Abstract
Wilm’s tumor 1 (WT1), a zinc-finger transcription factor and an epigenetic modifier, is frequently overexpressed in several hematologic disorders and solid tumors, and it has been proposed as diagnostic and prognostic marker of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). However, the exact role of WT1 in leukemogenesis and disease progression remains unclear. In this real-world evidence retrospective study, we investigated prognostic role of WT1-mRNA expression levels in AML and MDS patients and correlations with complete blood counts, flow cytometry counts, and molecular features. A total of 71 patients (AML, n = 46; and MDS, n = 25) were included in this study, and WT1 levels were assessed at diagnosis, during treatment and follow-up. We showed that WT1 expression levels were inversely correlated with normal hemopoiesis in both AML and MDS, and positively associated with blast counts. Flow cytometry was more sensitive and specific in distinguishing normal myeloid cells from neoplastic counterpart even just using linear parameters and CD45 expression. Moreover, we showed that a simple integrated approach combining blast counts by flow cytometry, FLT3 mutational status, and WT1 expression levels might be a useful tool for a better prognostic definition in both AML and MDS patients.
1. Introduction
Acute myeloid leukemia (AML) is a heterogeneous group of clonal aggressive hematologic malignancies characterized by differentiation block and increased proliferation of neoplastic cells of myeloid origins [1]. The presence of at least 20% myeloblasts in the bone marrow (BM) or peripheral blood (PB) is the main criterion of AML diagnosis as outlined in the 2016 World Health Organization (WHO) guidelines [1,2]. Exceptions are AML with specific cytogenetic abnormalities or nucleophosmin 1 (NPM1) mutated leukemias [3]. However, the 20% cut-off is arbitrary and is used for differential diagnosis with myelodysplastic syndromes (MDS), especially excess blasts 2 subtype [4]. MDS are a group of clonal premalignant hematological diseases characterized by ineffective hematopoiesis, progressive PB cytopenias, increased risk of developing AML, and poor overall survival (OS) [2]. MDS are heterogeneous in clinical presentation, cytogenetics and molecular signatures resulting in various outcomes with OS ranging from 5 years to 9 months [5]. Several genetic alterations frequently found in MDS can be present also in other hematological disorders and in healthy individuals because clonal hematopoiesis is commonly seen with aging [6,7]. Therefore, additional pathogenetic mechanisms are required for dysplastic hemopoiesis, and immune dysregulation can initiate or support dyspoiesis. Several studies have been investigating the prognostic impact of cytogenetic and molecular abnormalities in event-free survival (EFS) and OS of AML and MDS patients [8,9,10]. The European LeukemiaNet (ELN) has defined three risk categories in AML—favorable, intermediate, and adverse—based on the combination of specific genetic or chromosomal alterations, advanced age (>60 years old), or neutropenia [8]. Similarly, risk stratification of MDS patients is based on percentage of BM blasts, complete blood counts (CBC), and cytogenetic abnormalities [9,10].
Wilms’ tumor 1 (WT1), a zinc-finger transcription factor and an epigenetic modifier, has been proposed as prognostic marker of several solid and hematologic tumors because is frequently overexpressed in leukemias, lung, colon, or pancreatic cancers [11,12,13]. In physiological conditions, WT1 is expressed at basal levels in CD34+CD38− hematopoietic stem cells (HSCs) in the BM and is related to quiescence and stemness, while lineage-committed progenitors show undetectable WT1 levels [11]. Conversely, WT1 is frequently overexpressed in AML, MDS, and blast crisis of chronic myeloid leukemia, and expression levels are associated with increased blast counts, higher risk of progression and relapse, resistance to therapy, and poor OS [11,14,15,16,17].
In this study, we investigated the prognostic role of WT1 expression levels and of a combined phenotypic and molecular score in AML and MDS patients for risk stratification.
2. Materials and Methods
2.1. Patients and Therapeutic Regimens
Whole PB or BM specimens were collected in ethylenediaminetetraacetic acid (EDTA) tubes for WT1 expression level assessment or heparin tubes for immunophenotyping from patients after informed consent obtained in accordance with the Declaration of Helsinki [18]. A total of 71 patients were included in this retrospective study after received a diagnosis of AML or MDS, and chemotherapy as per international guidelines at the Hematology and Transplant Center, University Hospital ”San Giovanni di Dio e Ruggi d’Aragona” of Salerno, Italy. Risk stratification was calculated according to ELN or to the Revised International Prognostic Scoring System (IPSS-R) for AML or MDS, respectively [8,9,10]. International Working Group (IWG) consensus criteria were used to determine patients’ treatment response [19]. Clinical characteristics at baseline are summarized in Table 1 and Table 2.

Table 1.
AML patients’ characteristics at baseline.

Table 2.
MDS patients’ characteristics at baseline
Among AML patients (n = 46; mean age, 58 years old; range, 17–93; M/F, 26/20), 15 subjects received chemotherapy as per standard protocols with daunorubicin + cytarabine (Ara-C), FLANG (fludarabine + high-dose Ara-C + granulocyte colony-stimulating factor (G-CSF)), FLAG-IDA (FLAG plus idarubicin), or MEC (mitoxantrone, etoposide, and Ara-C); while 18 subjects were treated with hypomethylating agents, such as 5-azacitidine and decitabine, with or without venetoclax, a Bcl-2 inhibitor. Fifteen AML patients (eight in first CR, and seven in second CR) underwent to allogeneic hematopoietic stem cell transplantation (HSCT) after conditioning regimen with busulfan and melphalan. Among MDS patients (n = 25; mean age, 70 years old; range, 57–84; M/F, 17/8), five subjects were treated with lenalidomide because of del(5q), and 17 with hypomethylating agents with or without venetoclax.
2.2. WT1 Quantitative Assessment
WT1 expression levels were quantified by real-time polymerase chain reaction (RT-PCR) at diagnosis, during treatment and follow-up. Mononuclear cells were freshly isolated from PB or BM samples by density gradient centrifugation using Lymphoprep (Axis-Shield Density Gradient Media, Oslo, Norway) and subsequently subjected to RNA extraction using QIAamp RNA Blood Mini Kit (Qiagen, Hilden, Germany) following manufacturer’s instructions. After RNA quantification using a BioSpectrometer (Eppendorf, Hamburg, Germany), at least 1 µg of RNA was used for cDNA reverse transcription (Ipsogen RT Kit Qiagen). Subsequently, WT1-mRNA quantitative assessment was performed using an ELN-certified Ipsogen WT1 ProfilQuant Kit (Qiagen) following manufacturer’s instructions.
2.3. Flow Cytometry
For immunophenotyping, 50 µL of fresh heparinized whole PB or BM was stained with antibodies according to the manufacturers’ instructions. The following antibodies were used for PB immunophenotyping: CD56, CD45, CD34, CD19, CD11b, CD3, CD8, CD71, CD33, CD16, SmIg-kappa, and SmIg-lambda. For BM immunophenotyping, the following antibodies were employed: CD3, CD7, CD5, CD19, CD34, CD16, CD11b, CD13, CD14, CD56, CD45, CD33, HLA-DR, CD117, SmIg-kappa, and SmIg-lambda. CD45dim blast phenotype was further studied for CD19, CD20, CD34, CD56, CD5, CD117, CD33, CD16, CD11b, CD36, CD13, HLA-DR, CD64, CD4, CD5, CD7, CD14, CD10, CD15, CD11a, CD11c, CD45RA, CD45RO, CD61, CD42b, TdT, and MPO expression. Manufacturer’s characteristics of used antibodies are summarized in Table 3. After 20 min incubation at room temperature, red cell lysis was performed with IO Test Lysing Solution (Beckman Coulter, Brea, CA, United States), cells were washed twice with phosphate-buffered saline (PBS) (IsoFlow Sheath Fluid, Beckman Coulter), and then resuspended in 500 µL PBS for acquisition.

Table 3.
Antibodies for immunophenotyping
Samples were acquired on a Navios or Navios/EX cytometer (Beckman Coulter), equipped with blue (488 nm), green (532 nm), and red (633 nm) lasers. Instrument daily quality control was carried out using Flow-Check Pro Fluorospheres (Beckman Coulter), and external quality control by UK NEQAS for Leucocyte Immunophenotyping. Compensation was monthly checked by a Beckman Coulter’s Specialist using Flow-Set and compensation kit (Beckman Coulter). Samples were run using the same PMT voltages, and at least 50,000–200,000 events were recorded. Post-acquisition analysis was carried out using Navios Software v1.3, Navios EX Software v2.0, or Kaluza Analysis Flow Cytometry Software v2.1.1 (Beckman Coulter).
Cell populations were first identified based on linear parameter (forward scatter area, FSC-A) and CD45 expression, and lymphocytes, monocytes, granulocytes, and immature cells were gated. Lymphocytes were further studied for T (CD3 or CD5 or CD7, CD4, and CD8), B (CD19, SmIg-kappa, and SmIg-lambda), and NK cell (CD56 and CD16) markers. CD33, CD14, CD11b, and CD56 expression was investigated on monocytes with additional CD13, CD36, CD64, CD15, and CD16 assessment in case of monocyte frequency was increased or showed aberrant marker expression. Maturation profiling of CD33+CD56− granulocytes in the BM was carried using CD16 vs. CD11b expression. Normal CD34+ cells were gated for CD19, CD117, and CD33 for definition of lymphoid (CD19+) or myeloid (CD117+CD33+) progenitors. Hematogones were identified based on CD19, CD34, and CD45 expression (CD19+CD34−CD45+/−). CD45dim blasts were further investigated with specific surface and intracellular markers which were also employed for monitoring minimal residual disease (MRD).
2.4. Statistical Analysis
Data were analyzed using Prism (v.8.3.0; GraphPad software, La Jolla, CA, USA). For WT1 quantification, ABL was employed as housekeeping gene for data normalization, and WT1 levels were reported as WT1 copy number/104 ABL copies (normalized WT1 expression). Normal expression levels were considered <50 or <250 copies in PB or BM, respectively, as previously reported [16,17,18,19,20]. Normalized blast count (NBC) was calculated as following: NBC = (%CD34+ cells + %immature cells + %blasts)/%granulocytes, using frequencies measured by flow cytometry. During monitoring, WT1 was reported increased when levels were 2.5-fold higher than those documented at previous timepoint, while WT1 was considered decreased when levels were 0.5-fold lower than those at previous timepoint. For flow cytometry data, populations were reported as percent of positive cells. Pearson analysis was employed for studying correlations between WT1 levels and clinical and phenotypic features. Unpaired two-tailed t-tests for two group comparison and one-way analysis of variance (ANOVA) for three-group comparison were performed. Log-rank (Mantel-Cox) test was employed for survival analysis between groups. Chi-square test was employed for testing association between WT1 expression levels and cytogenetic abnormalities. Multivariate linear regression was used for investigation of association of WT1 levels with other clinical and biological features. A p < 0.05 was considered statistically significant.
3. Results
3.1. Correlation of WT1 Expression with Clinical, Phenotypic, and Molecular Features in AML
Whether to investigate associations between WT1-mRNA levels and AML disease severity and prognosis, WT1 expression was assessed at diagnosis, during therapy, and follow-up, and levels were correlated with complete blood counts (CBCs) and flow cytometer counts (percentage of granulocytes, percentage of CD34+ cells, and NBC). In addition, percentage of BM blasts identified by light microscopy or flow cytometry was correlated with normalized WT1 expression (Figure 1). No correlations with white blood cells (WBCs) were described (r = 0.1002; p = 0.2763), while a negative correlation was documented between normalized WT1 levels and platelets (r = −0.2858; p = 0.0016) or hemoglobin levels (r = −0.2205; p = 0.0155) (Figure 1A). In contrast with findings described for WBC, a negative correlation between normalized WT1 expression and granulocytes identified by flow cytometry was described (r = −0.3350; p = 0.0014). Moreover, WT1 levels correlated with percentage of CD34+ cells (r = 0.3221; p = 0.0089) and NBC (r = 0.3383; p = 0.0011) (Figure 1B). Mean percentage of BM blasts by light microscopy at diagnosis was 43.3 ± 27.4% (range, 15–88%), while mean blast count by flow cytometry was 31.35 ± 30.8% (range, 0–94%). A positive correlation with normalized WT1 levels was described for both light microscopy (r = 0.6032; p = 0.0063) and flow cytometry blast counts (r = 0.3578; p = 0.0348) (Figure 1C).
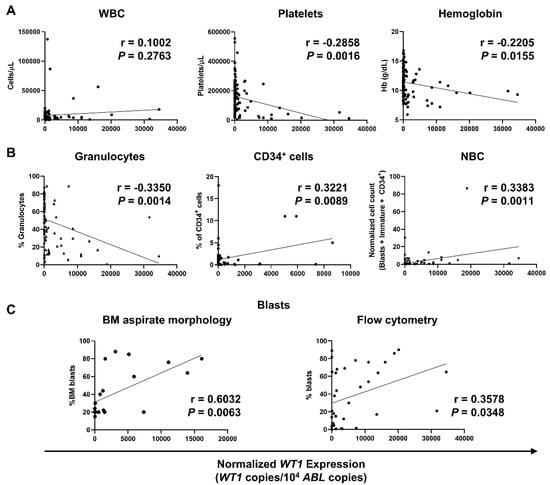
Figure 1.
Correlations of normalized WT1 levels with complete blood counts (CBCs), flow cytometry parameters, and percentage of blasts in AML patients. WT1 expression was normalized using ABL as housekeeping gene, and levels were reported as WT1 copies/104 ABL copies. (A) Pearson correlation analysis between normalized WT1 expression and CBCs, such as white blood cells (WBC), platelets, and hemoglobin (Hb) levels. (B) Correlations with percentage of granulocytes, CD34+ cells, and normalized blast count (NBC) by flow cytometry. (C) Correlations with bone marrow (BM) blasts identified by light microscopy (left) or by flow cytometry (right).
AML patients were then divided based on ELN risk category, and normalized WT1 levels were compared among groups. Mean normalized WT1 expression was 1718 ± 2551 copies (range, 3–7373 copies) in seven patients (15%) with ELN favorable risk, 3375 ± 7258 copies (range, 2–31,684 copies) in 25 subjects (54%) with intermediate risk, and 5960 ± 10,295 copies (range, 3–34,537 copies) in 13 (28%) ELN adverse risk patients. Despite patients with intermediate and adverse risk tended to have higher WT1 levels compared to subjects with favorable risk, no statistically significant differences were described (p = 0.4662).
Patients were then divided based on the presence of somatic mutations in FLT3 (fms like tyrosine kinase 3) or NPM1, and normalized WT1 expression was compared among groups. Higher WT1 levels were found in patients carrying somatic mutations in FLT3 internal tandem duplication (ITD) (mean ± SD, 11,240 ± 13,127 copies; range, 2–31,684 copies) compared to those with FLT3 wild type (mean ± SD, 2801 ± 6205 copies; range, 2–34,537 copies) (p = 0.0127). No differences were described between patients carrying somatic mutations in NPM1 (mean ± SD, 2982 ± 5445 copies; range, 3–6100 copies) and those with NPM1 wild type (mean ± SD, 4280 + 8651 copies; range, 2–34,537 copies) (p = 0.6728).
3.2. Prognostic Impact of WT1 Expression and Combined Score in AML
In order to investigate prognostic impact of normalized WT1 levels in AML, patients were first divided in two groups based on WT1 expression at diagnosis ≥ cut-offs or within normal ranges (Figure 2A). Patients with increased WT1 levels at diagnosis displayed a shorter OS compared to those with WT1 levels < cut-offs (11.7 vs. 92.4 months, WT1 ≥ cut-offs vs. WT1 < cut-offs, respectively; p = 0.0002; hazard ratio (HR), 4.305; 95% confidential interval (CI), 1.983 to 9.344). Patients were also divided based on NBC > 0.5 and OS was compared between groups (Figure 2B); however, no statistically significant variations were described between patients with NBC > 0.5 at diagnosis and those with NBC ≤ 0.5 (10.23 vs. 38.23 months, NBC > 0.5 vs. NBC ≤ 0.5, respectively; p = 0.2396; HR, 0.6442; 95%CI, 0.2754 to 1.507). We then combined WT1 expression levels and NBC at diagnosis, patients were divided in four groups, and OS was compared (Figure 2C). Patients with increased WT1 levels and NBC had the shortest OS compared with those with only elevated WT1 expression or NBC or compared with subjects with normal WT1 levels and NBC ≤ 0.5 (4.43 vs. 17.97 vs. 123.17 vs. 78.1 months, WT1 ≥ cut-off + NBC > 0.5 vs. WT1 ≥ cut-off + NBC ≤ 0.5 vs. WT1 < cut-off + NBC > 0.5 vs. WT1 < cut-off + NBC ≤ 0.5, respectively; p < 0.0001).
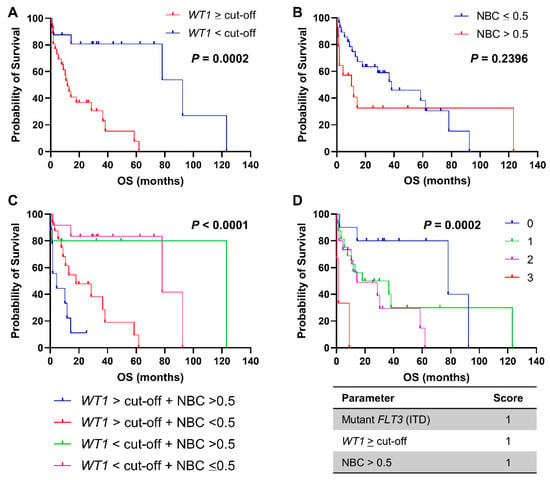
Figure 2.
Clinical outcomes of AML patients. Overall survival (OS) of AML patients is reported based on (A) normalized WT1 expression ≥ cut-off (50 copies in peripheral blood or 250 copies in bone marrow samples), (B) normalized blast count (NBC) > 0.5, or (C) combination of these two features. (D) A prognostic score was calculated based on the presence of mutant FLT3, WT1 ≥ cut-off, and NBC > 0.5, and OS were compared among groups. A p < 0.05 was considered statistically significant.
Next, a prognostic score was developed by assigning a value of 1 if mutant FLT3 ITD was present, WT1 expression levels was ≥ cut-offs, and/or NBC > 0.5 (Figure 2D). Patients were divided in four groups according to this simple combined phenotypic and molecular score ranging from 0 to 3, and OS was compared between risk categories. Patients with a score of 3 displayed the shortest OS compared to other groups (78.1 vs. 27.3 vs. 14.3 vs. 1.3 months, score 0 vs. score 1 vs. score 2 vs. score 3, respectively; p = 0.0002). In multivariate analysis, WT1 expression levels were significantly associated with the score (p = 0.0162), but not with NBC, FLT3 or NPM1 mutational status, cytogenetic abnormalities, sex, age, ELN risk stratification, or outcome.
3.3. Correlation of WT1 Expression with Clinical, Phenotypic, and Molecular Features in MDS Patients
Expression levels of WT1 at diagnosis, during treatment, and follow-up were correlated with CBCs, flow cytometric counts, and percentage of blasts in our MDS patients (Figure 3). Similar to that reported in the AML cohort, no correlations with WBCs were described (r = −0.094; p = 0.3634), while negative correlations between normalized WT1 levels and platelets (r = −0.2044; p = 0.0470) and hemoglobin levels (r = −0.3386; p = 0.0008) were confirmed in MDS patients (Figure 3A). Negative correlations between normalized WT1 expression and granulocytes identified by flow cytometry were also described in MDS subjects (r = −0.3664; p = 0.0506) as documented in AML patients. In addition, WT1 levels correlated with percentage of CD34+ cells (r = 0.8383; p < 0.0001) and NBC (r = 0.3700; p = 0.0482) (Figure 3B). Mean percentage of BM blasts by light microscopy at diagnosis was 6.6 ± 4.7% (range, 1–16%), and mean percentage of blasts by flow cytometry was 5.5 ± 7.8% (range, 0–38%). A positive correlation with normalized WT1 levels was described for flow cytometry blasts (r = 0.7019; p < 0.0001), while no correlations were documented between WT1 levels and percentage of blasts at diagnosis by light microscopy (r = 0.0428; p = 0.8579) (Figure 3C).
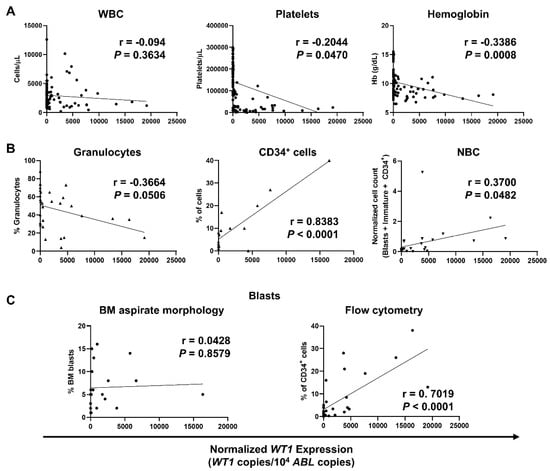
Figure 3.
Correlations of normalized WT1 levels with complete blood counts (CBCs), flow cytometry parameters, and percentage of blasts in MDS patients. WT1 expression was normalized using ABL as housekeeping gene, and levels were reported as WT1 copies/104 ABL copies. (A) Pearson correlation analysis between normalized WT1 expression and CBCs, such as white blood cells (WBC), platelets, and hemoglobin (Hb) levels. (B) Correlations with percentage of granulocytes, CD34+ cells, and normalized blast count (NBC) by flow cytometry. (C) Correlations with bone marrow (BM) blasts identified by light microscopy (left) or by flow cytometry (right).
Higher WT1 expression levels were documented in intermediate-2/high-risk MDS patients (mean ± SD, 3107 ± 4397 copies; range, 8–16,364 copies) compared to low-/intermediate-1 risk subjects (mean ± SD, 398.3 ± 770.6 copies; range, 0.4–2119 copies); however, no statistically significant variations were registered (p = 0.1266) likely because of the small number of patients in our MDS cohort.
3.4. Associations with Chromosomal Abnormalities
Associations between WT1 levels and cytogenetic abnormalities were investigated in both AML and MDS patients (Figure 4). In AML cohort, 39 subjects were evaluable for the presence of chromosomal abnormalities, and 12 of them (31%) had WT1 levels < cut-off at diagnosis, while remaining 27 subjects (69%) had increased levels (Figure 4A). Seven out of 12 AML patients with normal WT1 expression had normal karyotype (58%), and five one or more chromosomal abnormalities (42%). Similarly, 16 out 27 AML subjects with increased WT1 levels (59%) had normal karyotype, and 11 one or more chromosomal abnormalities (41%). In our MDS cohort, 19 subjects were evaluable for cytogenetic abnormalities (Figure 4B). Two out six patients with normal WT1 levels did not show chromosomal abnormalities (33%), and four had one or more abnormalities (67%). Similarly, four out of 13 MDS patients with increased WT1 levels had one chromosomal abnormality (31%), and nine one or more (69%). Therefore, no statistically significant differences were described in both AML and MDS cohorts by Chi-square test (p > 0.9999).
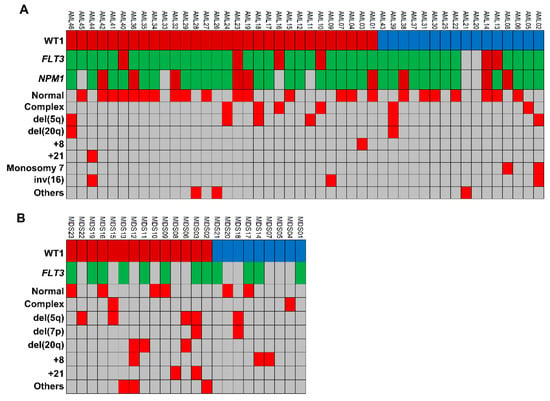
Figure 4.
Chromosomal abnormalities in our acute myeloid leukemia (AML) and myelodisplastic syndrome (MDS) patients. (A) AML and (B) MDS patients (UPN) were screened for the presence of cytogenetic abnormalities and divided based on WT1 expression levels at diagnosis (WT1 peripheral blood cut-off, 50 copies; and WT1 bone marrow cut-off, 250 copies). Normal expression WT1 levels are reported in blue, while increased in dark red. For each patient, cytogenetic alteration detected by karyotype or fluorescence in situ hybridization analysis are reported in red. Screening for FLT3 and NPM1 mutations was also performed and reported in green for wild type genes, or in red for mutant forms. Light grey is used when marker was not tested or not present.
3.5. Prognostic Impact of WT1 Expression in MDS
Whether to investigate prognostic impact of normalized WT1 levels in MDS, patients were divided in two groups based on WT1 expression at diagnosis (Figure 5A). No statistically significant differences in OS were described between patients with increased WT1 levels at diagnosis and those with WT1 levels < cut-offs (25.9 vs. 20.7 months, WT1 ≥ cut-offs vs. WT1 < cut-offs, respectively; p = 0.0.2837; HR, 1.815; 95% CI, 0.6454 to 5.105). Patients were also divided based on NBC > 0.5 and OS was compared between groups (Figure 5B). A slight shorter OS was documented in patients with NBC > 0.5 at diagnosis compared to those with NBC ≤ 0.5 (9 vs. 35.7 months, NBC > 0.5 vs. NBC ≤ 0.5, respectively; p = 0.0613; HR, 0.3827; 95% CI, 0.0964 to 1.518); however, statistical significance was not reached likely because of the small number of patients in each group (NBC ≤ 0.5, n = 17; NBC > 0.5, n = 5). We then divided patients in four groups based on the combination of WT1 expression levels and NBC at diagnosis, and OS was compared among groups (Figure 5C). Patients with increased WT1 levels and NBC had the shortest OS compared with those with elevated WT1 expression and NBC ≤ 0.5 or those subjects with normal WT1 levels and NBC ≤ 0.5 (9 vs. 36.7 vs. 20.7 months, WT1 ≥ cut-off + NBC > 0.5 vs. WT1 ≥ cut-off + NBC ≤ 0.5 vs. WT1 < cut-off + NBC ≤ 0.5, respectively; p = 0.1648). Statistical significance was not reached likely because of the small number of patients in each group (WT1 ≥ cut-off + NBC > 0.5, n = 5; WT1 ≥ cut-off + NBC ≤ 0.5, n = 8; WT1 < cut-off + NBC ≤ 0.5, n = 9). In multivariate analysis, no statistically significant associations were described.
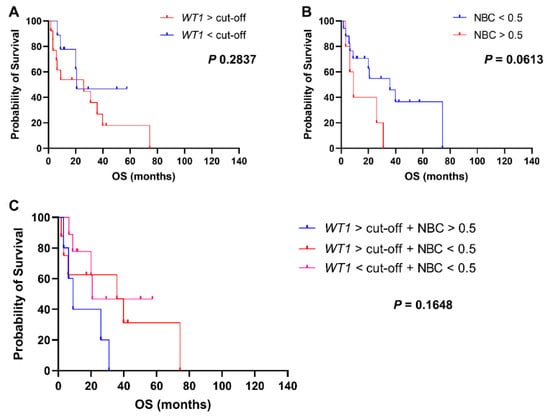
Figure 5.
Clinical outcomes of MDS patients. Overall survival (OS) of MDS patients is reported based on (A) normalized WT1 expression ≥ cut-off (50 copies in peripheral blood or 250 copies in bone marrow samples), (B) normalized blast count (NBC) > 0.5, or (C) combination of these two features.
4. Discussion
WT1 is expressed in normal hemopoiesis at basal levels while is overexpressed in the majority of AML [11]. Indeed, WT1 measurement by PCR has been proposed for monitoring minimal residual diseases in AML patients after allogeneic HSCT because WT1 levels strongly correlate with chimerism and disease relapse [21,22,23,24]. In addition, WT1 expression levels might also be used as prognostic factor in MDS [16,17]. In this retrospective real-world evidence study, we have investigated prognostic value of WT1 expression in AML and MDS, alone and in combination with risk categories, CBCs, flow cytometry counts, and molecular biology features.
In normal hemopoiesis, WT1 is expressed at basal levels in quiescent cells and is correlated with stemness as differentiated and mature cells express WT1 at very low levels [11,25,26]. Conversely, leukemic cells have increased WT1 levels, and monitoring of minimal residual diseases using WT1 quantification is a specific and sensitive biomarker of disease relapse or progression, especially after allogeneic HSCT [11,23,27,28]. In our study, we confirmed that WT1 inversely correlated with normal hemopoiesis as a negative correlation with platelet count and hemoglobin levels was described in both AML and MDS patients. For WBC, we showed that flow cytometry is more sensitive and specific to distinguish normal hemopoiesis from neoplastic counterpart, as WBC counted using an automated hemocytometer did not correlate with WT1 levels. By contrast, percentage of granulocytes identified by flow cytometry using linear parameters and CD45 expression negatively correlated with WT1 levels, while positively associated with CD34+, immature, and CD45dim cells which represent the neoplastic counterpart. BM blast count by light microscopy was also correlated with WT1 levels in AML, while not in MDS. Conversely, blast count by flow cytometry was positively associated with WT1 levels also in MDS patients, confirming flow cytometry as a more sensitive and specific tool for identification of leukemic cells compared to light microscopy that is operator dependent [29,30,31].
WT1 has been largely reported to be overexpressed in all types of leukemias both of myeloid and lymphoid origins, as well as during blast crisis of chronic myeloid leukemia, and increased WT1 levels are associated with poor outcomes because of higher incidence of relapse and resistance to standard chemotherapy [11,14,16,17]. Indeed, a 2-log reduction of WT1 levels after induction and consolidation therapy is related to a better outcome and a reduced relapse rate in AML patients [15]. Moreover, WT1 levels <50 copies/104 ABL copies in PB or <250 copies in BM are associated with complete remission (CR), while WT1 levels above the cut-offs are an early predictor of disease progression [15,20]. However, the exact role of WT1 in leukemogenesis and disease progression is still unclear. In addition, WT1 has been reported to be increased in MDS, especially in those patients with elevated blast counts and higher risk of AML development [11,27]. A limited number of studies proposes WT1 as a prognostic factor in MDS, as peripheral blood WT1-mRNA levels correlate with IPSS-R risk category and outcomes [16,17]. According to published data, we confirmed the prognostic role of WT1 expression in both AML and MDS patients, regardless type of disease and risk category. However, OS did not statistically differ between MDS patients with increased WT1 levels and those with normal levels, likely because of the small number of subjects included in each group.
Diagnosis and prognosis of MDS patients are still challenging and cannot rely on a single clinical, phenotypic, or molecular feature; indeed, prognostic scores are calculated based on cytogenetic abnormalities, BM blasts, and CBCs [8,9,32]. However, we showed that CBCs did not correlate with WT1-mRNA levels in our cohort, while flow cytometry counts were highly associated. Therefore, a better prognostic definition could be achieved by combining clinical, molecular, and flow cytometry features. Previous studies have shown the utility of flow cytometry scoring system for differential diagnosis and prognosis of MDS [29]. Here, we added evidence for inclusion of flow cytometry counts in diagnostic and prognostic definition of AML and MDS patients. Combination of WT1 expression levels, NBC, and FLT3 mutational status allowed a better risk stratification in AML patients. Similarly, risk stratification based only on WT1 levels and NBC might identify a subgroup of MDS subjects with a poorer prognosis. WT1 mutations are frequently found in de novo AML, especially in younger patients and in FLT3-ITD or CEBPA mutated leukemias [11,17,26,33,34]. Recently, mutant WT1 has been also associated with NPM1 as secondary mutations; while when WT1 occurs as a secondary mutation, the most common dominant alterations are in DNMT3A, which also negatively affects prognosis of AML patients, PHF6, FLT3, and CEBPA [26]. However, these comprehensive studies on genomic landscape of WT1 mutant AML do not shade lights on the role of WT1 in leukemogenesis, and it remains unclear if mutations in WT1 and associated genes are drivers or passengers. In our study, we focused on WT1 expression rather than mutational status. In addition, we correlated WT1-mRNA levels with the presence of cytogenetic abnormalities showing that there was no association between WT1 expression levels and cytogenetic alterations. These preliminary findings suggested that WT1 overexpression might not have effects on genomic instability favoring chromosomal alterations.
5. Conclusions
WT1-mRNA levels have been proposed as a diagnostic and prognostic marker of AML and MDS [11,16]. Indeed, WT1 levels can identify MDS with higher risk of AML development, relapsed/refractory AML, and early relapse after allogeneic stem cell transplantation [24]. WT1-mRNA expression is more sensitive and specific as hematological biomarker compared to WT1 mutational status [26]. Moreover, as we are improving our understanding of disease biology, prognostic definition of AML and MDS should consider clinical, phenotypic, and molecular features [8,9]. Here, we proposed a simple score based on WT1 expression levels, NBC by flow cytometry, and FLT3 mutational status for risk stratification of AML patients, while WT1 expression levels and NBC might identify a subgroup of MDS patients with poorer prognosis. However, our findings need further validation in larger cohorts and prospective studies.
Author Contributions
Conceptualization, V.G., and C.S.; Methodology, M.L., M.G., R.V., A.B., R.M., B.I., and A.P.; Clinical data, B.S., R.G., I.F., R.P., and F.D.; Formal analysis, V.G.; Data curation, N.M., and R.P.; Writing—original draft preparation, V.G.; Writing—review and editing V.G. and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Intramural Program of the Department of Medicine, Surgery and Dentistry, University of Salerno, Italy.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon request by the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Heuser, M.; Ofran, Y.; Boissel, N.; Brunet Mauri, S.; Craddock, C.; Janssen, J.; Wierzbowska, A.; Buske, C. ESMO Guidelines Committee. Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 697–712. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Estey, E.H. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am. J. Hematol. 2018, 93, 1267–1291. [Google Scholar] [CrossRef]
- Estey, E.; Thall, P.; Beran, M.; Kantarjian, H.; Pierce, S.; Keating, M. Effect of diagnosis (refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, or acute myeloid leukemia [AML]) on outcome of AML-type chemotherapy. Blood 1997, 90, 2969–2977. [Google Scholar] [CrossRef]
- Gangat, N.; Patnaik, M.M.; Begna, K.; Al Kali, A.; Litzow, M.R.; Ketterling, R.P.; Hanson, C.A.; Pardanani, A.D.; Tefferi, A. Survival trends in primary myelodysplastic syndromes: A comparative analysis of 1000 patients by year of diagnosis and treatment. Blood Cancer J. 2016, 6, e414. [Google Scholar] [CrossRef]
- Groarke, E.M.; Young, N.S. Aging and Hematopoiesis. Clin. Geriatr. Med. 2019, 35, 285–293. [Google Scholar] [CrossRef]
- Walter, M.J. What came first: MDS or AML? Blood 2015, 125, 1357–1358. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef]
- Montalban-Bravo, G.; Garcia-Manero, G. Myelodysplastic syndromes: 2018 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2018, 93, 129–147. [Google Scholar] [CrossRef]
- Rampal, R.; Figueroa, M.E. Wilms tumor 1 mutations in the pathogenesis of acute myeloid leukemia. Haematologica 2016, 101, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Oji, Y.; Miyoshi, S.; Maeda, H.; Hayashi, S.; Tamaki, H.; Nakatsuka, S.; Yao, M.; Takahashi, E.; Nakano, Y.; Hirabayashi, H.; et al. Overexpression of the Wilms’ tumor gene WT1 in de novo lung cancers. Int. J. Cancer. 2002, 100, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Oji, Y.; Nakamori, S.; Fujikawa, M.; Nakatsuka, S.; Yokota, A.; Tatsumi, N.; Abeno, S.; Ikeba, A.; Takashima, S.; Tsujie, M.; et al. Overexpression of the Wilms’ tumor gene WT1 in pancreatic ductal adenocarcinoma. Cancer Sci. 2004, 95, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, L.; Miething, C.; Maurer, U.; Brieger, J.; Karakas, T.; Weidmann, E.; Hoelzer, D. High levels of Wilms’ tumor gene (wt1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome. Blood 1997, 90, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Cilloni, D.; Renneville, A.; Hermitte, F.; Hills, R.K.; Daly, S.; Jovanovic, J.V.; Gottardi, E.; Fava, M.; Schnittger, S.; Weiss, T.; et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: A European LeukemiaNet study. J. Clin. Oncol. 2009, 27, 5195–5201. [Google Scholar] [CrossRef]
- Rautenberg, C.; Germing, U.; Pechtel, S.; Lamers, M.; Fischermanns, C.; Jäger, P.; Geyh, S.; Haas, R.; Kobbe, G.; Schroeder, T. Prognostic impact of peripheral blood WT1-mRNA expression in patients with MDS. Blood Cancer J. 2019, 9, 86. [Google Scholar] [CrossRef]
- Hou, H.A.; Huang, T.C.; Lin, L.I.; Liu, C.Y.; Chen, C.Y.; Chou, W.C.; Tang, J.L.; Tseng, M.H.; Huang, C.F.; Chiang, Y.C.; et al. WT1 mutation in 470 adult patients with acute myeloid leukemia: Stability during disease evolution and implication of its incorporation into a survival scoring system. Blood 2010, 115, 5222–5231. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, C.D.; Estey, E.; Pleyer, L.; Schuh, A.C.; Stein, E.M.; Tallman, M.S.; Wei, A. Time to repeal and replace response criteria for acute myeloid leukemia? Blood Rev. 2018, 32, 416–425. [Google Scholar] [CrossRef]
- Cilloni, D.; Saglio, G. WT1 as a universal marker for minimal residual disease detection and quantification in myeloid leukemias and in myelodysplastic syndrome. Acta Haematol. 2004, 112, 79–84. [Google Scholar] [CrossRef]
- Miyagi, T.; Ahuja, H.; Kubota, T.; Kubonishi, I.; Koeffler, H.P.; Miyoshi, I. Expression of the candidate Wilm’s tumor gene, WT1, in human leukemia cells. Leukemia. 1993, 7, 970–977. [Google Scholar]
- Miwa, H.; Beran, M.; Saunders, G.F. Expression of the Wilms’ tumor gene (WT1) in human leukemias. Leukemia 1992, 6, 405–409. [Google Scholar] [PubMed]
- Barragán, E.; Cervera, J.; Bolufer, P.; Ballester, S.; Martín, G.; Fernández, P.; Collado, R.; Sayas, M.J.; Sanz, M.A. Prognostic implications of Wilms’ tumor gene (WT1) expression in patients with de novo acute myeloid leukemia. Haematologica 2004, 89, 926–933. [Google Scholar] [PubMed]
- Kwon, M.; Martínez-Laperche, C.; Infante, M.; Carretero, F.; Balsalobre, P.; Serrano, D.; Gayoso, J.; Pérez-Corral, A.; Anguita, J.; Díez-Martín, J.L.; et al. Evaluation of minimal residual disease by real-time quantitative PCR of Wilms’ tumor 1 expression in patients with acute myelogenous leukemia after allogeneic stem cell transplantation: Correlation with flow cytometry and chimerism. Biol. Blood Marrow Transplant. 2012, 18, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Brieger, J.; Weidmann, E.; Fenchel, K.; Mitrou, P.S.; Hoelzer, D.; Bergmann, L. The expression of the Wilms’ tumor gene in acute myelocytic leukemias as a possible marker for leukemic blast cells. Leukemia 1994, 8, 2138–2143. [Google Scholar]
- Awada, H.; Durmaz, A.; Gurnari, C.; Kishtagari, A.; Zawit, M.; Pagliuca, S.; Visconte, V. Friend or foe? The case of Wilms’ Tumor 1 (WT1) mutations in acute myeloid leukemia. Blood Cells Mol. Dis. 2021, 88, 102549. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, H.; Ogawa, H.; Ohyashiki, K.; Ohyashiki, J.H.; Iwama, H.; Inoue, K.; Soma, T.; Oka, Y.; Tatekawa, T.; Oji, Y.; et al. The Wilms’ tumor gene WT1 is a good marker for diagnosis of disease progression of myelodysplastic syndromes. Leukemia 1999, 13, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Malagola, M.; Skert, C.; Ruggeri, G.; Turra, A.; Ribolla, R.; Cancelli, V.; Cattina, F.; Alghisi, E.; Bernardi, S.; Perucca, S.; et al. Peripheral blood WT1 expression predicts relapse in AML patients undergoing allogeneic stem cell transplantation. Biomed Res. Int. 2014, 2014, 123079. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, H.; Xiong, S.; Zhang, X.; Zhang, C.; Yang, D.; Zhang, J.; Zhai, Z. Simplified flow cytometry scoring for diagnosis and prognosis of myelodysplastic symptom. Am. J. Transl. Res. 2020, 12, 7449–7458. [Google Scholar]
- Hodes, A.; Calvo, K.R.; Dulau, A.; Maric, I.; Sun, J.; Braylan, R. The challenging task of enumerating blasts in the bone marrow. Semin. Hematol. 2019, 56, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Zini, G. How I investigate difficult cells at the optical microscope. Int. J. Lab. Hematol. 2020. [Google Scholar] [CrossRef]
- DeZern, A.E.; Sekeres, M.A. The challenging world of cytopenias: Distinguishing myelodysplastic syndromes from other disorders of marrow failure. Oncologist 2014, 19, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Renneville, A.; Boissel, N.; Zurawski, V.; Llopis, L.; Biggio, V.; Nibourel, O.; Philippe, N.; Thomas, X.; Dombret, H.; Preudhomme, C. Wilms tumor 1 gene mutations are associated with a higher risk of recurrence in young adults with acute myeloid leukemia: A study from the Acute Leukemia French Association. Cancer 2009, 115, 3719–3727. [Google Scholar] [CrossRef] [PubMed]
- Gaidzik, V.I.; Schlenk, R.F.; Moschny, S.; Becker, A.; Bullinger, L.; Corbacioglu, A.; Krauter, J.; Schlegelberger, B.; Ganser, A.; Döhner, H.; et al. German-Austrian AML Study Group. Prognostic impact of WT1 mutations in cytogenetically normal acute myeloid leukemia: A study of the German-Austrian AML Study Group. Blood 2009, 113, 4505–4511. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).