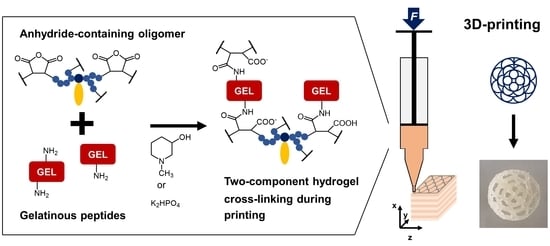
Extrusion-Printing of Multi-Channeled Two-Component Hydrogel Constructs from Gelatinous Peptides and Anhydride-Containing Oligomers
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of the Anhydride-Containing Oligomer
2.2. Manual Fabrication of Two-Component Hydrogels
2.3. 3D-Printing of Two-Component Hydrogels
2.4. Post-Fabrication Processing and Characterization of Two-Component Hydrogels
2.4.1. Washing and Lyophilization of Dried Hydrogels
2.4.2. Hydrogel Weight, Water Content and Leachables
2.4.3. Rheological Characterization of Fabricated Hydrogels
2.4.4. Stereomicroscopic Visualization
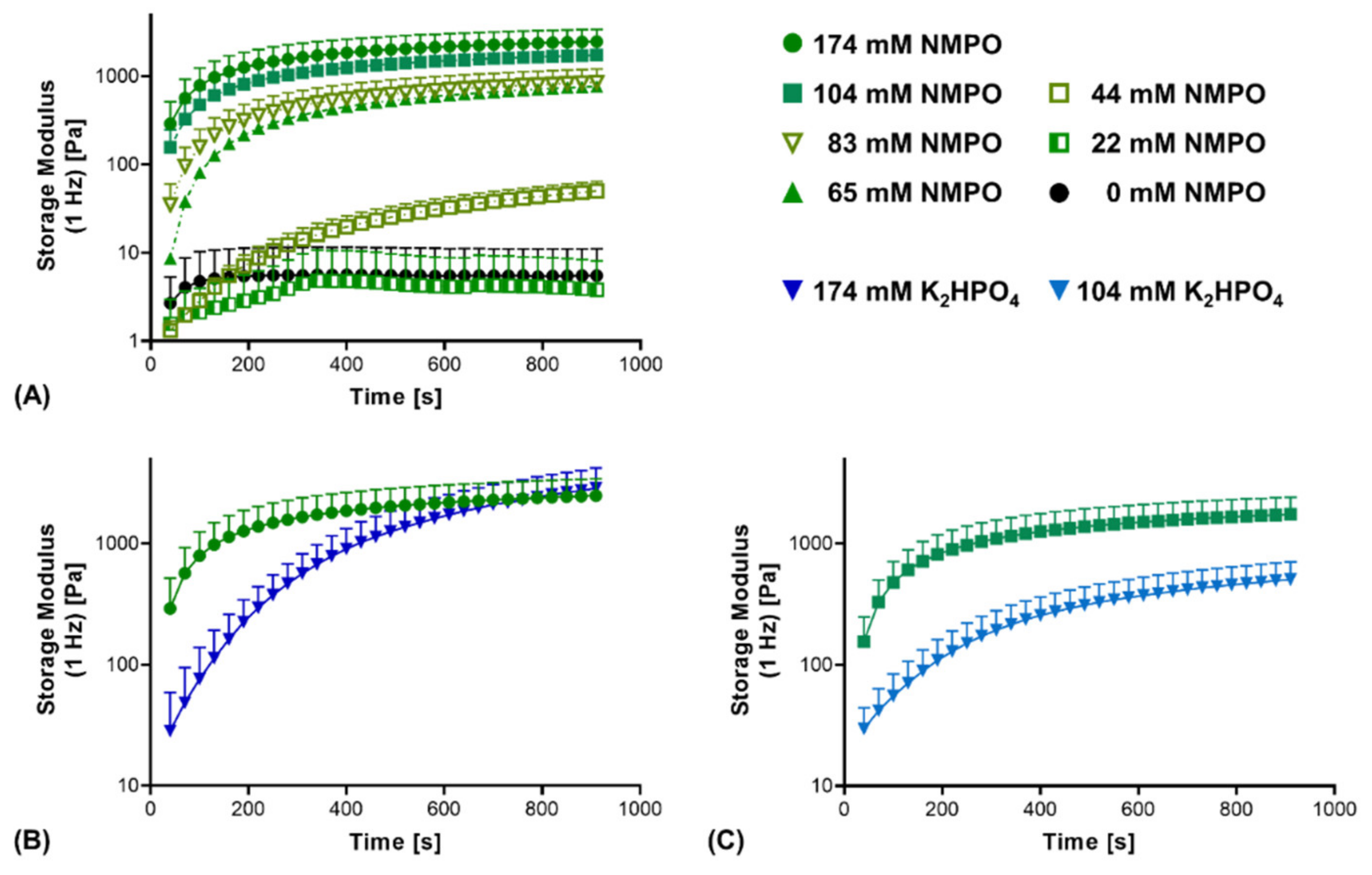
2.5. Kinetics of the Cross-Linking Reaction
2.6. Pre-Derivatization of Anhydride-Containing Oligomer
2.7. Degradation Analysis
2.8. µXCT Analysis of Degradation Samples
2.9. Fabrication under Aseptic Conditions and Analysis for Microbiological Contamination
2.10. Direct Contact Cell Culture of hASC on Two-Component Hydrogels
2.11. Indirect Cytocompatibility Testing
2.12. Statistical Analysis
3. Results and Discussion
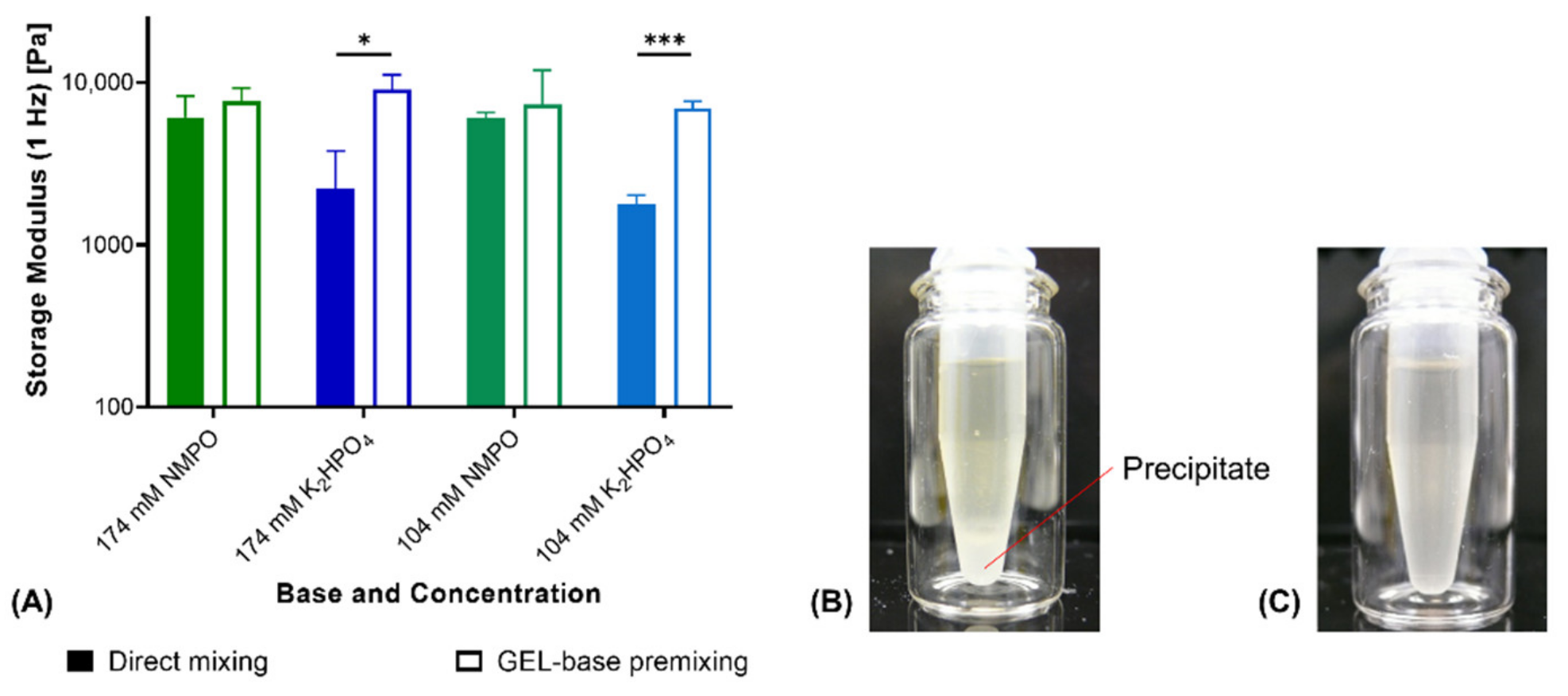
3.1. Two-Component Hydrogels with Inorganic Bases
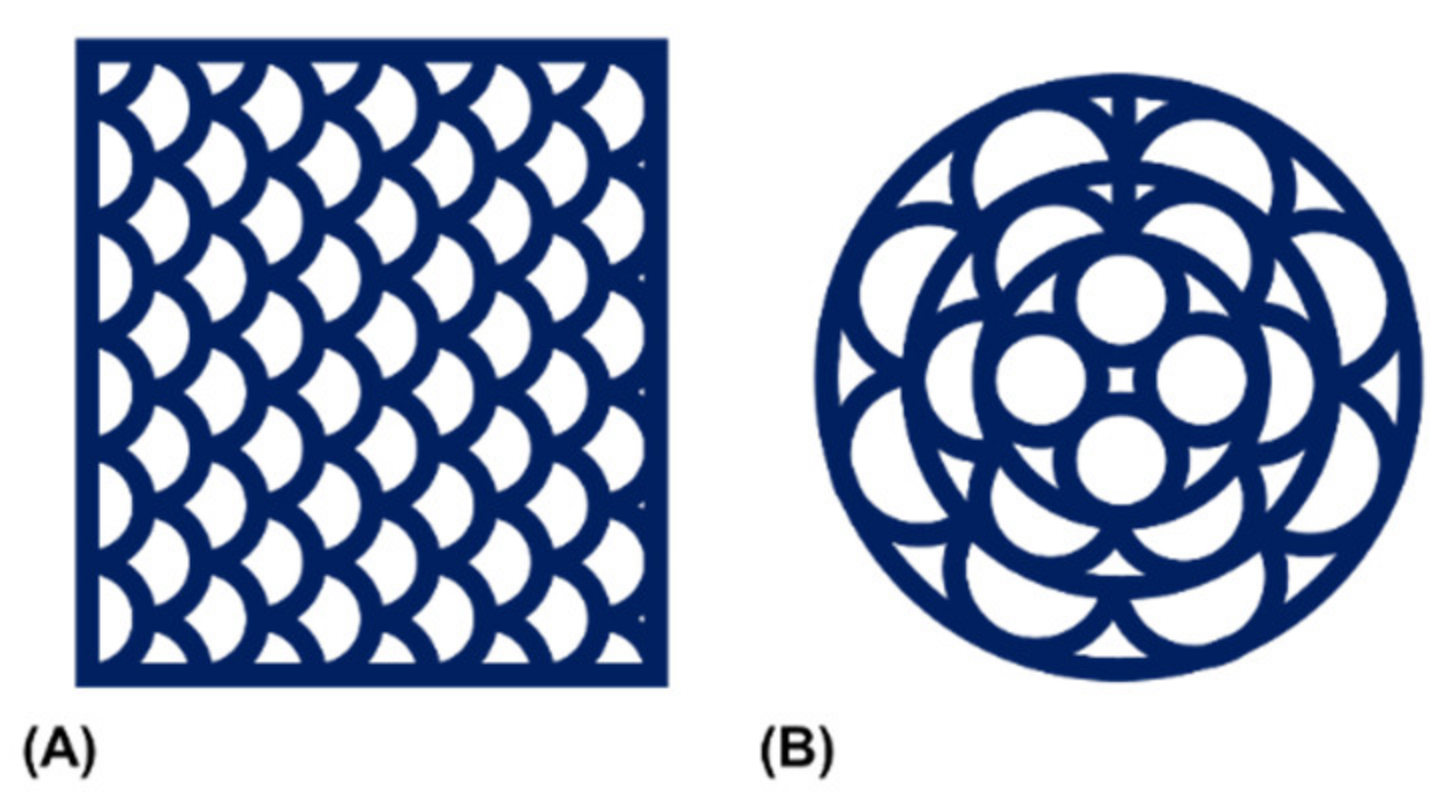
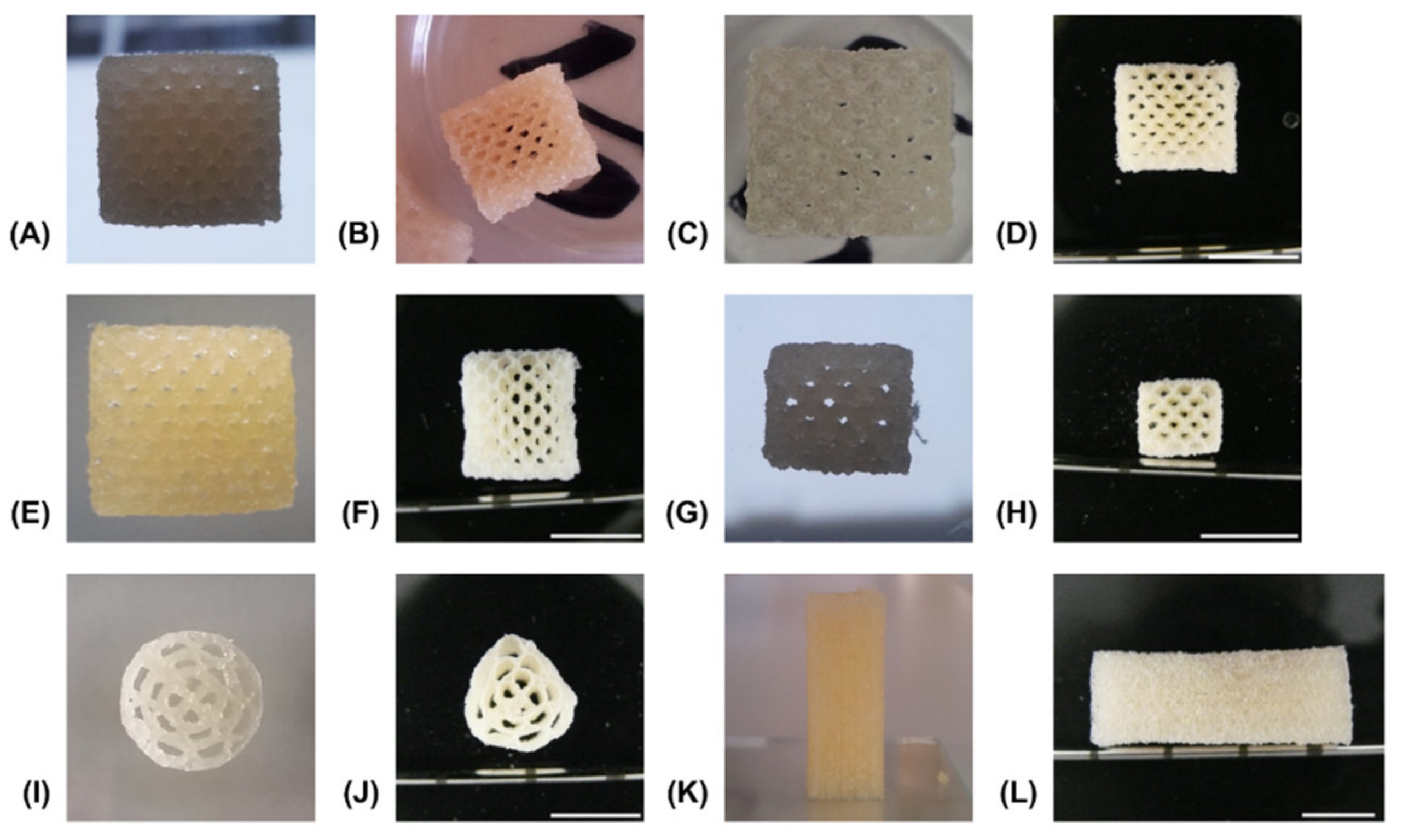
3.2. 3D-Printing of Two-Component Hydrogel
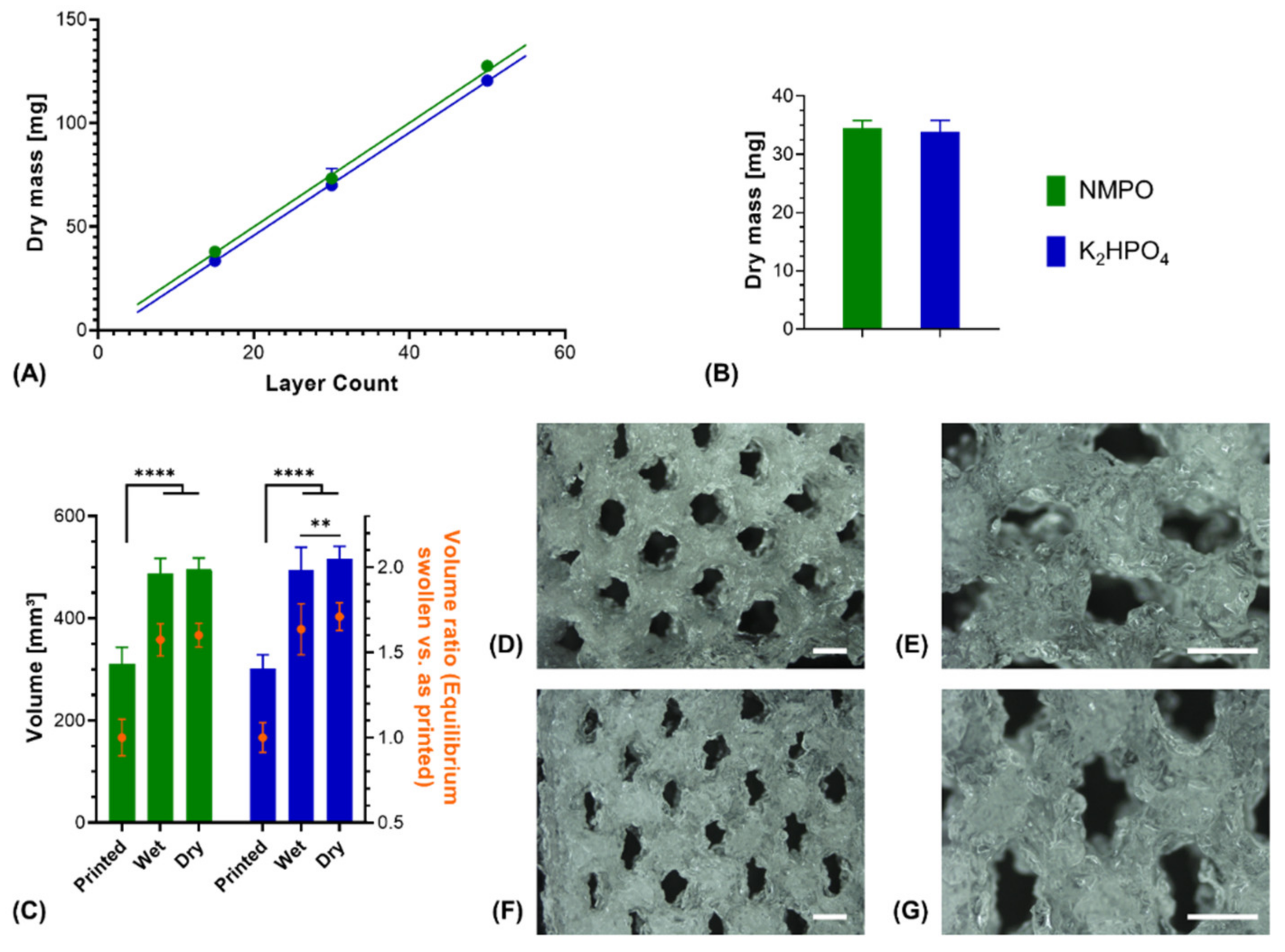
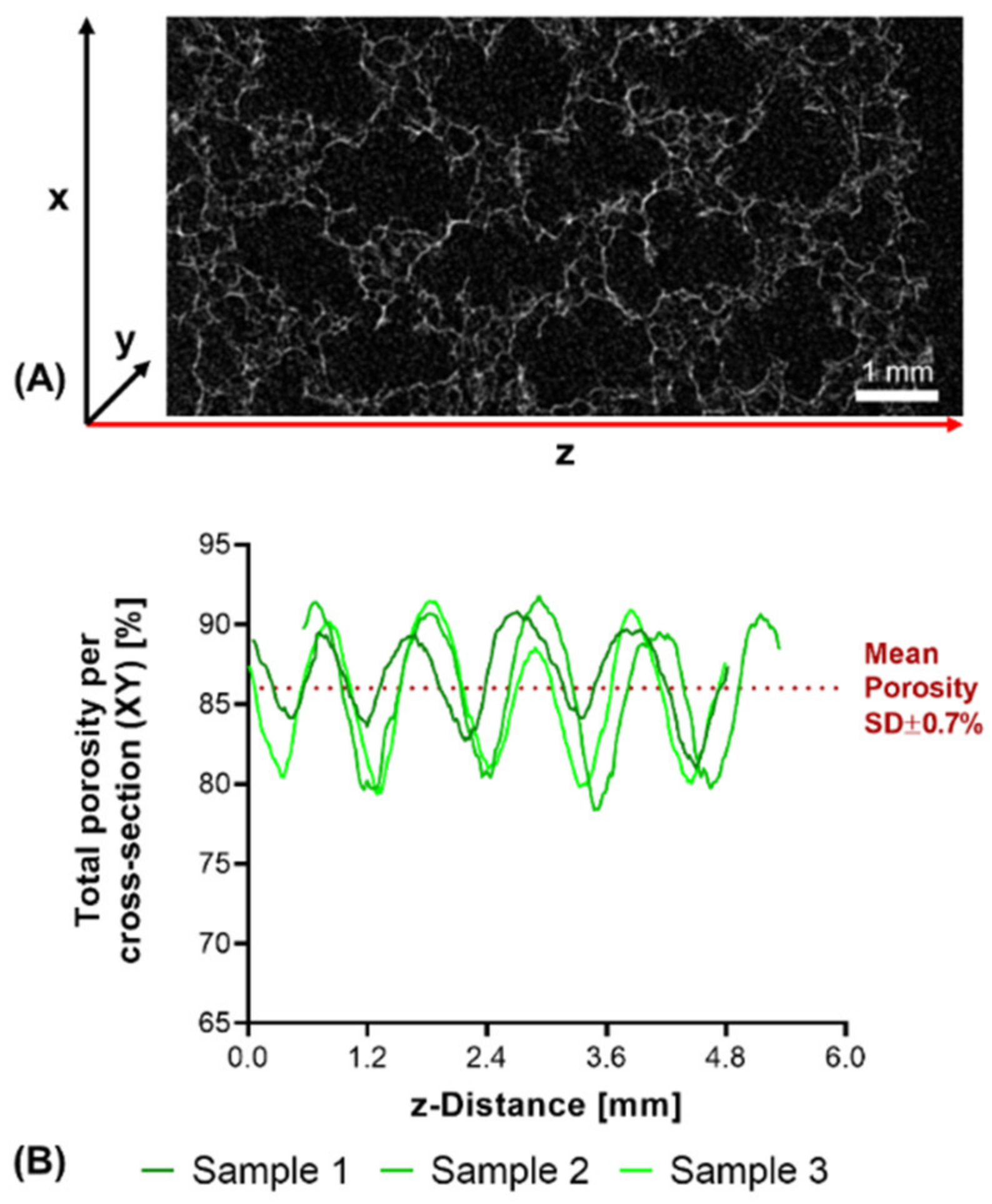
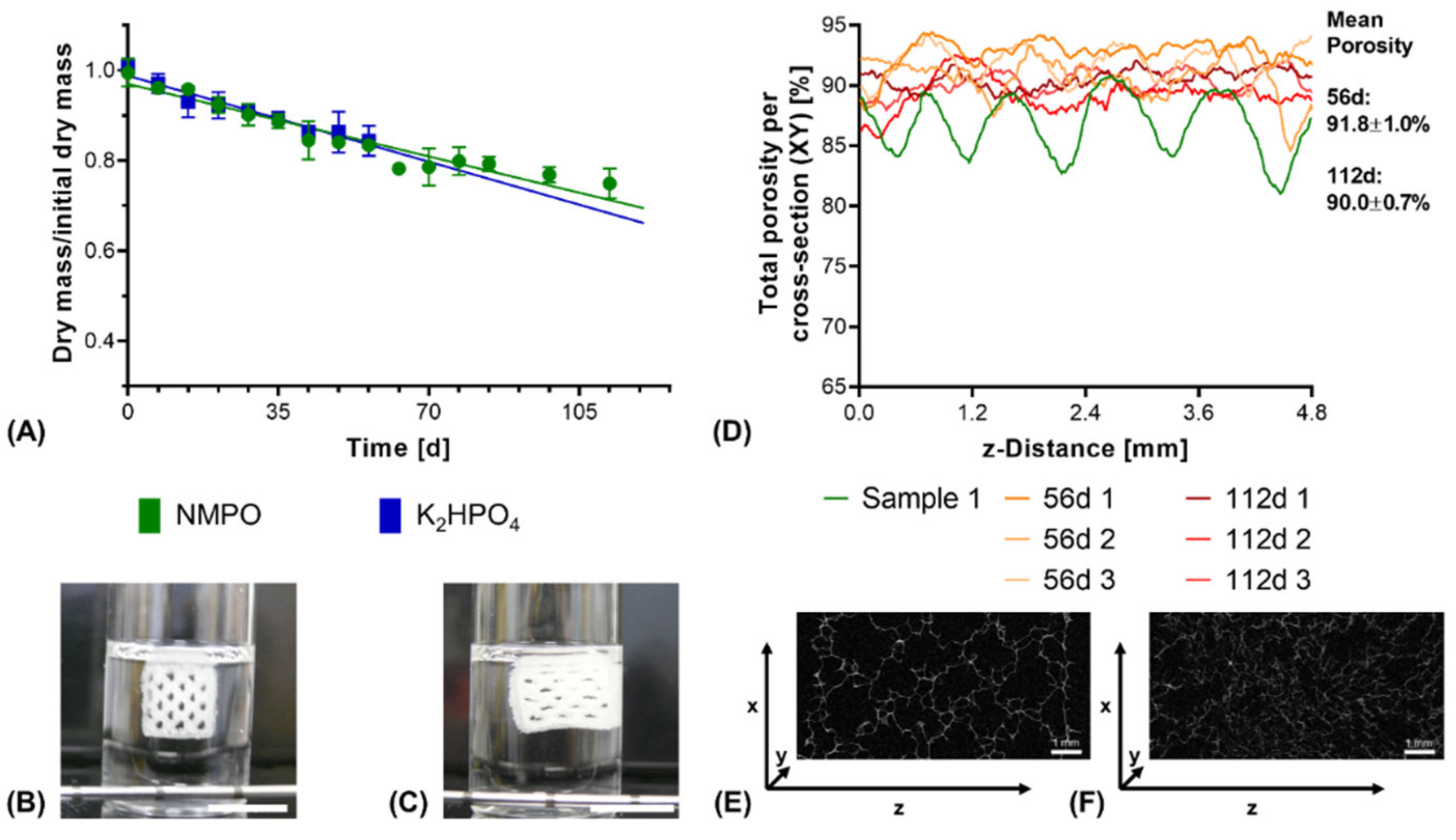
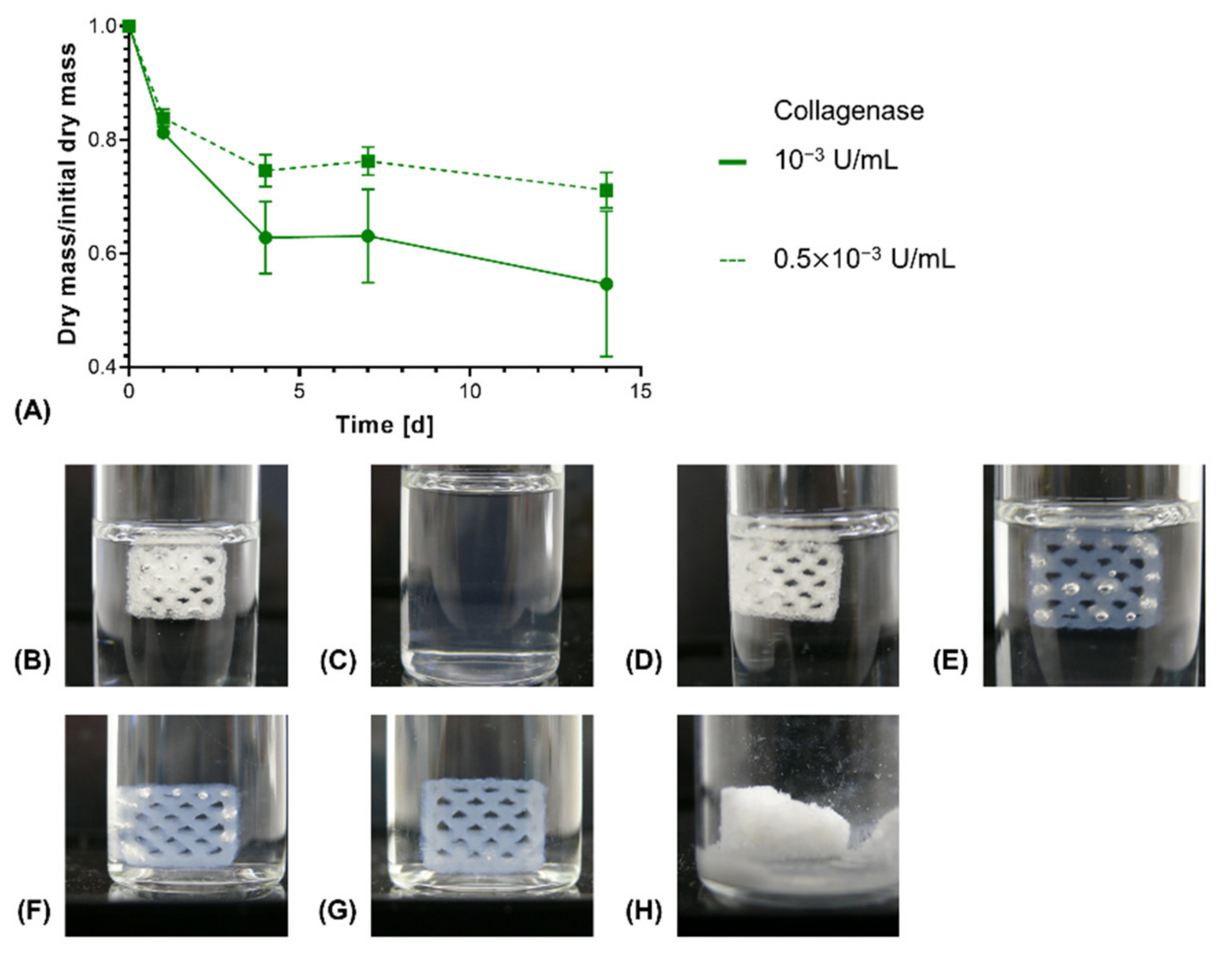
3.3. Degradation Study with Printed Constructs
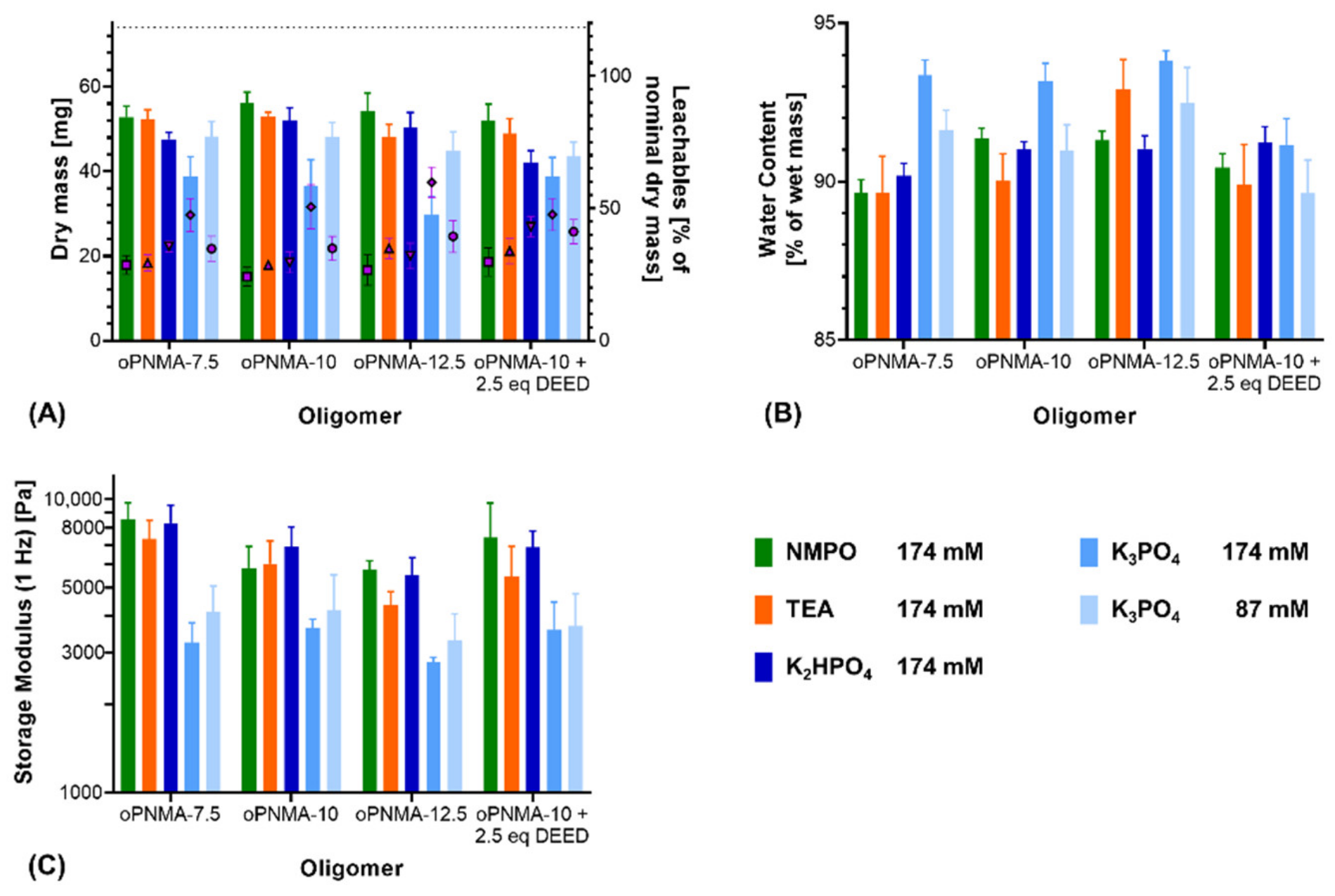
3.4. Varying the Cross-Linking Oligomers
3.5. Two-Component Hydrogel Printing under Aseptic Conditions
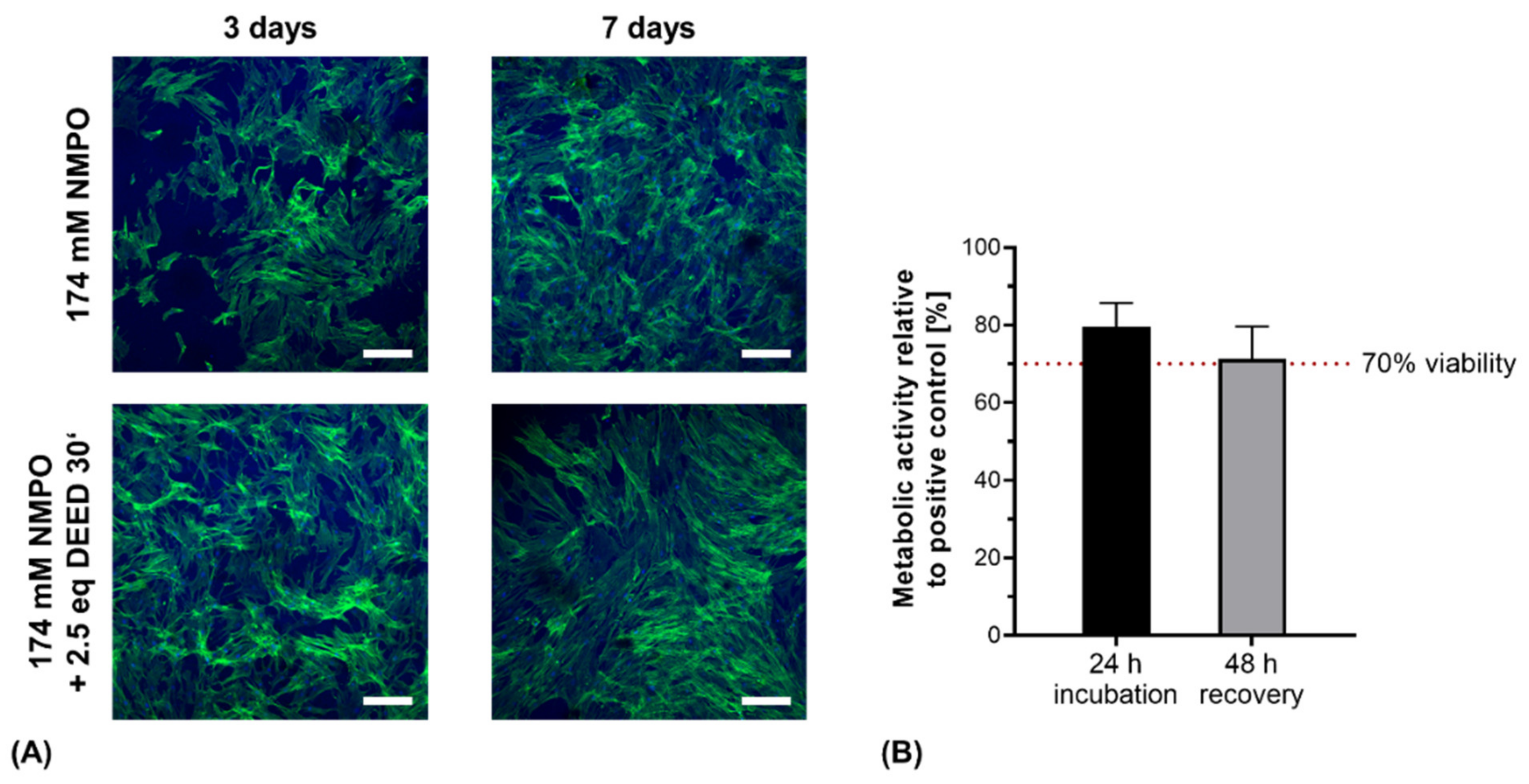
3.6. In Vitro Cell Direct Contact and Indirect Cytocompatibility Testing on Two-Component Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Houshyar, S.; Bhattacharyya, A.; Shanks, R. Peripheral Nerve Conduit: Materials and Structures. ACS Chem. Neurosci. 2019, 10, 3349–3365. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Tonda-Turo, C. Trends in the design of nerve guidance channels in peripheral nerve tissue engineering. Prog. Neurobiol. 2015, 131, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.R.; Oliveira, J.M.; Reis, R.L. Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit. Front. Bioeng. Biotechnol. 2019, 7, 337. [Google Scholar] [CrossRef]
- Yao, L.; de Ruiter, G.C.W.; Wang, H.; Knight, A.M.; Spinner, R.J.; Yaszemski, M.J.; Windebank, A.J.; Pandit, A. Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials 2010, 31, 5789–5797. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, K.M.; Hix, J.; Shapiro, E.M.; Sakamoto, J. The mechanics of scaling-up multichannel scaffold technology for clinical nerve repair. J. Mech. Behav. Biomed. Mater. 2019, 91, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, T.; Sundback, C.; Hunter, D.; Cheney, M.; Vacanti, J.P. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000, 6, 119–127. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, G.C.; Onyeneho, I.A.; Liang, E.T.; Moore, M.J.; Knight, A.M.; Malessy, M.J.A.; Spinner, R.J.; Lu, L.; Currier, B.L.; Yaszemski, M.J.; et al. Methods for in vitro characterization of multichannel nerve tubes. J. Biomed. Mater. Res. A 2008, 84, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Harding, A.J.; Albadawi, E.; Boissonade, F.M.; Haycock, J.W.; Claeyssens, F. Additive manufactured biodegradable poly(glycerol sebacate methacrylate) nerve guidance conduits. Acta Biomater. 2018, 78, 48–63. [Google Scholar] [CrossRef]
- Kroehne, V.; Heschel, I.; Schügner, F.; Lasrich, D.; Bartsch, J.W.; Jockusch, H. Use of a novel collagen matrix with oriented pore structure for muscle cell differentiation in cell culture and in grafts. J. Cell. Mol. Med. 2008, 12, 1640–1648. [Google Scholar] [CrossRef]
- Van Neerven, S.G.A.; Haastert-Talini, K.; Boecker, A.; Schriever, T.; Dabhi, C.; Claeys, K.; Deumens, R.; Brook, G.A.; Weis, J.; Pallua, N.; et al. Two-component collagen nerve guides support axonal regeneration in the rat peripheral nerve injury model. J. Tissue Eng. Regen. Med. 2017, 11, 3349–3361. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Truby, R.L.; Lewis, J.A. Printing soft matter in three dimensions. Nature 2016, 540, 371–378. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R. Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Seidel, J.; Ahlfeld, T.; Adolph, M.; Kümmritz, S.; Steingroewer, J.; Krujatz, F.; Bley, T.; Gelinsky, M.; Lode, A. Green bioprinting: Extrusion-based fabrication of plant cell-laden biopolymer hydrogel scaffolds. Biofabrication 2017, 9, 45011. [Google Scholar] [CrossRef]
- Tetsuka, H.; Shin, S.R. Materials and technical innovations in 3D printing in biomedical applications. J. Mater. Chem. B 2020, 8, 2930–2950. [Google Scholar] [CrossRef]
- Trachtenberg, J.E.; Placone, J.K.; Smith, B.T.; Piard, C.M.; Santoro, M.; Scott, D.W.; Fisher, J.P.; Mikos, A.G. Extrusion-Based 3D Printing of Poly(propylene fumarate) in a Full-Factorial Design. ACS Biomater. Sci. Eng. 2016, 2, 1771–1780. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Shapira, A.; Noor, N.; Asulin, M.; Dvir, T. Stabilization strategies in extrusion-based 3D bioprinting for tissue engineering. Appl. Phys. Rev. 2018, 5, 41112. [Google Scholar] [CrossRef]
- Chen, S.; Jang, T.-S.; Pan, H.M.; Jung, H.-D.; Sia, M.W.; Xie, S.; Hang, Y.; Chong, S.K.M.; Wang, D.; Song, J. 3D Freeform Printing of Nanocomposite Hydrogels through in situ Precipitation in Reactive Viscous Fluid. Int. J. Bioprint. 2020, 6, 258. [Google Scholar] [CrossRef]
- McCormack, A.; Highley, C.B.; Leslie, N.R.; Melchels, F.P.W. 3D Printing in Suspension Baths: Keeping the Promises of Bioprinting Afloat. Trends Biotechnol. 2020. [Google Scholar] [CrossRef]
- Cai, F.-F.; Heid, S.; Boccaccini, A.R. Potential of laponite incorporated oxidized alginate-gelatin (ADA-GEL) composite hydrogels for extrusion-based 3D printing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Collagen- vs. Gelatine-Based Biomaterials and Their Biocompatibility: Review and Perspectives. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; IntechOpen: London, UK, 2011; ISBN 978-953-307-661-4. [Google Scholar]
- Campiglio, C.E.; Contessi Negrini, N.; Farè, S.; Draghi, L. Cross-Linking Strategies for Electrospun Gelatin Scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef]
- Madhusudanan, P.; Raju, G.; Shankarappa, S. Hydrogel systems and their role in neural tissue engineering. J. R. Soc. Interface 2020, 17. [Google Scholar] [CrossRef]
- Echave, M.C.; Saenz del Burgo, L.; Pedraz, J.L.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef]
- Loth, T.; Hötzel, R.; Kascholke, C.; Anderegg, U.; Schulz-Siegmund, M.; Hacker, M.C. Gelatin-Based Biomaterial Engineering with Anhydride-Containing Oligomeric Cross-Linkers. Biomacromolecules 2014, 15, 2104–2118. [Google Scholar] [CrossRef]
- Chang, J.-Y.; Lin, J.-H.; Yao, C.-H.; Chen, J.-H.; Lai, T.-Y.; Chen, Y.-S. In vivo evaluation of a biodegradable EDC/NHS-cross-linked gelatin peripheral nerve guide conduit material. Macromol. Biosci. 2007, 7, 500–507. [Google Scholar] [CrossRef]
- Itoh, S.; Takakuda, K.; Kawabata, S.; Aso, Y.; Kasai, K.; Itoh, H.; Shinomiya, K. Evaluation of cross-linking procedures of collagen tubes used in peripheral nerve repair. Biomaterials 2002, 23, 4475–4481. [Google Scholar] [CrossRef]
- Lu, M.-C.; Hsiang, S.-W.; Lai, T.-Y.; Yao, C.-H.; Lin, L.-Y.; Chen, Y.-S. Influence of cross-linking degree of a biodegradable genipin-cross-linked gelatin guide on peripheral nerve regeneration. J. Biomater. Sci. Polym. Ed. 2007, 18, 843–863. [Google Scholar] [CrossRef] [PubMed]
- Loth, T.; Hennig, R.; Kascholke, C.; Hötzel, R.; Hacker, M.C. Reactive and stimuli-responsive maleic anhydride containing macromers—multi-functional cross-linkers and building blocks for hydrogel fabrication. React. Funct. Polym. 2013, 73, 1480–1492. [Google Scholar] [CrossRef]
- Kascholke, C.; Loth, T.; Kohn-Polster, C.; Moller, S.; Bellstedt, P.; Schulz-Siegmund, M.; Schnabelrauch, M.; Hacker, M.C. Dual-Functional Hydrazide-Reactive and Anhydride-Containing Oligomeric Hydrogel Building Blocks. Biomacromolecules 2017, 18, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Kohn, C.; Klemens, J.M.; Kascholke, C.; Murthy, N.S.; Kohn, J.; Brandenburger, M.; Hacker, M.C. Dual-component collagenous peptide/reactive oligomer hydrogels as potential nerve guidance materials—from characterization to functionalization. Biomater. Sci. 2016, 4, 1605–1621. [Google Scholar] [CrossRef]
- Kohn-Polster, C.; Bhatnagar, D.; Woloszyn, D.J.; Richtmyer, M.; Starke, A.; Springwald, A.H.; Franz, S.; Schulz-Siegmund, M.; Kaplan, H.M.; Kohn, J.; et al. Dual-Component Gelatinous Peptide/Reactive Oligomer Formulations as Conduit Material and Luminal Filler for Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2017, 18, 1104. [Google Scholar] [CrossRef]
- Koenig, A. Analysis of air voids in cementitious materials using micro X-ray computed tomography (µXCT). Constr. Build. Mater. 2020, 244, 118313. [Google Scholar] [CrossRef]
- Hofmeister, F. Zur Lehre von der Wirkung der Salze. Archiv für Experimentelle Pathologie und Pharmakologie. Archivf. Exp. Pathol. Pharmakol. 1888, 24, 247–260. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Metters, A.T.; Anseth, K.S.; Bowman, C.N. Fundamental studies of a novel, biodegradable PEG-b-PLA hydrogel. Polymer 2000, 41, 3993–4004. [Google Scholar] [CrossRef]
- Dhote, V.; Skaalure, S.; Akalp, U.; Roberts, J.; Bryant, S.J.; Vernerey, F.J. On the role of hydrogel structure and degradation in controlling the transport of cell-secreted matrix molecules for engineered cartilage. J. Mech. Behav. Biomed. Mater. 2013, 19, 61–74. [Google Scholar] [CrossRef]
- Nawaz, H.A.; Schröck, K.; Schmid, M.; Krieghoff, J.; Maqsood, I.; Kascholke, C.; Kohn-Polster, C.; Schulz-Siegmund, M.; Hacker, M.C. Injectable oligomer-cross-linked gelatine hydrogels via anhydride–amine-conjugation. J. Mater. Chem. B 2021. [Google Scholar] [CrossRef]
- Blaine, J.; Chonchol, M.; Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef]
- Thier, S.O. Potassium physiology. Am. J. Med. 1986, 80, 3–7. [Google Scholar] [CrossRef]
- Huber, T.; Najaf Zadeh, H.; Feast, S.; Roughan, T.; Fee, C. 3D Printing of Gelled and Cross-Linked Cellulose Solutions, an Exploration of Printing Parameters and Gel Behaviour. Bioengineering 2020, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Savoji, H.; Davenport Huyer, L.; Mohammadi, M.H.; Lun Lai, B.F.; Rafatian, N.; Bannerman, D.; Shoaib, M.; Bobicki, E.R.; Ramachandran, A.; Radisic, M. 3D Printing of Vascular Tubes Using Bioelastomer Prepolymers by Freeform Reversible Embedding. ACS Biomater. Sci. Eng. 2020, 6, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.J.; Lackington, W.A.; Hibbitts, A.J.; Matheson, A.; Alekseeva, T.; Stejskalova, A.; Roche, P.; O’Brien, F.J. A Physicochemically Optimized and Neuroconductive Biphasic Nerve Guidance Conduit for Peripheral Nerve Repair. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes-different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Qin, H.; Wang, J.; Wang, T.; Gao, X.; Wan, Q.; Pei, X. Preparation and Characterization of Chitosan/β-Glycerophosphate Thermal-Sensitive Hydrogel Reinforced by Graphene Oxide. Front. Chem. 2018, 6, 565. [Google Scholar] [CrossRef]
- Siemers, F. Nervenröhrchen in der Nervenchirurgie. Trauma Berufskrankh 2016, 18, 260–263. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Gao, W. Fabrication and characterization of 3D microtubular collagen scaffolds for peripheral nerve repair. J. Biomater. Appl. 2018, 33, 541–552. [Google Scholar] [CrossRef]
- Bond, M.D.; van Wart, H.E. Characterization of the individual collagenases from Clostridium histolyticum. Biochemistry 1984, 23, 3085–3091. [Google Scholar] [CrossRef]
- Van Doren, S.R. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 2015, 44–46, 224–231. [Google Scholar] [CrossRef]
- Chen, Z.; Du, T.; Tang, X.; Liu, C.; Li, R.; Xu, C.; Tian, F.; Du, Z.; Wu, J. Comparison of the properties of collagen-chitosan scaffolds after γ-ray irradiation and carbodiimide cross-linking. J. Biomater. Sci. Polym. Ed. 2016, 27, 937–953. [Google Scholar] [CrossRef]
- Helling, A.L.; Tsekoura, E.K.; Biggs, M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. In Vitro Enzymatic Degradation of Tissue Grafts and Collagen Biomaterials by Matrix Metalloproteinases: Improving the Collagenase Assay. ACS Biomater. Sci. Eng. 2017, 3, 1922–1932. [Google Scholar] [CrossRef]
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef]
- Sevim, K.; Pan, J. A model for hydrolytic degradation and erosion of biodegradable polymers. Acta Biomater. 2018, 66, 192–199. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, Q.; Shi, M.; Zhang, Y.; Tao, W.; Li, M. Effect of Pore Size on the Biodegradation Rate of Silk Fibroin Scaffolds. Adv. Mater. Sci. Eng. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Rawn, J.D. Amines and Amides. In Principles of Organic Chemistry; Ouellette, R.J., Ed.; Elsevier: Amsterdam, Switzerland, 2015; pp. 315–342. ISBN 9780128024447. [Google Scholar]
- Jansen, J.; Ghaffar, A.; van der Horst, T.N.S.; Mihov, G.; van der Wal, S.; Feijen, J.; Grijpma, D.W. Controlling the kinetic chain length of the crosslinks in photo-polymerized biodegradable networks. J. Mater. Sci. Mater. Med. 2013, 24, 877–888. [Google Scholar] [CrossRef]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef]
- Duffy, P.; McMahon, S.; Wang, X.; Keaveney, S.; O’Cearbhaill, E.D.; Quintana, I.; Rodríguez, F.J.; Wang, W. Synthetic bioresorbable poly-α-hydroxyesters as peripheral nerve guidance conduits; a review of material properties, design strategies and their efficacy to date. Biomater. Sci. 2019, 7, 4912–4943. [Google Scholar] [CrossRef]
- Bakarich, S.E.; Gorkin, R.; Gately, R.; Naficy, S.; Panhuis, M.I.H.; Spinks, G.M. 3D printing of tough hydrogel composites with spatially varying materials properties. Addit. Manuf. 2017, 14, 24–30. [Google Scholar] [CrossRef]
- Yassin, M.A.; Fuoco, T.; Mohamed-Ahmed, S.; Mustafa, K.; Finne-Wistrand, A. 3D and Porous RGDC-Functionalized Polyester-Based Scaffolds as a Niche to Induce Osteogenic Differentiation of Human Bone Marrow Stem Cells. Macromol. Biosci. 2019, 19, e1900049. [Google Scholar] [CrossRef]
- Mangini, V.; Maggi, V.; Trianni, A.; Melle, F.; de Luca, E.; Pennetta, A.; Del Sole, R.; Ventura, G.; Cataldi, T.R.I.; Fiammengo, R. Directional Immobilization of Proteins on Gold Nanoparticles Is Essential for Their Biological Activity: Leptin as a Case Study. Bioconjugate Chem. 2020, 31, 74–81. [Google Scholar] [CrossRef]
- Evans, H.L.; Carroll, L.; Aboagye, E.O.; Spivey, A.C. Bioorthogonal chemistry for 68Ga radiolabelling of DOTA-containing compounds: Biororthogonal chemistry for 68Ga. J. Label. Comp. Radiopharm. 2014, 57, 291–297. [Google Scholar] [CrossRef]
- Gouveia, B.G.; Rijo, P.; Gonçalo, T.S.; Reis, C.P. Good manufacturing practices for medicinal products for human use. J. Pharm. Bioallied Sci. 2015, 7, 87–96. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]


| NMPO | K2HPO4 | ||
|---|---|---|---|
| Post-Fabrication | Result | Post-Fabrication | Result |
| Standard protocol (SP) | No growth (0/6 samples) | Standard protocol (SP) | No growth (0/6 samples) |
| SP w/o washing and lyophilization | No growth (0/3 samples) | SP w/o washing and lyophilization | No growth (0/3 samples) |
| SP plus γ-sterilization | No growth (0/6 samples) | SP plus γ-sterilization | No growth (0/6 samples) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krieghoff, J.; Rost, J.; Kohn-Polster, C.; Müller, B.M.; Koenig, A.; Flath, T.; Schulz-Siegmund, M.; Schulze, F.-P.; Hacker, M.C. Extrusion-Printing of Multi-Channeled Two-Component Hydrogel Constructs from Gelatinous Peptides and Anhydride-Containing Oligomers. Biomedicines 2021, 9, 370. https://doi.org/10.3390/biomedicines9040370
Krieghoff J, Rost J, Kohn-Polster C, Müller BM, Koenig A, Flath T, Schulz-Siegmund M, Schulze F-P, Hacker MC. Extrusion-Printing of Multi-Channeled Two-Component Hydrogel Constructs from Gelatinous Peptides and Anhydride-Containing Oligomers. Biomedicines. 2021; 9(4):370. https://doi.org/10.3390/biomedicines9040370
Chicago/Turabian StyleKrieghoff, Jan, Johannes Rost, Caroline Kohn-Polster, Benno M. Müller, Andreas Koenig, Tobias Flath, Michaela Schulz-Siegmund, Fritz-Peter Schulze, and Michael C. Hacker. 2021. "Extrusion-Printing of Multi-Channeled Two-Component Hydrogel Constructs from Gelatinous Peptides and Anhydride-Containing Oligomers" Biomedicines 9, no. 4: 370. https://doi.org/10.3390/biomedicines9040370
APA StyleKrieghoff, J., Rost, J., Kohn-Polster, C., Müller, B. M., Koenig, A., Flath, T., Schulz-Siegmund, M., Schulze, F.-P., & Hacker, M. C. (2021). Extrusion-Printing of Multi-Channeled Two-Component Hydrogel Constructs from Gelatinous Peptides and Anhydride-Containing Oligomers. Biomedicines, 9(4), 370. https://doi.org/10.3390/biomedicines9040370