Ex Vivo Generation and Characterization of Human Hyaline and Elastic Cartilaginous Microtissues for Tissue Engineering Applications
Abstract
1. Introduction
2. Experimental Section
2.1. Cell Cultures
2.2. Fabrication of Molds
2.3. Fabrication of Agarose Microchips
2.4. Formation of Chondrogenic Microtissues or Organoids
- (a)
- HC-MT cultured under EM as control (HC-MT-EM);
- (b)
- HC-MT cultured with CM (HC-MT-CM);
- (c)
- EC-MT cultured with EM as control (EC-MT-EM);
- (d)
- EC-MT cultured with CM (EC-MT-CM).
2.5. Morphometric Analyses
2.6. Assessment of the Cell Viability and Metabolic Activity during MT Formation
2.7. Histological Analyses and Histochemical Analysis
2.8. Statistical Analysis
3. Results
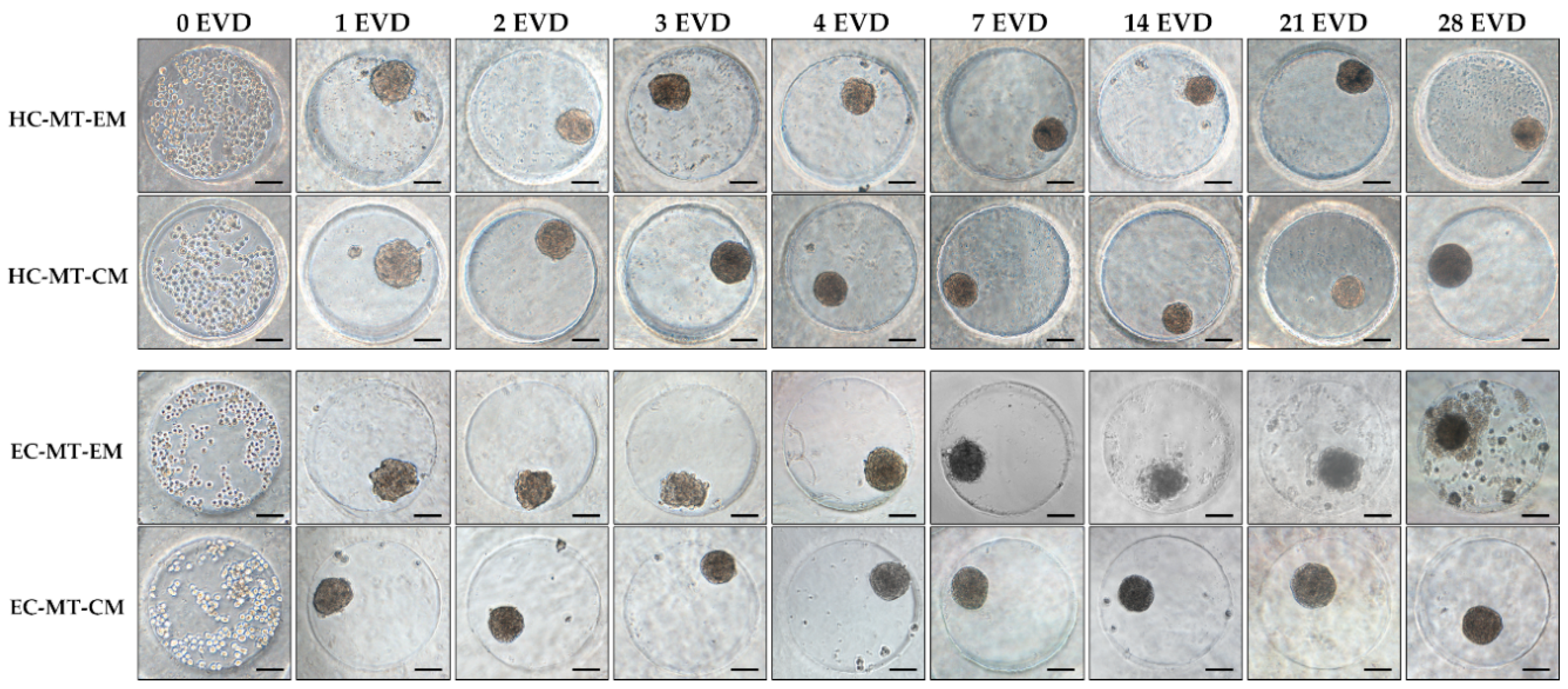
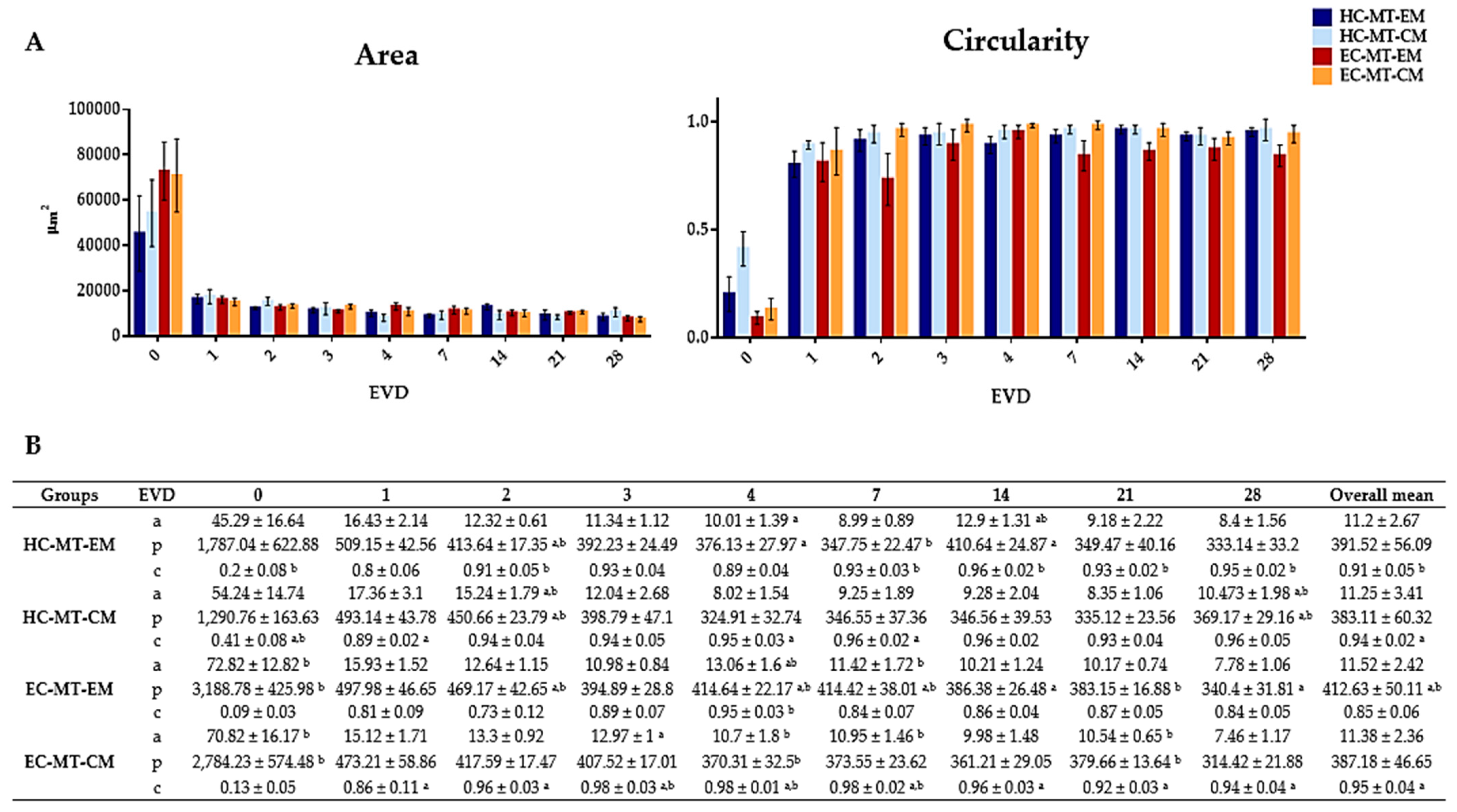
3.1. Microtissue Formation and Morphometric Analysis
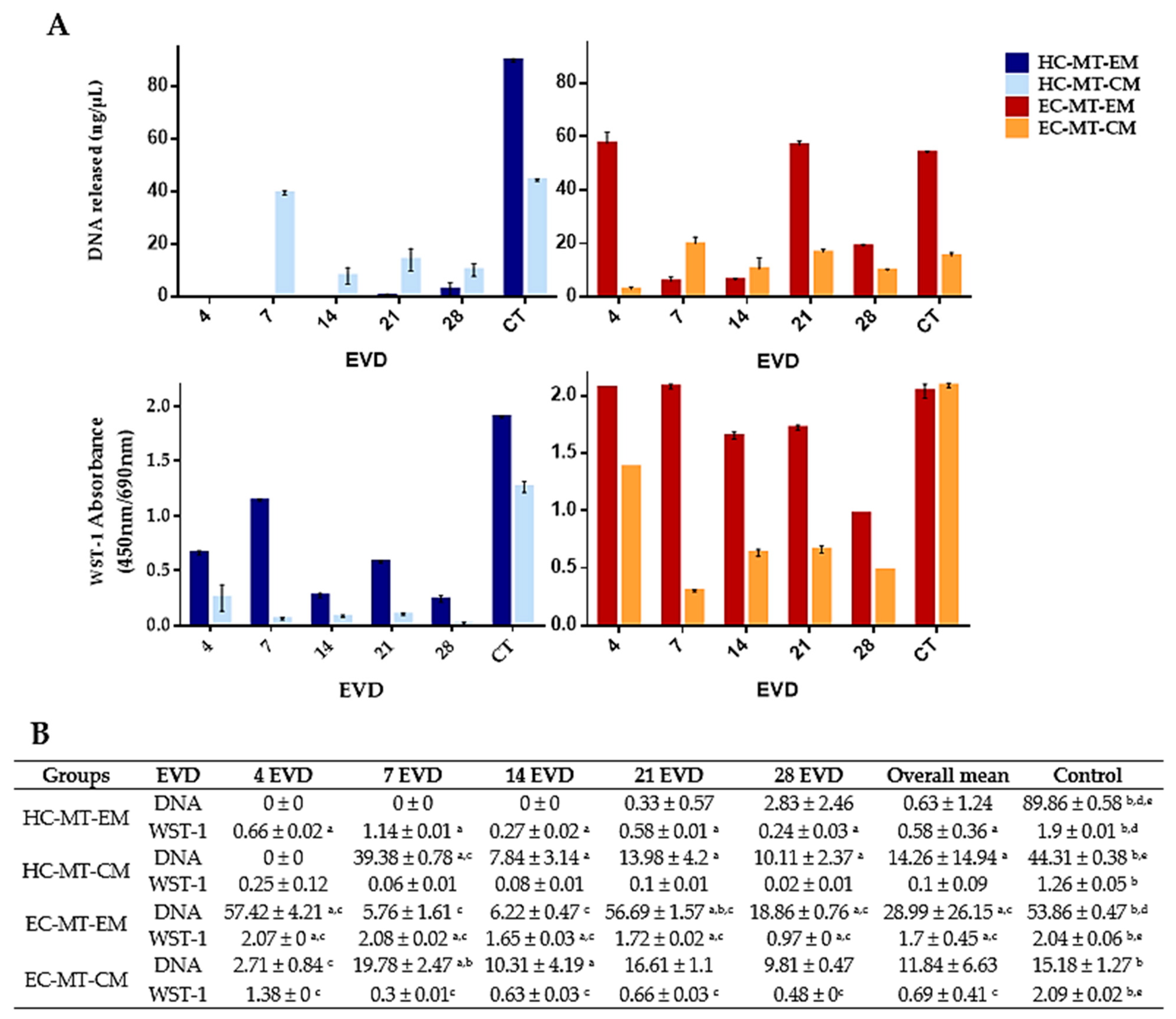
3.2. Cell Viability
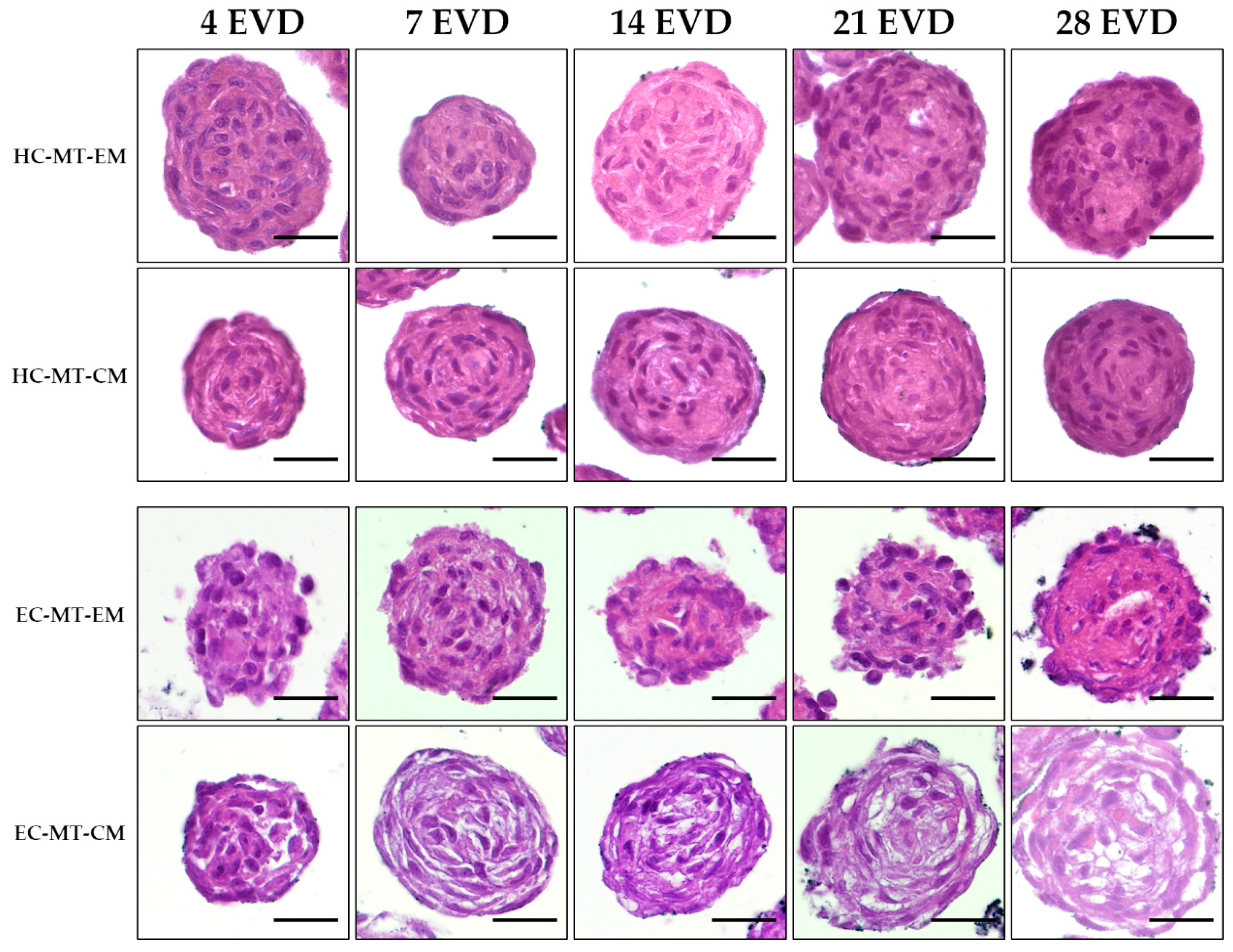
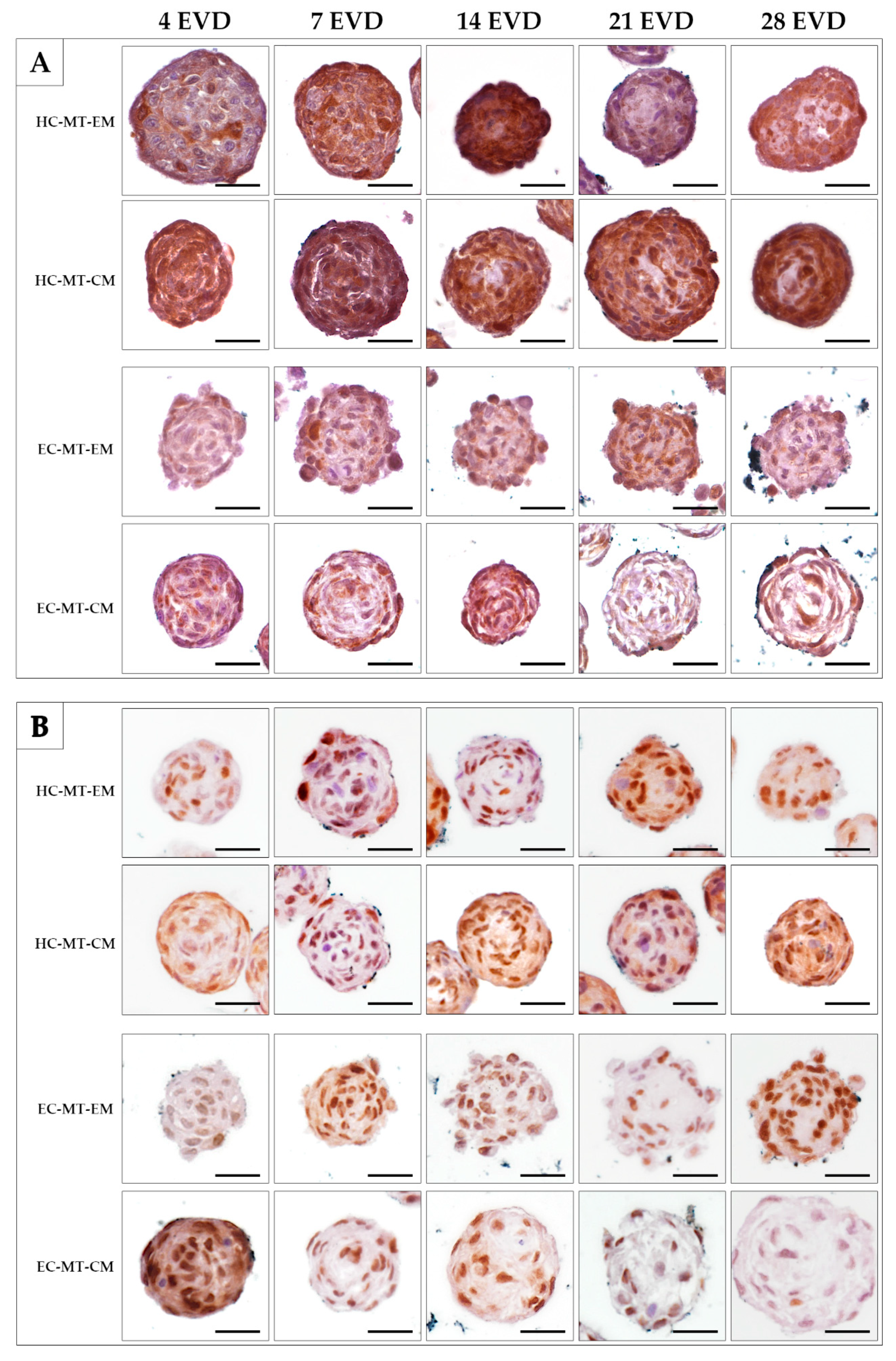
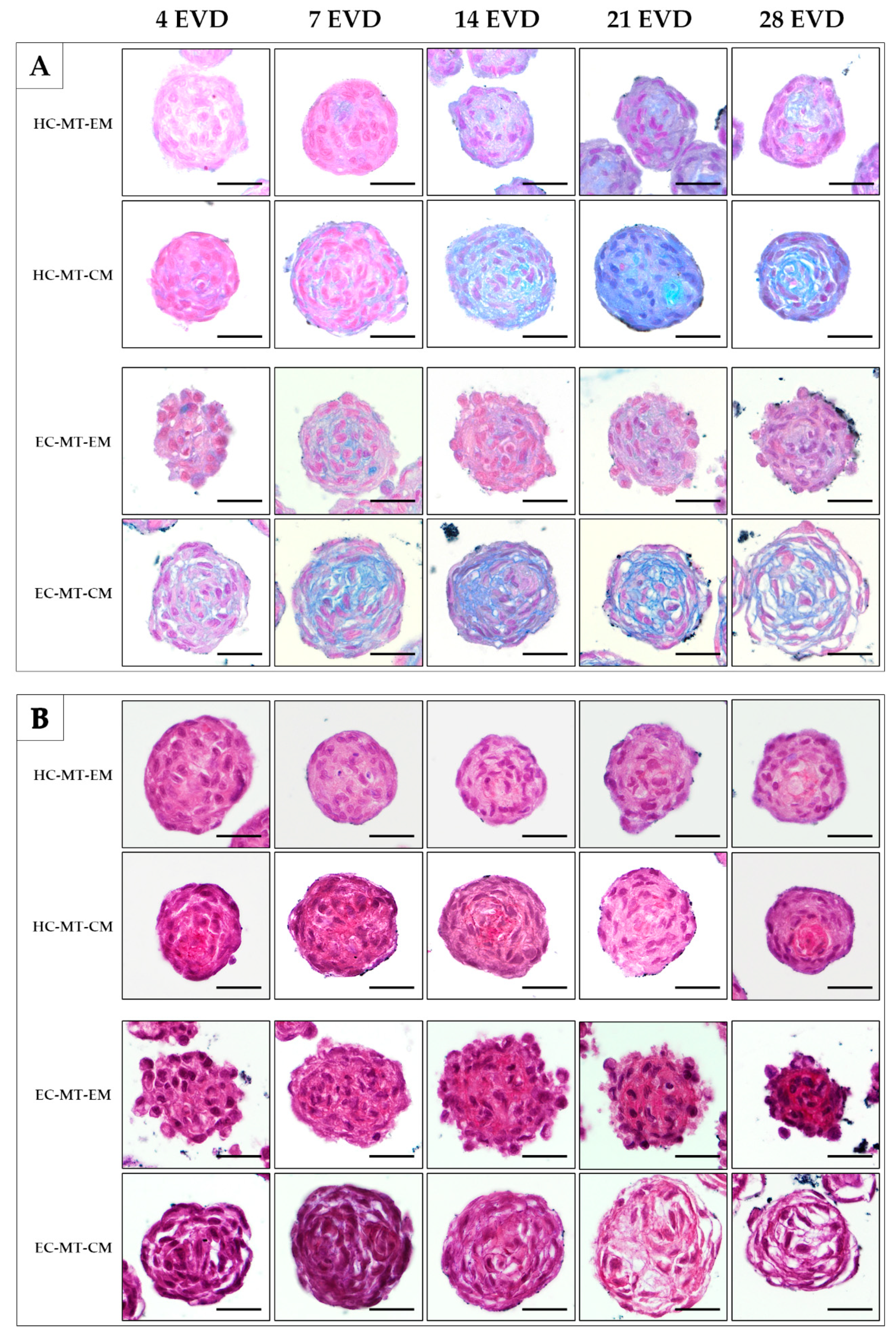
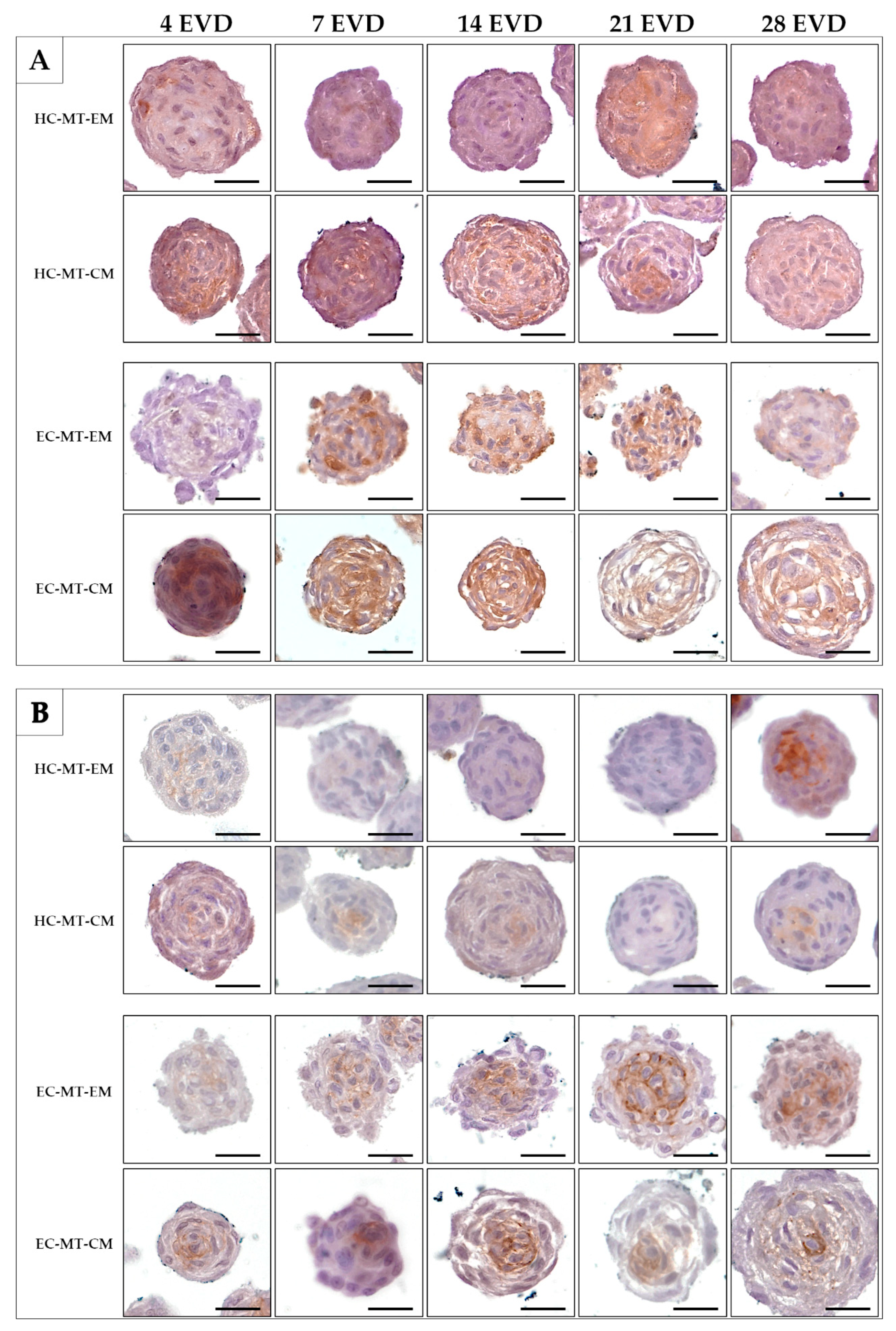
3.3. Histology of the Microtissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Alaminos, M.; Sánchez-Quevedo, M.D.C.; Muñoz-Ávila, J.I.; Serrano, D.; Medialdea, S.; Carreras, I.; Campos, A. Construction of a Complete Rabbit Cornea Substitute Using a Fibrin-Agarose Scaffold. Investig. Opthalmol. Vis. Sci. 2006, 47, 3311–3317. [Google Scholar] [CrossRef]
- Griffith, L.G. Tissue Engineering—Current Challenges and Expanding Opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef]
- Durand-Herrera, D.; Campos, F.; Jaimes-Parra, B.D.; Sánchez-López, J.D.; Fernández-Valadés, R.; Alaminos, M.; Campos, A.; Carriel, V. Wharton’s jelly-derived mesenchymal cells as a new source for the generation of microtissues for tissue engineering applications. Histochem. Cell Biol. 2018, 150, 379–393. [Google Scholar] [CrossRef]
- Carriel, V.; Alaminos, M.; Garzón, I.; Campos, A.; Cornelissen, M. Tissue engineering of the peripheral nervous system. Expert Rev. Neurother. 2014, 14, 301–318. [Google Scholar] [CrossRef]
- Mills, S.E. Histology for Pathologists, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; p. xi. [Google Scholar]
- Norris, J.A.; Stabile, K.J.; Jinnah, R.H. An introduction to tribology. J. Surg. Orthop. Adv. 2008, 17, 2–5. [Google Scholar] [PubMed]
- Melrose, J. The Importance of the Knee Joint Meniscal Fibrocartilages as Stabilizing Weight Bearing Structures Providing Global Protection to Human Knee-Joint Tissues. Cells 2019, 8, 324. [Google Scholar] [CrossRef]
- Mauck, R.L.; Burdick, J.A. Engineering Cartilage Tissue. In Molecular Cardiology; Springer International Publishing: Berlin/Heidelberg, Germany, 2010; pp. 493–520. [Google Scholar]
- García-Martínez, L.; Campos, F.; Godoy-Guzmán, C.; Sánchez-Quevedo, M.D.C.; Garzón, I.; Alaminos, M.; Campos, A.; Carriel, V. Encapsulation of human elastic cartilage-derived chondrocytes in nanostructured fibrin-agarose hydrogels. Histochem. Cell Biol. 2016, 147, 83–95. [Google Scholar] [CrossRef]
- O’Driscoll, S.W. Current Concepts Review-The Healing and Regeneration of Articular Cartilage. J. Bone Jt. Surg. 1998, 80, 1795–1812. [Google Scholar] [CrossRef]
- Basad, E.; Wissing, F.R.; Fehrenbach, P.; Rickert, M.; Steinmeyer, J.; Ishaque, B. Matrix-induced autologous chondrocyte implantation (MACI) in the knee: Clinical outcomes and challenges. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3729–3735. [Google Scholar] [CrossRef]
- Nam, Y.; Rim, Y.A.; Lee, J.; Ju, J.H. Current Therapeutic Strategies for Stem Cell-Based Cartilage Regeneration. Stem Cells Int. 2018, 2018, 1–20. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Huang, B.-R.; Chen, H.-C.; Shih, C.-P.; Chang, W.-K.; Tsai, Y.-L.; Lin, Y.-Y.; Tsai, W.-C.; Wang, C.-H. Surgical Results of Retrograde Mastoidectomy with Primary Reconstruction of the Ear Canal and Mastoid Cavity. BioMed Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Jiang, H.; Yin, Z.; Liu, Y.; Zhang, Q.; Zhang, C.; Pan, B.; Zhou, J.; Zhou, X.; Sun, H.; et al. In Vitro Regeneration of Patient-specific Ear-shaped Cartilage and Its First Clinical Application for Auricular Reconstruction. EBioMedicine 2018, 28, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Firmin, F. State-of-the-Art Autogenous Ear Reconstruction in Cases of Microtia. Adv. Otorhinolaryngol. 2010, 68, 25–52. [Google Scholar] [CrossRef]
- Minas, T. The role of cartilage repair techniques, including chondrocyte transplantation, in focal chondral knee damage. Instr. Course Lect. 1999, 48, 629–643. [Google Scholar]
- Lamplot, J.D.; Schafer, K.A.; Matava, M.J. Treatment of Failed Articular Cartilage Reconstructive Procedures of the Knee: A Systematic Review. Orthop. J. Sports Med. 2018, 6, 2325967118761871. [Google Scholar] [CrossRef]
- Sridharan, B.; Lin, S.M.; Hwu, A.T.; Laflin, A.D.; Detamore, M.S. Stem Cells in Aggregate Form to Enhance Chondrogenesis in Hydrogels. PLoS ONE 2015, 10, e0141479. [Google Scholar] [CrossRef] [PubMed]
- Berneel, E.; Philips, C.; Declercq, H.; Cornelissen, R. Redifferentiation of High-Throughput Generated Fibrochondrocyte Mi-cro-Aggregates: Impact of Low Oxygen Tension. Cells Tissues Organs 2016, 202, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, M.; Boccafoschi, F.; Leigheb, M.; Bianchi, A.E.; Cannas, M. Chondrogenic induction of human mesenchymal stem cells using combined growth factors for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2011, 6, 205–213. [Google Scholar] [CrossRef]
- Leijten, J.; Teixeira, L.S.M.; Bolander, J.; Ji, W.; Vanspauwen, B.; Lammertyn, J.; Schrooten, J.; Luyten, F.P. Bioinspired seeding of biomaterials using three dimensional microtissues induces chondrogenic stem cell differentiation and cartilage formation under growth factor free conditions. Sci. Rep. 2016, 6, 36011. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.S.M.; Leijten, J.C.H.; Sobral, J.; Jin, R.A.; Van Apeldoorn, A.; Feijen, J.; Van Blitterswijk, C.; Dijkstra, P.J.; Karperien, M. High throughput generated micro-aggregates of chondrocytes stimulate cartilage formation in vitro and in vivo. Eur. Cells Mater. 2012, 23, 387–399. [Google Scholar] [CrossRef]
- Lehmann, M.; Martin, F.; Mannigel, K.; Kaltschmidt, K.; Sack, U.; Anderer, U. Three-dimensional scaffold-free fusion culture: The way to enhance chondrogenesis of in vitro propagated human articular chondrocytes. Eur. J. Histochem. 2013, 57, e31. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, J.; Zhang, X.; Li, H.; Jiang, L.; Qin, J. Hypoxia combined with spheroid culture improves cartilage specific function in chondrocytes. Integr. Biol. 2015, 7, 289–297. [Google Scholar] [CrossRef]
- Achilli, T.-M.; Meyer, J.; Morgan, J.R. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin. Biol. Ther. 2012, 12, 1347–1360. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Zorlutuna, P.; Annabi, N.; Camci-Unal, G.; Nikkhah, M.; Cha, J.M.; Nichol, J.W.; Manbachi, A.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabricated Biomaterials for Engineering 3D Tissues. Adv. Mater. 2012, 24, 1782–1804. [Google Scholar] [CrossRef]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; de Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.M.; Napolitano, A.P.; Youssef, J.; Morgan, J.R. Rods, tori, and honeycombs: The directed self-assembly of microtissues with prescribed microscale geometries. FASEB J. 2007, 21, 4005–4012. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.P.; Chai, P.; Dean, D.M.; Morgan, J.R. Dynamics of the Self-Assembly of Complex Cellular Aggregates on Micromolded Nonadhesive Hydrogels. Tissue Eng. 2007, 13, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.P.; Dean, D.M.; Man, A.J.; Youssef, J.; Ho, D.N.; Rago, A.P.; Lech, M.P.; Morgan, J.R. Scaffold-free three-dimensional cell culture utilizing micromolded nonadhesive hydrogels. Biotechniques 2007, 43, 494–500. [Google Scholar] [CrossRef]
- Syu, W.-Z.; Chen, S.-G.; Chan, J.Y.-H.; Wang, C.-H.; Dai, N.-T.; Huang, S.-M. The Potential of Acellular Dermal Matrix Combined with Neural Stem Cells Induced From Human Adipose-Derived Stem Cells in Nerve Tissue Engineering. Ann. Plast. Surg. 2019, 82, S108–S118. [Google Scholar] [CrossRef]
- Budel, L.; Djabali, K. Rapid isolation and expansion of skin-derived precursor cells from human primary fibroblast cultures. Biol. Open 2017, 6, 1745–1755. [Google Scholar] [CrossRef]
- Hynds, R.E.; Bonfanti, P.; Janes, S.M. Regenerating human epithelia with cultured stem cells: Feeder cells, organoids and beyond. EMBO Mol. Med. 2017, 10, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Bonhome-Espinosa, A.B.; Campos, F.; Durand-Herrera, D.; Sánchez-López, J.D.; Schaub, S.; Durán, J.D.; Lopez-Lopez, M.T.; Carriel, V. In Vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103619. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.; Bonhome-Espinosa, A.B.; García-Martínez, L.; Durán, J.D.G.; López-López, M.T.; Alaminos, M.; Sánchez-Quevedo, M.C.; Carriel, V. Ex Vivo characterization of a novel tissue-like cross-linked fibrin-agarose hydrogel for tissue engineering applications. Biomed. Mater. 2016, 11, 055004. [Google Scholar] [CrossRef] [PubMed]
- Garzón, I.; Pérez-Köhler, B.; Garrido-Gómez, J.; Carriel, V.; Nieto-Aguilar, R.; Martín-Piedra, M.A.; García-Honduvilla, N.; Buján, J.; Campos, A.; Alaminos, M. Evaluation of the Cell Viability of Human Wharton’s Jelly Stem Cells for Use in Cell Therapy. Tissue Eng. Part C Methods 2012, 18, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Martin-Piedra, M.A.; Garzon, I.; Oliveira, A.C.; Alfonso-Rodriguez, C.A.; Carriel, V.; Scionti, G.; Alaminos, M. Cell viability and proliferation capability of long-term human dental pulp stem cell cultures. Cytotherapy 2014, 16, 266–277. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Alfonso-Rodríguez, C.; Monsalve-Guil, L.; España-López, A.; Jiménez-Guerra, A.; Garzón, I.; Alaminos, M.; Gil, F. Relevant aspects in the surface properties in titanium dental implants for the cellular viability. Mater. Sci. Eng. C 2016, 64, 1–10. [Google Scholar] [CrossRef]
- Campos, F.; Bonhome-Espinosa, A.B.; Vizcaino, G.; Rodriguez, I.A.; Durand-Herrera, D.; López-López, M.T.; Sánchez-Montesinos, I.; Alaminos, M.; Sánchez-Quevedo, M.C.; Carriel, V. Generation of genipin cross-linked fibrin-agarose hydrogel tissue-like models for tissue engineering applications. Biomed. Mater. 2018, 13, 025021. [Google Scholar] [CrossRef]
- Carriel, V.G.I. Aplicación del método de bloque celular para evaluar la población de fibroblastos de mucosa oral en ingeniería tisular. Actualidad Medica 2009, 94, 7–11. [Google Scholar]
- Chato-Astrain, J.; Campos, F.; Roda, O.; Miralles, E.; Durand-Herrera, D.; Sáez-Moreno, J.A.; García-García, S.; Alaminos, M.; Campos, A.; Carriel, V. In Vivo Evaluation of Nanostructured Fibrin-Agarose Hydrogels With Mesenchymal Stem Cells for Peripheral Nerve Repair. Front. Cell. Neurosci. 2018, 12, 501. [Google Scholar] [CrossRef]
- Vela-Romera, A.; Carriel, V.; Martín-Piedra, M.A.; Aneiros-Fernández, J.; Campos, F.; Chato-Astrain, J.; Prados-Olleta, N.; Campos, A.; Alaminos, M.; Garzón, I. Characterization of the human ridged and non-ridged skin: A comprehensive histological, histochemical and immunohistochemical analysis. Histochem. Cell Biol. 2019, 151, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Armiento, A.; Stoddart, M.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, C.; Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann. Phys. Rehabil. Med. 2016, 59, 139–144. [Google Scholar] [CrossRef]
- Sart, S.; Tsai, A.-C.; Li, Y.; Ma, T. Three-Dimensional Aggregates of Mesenchymal Stem Cells: Cellular Mechanisms, Biological Properties, and Applications. Tissue Eng. Part B Rev. 2014, 20, 365–380. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Chang, Y.-H.; Yeh, Y.-C.; Chen, C.-H.; Lin, K.M.; Huang, C.-C.; Chang, Y.; Sung, H.-W. The use of injectable spherically symmetric cell aggregates self-assembled in a thermo-responsive hydrogel for enhanced cell transplantation. Biomaterials 2009, 30, 5505–5513. [Google Scholar] [CrossRef]
- De Moor, L.; Beyls, E.; Declercq, H. Scaffold Free Microtissue Formation for Enhanced Cartilage Repair. Ann. Biomed. Eng. 2019, 48, 298–311. [Google Scholar] [CrossRef]
- Leenaars, C.H.C.; Kouwenaar, C.; Stafleu, F.R.; Bleich, A.; Ritskes-Hoitinga, M.; De Vries, R.B.M.; Meijboom, F.L.B. Animal to human translation: A systematic scoping review of reported concordance rates. J. Transl. Med. 2019, 17, 1–22. [Google Scholar] [CrossRef]
- Kamisan, N.; Naveen, S.V.; Ahmad, R.E.; Tunku, K. Chondrocyte density, proteoglycan content and gene expressions from native cartilage are species specific and not dependent on cartilage thickness: A comparative analysis between rat, rabbit and goat. BMC Veter. Res. 2013, 9, 62. [Google Scholar] [CrossRef]
- Yanaga, H.; Imai, K.; Koga, M.; Yanaga, K. Cell-Engineered Human Elastic Chondrocytes Regenerate Natural Scaffold In Vitro and Neocartilage with Neoperichondrium in the Human Body Post-Transplantation. Tissue Eng. Part A 2012, 18, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.J.; Lloyd, M.S. Tissue Engineering Strategies for Auricular Reconstruction. J. Craniofacial Surg. 2017, 28, 2007–2011. [Google Scholar] [CrossRef]
- De Chalain, T.; Phillips, J.H.; Hinek, A. Bioengineering of elastic cartilage with aggregated porcine and human auricular chondrocytes and hydrogels containing alginate, collagen, and-elastin. J. Biomed. Mater. Res. 1999, 44, 280–288. [Google Scholar] [CrossRef]
- Okubo, R.; Asawa, Y.; Watanabe, M.; Nagata, S.; Nio, M.; Takato, T.; Hikita, A.; Hoshi, K. Proliferation medium in three-dimensional culture of auricular chondrocytes promotes effective cartilage regeneration in vivo. Regen. Ther. 2019, 11, 306–315. [Google Scholar] [CrossRef]
- Diaz-Romero, J.; Gaillard, J.P.; Grogan, S.P.; Nesic, D.; Trub, T.; Mainil-Varlet, P. Immunophenotypic analysis of human articular chondrocytes: Changes in surface markers associated with cell expansion in monolayer culture. J. Cell. Physiol. 2004, 202, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Brent, B. Microtia repair with rib cartilage grafts. Clin. Plast. Surg. 2002, 29, 257–271. [Google Scholar] [CrossRef]
- Yanaga, H.; Yanaga, K.; Imai, K.; Koga, M.; Soejima, C.; Ohmori, K. Clinical Application of Cultured Autologous Human Auricular Chondrocytes with Autologous Serum for Craniofacial or Nasal Augmentation and Repair. Plast. Reconstr. Surg. 2006, 117, 2019–2030. [Google Scholar] [CrossRef]
- Lin, R.-Z.; Chang, H.-Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Jiang, J.; Yanase, T.; Nishi, Y.; Morgan, J.R. Connexon-mediated cell adhesion drives microtissue self-assembly. FASEB J. 2010, 25, 255–264. [Google Scholar] [CrossRef]
- Cheng, B.; Lian, J.; Yang, H.; Wang, L.; Yu, H.; Bi, J.; Gao, Y.; Chen, S.; Wang, M.; Feng, Z. Neural cell adhesion molecule regulates chondrocyte hypertrophy in chondrogenic differentiation and experimental osteoarthritis. Stem Cells Transl. Med. 2019, 9, 273–283. [Google Scholar] [CrossRef]
- Mayan, M.D.; Carpintero-Fernandez, P.; Gago-Fuentes, R.; Martinez-De-Ilarduya, O.; Wang, H.-Z.; Valiunas, V.; Brink, P.; Blanco, F.J. Human Articular Chondrocytes Express Multiple Gap Junction Proteins. Am. J. Pathol. 2013, 182, 1337–1346. [Google Scholar] [CrossRef]
- Delise, A.M.; Tuan, R.S. Analysis of N-cadherin function in limb mesenchymal chondrogenesis in vitro. Dev. Dyn. 2002, 225, 195–204. [Google Scholar] [CrossRef]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. 2009, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Foldager, C.B.; Toh, W.S.; Gomoll, A.H.; Olsen, B.R.; Spector, M. Distribution of Basement Membrane Molecules, Laminin and Collagen Type IV, in Normal and Degenerated Cartilage Tissues. Cartilage 2013, 5, 123–132. [Google Scholar] [CrossRef]
- Kozel, B.A.; Rongish, B.J.; Czirok, A.; Zach, J.; Little, C.D.; Davis, E.C.; Knutsen, R.H.; Wagenseil, J.E.; Levy, M.A.; Mecham, R.P. Elastic fiber formation: A dynamic view of extracellular matrix assembly using timer reporters. J. Cell. Physiol. 2006, 207, 87–96. [Google Scholar] [CrossRef]
- Isogai, N.; Kusuhara, H.; Ikada, Y.; Ohtani, H.; Jacquet, R.; Hillyer, J.; Lowder, E.; Landis, W.J. Comparison of Different Chondrocytes for Use in Tissue Engineering of Cartilage Model Structures. Tissue Eng. 2006, 12, 691–703. [Google Scholar] [CrossRef]
- Salinas, E.Y.; Hu, J.C.; Athanasiou, K. A Guide for Using Mechanical Stimulation to Enhance Tissue-Engineered Articular Cartilage Properties. Tissue Eng. Part B Rev. 2018, 24, 345–358. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Porras, D.; Durand-Herrera, D.; Paes, A.B.; Chato-Astrain, J.; Verplancke, R.; Vanfleteren, J.; Sánchez-López, J.D.; García-García, Ó.D.; Campos, F.; Carriel, V. Ex Vivo Generation and Characterization of Human Hyaline and Elastic Cartilaginous Microtissues for Tissue Engineering Applications. Biomedicines 2021, 9, 292. https://doi.org/10.3390/biomedicines9030292
Sánchez-Porras D, Durand-Herrera D, Paes AB, Chato-Astrain J, Verplancke R, Vanfleteren J, Sánchez-López JD, García-García ÓD, Campos F, Carriel V. Ex Vivo Generation and Characterization of Human Hyaline and Elastic Cartilaginous Microtissues for Tissue Engineering Applications. Biomedicines. 2021; 9(3):292. https://doi.org/10.3390/biomedicines9030292
Chicago/Turabian StyleSánchez-Porras, David, Daniel Durand-Herrera, Ana B. Paes, Jesús Chato-Astrain, Rik Verplancke, Jan Vanfleteren, José Darío Sánchez-López, Óscar Darío García-García, Fernando Campos, and Víctor Carriel. 2021. "Ex Vivo Generation and Characterization of Human Hyaline and Elastic Cartilaginous Microtissues for Tissue Engineering Applications" Biomedicines 9, no. 3: 292. https://doi.org/10.3390/biomedicines9030292
APA StyleSánchez-Porras, D., Durand-Herrera, D., Paes, A. B., Chato-Astrain, J., Verplancke, R., Vanfleteren, J., Sánchez-López, J. D., García-García, Ó. D., Campos, F., & Carriel, V. (2021). Ex Vivo Generation and Characterization of Human Hyaline and Elastic Cartilaginous Microtissues for Tissue Engineering Applications. Biomedicines, 9(3), 292. https://doi.org/10.3390/biomedicines9030292







