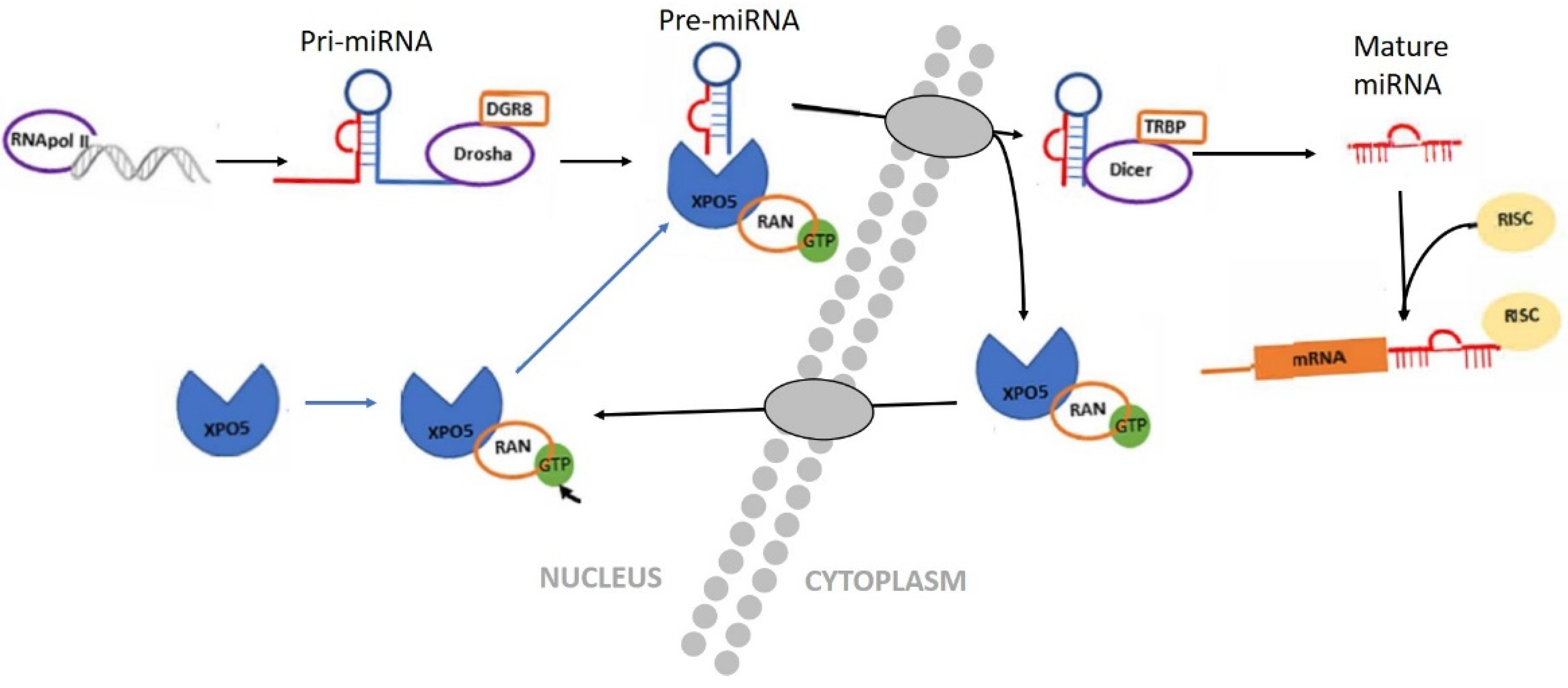
3.1. Biogenesis of miRNAs
3.2. Biological Functions of miRNAs
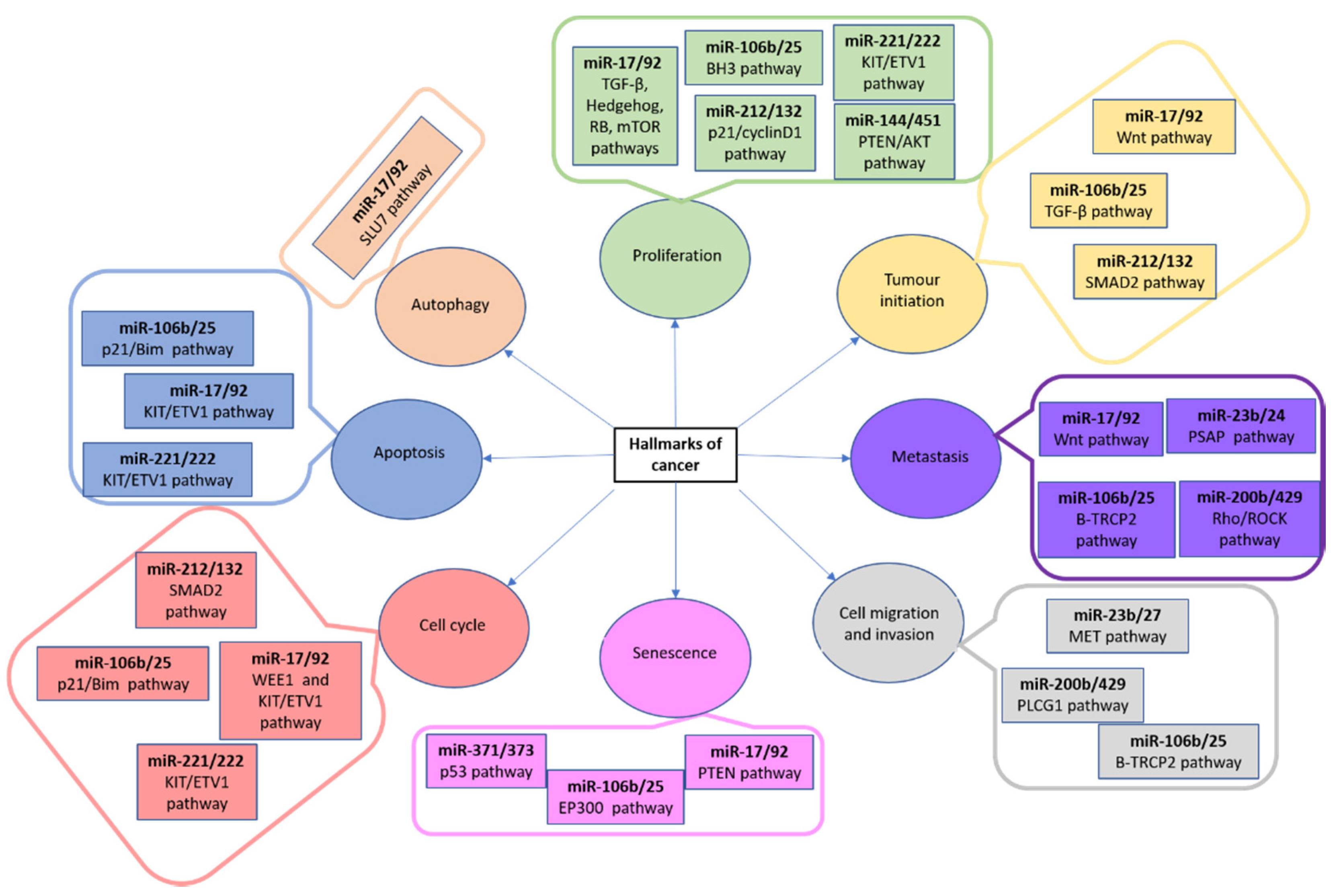
3.3. MiRNAs and Human Osteosarcoma
4. Possible Applications of miRNAs in Human Osteosarcoma
4.1. MiRNAs as Potential Biomarkers
4.2. MiRNAs and OS Chemoresistance
4.3. MiRNAs as Targets for OS Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, M.E. Update on Survival in Osteosarcoma. Orthop. Clin. N. Am. 2016, 47, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Smrke, A.; Anderson, P.; Gulia, A.; Gennatas, S.; Huang, P.; Jones, R. Future Directions in the Treatment of Osteosarcoma. Cells 2021, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Kushlinskii, N.E.; Fridman, M.V.; Braga, E.A. Long Non-Coding RNAs as Competitive Endogenous RNAs in Osteosarcoma. Mol. Biol. 2020, 54, 776–801. [Google Scholar] [CrossRef]
- Bielack, S.S.; Kempf-Bielack, B.; Branscheid, D.; Carrle, D.; Friedel, G.; Helmke, K.; Kevric, M.; Jundt, G.; Kühne, T.; Maas, R.; et al. Second and Subsequent Recurrences of Osteosarcoma: Presentation, Treatment, and Outcomes of 249 Consecutive Cooperative Osteosarcoma Study Group Patients. J. Clin. Oncol. 2009, 27, 557–565. [Google Scholar] [CrossRef]
- Aljubran, A.H.; Griffin, A.; Pintilie, M.; Blackstein, M. Osteosarcoma in adolescents and adults: Survival analysis with and without lung metastases. Ann. Oncol. 2009, 20, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Osaki, M.; Okada, F. MicroRNA-Based Diagnosis and Treatment of Metastatic Human Osteosarcoma. Cancers 2019, 11, 553. [Google Scholar] [CrossRef]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.S.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef]
- Basile, P.; Greengard, E.; Weigel, B.; Spector, L. Prognostic Factors for Development of Subsequent Metastases in Localized Osteosarcoma: A Systematic Review and Identification of Literature Gaps. Sarcoma 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Sadykova, L.R.; Ntekim, A.I.; Muyangwa-Semenova, M.; Rutland, C.S.; Jeyapalan, J.N.; Blatt, N.; Rizvanov, A.A. Epidemiology and Risk Factors of Osteosarcoma. Cancer Investig. 2020, 38, 259–269. [Google Scholar] [CrossRef]
- Rickel, K.; Fang, F.; Tao, J. Molecular genetics of osteosarcoma. Bone 2017, 102, 69–79. [Google Scholar] [CrossRef]
- Jafari, F.; Javdansirat, S.; Sanaie, S.; Naseri, A.; Shamekh, A.; Rostamzadeh, D.; Dolati, S. Osteosarcoma: A comprehensive review of management and treatment strategies. Ann. Diagn. Pathol. 2020, 49, 151654. [Google Scholar] [CrossRef]
- Wittig, J.C.; Bickels, J.; Priebat, D.; Jelinek, J.; Kellar-Graney, K.; Shmookler, B.; Malawer, M.M. Osteosarcoma: A multidisciplinary approach to diagnosis and treatment. Am. Fam. Physician 2002, 65, 1123–1132. [Google Scholar] [PubMed]
- Chindamo, G.; Sapino, S.; Peira, E.; Chirio, D.; Gonzalez, M.C.; Gallarate, M. Bone Diseases: Current Approach and Future Perspectives in Drug Delivery Systems for Bone Targeted Therapeutics. Nanomaterials 2020, 10, 875. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Melo-Alvim, C.; Lopes-Brás, R.; Esperança-Martins, M.; Costa, L. Osteosarcoma Pathogenesis Leads the Way to New Target Treatments. Int. J. Mol. Sci. 2021, 22, 813. [Google Scholar] [CrossRef]
- He, X.; Gao, Z.; Xu, H.; Zhang, Z.; Fu, P. A meta-analysis of randomized control trials of surgical methods with osteosarcoma outcomes. J. Orthop. Surg. Res. 2017, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; McManus, M.M.; Hughes, D.P.M. Understanding the Biology of Bone Sarcoma from Early Initiating Events through Late Events in Metastasis and Disease Progression. Front. Oncol. 2013, 3, 230. [Google Scholar] [CrossRef]
- Gourbault, O.; Llobat, L. MicroRNAs as Biomarkers in Canine Osteosarcoma: A New Future? Vet. Sci. 2020, 7, 146. [Google Scholar] [CrossRef]
- Chen, X. A MicroRNA as a Translational Repressor of APETALA2 in Arabidopsis Flower Development. Science 2004, 303, 2022–2025. [Google Scholar] [CrossRef]
- Doench, J.G. Specificity of microRNA target selection in translational repression. Genes Dev. 2004, 18, 504–511. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Miller, N.; Kerin, M.J. MiRNAs as biomarkers and therapeutic targets in cancer. Curr. Opin. Pharmacol. 2010, 10, 543–550. [Google Scholar] [CrossRef]
- Macfarlane, L.-A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.N.; Han, J.; Siomi, M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Ballarino, M.; Pagano, F.; Girardi, E.; Morlando, M.; Cacchiarelli, D.; Marchioni, M.; Proudfoot, N.J.; Bozzoni, I. Coupled RNA Processing and Transcription of Intergenic Primary MicroRNAs. Mol. Cell. Biol. 2009, 29, 5632–5638. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lee, Y.; Yeom, K.-H.; Kim, Y.-K.; Jin, H.; Kim, V.N. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004, 18, 3016–3027. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Rådmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nat. Cell Biol. 2003, 425, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Morlando, M.; Ballarino, M.; Gromak, N.; Pagano, F.; Bozzoni, I.; Proudfoot, N.J. Primary MicroRNA Transcripts are Processed Co-Transcriptionally. Nat. Struct. Mol. Biol. 2008, 15, 902–909. [Google Scholar] [CrossRef]
- Bohnsack, M.T.; Czaplinski, K.; Görlich, D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 2004, 10, 185–191. [Google Scholar] [CrossRef]
- Lund, E.; Güttinger, S.; Calado, A.; Dahlberg, J.E.; Kutay, U. Nuclear Export of MicroRNA Precursors. Science 2004, 303, 95–98. [Google Scholar] [CrossRef]
- Yi, R.; Qin, Y.; Macara, I.G.; Cullen, B.R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003, 17, 3011–3016. [Google Scholar] [CrossRef]
- Hutvágner, G.; McLachlan, J.; Pasquinelli, A.E.; Bálint, É.; Tuschl, T.; Zamore, P.D. A Cellular Function for the RNA-Interference Enzyme Dicer in the Maturation of the let-7 Small Temporal RNA. Science 2001, 293, 834–838. [Google Scholar] [CrossRef]
- Ketting, R.F.; Fischer, S.E.; Bernstein, E.; Sijen, T.; Hannon, G.J.; Plasterk, R.H. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001, 15, 2654–2659. [Google Scholar] [CrossRef] [PubMed]
- Altuvia, Y. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res. 2005, 33, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Kabekkodu, S.P.; Shukla, V.; Varghese, V.K.; Souza, J.D.; Chakrabarty, S.; Satyamoorthy, K. Clustered miRNAs and their role in biological functions and diseases. Biol. Rev. 2018, 93, 1955–1986. [Google Scholar] [CrossRef]
- Mogilyansky, E.; Rigoutsos, I. The miR-17/92 cluster: A comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013, 20, 1603–1614. [Google Scholar] [CrossRef]
- Yan, S.; Jiao, K. Functions of miRNAs during Mammalian Heart Development. Int. J. Mol. Sci. 2016, 17, 789. [Google Scholar] [CrossRef]
- Kumar, P.; Luo, Y.; Tudela, C.; Alexander, J.M.; Mendelson, C.R. The c-Myc-Regulated MicroRNA-17∼92 (miR-17∼92) and miR-106a∼363 Clusters Target hCYP19A1 and hGCM1 To Inhibit Human Trophoblast Differentiation. Mol. Cell. Biol. 2013, 33, 1782–1796. [Google Scholar] [CrossRef]
- Ventura, A.; Young, A.G.; Winslow, M.M.; Lintault, L.; Meissner, A.; Erkeland, S.J.; Newman, J.; Bronson, R.T.; Crowley, D.; Stone, J.R.; et al. Targeted Deletion Reveals Essential and Overlapping Functions of the miR-17∼92 Family of miRNA Clusters. Cell 2008, 132, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Srinivasan, L.; Calado, D.P.; Patterson, H.C.; Zhang, B.; Wang, J.; Henderson, J.M.; Kutok, J.L.; Rajewsky, K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat. Immunol. 2008, 9, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Baumjohann, D.; Kageyama, R.; Clingan, J.M.; Morar, M.M.; Patel, S.; De Kouchkovsky, D.; Bannard, O.; Bluestone, J.A.; Matloubian, M.; Ansel, K.M.; et al. The microRNA cluster miR-17∼92 promotes TFH cell differentiation and represses subset-inappropriate gene expression. Nat. Immunol. 2013, 14, 840–848. [Google Scholar] [CrossRef]
- Doebele, C.; Bonauer, A.; Fischer, A.; Scholz, A.; Reiss, Y.; Urbich, C.; Hofmann, W.-K.; Zeiher, A.M.; Dimmeler, S. Members of the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood 2010, 115, 4944–4950. [Google Scholar] [CrossRef]
- Hajarnis, S.; Lakhia, R.; Yheskel, M.; Williams, D.; Sorourian, M.; Liu, X.; Aboudehen, K.; Zhang, S.; Kersjes, K.; Galasso, R.; et al. microRNA-17 family promotes polycystic kidney disease progression through modulation of mitochondrial metabolism. Nat. Commun. 2017, 8, 14395. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Huang, R.; Liu, S.; Wu, W.; Su, A.; Li, R.; Liu, X.; Lei, Y.; Sun, H.; Liu, X.; et al. A positive feedback loop of SIRT1 and miR17HG promotes the repair of DNA double-stranded breaks. Cell Cycle 2019, 18, 2110–2123. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.H.; Mitchell, D.A.; McGowan, S.D.; Evanoff, R.; Griswold, M.D. Two miRNA Clusters, Mir-17-92 (Mirc1) and Mir-106b-25 (Mirc3), Are Involved in the Regulation of Spermatogonial Differentiation in Mice1. Biol. Reprod. 2012, 86, 72. [Google Scholar] [CrossRef] [PubMed]
- Brett, J.O.; Renault, V.M.; Rafalski, V.A.; Webb, A.E.; Brunet, A. The microRNA cluster miR-106b~25 regulates adult neural stem/progenitor cell proliferation and neuronal differentiation. Aging 2011, 3, 108–124. [Google Scholar] [CrossRef]
- Bronze-Da-Rocha, E. MicroRNAs Expression Profiles in Cardiovascular Diseases. BioMed Res. Int. 2014, 2014, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Pinton, P. Mitochondrial calcium uniporter, MiRNA and cancer. Commun. Integr. Biol. 2013, 6, e23818. [Google Scholar] [CrossRef]
- Conkrite, K.; Sundby, M.; Mukai, S.; Thomson, J.M.; Mu, D.; Hammond, S.M.; MacPherson, D. miR-17 92 cooperates with RB pathway mutations to promote retinoblastoma. Genes Dev. 2011, 25, 1734–1745. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, P.; Wang, Q.; Wang, B.; Mu, J.; Zhuang, X.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H.-G. Quantitatively Controlling Expression of miR-17∼92 Determines Colon Tumor Progression in a Mouse Tumor Model. Am. J. Pathol. 2014, 184, 1355–1368. [Google Scholar] [CrossRef]
- Hong, L.; Lai, M.; Chen, M.; Xie, C.; Liao, R.; Kang, Y.J.; Xiao, C.; Hu, W.Y.; Han, J.; Sun, P. The miR-17-92 Cluster of MicroRNAs Confers Tumorigenicity by Inhibiting Oncogene-Induced Senescence. Cancer Res. 2010, 70, 8547–8557. [Google Scholar] [CrossRef]
- Brockway, S.; Zeleznik-Le, N.J. WEE1 is a validated target of the microRNA miR-17-92 cluster in leukemia. Cancer Genet. 2015, 208, 279–287. [Google Scholar] [CrossRef][Green Version]
- Gits, C.; Van Kuijk, P.F.; Jonkers, M.B.E.; Boersma, A.W.M.; Van Ijcken, W.F.; Wozniak, A.; Sciot, R.; Rutkowski, P.; Schoffski, P.; Taguchi, T.; et al. MiR-17-92 and miR-221/222 cluster members target KIT and ETV1 in human gastrointestinal stromal tumours. Br. J. Cancer 2013, 109, 1625–1635. [Google Scholar] [CrossRef]
- Urtasun, R.; Elizalde, M.; Azkona, M.; Latasa, M.U.; García-Irigoyen, O.; Uriarte, I.; Fernández-Barrena, M.G.; Vicent, S.; Alonso, M.M.; Muntané, J.; et al. Splicing regulator SLU7 preserves survival of hepatocellular carcinoma cells and other solid tumors via oncogenic miR-17-92 cluster expression. Oncogene 2016, 35, 4719–4729. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, W.; Neo, T.W.; Aung, M.O.; Wasser, S.; Lim, S.G.; Tan, T.M. Role of themiR-106b-25microRNA cluster in hepatocellular carcinoma. Cancer Sci. 2009, 100, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Iwanaga, R.; Drasin, D.J.; Micalizzi, D.S.; Vartuli, R.L.; Tan, A.-C.; Ford, H.L. The miR-106b-25 cluster targets Smad7, activates TGF-β signaling, and induces EMT and tumor initiating cell characteristics downstream of Six1 in human breast cancer. Oncogene 2012, 31, 5162–5171. [Google Scholar] [CrossRef]
- Savita, U.; Karunagaran, D. MicroRNA-106b-25 cluster targets β-TRCP2, increases the expression of Snail and enhances cell migration and invasion in H1299 (non small cell lung cancer) cells. Biochem. Biophys. Res. Commun. 2013, 434, 841–847. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, Y.; Yang, M.; Jat, P.; Li, K.; Lombardo, Y.; Xiong, D.; Coombes, R.C.; Raguz, S.; Yagüe, E. The miR-106b∼25 cluster promotes bypass of doxorubicin-induced senescence and increase in motility and invasion by targeting the E-cadherin transcriptional activator EP300. Cell Death Differ. 2014, 21, 462–474. [Google Scholar] [CrossRef]
- Kan, T.; Sato, F.; Ito, T.; Matsumura, N.; David, S.; Cheng, Y.; Agarwal, R.; Paun, B.C.; Jin, Z.; Olaru, A.V.; et al. The miR-106b-25 Polycistron, Activated by Genomic Amplification, Functions as an Oncogene by Suppressing p21 and Bim. Gastroenterology 2009, 136, 1689–1700. [Google Scholar] [CrossRef]
- Baumhoer, D.; Zillmer, S.; Unger, K.; Rosemann, M.; Atkinson, M.J.; Irmler, M.; Beckers, J.; Siggelkow, H.; Von Luettichau, I.; Jundt, G.; et al. MicroRNA profiling with correlation to gene expression revealed the oncogenic miR-17-92 cluster to be up-regulated in osteosarcoma. Cancer Genet. 2012, 205, 212–219. [Google Scholar] [CrossRef]
- Yang, H.; Peng, Z.; Liang, M.; Zhang, Y.; Wang, Y.; Huang, T.; Jiang, Y.; Jiang, B.; Wang, Y. The miR-17-92 cluster/QKI2/β-catenin axis promotes osteosarcoma progression. Oncotarget 2018, 9, 25285–25293. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, H.; Tian, Q.; Liu, Y.; Weng, Y. Upregulation of microRNA-17-92 cluster associates with tumor progression and prognosis in osteosarcoma. Neoplasma 2014, 61, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Viera, G.M.; Salomao, K.B.; De Sousa, G.R.; Baroni, M.; Delsin, L.E.A.; Pezuk, J.A.; Brassesco, M.S. miRNA signatures in childhood sarcomas and their clinical implications. Clin. Transl. Oncol. 2019, 21, 1583–1623. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Han, H.-J.; Kohwi-Shigematsu, T. Tissue-specific nuclear architecture and gene expession regulated by SATB1. Nat. Genet. 2003, 34, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, B.; Fu, Y.; He, M.; Wang, J.; Shen, P.; Bai, L. miR-23a suppresses proliferation of osteosarcoma cells by targeting SATB1. Tumor Biol. 2015, 36, 4715–4721. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Meng, C.; Shao, Z.; Wang, H.; Yang, S. MiR-23a Functions as a Tumor Suppressor in Osteosarcoma. Cell. Physiol. Biochem. 2014, 34, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Di, R.; Wang, L. MicroRNA-23a enhances migration and invasion through PTEN in osteosarcoma. Cancer Gene Ther. 2015, 22, 351–359. [Google Scholar] [CrossRef]
- Yuan, G.; Zhao, Y.; Wu, D.; Gao, C.; Jiao, Z. miRNA-20a upregulates TAK1 and increases proliferation in osteosarcoma cells. Futur. Oncol. 2018, 14, 461–469. [Google Scholar] [CrossRef]
- Zhuo, W.; Ge, W.; Meng, G.; Jia, S.; Zhou, X.; Liu, J. MicroRNA-20a promotes the proliferation and cell cycle of human osteosarcoma cells by suppressing early growth response 2 expression. Mol. Med. Rep. 2015, 12, 4989–4994. [Google Scholar] [CrossRef]
- Pu, Y.; Yi, Q.; Zhao, F.; Wang, H.; Cai, W.; Cai, S. MiR-20a-5p represses multi-drug resistance in osteosarcoma by targeting the KIF26B gene. Cancer Cell Int. 2016, 16, 1–15. [Google Scholar] [CrossRef]
- Jiang, R.; Zhang, C.; Liu, G.; Gu, R.; Wu, H. MicroRNA-107 Promotes Proliferation, Migration, and Invasion of Osteosarcoma Cells by Targeting Tropomyosin 1. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 1409–1419. [Google Scholar] [CrossRef]
- Rossini, M.; Gatti, D.; Adami, S. Involvement of WNT/β-catenin Signaling in the Treatment of Osteoporosis. Calcif. Tissue Int. 2013, 93, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C.; Liu, J.X.; Shao, Z.W.; Pu, F.F.; Wang, B.C.; Wu, Q.; Zhang, Y.K.; Zeng, X.L.; Guo, X.D.; Yang, S.H.; et al. In vitro effect of microRNA-107 targeting Dkk-1 by regulation of Wnt/β-catenin signaling pathway in osteosarcoma. Medicine 2017, 96, e7245. [Google Scholar] [CrossRef]
- Namløs, H.M.; Meza-Zepeda, L.A.; Barøy, T.; Østensen, I.H.G.; Kresse, S.H.; Kuijjer, M.L.; Serra, M.; Bürger, H.; Cleton-Jansen, A.M.; Myklebost, O. Modulation of the Osteosarcoma Expression Phenotype by MicroRNAs. PLoS ONE 2012, 7, e48086. [Google Scholar] [CrossRef]
- Wu, F.; Jiang, X.; Wang, Q.; Lu, Q.; He, F.; Li, J.; Li, X.; Jin, M.; Xu, J. The impact of miR-9 in osteosarcoma. Medicine 2020, 99, e21902. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.H.; Yang, Y.L.; Han, S.M.; Wu, Z.H. MicroRNA-9 expression is a prognostic biomarker in patients with osteosarcoma. World J. Surg. Oncol. 2014, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, J.; Tian, S.; Luo, J. miR‑9 depletion suppresses the proliferation of osteosarcoma cells by targeting p16. Int. J. Oncol. 2019, 54, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Fei, D.; Li, Y.; Zhao, D.; Zhao, K.; Dai, L.; Gao, Z. Serum miR-9 as a prognostic biomarker in patients with osteosarcoma. J. Int. Med. Res. 2014, 42, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Fang, J.; Pang, Y.; Zheng, J. Prognostic value of the microRNA-29 family in patients with primary osteosarcomas. Med. Oncol. 2014, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Geng, P.; Shi, L.; Wang, Q.; Wang, P. miR-29 promotes osteosarcoma cell proliferation and migration by targeting PTEN. Oncol. Lett. 2018, 17, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Liu, P.; Yang, S.; Ye, S.; Xu, W.; Liu, X. A three-plasma miRNA signature serves as novel biomarkers for osteosarcoma. Med. Oncol. 2012, 30, 340. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Jin, Z.; Zhou, F.; Zhang, Y.-Q.; Zhuang, Y. The expression significance of serum MiR-21 in patients with osteosarcoma and its relationship with chemosensitivity. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2989–2994. [Google Scholar]
- Geng, Y.; Zhao, S.; Jia, Y.; Xia, G.; Li, H.; Fang, Z.; Zhang, Q.; Tian, R. miR‑95 promotes osteosarcoma growth by targeting SCNN1A. Oncol. Rep. 2020, 43, 1429–1436. [Google Scholar] [CrossRef]
- Liu, X.; Ma, W.; Ma, J.; Xiao, L.; Hao, D. Upregulation of miR‑95-3p inhibits growth of osteosarcoma by targeting HDGF. Pathol. Res. Pr. 2019, 215, 152492. [Google Scholar] [CrossRef]
- Niu, J.; Sun, Y.; Guo, Q.; Niu, D.; Liu, B. Serum miR-95-3p is a diagnostic and prognostic marker for osteosarcoma. SpringerPlus 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Shi, L.; Xie, C.; Zhu, J.; Chen, X. Downregulation of serum miR-194 predicts poor prognosis in osteosarcoma patients. Ann. Diagn. Pathol. 2020, 46, 151488. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, L.S.; Yuan, W.; Wu, Z.; Wang, H.B.; Xu, T.; Sun, J.C.; Cheng, K.F.; Shi, J.G. Low miR-34a and miR-192 are associated with unfavorable prognosis in patients suffering from osteosarcoma. Am. J. Transl. Res. 2015, 7, 111–119. [Google Scholar]
- Xi, L.; Zhang, Y.; Kong, S.; Liang, W. miR-34 inhibits growth and promotes apoptosis of osteosarcoma in nude mice through targetly regulating TGIF2 expression. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; He, A.; Zhang, Q.; Tao, C. miR-126 inhibits cell growth, invasion, and migration of osteosarcoma cells by downregulating ADAM-9. Tumor Biol. 2014, 35, 12645–12654. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, Z.Y.; Shi, L.; Yuan, W.D. Tissue microRNA-126 expression level predicts outcome in human osteosarcoma. Diagn. Pathol. 2015, 10, 116. [Google Scholar] [CrossRef]
- Luo, X.; Tang, J.; Xuan, H.; Liu, J.; Li, X. Identification and Validation of a Potent Multi-miRNA Signature for Prediction of Prognosis of Osteosarcoma Patients. Med. Sci. Monit. 2020, 26, e919272-1. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Li, B.; Sun, L.; An, C. MicroRNA-153 Inhibits Osteosarcoma Cells Proliferation and Invasion by Targeting TGF-β2. PLoS ONE 2015, 10, e0119225. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Yi, X.; Lv, X.; Liang, R.; Zan, N.; Su, X. MiR-212 promotes proliferation and inhibits apoptosis of osteosarcoma cells via regulating hedgehog signaling pathway. J. BUON 2020, 25, 2086–2091. [Google Scholar]
- Huang, X.; Tang, F.; Weng, Z.; Zhou, M.; Zhang, Q. MiR-591 functions as tumor suppressor in breast cancer by targeting TCF4 and inhibits Hippo-YAP/TAZ signaling pathway. Cancer Cell Int. 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Huh, J.H.; Kim, T.H.; Kim, K.; Song, J.A.; Jung, Y.J.; Jeong, J.Y.; Lee, M.J.; Kim, Y.K.; Lee, D.H.; An, H.J. Dysregulation of miR-106a and miR-591 confers paclitaxel resistance to ovarian cancer. Br. J. Cancer 2013, 109, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, Z.; Zhu, X.; Xu, R.; Xu, Y. miR-29 Family Inhibits Resistance to Methotrexate and Promotes Cell Apoptosis by Targeting COL3A1 and MCL1 in Osteosarcoma. Med. Sci. Monit. 2018, 24, 8812–8821. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, J.Y.; Kim, M.S.; Vares, G.; Ohno, T.; Takahashi, A.; Uzawa, A.; Seo, S.J.; Sai, S. Molecular mechanisms underlying the enhancement of carbon ion beam radiosensitivity of osteosarcoma cells by miR-29b. Am. J. Cancer Res. 2020, 10, 4357–4371. [Google Scholar] [PubMed]
- Jiang, L.; He, A.; He, X.; Tao, C. MicroRNA-126 enhances the sensitivity of osteosarcoma cells to cisplatin and methotrexate. Oncol. Lett. 2015, 10, 3769–3778. [Google Scholar] [CrossRef]
- Pu, Y.; Zhao, F.; Wang, H.; Cai, W.; Gao, J.; Li, Y.; Cai, S. MiR-34a-5p promotes the multi-drug resistance of osteosarcoma by targeting the CD117 gene. Oncotarget 2016, 7, 28420–28434. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, R.H.; Tseng, K.F.; Li, K.P.; Lu, Z.G.; Liu, Y.; Han, K.; Gan, Z.H.; Lin, S.C.; Hu, H.Y.; et al. Sirolimus induces apoptosis and reverses multidrug resistance in human osteosarcoma cells in vitro via increasing microRNA-34b expression. Acta Pharmacol. Sin. 2016, 37, 519–529. [Google Scholar] [CrossRef]
- Li, E.Y.; Zhao, P.J.; Jian, J.; Yin, B.Q.; Sun, Z.Y.; Xu, C.X.; Tang, Y.C.; Wu, H. Vitamin B1 and B12 mitigates neuron apoptosis in cerebral palsy by augmenting BDNF expression through MALAT1/miR-1 axis. Cell Cycle 2019, 18, 2849–2859. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Chen, Q.; Wang, W.; Ling, Y.; Yan, Y.; Xia, P. Hepatocyte-derived extracellular vesicles promote endothelial inflammation and atherogenesis via microRNA-1. J. Hepatol. 2020, 72, 156–166. [Google Scholar] [CrossRef]
- Lv, L.; Zheng, N.; Zhang, L.; Li, R.; Li, Y.; Yang, R.; Li, C.; Fang, R.; Shabanova, A.; Li, X.; et al. Metformin ameliorates cardiac conduction delay by regulating microRNA-1 in mice. Eur. J. Pharmacol. 2020, 881, 173131. [Google Scholar] [CrossRef] [PubMed]
- Mondejar-Parreño, G.; Callejo, M.; Barreira, B.; Morales-Cano, D.; Esquivel-Ruiz, S.; Moreno, L.; Cogolludo, A.; Perez-Vizcaino, F. miR-1 is increased in pulmonary hypertension and downregulates Kv1.5 channels in rat pulmonary arteries. J. Physiol. 2018, 597, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, C.; Wu, Z.; Wang, Y.; Yin, W.; Lin, Y.; Zhou, L.; Lu, J. Upregulation of microRNA‑1 inhibits proliferation and metastasis of breast cancer. Mol. Med. Rep. 2020, 22, 454–464. [Google Scholar] [CrossRef]
- Wu, Y.; Pu, N.; Su, W.; Yang, X.; Xing, C. Downregulation of miR-1 in colorectal cancer promotes radioresistance and aggressive phenotypes. J. Cancer 2020, 11, 4832–4840. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N.; Fu, J.; Zhou, W. Long noncoding RNA HOTAIR promotes medulloblastoma growth, migration and invasion by sponging miR-1/miR-206 and targeting YY1. Biomed. Pharmacother. 2020, 124, 109887. [Google Scholar] [CrossRef] [PubMed]
- Sheervalilou, R.; Lotfi, H.; Shirvaliloo, M.; Sharifi, A.; Nazemiyeh, M.; Zarghami, N. Circulating MiR-10b, MiR-1 and MiR-30a Expression Profiles in Lung Cancer: Possible Correlation with Clinico-Pathologic Characteristics and Lung Cancer Detection. Int. J. Mol. Cell Med. 2019, 8, 118–129. [Google Scholar]
- Fujii, R.; Osaka, E.; Sato, K.; Tokuhashi, Y. MiR-1 Suppresses Proliferation of Osteosarcoma Cells by Up-regulating p21 via PAX3. Cancer Genom. Proteom. 2019, 16, 71–79. [Google Scholar] [CrossRef]
- Niu, J.; Sun, Y.; Guo, Q.; Niu, D.; Liu, B. miR-1 Inhibits Cell Growth, Migration, and Invasion by Targeting VEGFA in Osteosarcoma Cells. Dis. Markers 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, Z.; Xu, J.; Chen, C.; Ge, Q.; Li, P.; Wei, D.; Wu, Z.; Sun, X. Micro RNA -134 inhibits osteosarcoma angiogenesis and proliferation by targeting the VEGFA/VEGFR 1 pathway. FEBS J. 2018, 285, 1359–1371. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, L.; Jiao, Y.; Zhou, Y.; Ge, Q.; Li, P.; Sun, X.; Lv, Z. miR-134 inhibits osteosarcoma cell invasion and metastasis through targeting MMP 1 and MMP 3 in vitro and in vivo. FEBS Lett. 2019, 593, 1089–1101. [Google Scholar] [CrossRef]
- Thayanithy, V.; Sarver, A.L.; Kartha, R.V.; Li, L.; Angstadt, A.Y.; Breen, M.; Steer, C.J.; Modiano, J.F.; Subramanian, S. Perturbation of 14q32 miRNAs-cMYC gene network in osteosarcoma. Bone 2012, 50, 171–181. [Google Scholar] [CrossRef]
- Chen, B.; Huang, Z.; Zhang, Y.; Chen, Y.; Li, Z. MicroRNA-145 Suppresses Osteosarcoma Metastasis via Targeting MMP16. Cell. Physiol. Biochem. 2015, 37, 2183–2193. [Google Scholar] [CrossRef]
- Wu, G.; Yu, W.; Zhang, M.; Yin, R.; Wu, Y.; Liu, Q. MicroRNA-145-3p suppresses proliferation and promotes apotosis and autophagy of osteosarcoma cell by targeting HDAC4. Artif. Cells Nanomed. Biotechnol. 2018, 46, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Zhang, J.; Yuan, T.; Ma, B. miR-145 inhibits osteosarcoma cells proliferation and invasion by targeting ROCK1. Tumor Biol. 2014, 35, 7645–7650. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wu, Q.; Xing, X.; Wei, Y.; Shao, Z. MicroRNA-145 targets vascular endothelial growth factor and inhibits invasion and metastasis of osteosarcoma cells. Acta Biochim. Biophys. Sin. 2012, 44, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Y.; Tang, J.; Wang, P.; Li, L.; Yan, X.; Zheng, X.; Ren, S.; Zhang, M.; Xu, M. The Prognostic Value and Regulatory Mechanisms of microRNA-145 in Various Tumors: A Systematic Review and Meta-analysis of 50 Studies. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tang, M.; Lin, L.; Cai, H.; Tang, J. MicroRNA-145 downregulation associates with advanced tumor progression and poor prognosis in patients suffering osteosarcoma. Oncol. Targets Ther. 2013, 6, 833. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, J.; Zou, Y.; Jiang, D. Honokiol suppresses proliferation and induces apoptosis via regulation of the miR‑21/PTEN/PI3K/AKT signaling pathway in human osteosarcoma cells. Int. J. Mol. Med. 2018, 41, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Kang, Y.-K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.-L.; Kim, T.-Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: A first-in-man, phase 1, open-label, dose-escalation study. Lancet Oncol. 2017, 18, 1386–1396. [Google Scholar] [CrossRef]
- van der Ree, M.H.; de Vree, J.M.; Stelma, F.; Willemse, S.; van der Valk, M.; Rietdijk, S.; Molenkamp, R.; Schinkel, J.; van Nuenen, A.C.; Beuers, U.; et al. Safety, tolerability, and antiviral effect of RG-101 in patients with chronic hepatitis C: A phase 1B, double-blind, randomised controlled trial. Lancet 2017, 389, 709–717. [Google Scholar] [CrossRef]
- Van Der Ree, M.H.; Van Der Meer, A.J.; Van Nuenen, A.C.; De Bruijne, J.; Ottosen, S.; Janssen, H.L.; Kootstra, N.A.; Reesink, H.W. Miravirsen dosing in chronic hepatitis C patients results in decreased microRNA-122 levels without affecting other microRNAs in plasma. Aliment. Pharmacol. Ther. 2016, 43, 102–113. [Google Scholar] [CrossRef] [PubMed]
| Target | miRNAs |
|---|---|
| Aurora-B | Let-7 |
| ESR1 | miR-1 |
| Bcl-2 | miR-15a, miR-34a, miR-125b, miR-143, miR-190b, miR-326, miR-449a |
| IGF system | miR-16, miR-26a, miR-133a, miR-150, miR-497 |
| MED27 | miR-18a |
| TAK1 | miR-20a |
| VEGF | miR20b, miR-29b, miR-205, miR-410 |
| HMG system | miR-22, miR-106a-5p, miR-142-3p, miR-505 |
| SATB1 | miR-23a, miR-376a-3p |
| LPAATβ | miR-24 |
| SOX4, SOX9 or SOX2 | miR-25, miR-25-3p, miR-132, miR-188, miR-212, miR-32 (SOX9), miR-336 (SOX2) |
| PFKFB3 | miR-26 |
| PTEN | miR-30a |
| HIF1 | miR-33b, miR-20b, miR186 |
| MARCKS | miR-34c-3p |
| EZRIN | miR-96, miR-144, miR-150 |
| TNFAIP | miR-15a, miR-99a, |
| PI3K-AKT | miR-100, miR-497 |
| mTOR | miR-101 |
| Β-catenin | miR-107, miR-184 |
| TGF β | miR-124, miR-153, miR-422a |
| TGF-α | miR-205, miR-376a, miR-376c, miR-422a, |
| ANXA2 | miR-206 |
| ROR2 | miR-208b |
| ROCK1 | miR-129-5p, miR-139, miR-144, miR-145, miR-148a, miR-150, miR-198, miR-214-5p |
| CDK14 | miR-216a |
| CDK6 | miR-377, miR-494, miR-3928, miR-494 |
| PDK1 | miR-379 |
| LRH-1 | miR-381, miR-451 |
| ELF2 | miR-409-3p |
| ZEB1 | miR-126, miR-130a, miR-141, miR-200b, miR-429, miR-643 |
| SETD8 | miR-127-3p |
| Sirt1 | miR-133b, miR-138, miR-204 |
| c-Myc | miR-135b, miR-449c |
| MTDH | miR-136 |
| FXYD6 | miR-137 |
| HDAC4 | miR-140 |
| GLI2 | miR-141-3p, miR-202 |
| FASN | miR-142-3p |
| FOSL2 | miR-143-3p |
| E2F1 | miR-320 |
| E2F3 | miR-152, miR-874 |
| Wnt system | miR-154, miR-184, miR-217 |
| TIAM1 | miR-182 |
| LRP6 | miR-183 |
| ZERB2 | miR-187 |
| TCF7 | miR-192 |
| CDH2 | miR-194 |
| NKD1 | miR-195-5p |
| VANGL-2 | miR-199-3p |
| PMP22 | miR-200bc/429 |
| RAB22A | miR-203 |
| ANXA2 | miR-206 |
| ROR2 | miR-208b |
| Hsp90B1 | miR-223 |
| Ect-2 | miR-223 |
| Rac-1 | miR-224 |
| ADAM9 | miR-302a |
| LDHA | miR-323a-3p |
| Rab10 | miR-329 |
| Bmi-1 (or BMI1) | miR-330-3p, miR-452 |
| Survivin | miR-335 |
| AEG-1 | miR-342-3p, miR-448, miR-506 |
| KRAS | miR-365 |
| FOXM1 | miR-370 |
| FOXP4 | miR-491-5p |
| SATB1 | miR-376a-3p |
| LRH-1 | miR-451 |
| c-Met | miR-454, miR-613 |
| PKC | miR-486 |
| HMGA2 | miR-490-3p |
| HMGB1 | miR-505 |
| HMGN5 | miR-495 |
| TSPAN1 | miR-491-3p |
| PAK6 | miR-492 |
| ARL2 | miR-497-5p |
| FGF2 | miR-503 |
| AKT-1 (or Akt) | miR-520a-3p, miR-564 |
| MMP8 | miR-539 |
| ITGAV | miR-548c-3p |
| KLF5 | miR-590-5p |
| PDGFB | miR-598 |
| YAP-1 | miR-625 |
| PIM1 | miR-638 |
| CYC1 | miR-661 |
| RAB23 | miR-665 |
| BCL9 | miR-1301 |
| ERBB3 | miR-3928 |
| Target | miRNAs |
|---|---|
| GCIP | miR-9 |
| KLF4 | miR-10b |
| PTEN | miR-17, miR-21, miR-23a, miR-92a, miR-93, miR-196a, miR-214, miR-221, miR-1908 |
| BRCC2 | miR-17-5p, miR-603 |
| SOCS6 | miR-19 |
| EGR2 | miR-20a |
| ING5 | miR-27-3p |
| TET1 | miR-27a-3p |
| TGF-β | miR-29 |
| RASSF1A | miR-81a |
| RECK | miR-92b |
| CDKN1 | miR95-3p |
| VNN2 | miR-106a |
| PI3K | miR-106b |
| TPM1 | miR-107 |
| PPARγ | miR-130b |
| CDC14A | miR-131a |
| ROCK1 | miR-138 |
| BMP9 | miR-149 |
| HBP1 | miR-155 |
| RIPK1 | miR-155-5p |
| CFIm25 | miR-181a |
| Chk2 | miR-191 |
| p21 | miR-93, miR-95-3p |
| p27 | miR-199a-5p |
| BRD7 | miR-300 |
| DAB2IP | miR-367 |
| FOXO1 and FOXO4 | miR-374a, miR-660, miR-664 (FOXO4) |
| KLF9 | miR-378 |
| MTSS1 | miR-411 |
| Bim | miR-488 |
| TP53INP1 | miR-504 |
| VANGL-2 | miR-542-3p, miR-542-5p |
| MAP3K9 | miR-1247 |
| AGTR1 | miR-1248 |
| SRSF3 | miR-1908-5p |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llobat, L.; Gourbault, O. Role of MicroRNAs in Human Osteosarcoma: Future Perspectives. Biomedicines 2021, 9, 463. https://doi.org/10.3390/biomedicines9050463
Llobat L, Gourbault O. Role of MicroRNAs in Human Osteosarcoma: Future Perspectives. Biomedicines. 2021; 9(5):463. https://doi.org/10.3390/biomedicines9050463
Chicago/Turabian StyleLlobat, Lola, and Olivia Gourbault. 2021. "Role of MicroRNAs in Human Osteosarcoma: Future Perspectives" Biomedicines 9, no. 5: 463. https://doi.org/10.3390/biomedicines9050463
APA StyleLlobat, L., & Gourbault, O. (2021). Role of MicroRNAs in Human Osteosarcoma: Future Perspectives. Biomedicines, 9(5), 463. https://doi.org/10.3390/biomedicines9050463







