Abstract
Phosphodiesterase type 5 (PDE5) inhibitors such as Viagra® (sildenafil citrate) have demonstrated efficacy in the treatment of erectile dysfunction (ED) by inducing cyclic guanosine monophosphate (cGMP) elevation followed by vasodilation and increased blood flow. It also exerts minor inhibitory action against PDE6, which is present exclusively in rod and cone photoreceptors. The effects of sildenafil on the visual system have been investigated in a wide variety of clinical and preclinical studies due to the fact that a high dose of sildenafil may cause mild and transient visual symptoms in some patients. A literature review was performed using PubMed, Cochrane Library and Clinical Trials databases from 1990 up to 2020, focusing on the pathophysiology of visual disorders induced by sildenafil. The aim of this review was not only to gather and summarize the information available on sildenafil clinical trials (CTs), but also to spot subpopulations with increased risk of developing undesirable visual side effects. This PDE inhibitor has been associated with transient and reversible ocular side effects, including changes in color vision and light perception, blurred vision, photophobia, conjunctival hyperemia and keratitis, and alterations in the electroretinogram (ERG). Sildenafil may induce a reversible increase in intraocular pressure (IOP) and a few case reports suggest it is involved in the development of nonarteritic ischemic optic neuropathy (NAION). Reversible idiopathic serous macular detachment, central serous retinopathy and ERG disturbances have been related to the significant impact of sildenafil on retinal perfusion. So far, sildenafil does not seem to cause permanent toxic effects on chorioretinal tissue and photoreceptors as long as the therapeutic dose is not exceeded and is taken under a physician’s direction to treat a medical condition. However, the recreational use of sildenafil can lead to harmful side effects, including vision changes.
1. Introduction
1.1. Phototransduction Cascade
The phototransduction process is a G-protein mediated signaling cascade where rod or cone opsins couple photon absorption to current flow at the photoreceptor outer segment plasma membrane [1]. In the dark, cyclic guanosine monophosphate (cGMP), which is at a relatively high concentration in the photoreceptor outer segment, binds and maintains cyclic nucleotide-gated (CNG) channels in the plasma membrane in an open state, resulting in an influx of Na+ and Ca2+ ions into the cytosol. To maintain Ca2+ homeostasis within the photoreceptor, K+ and Ca2+ are, in parallel, continuously extruded via the potassium-dependent sodium-calcium exchanger (NCKX). This constant inward current, referred to as the dark current, causes photoreceptor depolarization and glutamate release at the synaptic terminal, inhibiting postsynaptic second-order neurons (bipolar cells). Absorption of photons by rhodopsin leads to the sequential activation of G-protein transducin and phosphodiesterase 6 (PDE6), responsible for the hydrolysis of cGMP and the consequent closure of CNG channels. This interrupts the dark current, resulting in the hyperpolarization of outer segments due to the continued activity of NCKX. As a result, the generation of this electro-chemical signal halts the release of neurotransmitters at the photoreceptor axon terminal and the visual signal is propagated to postsynaptic cells [1].
The role of cGMP as a second messenger is key in the regulation of phototransduction since the whole signaling cascade depends on the balance between its synthesis by retinal guanylyl cyclase (GC) and its hydrolysis by PDE6. Thus, it seems obvious that the disruption of cGMP metabolism implies serious consequences for visual functioning, including photoreceptor toxicity and cell death (reviewed in [2]). Processes such as retinal oxidative stress entail the generation of reactive nitrogen intermediates such as nitric oxide (NO), a second messenger that stimulates retinal GC, increasing free cGMP levels [3]. Likewise, genetic mutations are also involved in the pathological intracellular concentrations of cGMP. For instance, some forms of inherited retinal degeneration such as retinitis pigmentosa, Leber congenital amaurosis, or cone–rod dystrophies are related to increased cGMP levels (reviewed in [4]). Moreover, pharmacologically targeting the cGMP pathway has been postulated as a novel and interesting therapeutic approach for the treatment of inherited retinal degenerations [5]. Although the mechanisms linking elevated cGMP to photoreceptor demise have not been completely elucidated yet, two targets of cGMP, whose overactivation contributes to rod cell death, have been proposed: protein kinase G (PKG) and CNG channels [6].
It is known that the NO/GC/cGMP/PKG signaling pathway is functional and widely distributed in specific cell types of both the internal and external retina of mice [3]. Studies performed in the murine models of retinitis pigmentosa rd1 and rd10, which carry loss-of-function mutations in the beta subunit of rod PDE6 [7], have shown that high cGMP levels during retinal degeneration trigger an overactivation of PKG, which contributes to photoreceptor death [8]. Although cGMP-dependent phosphorylation of PKG in photoreceptors has already been demonstrated in 1977 [9], Paquet-Durand’s study was the first to link excessive PKG activity directly to cell death [8]. On the other hand, as mentioned above, cGMP regulates the opening of the CNG channels present in the plasma membrane of the photoreceptor outer segment. Therefore, excessive cGMP alters Ca2+ homeostasis, impairing the function of Ca2+-dependent phototransduction proteins such as recoverin and guanylyl cyclase-activating proteins (GCAPs), and even triggering photoreceptor death via the calpain signaling pathway [1,10] (Figure 1).
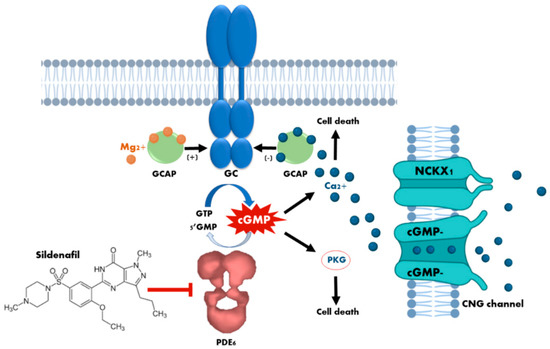
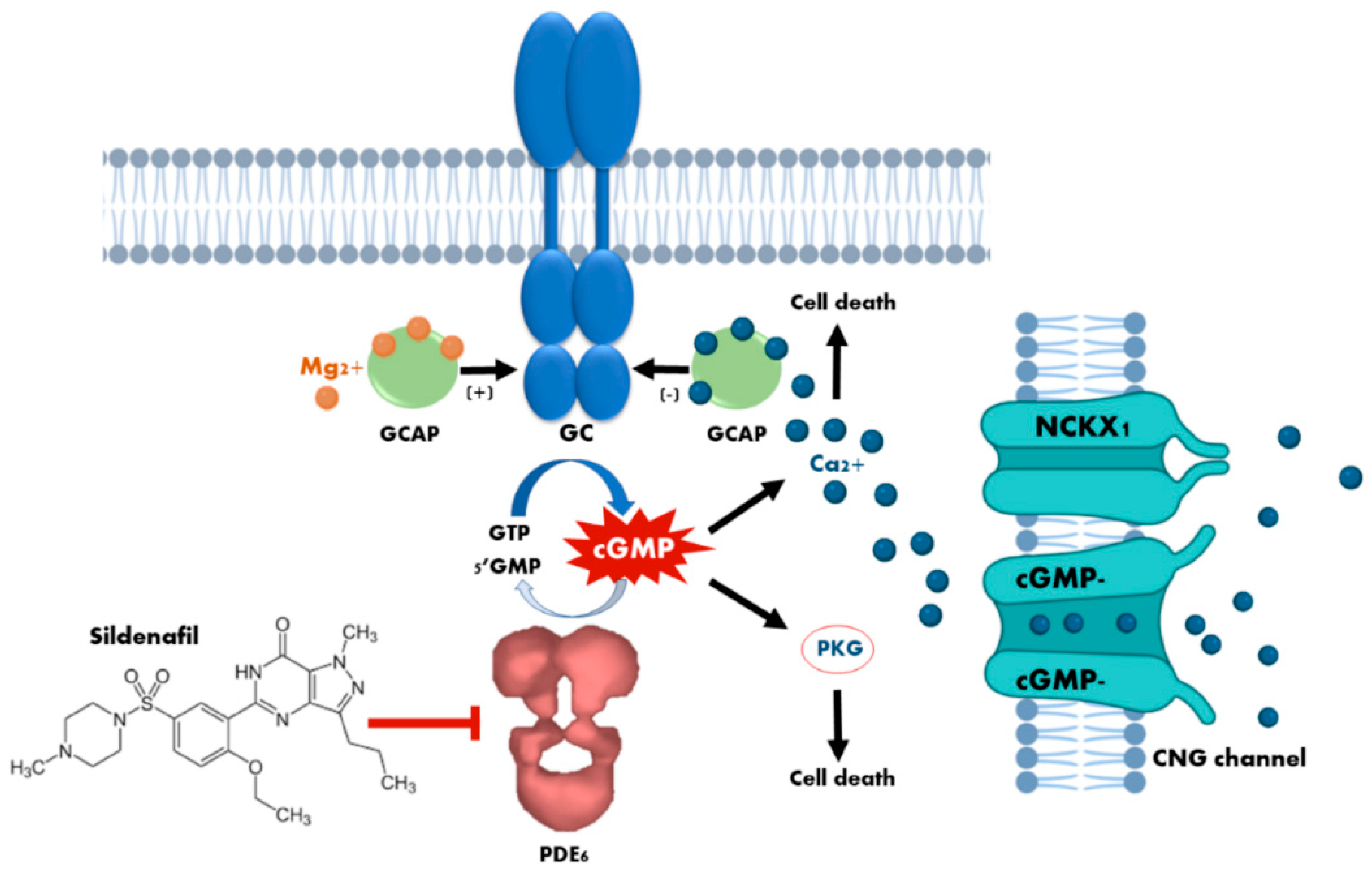
Figure 1.
Cytotoxicity mechanisms of cyclic guanosine monophosphate (cGMP) in photoreceptors. The loss of phosphodiesterase (PDE6) function, or the prolonged activation of retinal guanylyl cyclase (GC) due to dominant mutations in the guanylyl cyclase-activating proteins (GCAPs), leads to an increase in the concentration of cytosolic cGMP which, in turn, causes continuous stimulation of the of protein kinase G (PKG) and an excessive influx of Ca2+ through the sustained opening of cyclic nucleotide-gated (CNG) channels. Both events have been demonstrated to cause photoreceptor cell death. The curved blue arrow represents cGMP synthesis by GC enzyme, whereas the curved white arrow represents cGMP hydrolisis by PDE6 enzyme. The blunt-end arrow represents the inhibition of PDE6 by sildenafil.
1.2. Phosphodiesterases and Inhibitors
Phosphodiesterases are a family of enzymes that regulate intracellular levels of the second messengers cAMP and cGMP. Although phosphodiesterases are found in every cell in the body, the distribution of isoenzymes varies between tissues. For instance, PDE5 is expressed in vascular smooth muscles (prominently expressed in the penis corpus cavernosum), skeletal muscles, and many other tissues including kidney, pancreas, heart, lung, liver, brain, placenta and various gastrointestinal tissues [11]. By contrast, PDE6 is present exclusively in retinal photoreceptors [12]. The PDE6 family consists of three genes (PDE6A, PDE6B and PDE6C) that encode three catalytic subunits (α, β and α′, respectively). The α and β subunits are expressed predominantly in rods, whereas the α′ subunit is expressed in cones [13]. cGMP binds to PDE6 through two GAF domains (GAF-A and GAF-B) at the amino-terminal end of the enzyme. The structural similarity of PDE isoenzymes catalytic domains results in poor specificity of inhibitory drugs. In this sense, it is worth mentioning that first-generation PDE5 inhibitors such as sildenafil (Viagra®), vardenafil (Levitra®), and tadalafil (Cialis®) are highly selective for PDE5 and represent the first successful application of PDE inhibition therapy to an individual isoenzyme. Nonetheless, despite this high selectivity, each of these drugs inhibits other PDE isoenzymes to some extent. Sildenafil and vardenafil, for example, have shown only 10 and 15 times lower specificity for PDE6 than for PDE5, respectively (reviewed in [14]), which could be explained by the fact that the kinetic and catalytic properties of PDE6 are very similar to those of PDE5 [15,16,17].
PDE5 inhibitors have been used for the treatment of erectile dysfunction (ED), which is a form of peripheral vascular disease that impairs men’s abilities to achieve and maintain an erection, and have become some of the best-selling medications worldwide. Sildenafil and its analogues operate by increasing the cGMP levels because they occupy the active site of PDE5 and prevent cGMP catalysis. cGMP acts as a powerful smooth muscle relaxant that promotes blood flow to the corpus cavernosum, facilitating penis erection [18]. Apart from their use as drugs to treat ED, the US Food and Drug Administration also approved the use of sildenafil and their analogues for the treatment of pulmonary arterial hypertension (PAH) in 2005. These inhibitors offer the possibility of improving the patient’s quality of life, as well as being candidate drugs for palliative therapy [19]. In addition to the three drugs mentioned above, the second-generation inhibitor avanafil (Stendra®) became internationally available in 2013. Avanafil exhibited 100 times lower specificity for PDE6 than for PDE5, presumably reducing the potential side effects derived from the nonselective inhibition of PDE6 by sildenafil and vardenafil (reviewed in [14]). Other second-generation (udenafil and mirodenafil) or third-generation (lodenafil, SLX-2101, JNJ-10280205, and JNJ-10287069) PDE5 inhibitors have been either approved and introduced into the market in some parts of the world or are at the final stages of their clinical development. Udenafil (Zydena) is only available in some Asian countries and Russia, mirodenafil (Mvix) is commercialized in various Asian countries and lodenafil (Helleva) is sold in Brazil [20]. All of them have been trialed in tablet formulations at different doses, whose broadest range spans from 25 to 200 mg [21]. Several studies indicate that, in general terms, PDE5 inhibitors are well tolerated and their side effects are few, mild and very similar among the different compounds studied, except for tadalafil, which caused a higher incidence of myalgia (Table 1). Many of the side effects are due to the vasoactivity of these compounds, given the expression of PDE5 in vascular smooth muscles. The most common reported dose-dependent adverse events include headache, flushing, nasal congestion, facial and ocular hyperemia, myalgia, back pain and dyspepsia [22,23,24].

Table 1.
Common side effects of Phosphodiesterase type 5 (PDE5) inhibitors.
The occurrence of side effects increases with both serum levels and exposure time to the PDE5 inhibitor [25]. To overcome these issues, novel drug formulations that improve the safety and efficacy profile of the drug are under development. Despite the side effects, oral administration of PDE5 inhibitors (tablets, oral solution or orodispersible tablets) is nowadays considered the first-line therapy for ED. A second-line treatment consists of invasive procedures such as intracavernosal injections with vasogenic drugs such as alprostadil (synthetic prostaglandin E1), papaverine or phentolamine, as well as intraurethral alprostadil suppositories and vacuum erection devices. These show a more favorable systemic side effect profile compared to oral pharmacotherapy [26] and, despite being invasive, they are widely used. To avoid invasive techniques and, at the same time, minimize systemic side effects, topical formulations (alprostadil and sildenafil topical cream) constitute a promising alternative, as they can be applied locally and are safe and easy to use [21,27,28]. Additionally, solid lipid nanoparticles in hydrogel films for the transdermal local delivery of avanafil have been assayed in vitro and ex vivo with success [29]. Moreover, emerging medications and procedures are currently under investigation for the treatment of ED in both preclinical and clinical settings, including non-PDE5 inhibitor oral drugs such as melanocortin receptor antagonists (subcutaneous melanocortin analogue (PT-141)), rho-kinase inhibitors (SAR407899), and soluble GC activator (BAY60-4552 and BAY 60-2770) [21,27,28]. Additionally under consideration are: regeneration therapy involving stem cell injection; gene therapy where the genetic material can be easily injected into the penis; low-intensity extracorporeal shock wave therapy; low-intensity pulse ultrasound; platelet-rich plasma injections [21,28,30]. Finally, the use of nanotechnology for drug delivery is being studied in murine models for all delivery methods (oral, topical, and intracavernosal) as a way to either enhance bioavailability or to improve and promote the local effects of medications [6].
1.3. Side Effects of Sildenafil
Of the above mentioned drugs, sildenafil is the one that has exhibited a higher incidence of visual side effects, given that it is only 10-fold more potent on PDE5 than on PDE6 [11,31]. As an example, numerous case reports describing ocular side effects associated with the consumption of sildenafil can be found in the medical literature (Table 2) [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. Its nature of use, its frequent use, a possible overdosage beyond the recommended optimal dose and the advanced age of the patient with frequent associated vascular pathologies makes it necessary to mention some of the adverse effects observed by the intake of sildenafil. Sildenafil administered orally is rapidly absorbed and maximum plasma concentrations occur within 30–120 min. In Spain, the Centre for Information on Medicines reports that the recommended dose for patients with PAH is 20 mg three times a day. In contrast, for the treatment of ED, it should only be used before sexual relations, with an optimum single dose of 50 mg once a day for adults. The dose can be reduced to 25 mg or increased to 100 mg a day (maximum single dose) depending on individual tolerance and efficacy. However, the consumer could alter this prescription by taking a dose above the recommended level to achieve good results. Because of this, there is a need for control since an intake of 100 mg increases its toxicity five-fold [38]. Additionally, sildenafil pharmacokinetics can be modified by the concomitant use of other drugs such as inhibitors of the cytochrome P450 (CYP) 3A4 (e.g., macrolide antibiotics, calcium channel blockers, etc.) [51], which is the main enzyme responsible for its hepatic metabolization. Inhibition of CYP3A4 would elevate the plasma concentration of sildenafil, thereby also increasing the likelihood of unwanted side effects. These key drug-metabolizing enzymes often display genetic polymorphisms that contribute to the individual variability in drug safety and efficacy among patients and may represent a risk of drug–drug interactions [52].

Table 2.
Summary of case reports/series on sildenafil-induced visual side effects published on the last decade (2010–2020).
Overall, the most common adverse effects of sildenafil are strongly associated with its pharmacological nature as an inhibitor of PDE5 (headache, nasal congestion, ageing and dyspepsia) and as a weak inhibitor of PDE6 (visual impairment), being dose-dependent and observed in 6–18% of men taking sildenafil [53]. In this sense, visual side effects were reported in 3–11% of men taking 25–100 mg of sildenafil, 50% of men taking 200 mg and 100% of men taking 600 or 800 mg [31,54,55,56] (center for drug evaluation). Although subjective visual changes are common, studies on healthy volunteers [55,57], men with ED [54,58] and patients with previous visual pathologies such as age-related macular degeneration (AMD) [56] who were taking sildenafil either as a single dose [55,56,57] or following a long-term treatment [54,58] have not found significant differences in psychophysical testing of visual function, except for color discrimination, predominantly in the blue–green range, in some studies [59]. The effects on retinal function are shown as modest and transient visual symptoms, commonly reported as blue vision, increased sensitivity to lights and blurred vision, more often at high doses [41,60]. Karaarslan’s study has reported visual symptoms up to 21 days after taking sildenafil [41]. Although the cause of blue-tinted vision is unknown, it is thought that it can be related to PDE6 inhibition in the retina [61] but data are nonconclusive [62]. Because PDE5 is expressed in the endothelial and smooth muscle cells of the choroidal and retinal vessels, sildenafil may affect ocular blood flow [63]; thus, it is reasonable to think that may cause other visual symptoms apart from those derived from the nonselective inhibition of PDE6 [11,64]. In fact, severe effects such as an increase in intraocular pressure (IOP) [65,66,67], retinal and choroidal vasodilation and altered blood flow [68,69], and nonarteritic anterior ischemic optic neuropathy (NAION) [45,70,71] have been reported as a consequence of the intake of sildenafil. Since many of the symptoms are dose-dependent, further studies are needed to establish the dose above which adverse effects occur in sildenafil users.
The purpose of this literature review was to gather and summarize the information available on sildenafil clinical trials (CTs), focusing on the possible adverse effects related to different aspects of visual health.
2. Results
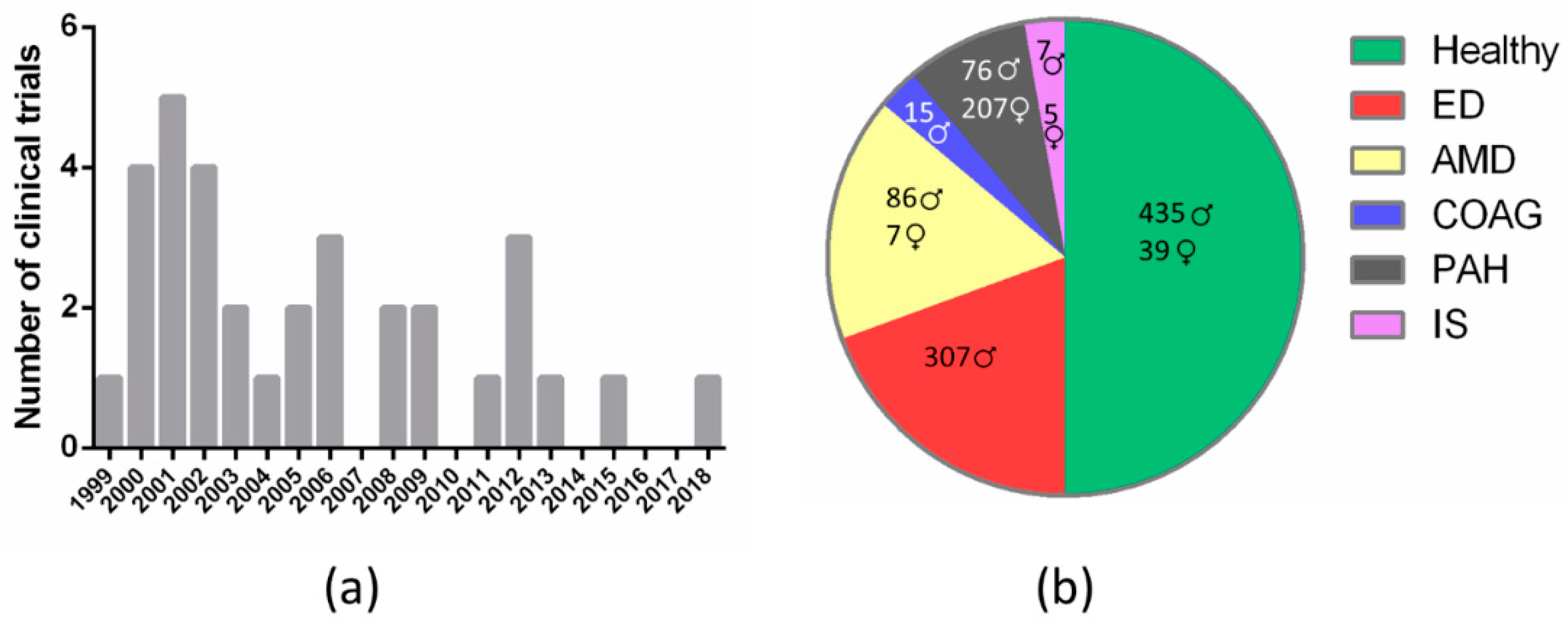
Given that sildenafil nonselective inhibition of retinal PDE6 results in visual disturbances, several reports have questioned the ocular safety of this drug over the last two decades [11,53,64,72,73]. Accordingly, several CTs have been conducted to evaluate the incidence of sildenafil-associated visual side effects, as well as its safety. Specifically, between the years 1999 and 2020, in the Cochrane Library (www.cochranelibrary.com, accessed on 20 December 2020) we retrieved 2001 entries including the term “sildenafil” in the title, abstract or keywords, of which we curated contents and selected all those results related with diverse features of the eye’s anatomy and physiology. The first CT on this topic “The effects of sildenafil citrate (Viagra®) on color discrimination in volunteers and patients with erectile dysfunction” (CN-00675062) was published in 1999 and over the following decade (2000–2009) a total of twenty-six CTs were conducted to assess adverse effects of sildenafil regarding visual health. Later on, the number of CTs declined and in the 2010–2019 decade only seven trials were registered (Figure 2a and Table 3). Studies carried out on healthy volunteers comprised most of the clinical trials (50%), although studies on ED or AMD patients were also widely represented (approximately 15% each). The remaining 20% is divided between PAH (10%), chronic open-angle glaucoma (COAG) (5%), and ischemic stroke (IS) (5%) patients (Figure 2b and Table 3).
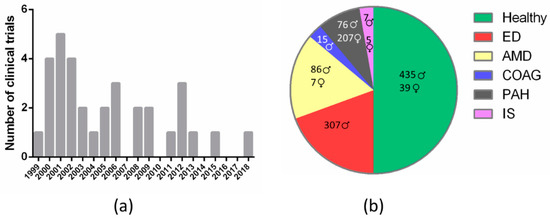
Figure 2.
Classification of clinical trials that assess sildenafil effects on visual health according to: (a) year of publication (frequency histogram); (b) medical condition (pie chart; results are disaggregated by gender). ED, erectile dysfunction; AMD, age-related macular degeneration; COAG, chronic open-angle glaucoma; PAH, pulmonary arterial hypertension; IS, ischemic stroke.

Table 3.
Summary of studies designed to assess the effects of sildenafil on vision.
Of the thirty-five registered CTs found on this subject in the US National Library of Medicine databases PubMed (www.pubmed.ncbi.nlm.nih.gov, accessed on 20 December 2020) and ClinicalTrials (www.clinicaltrials.gov, accessed on 20 December 2020), and in the Cochrane Library, twenty-six performed ophthalmic examinations, of which seven assessed ocular anatomy (external inspection of the eye, slit-lamp and fundoscopy), thirteen evaluated IOP, and six measured blood flow. Regarding visual function and perception, eleven CTs evaluated visual acuity (Snellen test), seven assessed contrast sensitivity (Pelli–Robson test), seven measured static perimetry (Humphrey visual field test), four performed electroretinogram (ERG), and nine assayed color perception. In some of the trials (five), the most common transient and subjective visual adverse events, derived from sildenafil consumption, were also evaluated. These included color perception distortion (blue color tinge, color discrimination alterations and tritanopia), changes in sensitivity to light, and blurred vision. In two of the CTs (NCT00461565, NCT01830790), the main outcomes were not publicly available (Table 3).
In general, long-term CTs mainly evaluate the effects of chronic sildenafil uptake on retinal function in patients with a previous pathology such as ED, early-stage AMD, or PAH. By contrast, studies that assessed the effects of a single acute dose of sildenafil were mostly carried out in healthy individuals. The frequency of ED increases dramatically with age and in the presence of cardiovascular risk factors. However, quantitative studies of side effects in both healthy volunteers and patients have produced mixed results.
2.1. Ophthalmologic Examination
2.1.1. Ocular Anatomy
Seven studies examined ocular anatomy (anterior and posterior chambers, lens and fundus) using a slit-lamp and/or a fundoscope (or ophthalmoscope). Most CTs were carried out on patients with previous pathologies such as ED [54,58,87,91], with the exception of the studies of Dündar et al. [57] and Yajima et al. [75], which were conducted on healthy volunteers. No study revealed clinically important and significant differences compared with placebo or baseline. Nevertheless, novel studies that question these results are emerging. In a case-control report, which included patients with PAH under chronic sildenafil medication and patients not taking the drug, 33% of the medicated patients showed severe bilateral keratitis. Connective tissue abnormalities are often present in PAH patients but this condition might be exacerbated with the use of sildenafil [69]. In a recent retrospective case report, carried out on seventeen men with ED taking sildenafil for the first time, pupil diameter assessment revealed symmetrical pupillary dilation for all patients, although approximately half of the subjects exhibited abnormally dilated pupils [41]. These current findings make it advisable to refer patients with chronic pathologies who are about to undergo sildenafil treatment for routine ophthalmic assessment with emphasis on the ocular surface evaluation.
2.1.2. Intraocular Pressure
Sildenafil inhibition of PDE5 leads to an increase in the concentration of cGMP, which, in animals, is known to lower IOP [98]. However, no clinically important acute adverse effects of sildenafil on IOP have been reported after 50–150 mg administration on a single [58,75,85] or on two separate doses [77,81,99]. Metelitsina et al. examined the foveal choroidal blood flow in men with AMD and, despite finding a decrease in blood pressure after 30 min of a single sildenafil dose, there were no relevant changes in IOP [86]. Regarding long-term studies, Dündar et al. assessed IOP after 3 months of sildenafil regular use in a group of men with ED and no significant changes were found in this case neither [54]. Likewise, no evident effect on IOP was found after chronic sildenafil administration over 2–4 years for the treatment of ED [91] or PAH [94]. On the other hand, Gerometta et al. studied the effect of 100 mg of sildenafil uptake as a single dose and detected an acute transient IOP increase 60 min later [64]. A possible explanation is that this transient rise could be due to an increase in choroidal volume induced by PDE5 inhibition (as mentioned above, PDE5 is expressed in endothelial cells of choroidal vessels) and might be of importance for patients chronically treated with sildenafil, especially glaucoma patients or individuals at high risk of developing the disease. Finally, Grunwald et al. studied the effect of sildenafil at the maximum therapeutic dose of 100 mg in patients with COAG and did not observe statistical nor clinical significant acute alterations in IOP, similarly to findings in placebo control subjects [78]. Therefore, most published reports have not demonstrated an association between sildenafil administration and IOP elevation, considering transient IOP elevations as coincidental.
2.1.3. Ocular Blood Flow
Sildenafil has a strong systemic vasodilating effect and it is known to decrease systemic blood pressure, which could lead to a decrease in choroidal blood flow [100]. However, since the choroid is a vascular tissue, similar to the corpus cavernosum, sildenafil could also have a strong vasodilatory effect, resulting in increased choroidal [101] and ciliary body perfusion via an increment in the posterior ciliary artery flow as a result of vascular smooth muscle relaxation [102]. The ophthalmic artery is the most responsive ocular artery after PDE5 inhibitors administration. Foresta et al. studied the acute effects of 100 mg sildenafil on the ophthalmic artery blood flow velocity and showed that sildenafil increased the flux in a time-dependent manner [63]. Dündar et al. evaluated the effect of a 50 mg dose of sildenafil in retrobulbar hemodynamics. They observed a significant increase in ophthalmic artery peak systolic velocity and in end-diastolic velocity that could be interpreted as an increase in volumetric blood flow [57]. Additionally, Sponsel et al. reported a significant increase in pulsatile choroidal blood flow 110 min after administration of a unique 50 mg dose of sildenafil [103], though the authors did not detect changes in retinal blood flow, neither in the central retinal nor in the temporal short posterior ciliary artery, in accordance with other studies [77,88,104]. Using laser Doppler flowmetry, Grunwald et al. did not find any significant changes in optic nerve head or foveolar choroidal blood flow neither 1 nor 5 h after sildenafil intake [77]. Therefore, although central retinal artery velocities were not changed, dilations of intraocular vasculature resulted in an increase in the mean ocular blood flow after sildenafil intake [84].
Most studies suggest an increase in choroidal blood flow velocity, with a lesser effect on the retinal vasculature in healthy subjects. This may be due to the production of NO, which plays a key role in the local regulation of ocular blood flow, and probably as a consequence of PDE5 inhibition in smooth muscle cells in a time-dependent manner [85]. It is worth noting that Dündar et al. also assessed the effects of sildenafil on ocular hemodynamics of healthy subjects in the long-term and reported no significant changes with chronic use, reflecting a mere temporary vasodilator effect without altering the orbital vasculature [54,87]. Nevertheless, these effects might have clinical consequences and may constitute a risk for ocular ischemia in patients with previous choroidal circulation problems, as is the case of central serous chorioretinopathy or AMD. In fact, there are reports regarding the development of unilateral and bilateral chorioretinopathy upon the use of sildenafil that resolved spontaneously [105]. PDE5 inhibitors are classified as only a possible risk factor for the development of central serous chorioretinopathy though [105], given that no strong evidence has been found in any of the studies performed to date [106]. In AMD, degeneration of the choroid and choroidal microcirculation (choriocapillaris) occurs with age [107] and choroidal blood flow is decreased [108]. As mentioned previously, choroidal blood flow and thickness may increase in response to sildenafil intake, thus sildenafil treatment is suggested as a means of increasing choroidal perfusion so that some CT-treated AMD patients with systemic sildenafil [97]. Birch et al. examined the acute effect of sildenafil administration in patients with early-stage AMD and observed no significantly or clinically relevant changes in visual function [56]. Furthermore, individuals taking sildenafil showed similar vasodilatation values of major retinal veins as the placebo group [88] and no statistically significant changes were detected in the foveolar choroidal circulation of AMD patients [86]. Finally, Coleman et al. evaluated the effect of sildenafil over 2 years in patients with macular degeneration or macular dystrophy and observed maintenance or even an improvement in the photoreceptor layer, concluding that sildenafil is a safe treatment for AMD that offers significant potential for vision retention and recovery [97]. Additionally, several case reports of NAION have been described in patients receiving sildenafil [109] but the relationship of ischemia to drug intake is not clear.
2.2. Visual Function and Perception
2.2.1. Visual Acuity
Eleven CTs evaluated the best-corrected visual acuity (BCVA) following sildenafil administration in healthy people [56,57,76,91], AMD [86,90,97], ED [54,58,87,91] and PAH patients [93,94]. The main finding of both acute trials and open-label extension studies was that chronic oral sildenafil treatment did not seem to result in any significant loss of visual acuity. Furthermore, Coleman et al. observed that participants with best vitelliform eruptive macular degeneration remained not only visually stable but a significant improvement of BCVA was also reported [97]. These findings are in agreement with reports in the literature that describe no significant clinical changes in several test scores such as visual acuity and color vision in PAH patients under a chronic sildenafil routine [69]. Conversely, we have found a recent case report of a 37-year-old woman with a history of primary PAH and a 5-year history of oral sildenafil intake who developed outer macular atrophy and exhibited a severe reduction in visual acuity in her left eye [50]. This research is the first one showing an association between long-term use of sildenafil and severe ocular side effects. Thus, it is necessary to warn about the chronic use of sildenafil and its potential risk of adverse visual outcomes.
2.2.2. Color Vision or Discrimination
Nine CTs evaluated color discrimination using different tests. Five studies were carried out on healthy volunteers [55,59,62,79,91], two in AMD [56,90] and three in ED patients [58,59,91]. Most CTs showed no alterations in color vision after taking sildenafil. Supporting these findings, in a recent study where the color vision was assessed upon chronic use of sildenafil in PAH patients, no significant effects were found [69]. However, the remaining CTs failed to confirm these outcomes. In a double-blind placebo-controlled trial, possible acute effects in color discrimination from 1 to 36 h after taking sildenafil (50–200 mg) were assessed in 16 healthy volunteers. Color perception was measured with Farnsworth–Munsell 100-Hue test (FM-100-Hue). A statistically significant and transient increase in FM 100-Hue error scores was noted at 1 h (100 and 200 mg doses) and 2 h (200 mg) after the consumption of sildenafil. Impaired blue–green color discrimination (induced tritanopia) was detected and the error scores correlated with plasma sildenafil concentration [59]. Similar results were found in a case-control unmasked study, where color perception was measured with the Lanthony desaturated Panel D-15 test. Compared with controls, a higher percentage of the subjects who were taking sildenafil committed more errors from the baseline to the 1-hour testing session. This difference was slightly and statistically significant [79]. Recently, it was reported the case of a 57-year-old man who, upon taking a single 100 mg dose of sildenafil for radical prostatectomy, experienced a sensation of unusual brightness of incoming visual stimulation combined with abnormal color vision that persisted beyond 5 h. These effects fully resolved within 7 days after discontinuing sildenafil [37]. Similarly, in a recent case-series study with 17 ED participants who took a single 100 mg sildenafil oral dose for the first time, visual color disturbances were reported by more than 75% of subjects. They did not reach the 90-point normal threshold in color vision assessment, where 5 of these patients had at least one score indicative of a definite impairment [41]. Such cases suggest that a relatively small subpopulation of people are at risk of disturbingly intense side effects upon intake of PDE inhibitors, which supports the practice of starting on a modest dose when prescribed this kind of drugs.
2.2.3. Contrast Sensitivity
Seven CTs evaluated contrast sensitivity in healthy people [62,80,82] and AMD [86,97], ED [58], and PAH patients [94] after sildenafil administration. Two of those studies revealed changes in contrast sensitivity. In a randomized, double-blind, placebo-controlled trial, using a monitor-based color vision test based on a luminance noise technique, cones with different wavelengths were selectively stimulated. Despite the fact that very small, nonsignificant threshold differences from predose baselines were found between the sildenafil and placebo groups for all three cone types, a statistically significant increase in sensitivity was observed during transient tritanopia, which correlated with sildenafil’s peak plasma concentration [62]. Similarly, in a randomized, double-masked placebo-controlled trial carried out in four healthy individuals who took a single oral dose of 50 mg of sildenafil, an 80% increase in contrast sensitivity compared with baseline was reported [80]. These findings are in the same direction as Karaaslan’s study, where 35% of individuals experienced a transient contrast sensitivity impairment and one individual experienced disability that spontaneously disappeared within 5 days [41]. No statistically or clinically significant differences were reported in the other CTs [58,82,86,94,97]. It is worth noting about Coleman’s study that, despite no significant changes in the Pelli–Robson chart test, all participants self-reported improvements in contrast sensitivity and the best vitelliform eruptive macular degeneration patients could see the chart with both eyes. However, the positive effects found in the studies were fully reversible within a few days.
2.2.4. Humphrey Perimetry Test
The 30-2 program on the Humphrey visual field (HVF) analyzer was used to assess the visual field in seven CTs. Three of them were carried out in healthy volunteers [55,74,91], one in AMD [56], one in PAH [94] and two in ED patients [87,91]. In a randomized prospective case-control study, HVF was carried out in the right and left eyes of eight healthy volunteers with both white-on-white (W/W) and blue-on-yellow (B/Y) protocols. One individual who experienced systemic side effects (headache, dizziness, nausea) also developed quadrantanopic field defects, more pronounced on B/Y than on W/W, between 1 and 2 h after taking a single dose of sildenafil [55,74]. No significant or clinical changes were reported in the rest of the CTs. These favorable results suggest that acute sildenafil administration, as well as long-term intake, is not associated with a compromised visual function such as the static perimetry assessment. Despite this, it is clear that more acute and long-term studies are desirable to investigate possible functional/structural effects of this drug both in healthy people and people with different pathologies.
2.2.5. Visual Disturbances: Light Sensitivity, Blurred Vision and Blue Color Tinge
Five CTs evaluated the presence or absence of the most common visual adverse effects such as light sensitivity changes, blurred vision and blue-tinted vision in healthy subjects [62,79], in AMD [56], ED [58] and PAH patients [94]. Luu et al. reported that 8 out of 14 healthy volunteers who received a single oral dose of 200 mg of sildenafil experienced varied subjective visual disturbances, while the placebo control subjects reported no visual disturbances [79]. The visual adverse effects frequently occurred within 1 to 2 h after drug consumption, highlighting an increased light sensitivity (in 5 out of 14 individuals). Other adverse effects included blue-tinted vision, red and blue speckled vision and blurred vision, which were reported by only one subject each. All these visual transient disturbances appeared to be dose-dependent. In the same direction, a double-masked, open-label trial, described mild to moderate-severe adverse transient events, although the incidence was low in all participants with the exception of one individual (in the sildenafil 80 mg group) who developed severe photophobia 72 days after the start of the study [94]. Slight incidence (<7%) of chromatopsia, cyanopsia, photophobia, and visual disturbances after administration of 80 mg of sildenafil three times daily were reported. It is well known that, generally, these visual disturbances resolve within 5 h. A recent case-series study with 17 ED patients reported visual disturbances that persisted more than 24 h in response to a single 100 mg dose of sildenafil [41]. More than 50% of participants exhibited some degree of clinical photophobia, including one severe and one very severe presentation. A high dose of sildenafil (maximum recommended therapeutic dose for ED) may be the cause for the extended durations of visual secondary effects.
2.2.6. Scotopic and Photic (ERG) Responses
Sildenafil intake can be expected to inhibit the phototransduction process, thus inducing changes in the ERG. However, only minimal changes were observed at sildenafil doses ranging from 50 to 200 mg compared with placebo [110]. Indeed, the assessment of the effect of 100 mg of sildenafil in healthy subjects [111] and in ED patients [112] 1 h after oral intake showed a reversible transient decrease in the amplitude of the a- and b-waves (rod-driven). In the ED plus sildenafil group, the treatment also increased full-field ERG implicit times of the scotopic b-wave that were not considered clinically significant [112]. No significant changes in implicit times were observed in healthy subjects [111]. Conversely, other studies reported transient, modest, dose-related increased photopic, but not scotopic, implicit times with a cone function slightly depressed in the macula and the periphery in healthy individuals receiving a single 100 or 200 mg dose of sildenafil [79,113]. Jägle et al. suggested from their ERG results that, when receiving a single 100 mg dose of sildenafil, inner retinal function was affected and prolonged implicit times of rods and cones showed no significant differences, whereas rod responses 1 h after sildenafil intake were raised too [62], contrary to previous reports about significant, transient reductions in the maximum response amplitudes of a- and b-waves [111,114]. Similarly, other studies supported a higher rod sensitivity and a higher rod response to light stimuli, as recorded by ERG 1 and 2 h after the intake of 50 or 100 mg of sildenafil. These findings are consistent with the weak PDE6 inhibition induced by sildenafil [114]. However, all these acute effects on the ERG are not clinically significant in terms of altered light sensitivity or visual function. Cordell et al. studied chronic PDE5 inhibition over 3 to 6 months on a daily basis of 50 mg of sildenafil and demonstrated no evidence of increased implicit times or decreased ERG amplitudes [91]. Furthermore, Zoumalan et al. studied the chronic daily use of sildenafil at higher doses (120–300 mg) for 1–4 years and did not find any toxic effect, only a modest lengthening of cone implicit time that seemed to be restored a few hours later, indicating that any possible retinal toxicity or visual disturbances of sildenafil may be reversible in the short term [115]. These inconsistencies in the effects in ERG recordings may be due, in part, to the use of different doses and group heterogeneity. Taken together, the ERG results suggest that sildenafil doses of 25 or 50 mg entail minimal visual side effects, and at maximum therapeutic doses, sildenafil can cause acute and transient changes in rod and/or cone function without a practical effect on visual performance.
3. Discussion
A growing body of evidence points to cGMP as one of the main players in inherited retinal diseases and oxidative stress-induced retinal degeneration. Therefore, it seems reasonable to think that the disruption of retinal cGMP concentration and subsequent Ca2+ homeostasis can be detrimental to photoreceptor survival. PDE5 inhibitors such as sildenafil are often used for the treatment of ED and PAH. Although sildenafil exhibits a high selectivity for PDE5, in high doses it is also capable of binding and inhibiting PDE6 nonselectively. Inhibition of both isoenzymes, PDE5 and PDE6, is the main cause of sildenafil visual side effects. PDE5 is expressed in some ocular tissues such as the endothelial cells of retinal and choroidal vessels. PDE6 is exclusively expressed in photoreceptors and its inhibition directly alters the phototransduction cascade due to an increase in cGMP levels. This idea has prompted many scientists and researchers to conduct CT to evaluate the safety and the visual side effects of sildenafil. From 1999, the moment when the first CT assessing visual parameters after sildenafil uptake was published, many other studies have been released and, although visual disturbances have been extensively reported, all of them seem to be transient and mild. However, many case reports regarding ocular side effects linked to sildenafil consumption have recently arisen in the medical literature (see Table 2) [34,37,38,40,41,61,106,116]. It seems that a small subset of individuals experience more severe effects either with a low dose but chronic use of sildenafil (as for the treatment of PAH) or with a high dose but sporadic use of the drug (as for the treatment of ED). Among the different factors that could influence individual sensitivity to sildenafil are gene polymorphisms of CYP3A4 and CYP2C9, the two major sildenafil-matabolizing hepatic enzymes [53]. This draws attention to the necessity of designing and conducting novel CTs where other populations are also represented. For instance, the majority of the CTs were carried out in small group samples and preferentially in males (see Figure 2b). It is obvious that ED affects only males; however, PAH or AMD affect both males and females and, therefore, it is interesting and necessary that both genders are equally represented in the CTs.
Additionally, evidence from preclinical studies carried out in animal models of human retinal diseases suggests that sildenafil consumption can be detrimental in some cases [5,117,118,119,120,121]. For instance, Nivinson-Smith et al. tested the effects of sildenafil on visual function in mice heterozygous for the rd1 mutation, which affects the PDE6 β-subunit [117]. The rd1 mutation causes autosomal recessive retinitis pigmentosa, thereby carriers of the mutation do not display a disease phenotype. cGMP metabolism is altered in these individuals, rendering them more susceptible to retinal degeneration from external metabolic or oxidative stress. In their study, Nivinson-Smith et al. showed that sildenafil caused a significant dose-dependent decrease in photoreceptor ERG responses of wild type mice, which recovered within 48 h. However, decreased photoreceptor ERG responses of heterozygous rd1 mice (Pde6b+/rd1) did not resolve until two weeks postadministration of the drug [117]. Behn et al. obtained very similar results using heterozygous PDE6 γ-subunit knockout mice (Pde6g+/tm1), another murine model of autosomal recessive retinitis pigmentosa [118]. Likewise, Pierce et al. administered a high dose of sildenafil citrate to dogs heterozygous for a functionally null mutation in PDE6 α-subunit (Pde6a) over a 4-month period. Despite the low number of animals used in their experiment, the results were statistically significant, showing that sildenafil-treated Pde6a+/− dogs exhibited thinner outer nuclear layers and lower photoreceptor counts than untreated Pde6a+/− dogs [119]. These data become especially relevant if we take into account that approximately 1 in 50 people are likely to be carriers of recessive traits leading to retinal degeneration. To date, no studies have been conducted in retinitis pigmentosa/cone-dystrophy patients or even in individuals who have normal vision but carry one allele for the disease. In a different paradigm, Eltony and Abdelhameed investigated the effect of chronic daily use of sildenafil on the histology of the retina and optic nerve of adult male rats and showed that sildenafil caused microglia activation, vacuolation and congested blood capillaries with apoptotic endothelial and pericytic cells, although partial recovery was observed after drug withdrawal [120]. Similar results were reported by Vatansever et al., who treated adult male rats with sildenafil for 4 weeks and observed dilatation and congestion in the choroidal vasculature, although no major changes were detected in retinal cytoarchitecture [121]. Therefore, in order to precisely exclude possible risks in these groups, it would be advisable to perform more research both at the preclinical and clinical levels.
Important regulatory agencies such as the U.S. Food and Drug Administration (FDA, www.fda.gov, accessed on 20 December 2020) and the European Medicines Agency (EMA, www.ema.europa.eu, accessed on 20 December 2020), and associations of eye physicians and surgeons such as the American Academy of Ophthalmology (www.aao.org, accessed on 20 December 2020) warn about the lack of controlled clinical data on the safety of sildenafil in patients with retinitis pigmentosa or with a family history of the disease. Thereby, it is essential that general practitioners supervise the treatment of a medical condition such as ED and guarantee a safe use of PDE5 inhibitors. The possibility of illegally purchasing online sildenafil and their analogues brings up relevant issues such as the risks linked to the irrational use of medicines. This is one of the factors that has prompted some countries to consider the reclassification of sildenafil from prescription-only medicine to a pharmacy medicine. Among the countries whose regulatory authorities have already taken that step are New Zealand in 2014 (Medicines and Medical Devices Safety Authority, Medsafe); the United Kingdom in 2017 (Medicines and Healthcare products Regulatory Agency, MHRA); Norway in 2019 (Norwegian Medicines Agency, NoMA); Ireland in 2020 (Health Products Regulatory Authority, HPRA). Although in these countries sildenafil can be sold without prescription, pharmacists receive specific training so they can provide proper guidance and request patients who answer some of the questions in the affirmative to contact their general practitioner for further assessment [122]. The aim of this practice is to lower the burden on general practitioners and, at the same time, to make the medication accessible while still keeping the risk of misuse and side effects low.
Finally, alternatives that minimize unwanted side effects should be pursued by scientists in general and by the pharmaceutical industry in particular. These may include, among others, the design, screening and development of drugs highly selective for PDE5 with no inhibitory effects on other PDE isoenzymes [123]; the investigation of new formulations that improve bioavailability [124]; the search of novel drug-delivery systems that allow a local vs. systemic application [29]; or the advancement in the field of pharmacogenomics, which would contribute to the implementation of a more precise and personalized medicine.
In conclusion, from the literature review we can affirm that visual side effects derived from the consumption of sildenafil are generally mild and transient, but the cessation of sildenafil therapy is advised if certain rare conditions such as central serous chorioretinopathy or NAION appear. Moreover, caution should be taken in patients with a family history of retinal dystrophy because available evidence in animal research supports the hypothesis that carriers of some recessive alleles are more sensitive to sildenafil toxicity.
Author Contributions
Conceptualization, V.G.-V.; investigation, E.A., G.E. and V.G.-V.; data curation, E.A. and G.E.; figures/tables preparation, G.E and E.A.; writing—original draft preparation, E.A., G.E., and V.G.-V.; writing—review and editing, V.G.-V.; supervision, V.G.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, because of the retrospective nature of the study, using information that was freely available in the public domain.
Informed Consent Statement
Patient consent was waived because of the retrospective nature of the study, involving clinical data that were properly anonymized at the time of original data collection.
Acknowledgments
We acknowledge Andrea Page Arribas for assistance with manuscript preparation; figures were created with BioRender and GraphPad Prism. References were formatted with Mendeley Web.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AMD | Age-related macular degeneration |
| BCVA | Best-corrected visual acuity |
| CDU | Color Doppler ultrasonography |
| CNG | Cyclic nucleotide-gated (channel) |
| COAG | Chronic open-angle glaucoma |
| CT | Clinical trial |
| CYP | Cytochrome P450 |
| ED | Erectile dysfunction |
| ERG | Electroretinogram |
| cGMP | Cyclic guanosine monophosphate |
| FM | Farnsworth-Munsell (test) |
| GC | Guanylyl cyclase |
| GCAP | Guanylyl cyclase-activating protein |
| HVF | Humphrey visual field (test) |
| IS | Ischemic stroke |
| IOP | Intraocular pressure |
| NAION | Nonarteritic anterior ischemic optic neuropathy |
| NCKX | Potassium-dependent sodium-calcium exchanger |
| NO | Nitric oxide |
| PAH | Pulmonary arterial hypertension |
| PDE | Phosphodiesterase |
| PKG | Protein kinase G |
References
- Vinberg, F.; Chen, J.; Kefalov, V.J. Regulation of calcium homeostasis in the outer segments of rod and cone photoreceptors. Prog. Retin. Eye Res. 2018, 67, 87–101. [Google Scholar] [CrossRef]
- Power, M.; Das, S.; Schütze, K.; Marigo, V.; Ekström, P.; Paquet-Durand, F. Cellular mechanisms of hereditary photoreceptor degeneration – Focus on cGMP. Prog. Retin. Eye Res. 2020, 74, 100772. [Google Scholar] [CrossRef]
- Blom, J.; Giove, T.; Deshpande, M.; Eldred, W.D. Characterization of nitric oxide signaling pathways in the mouse retina. J. Comp. Neurol. 2012, 520, 4204–4217. [Google Scholar] [CrossRef]
- Tolone, A.; Belhadj, S.; Rentsch, A.; Schwede, F.; Paquet-Durand, F. The cGMP pathway and inherited photoreceptor degeneration: Targets, compounds, and biomarkers. Genes 2019, 10, 453. [Google Scholar] [CrossRef]
- Yang, P.; Lockard, R.; Titus, H.; Hiblar, J.; Weller, K.; Wafai, D.; Weleber, R.G.; Duvoisin, R.M.; Morgans, C.W.; Pennesi, M.E. Suppression of cGMP-dependent photoreceptor cytotoxicity with mycophenolate is neuroprotective in murine models of retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2020, 61. [Google Scholar] [CrossRef]
- Wang, T.; Tsang, S.H.; Chen, J. Two pathways of rod photoreceptor cell death induced by elevated cGMP. Hum. Mol. Genet. 2017, 26, 2299–2306. [Google Scholar] [CrossRef]
- Chang, B.; Hawes, N.L.; Pardue, M.T.; German, A.M.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Rengarajan, K.; Boyd, A.P.; Sidney, S.S.; et al. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vision Res. 2007, 47, 624–633. [Google Scholar] [CrossRef]
- Paquet-Durand, F.; Hauck, S.M.; Van Veen, T.; Ueffing, M.; Ekström, P. PKG activity causes photoreceptor cell death in two retinitis pigmentosa models. J. Neurochem. 2009, 108, 796–810. [Google Scholar] [CrossRef]
- Lolley, R.; Farber, D.; Rayborn, M.; Hollyfield, J. Cyclic GMP accumulation causes degeneration of photoreceptor cells: Simulation of an inherited disease. Science 1977, 196, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Michalakis, S.; Becirovic, E.; Biel, M. Retinal cyclic nucleotide-gated channels: From pathophysiology to therapy. Int. J. Mol. Sci. 2018, 19, 749. [Google Scholar] [CrossRef]
- Bischoff, E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int. J. Impot. Res. 2004, 16, S11–S14. [Google Scholar] [CrossRef]
- Boswell-Smith, V.; Spina, D.; Page, C.P. Phosphodiesterase inhibitors. Br. J. Pharmacol. 2006, 147, 252–257. [Google Scholar] [CrossRef]
- Conti, M.; Jin, S.-L.C. The Molecular Biology of Cyclic Nucleotide Phosphodiesterases. In Progress in Nucleic Acid Research and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 63, pp. 1–38. [Google Scholar]
- Zucchi, A.; Costantini, E.; Scroppo, F.I.; Silvani, M.; Kopa, Z.; Illiano, E.; Petrillo, M.G.; Cari, L.; Nocentini, G. The first-generation phosphodiesterase 5 inhibitors and their pharmacokinetic issue. Andrology 2019, 7, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Manganiello, V.C.; Murata, T.; Taira, M.; Belfrage, P.; Degerman, E. Diversity in Cyclic Nucleotide Phosphodiesterase Isoenzyme Families. Arch. Biochem. Biophys. 1995, 322, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Beavo, J.A. Cyclic nucleotide phosphodiesterases: Functional implications of multiple isoforms. Physiol. Rev. 1995, 75, 725–748. [Google Scholar] [CrossRef]
- Cote, R.H. Characteristics of Photoreceptor PDE (PDE6): Similarities and differences to PDE5. Int. J. Impot. Res. 2004, 16, S28–S33. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.R.; Kim, H.K.; Park, J.K. Penile Erection Induced by Scoparone from Artemisia capillaris through the Nitric Oxide-Cyclic Guanosine Monophosphate Signaling Pathway. World J. Mens. Health 2017, 35, 196. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, S.; Khraisha, O.; Al Madani, M.; Treece, J.; Baumrucker, S.J.; Paul, T.K. Sildenafil for Pulmonary Arterial Hypertension. Am. J. Ther. 2019, 26, e520–e526. [Google Scholar] [CrossRef]
- Hatzimouratidis, K.; Salonia, A.; Adaikan, G.; Buvat, J.; Carrier, S.; El-Meliegy, A.; McCullough, A.; Torres, L.O.; Khera, M. Pharmacotherapy for Erectile Dysfunction: Recommendations From the Fourth International Consultation for Sexual Medicine (ICSM 2015). J. Sex. Med. 2016, 13, 465–488. [Google Scholar] [CrossRef]
- Grice, P.T.; Liu, J.; Gabrielson, A.T.; Pearce, I.; Bivalacqua, T.J.; Modgil, V. Drug delivery options and therapeutic advances in the management of erectile dysfunction. Expert Opin. Drug Deliv. 2020, 17, 1259–1268. [Google Scholar] [CrossRef]
- Ferguson, J.E.; Carson, C.C. Phosphodiesterase type 5 inhibitors as a treatment for erectile dysfunction: Current information and new horizons. Arab J. Urol. 2013, 11, 222–229. [Google Scholar] [CrossRef]
- Anderson, K. PDE5 inhibitors-pharmacology and clinical applications 20 years after sildenafil discovery. Br. J. Pharmacol. 2018, 175, 2554–2565. [Google Scholar] [CrossRef]
- Corona, G.; Rastrelli, G.; Burri, A.; Jannini, E.A.; Maggi, M. The safety and efficacy of Avanafil, a new 2nd generation PDE5i: Comprehensive review and meta-analysis. Expert Opin. Drug Saf. 2016, 15, 237–247. [Google Scholar] [CrossRef]
- Taylor, J.; Baldo, O.B.; Storey, A.; Cartledge, J.; Eardley, I. Differences in side-effect duration and related bother levels between phosphodiesterase type 5 inhibitors. BJU Int. 2009, 103, 1392–1395. [Google Scholar] [CrossRef]
- Belew, D.; Klaassen, Z.; Lewis, R.W. Intracavernosal Injection for the Diagnosis, Evaluation, and Treatment of Erectile Dysfunction: A Review. Sex. Med. Rev. 2015, 3, 11–23. [Google Scholar] [CrossRef]
- Patel, C.K.; Bennett, N. Advances in the treatment of erectile dysfunction: What’s new and upcoming? F1000Research 2016, 5, 369. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cho, M.C.; Cho, S.Y.; Chung, H.; Rajasekaran, M.R. Novel Emerging Therapies for Erectile Dysfunction. World J. Mens. Health 2020, 38, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Ahmed, O.A.A.; Fahmy, U.A.; Ahmed, T.A. Solid lipid nanoparticles for transdermal delivery of avanafil: Optimization, formulation, in-vitro and ex-vivo studies. J. Liposome Res. 2016, 26, 288–296. [Google Scholar] [CrossRef]
- Patel, N.B.; Lim, M.; Gajjar, A.; Evans, K.B.; Harwerth, R.S. Age-Associated Changes in the Retinal Nerve Fiber Layer and Optic Nerve Head. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5134–5143. [Google Scholar] [CrossRef]
- Goldstein, I.; Burnett, A.L.; Rosen, R.C.; Park, P.W.; Stecher, V.J. The Serendipitous Story of Sildenafil: An Unexpected Oral Therapy for Erectile Dysfunction. Sex. Med. Rev. 2019, 7, 115–128. [Google Scholar] [CrossRef]
- Felekis, T.; Asproudis, I.; Katsanos, K.; Tsianos, E. A case of nonarteritic anterior ischemic optic neuropathy of a male with family history of the disease after receiving sildenafil. Clin. Ophthalmol. 2011, 5, 1443–1445. [Google Scholar] [CrossRef] [PubMed]
- Moschos, M.M.; Margetis, I. Bilateral Simultaneous Anterior Ischemic Optic Neuropathy Associated with Sildenafil. Case Rep. Ophthalmol. 2011, 2, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Yu, Y.J.; Liu, X.P.; Liu, N.P. Visual impairment with possible macular changes after a high dose of sildenafil in a healthy young woman. Int. J. Ophthalmol. 2017, 11, 340–342. [Google Scholar] [CrossRef]
- Papageorgiou, E.; Xanthou, F.; Fili, P.; Tsironi, E.E.; Androudi, S. Multimodal retinal imaging in a case of an unsuccessful suicide attempt with sildenafil. Clin. Toxicol. 2018, 56, 798–800. [Google Scholar] [CrossRef]
- Rickmann, A.; Macek, M.A.; Szurman, P.; Boden, K. Acute monocular loss of vision: Differential diagnostic considerations apart from the internistic etiological clarification. Ophthalmologe 2018, 115, 676–679. [Google Scholar] [CrossRef]
- Rosen, S.M.; Kaja, S.; De Alba, F. Association of Transient Colorblindness with Sildenafil and Tadalafil. JAMA Ophthalmol. 2019, 137, 117–118. [Google Scholar] [CrossRef]
- Yanoga, F.; Gentile, R.C.; Chui, T.Y.P.; Freund, K.B.; Fell, M.; Dolz-Marco, R.; Rosen, R.B. Sildenafil Citrate Induced Retinal Toxicity-Electroretinogram, Optical Coherence Tomography, and Adaptive Optics Findings. Retin. Cases Brief Rep. 2018, 12, S33–S40. [Google Scholar] [CrossRef]
- Brader, H.S.; Athappilly, G.K.; Loewenstein, J. Retinal Toxicity Associated with Excessive Sildenafil Ingestion. JAMA Ophthalmol. 2019, 137, 326–328. [Google Scholar] [CrossRef]
- Mohammadpour, M.; Khodaparast, M.; Khorrami-Nejad, M. Central serous chorioretinopathy following ingestion of sildenafil citrate. Clin. Optom. 2019, 11, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Karaarslan, C. Ocular Side Effects of Sildenafil That Persist Beyond 24 h—A Case Series. Front. Neurol. 2020, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, A.; Warner, J. Case of Bilateral Sequential Nonarteritic Ischemic Optic Neuropathy After Rechallenge With Sildenafil. J. Neuroophthalmol. 2018, 38, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Izadi, S.; De Silva, S.R.; Sculfor, D.; Benjamin, L.; Downes, S.M. Persistant bilateral relative central scotomas induced by taking an excessive dose of sildenafil. Acta Ophthalmol. 2012, 90, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, A.; Faraoni, A.; Menchini, F.; Lanzetta, P. Bilateral simultaneous nonarteritic anterior ischemic optic neuropathy after ingestion of Sildenafil for erectile dysfunction. Case Rep. Med. 2012, 2012, 747658. [Google Scholar] [CrossRef] [PubMed]
- Gaffuri, M.; Cristofaletti, A.; Mansoldo, C.; Biban, P. Acute onset of bilateral visual loss during sildenafil therapy in a young infant with congenital heart disease. BMJ Case Rep. 2014, 1–3. [Google Scholar] [CrossRef]
- Karli, S.Z.; Liao, S.D.; Carey, A.R.; Lam, B.L.; Wester, S.T. Optic neuropathy associated with the use of over-the-counter sexual enhancement supplements. Clin. Ophthalmol. 2014, 8, 2171–2175. [Google Scholar] [CrossRef]
- Matheeussen, V.; Maudens, K.E.; Anseeuw, K.; Neels, H. A non-fatal self-poisoning attempt with sildenafil. J. Anal. Toxicol. 2015, 39, 572–576. [Google Scholar] [CrossRef]
- Coca, M.N.; Morgan, M.L.; Gupta, P.; Elkeeb, A.; Lee, A.G. Bilateral posterior ischemic optic neuropathy associated with the use of Sildenafil for pulmonary hypertension. Can. J. Ophthalmol. 2016, 51, e96–e99. [Google Scholar] [CrossRef]
- Jayadev, C.; Ramasastry, P.; Gul, A.; Vinekar, A. Possible Role of Sildenafil Citrate in the Recurrence of Neovascularization in Laser-regressed Aggressive Posterior ROP. Indian Pediatr. 2016, 53, S155–S156. [Google Scholar]
- Sajjad, A.; Weng, C.Y. Vision loss in a patient with primary pulmonary hypertension and long-term use of sildenafil. Retin. Cases Br. Rep. 2017, 11, 325–328. [Google Scholar] [CrossRef]
- Dresser, G.K.; Spence, J.D.; Bailey, D.G. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin. Pharmacokinet. 2000, 38, 41–57. [Google Scholar] [CrossRef]
- Tang, P.F.; Zheng, X.; Hu, X.X.; Yang, C.C.; Chen, Z.; Qian, J.C.; Cai, J.P.; Hu, G.X. Functional measurement of CYP2C9 and CYP3A4 allelic polymorphism on sildenafil metabolism. Drug Des. Devel. Ther. 2020, 14, 5129–5141. [Google Scholar] [CrossRef]
- Moschos, M.M.; Nitoda, E. Pathophysiology of visual disorders induced by phosphodiesterase inhibitors in the treatment of erectile dysfunction. Drug Des. Devel. Ther. 2016, 10, 3407–3413. [Google Scholar] [CrossRef] [PubMed]
- Dündar, S.O.; Dayanir, Y.; Topaloğlu, A.; Dündar, M.; Koçak, I. Effect of sildenafil on ocular hemodynamics in 3 months regular use. Int. J. Impot. Res. 2006, 18, 282–286. [Google Scholar] [CrossRef] [PubMed]
- McCulley, T.J.; Lam, B.L.; Marmor, M.F.; Hoffman, K.B.; Luu, J.K.; Feuer, W.J. Acute effects of sildenafil (viagra) on blue-on-yellow and white-on-white Humphrey perimetry. J. Neuroophthalmol. 2000, 20, 227–228. [Google Scholar] [CrossRef]
- Birch, D.G.; Toler, S.M.; Swanson, W.H.; Fish, G.E.; Laties, A.M. A double-blind placebo-controlled evaluation of the acute effects of sildenafil citrate (VIAGRA) on visual function in subjects with early-stage age-related macular degeneration. Am. J. Ophthalmol. 2002, 133, 665–672. [Google Scholar] [CrossRef]
- Dündar, S.O.; Dündar, M.; Koçak, I.; Dayanir, Y.; Özkan, S.B. Effect of sildenafil on ocular haemodynamics. Eye 2001, 15, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, E.; Koppiker, N.P.; Smith, M.D.; Constable, I.; Littlewood, R.S.B. The effects of long-term sildenafil treatment on ocular safety in patients with erectile dysfunction. Investig. Ophthalmol. Vis. Sci. 2000, 41, S592, Association for Research in Vision and Ophthalmology Annual Meeting, abstract 3147. [Google Scholar]
- Laties, A.M.; Ellis, P.M.J. The effets of sildenafil citrate (Viagra®) on color discrimination in volunteers and patients with erectile dysfunction. Investig. Ophthalmol. Vis. Sci. 1999, 40. Association for Research in Vision and Ophthalmology Annual Meeting, abstract 3660. [Google Scholar]
- Lee, C.H.; Yoon, J.S.; Ji, E. A Case Report of Cyanopsia after Taking Sildenafil. Korean J. Clin. Pharm. 2020, 30, 59–64. [Google Scholar] [CrossRef]
- Omori, K.; Kotera, J. Overview of PDEs and their regulation. Circ. Res. 2007, 100, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Jägle, H.; Jägle, C.; Sérey, L.; Yu, A.; Rilk, A.; Sadowski, B.; Besch, D.; Zrenner, E.; Sharpe, L.T. Visual short-term effects of viagra: Double-blind study in healthy young subjects. Am. J. Ophthalmol. 2004, 137, 842–849. [Google Scholar] [CrossRef]
- Foresta, C.; Caretta, N.; Zuccarello, D.; Poletti, A.; Biagioli, A.; Caretti, L.; Galan, A. Expression of the PDE5 enzyme on human retinal tissue: New aspects of PDE5 inhibitors ocular side effects. Eye 2008, 22, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kerr, N.M.; Danesh-Meyer, H.V. Phosphodiesterase inhibitors and the eye. Clin. Exp. Ophthalmol. 2009, 37, 514–523. [Google Scholar] [CrossRef]
- Gerometta, R.; Alvarez, L.J.; Candia, O.A. Effect of sildenafil citrate on intraocular pressure and blood pressure in human volunteers. Exp. Eye Res. 2011, 93, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.; Taghavi Tabrizi, Y.; Mokhtaree, M. Effect of periodic sildenafil dosage on intraocular pressure in patients with erectile dysfunction. Electron. Physician 2017, 9, 5229–5232. [Google Scholar] [CrossRef]
- Baker, J.C.; Fintelmann, R.; Sharifi, R.; Lee, M. Precautions and monitoring of patients taking phosphodiesterase type 5 inhibitors who are at risk of increased intraocular pressure. Drugs Aging 2019, 36, 991–997. [Google Scholar] [CrossRef]
- Berrones, D.; Salcedo-Villanueva, G.; Morales-Cantón, V.; Velez-Montoya, R. Changes in retinal and choroidal vascular blood flow after oral sildenafil: An optical coherence tomography angiography study. J. Ophthalmol. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Matieli, L.; Berezovsky, A.; Salomão, S.R.; Allemann, N.; Martins, E.N.; Hirai, F.E.; Ota-Arakaki, J.; Morales, M.S.A.; de Freitas, D. Ocular toxicity assessment of chronic sildenafil therapy for pulmonary arterial hypertension. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhu, L.; Zhong, J.; Zeng, G.; Deng, T. The association between phosphodiesterase type 5 inhibitor use and risk of non-arteritic anterior ischemic optic neuropathy: A systematic review and meta-analysis. Sex. Med. 2018, 6, 185–192. [Google Scholar] [CrossRef]
- Prat, N.M.; Sánchez-Dalmau, B.F.; Foroozan, R. Not Just For Men. Surv. Ophthalmol. 2011, 56, 173–177. [Google Scholar] [CrossRef]
- Marmor, M.F.; Kessler, R. Sildenafil (Viagra) and Ophthalmology. Surv. Ophthalmol. 1999, 44, 153–162. [Google Scholar] [CrossRef]
- Azzouni, F.; Abu, S.K. Are phosphodiesterase type 5 inhibitors associated with vision-threatening adverse events? a critical analysis and review of the literature. J. Sex. Med. 2011, 8, 2894–2903. [Google Scholar] [CrossRef]
- Hoffman, K.B.; McCulley, T.J.; Lam, B.L.; Marmor, M.F.; Luu, J.L.; Feuer, W. The effect of sildenafil (viagra) on Humphrey visual field testing. Investig. Ophthalmol. Vis. Sci. 2000, 41, 180. [Google Scholar]
- Yajima, T.; Yajima, Y.; Koppiker, N.; Grunwald, J.E.; Laties, A.M. No clinically important effects on intraocular pressure after short-term administration of sildenafil citrate (Viagra). Am. J. Ophthalmol. 2000, 129, 675–676. [Google Scholar] [CrossRef]
- Dündar, M.; Koçak, I.; Dündar, S.O.; Erol, H. Evaluation of side effects of sildenafil in group of young healthy volunteers. Int. Urol. Nephrol. 2001, 32, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, J.E.; Siu, K.K.; Jacob, S.S.; Dupont, J. Effect of sildenafil citrate (Viagra) on the ocular circulation. Am. J. Ophthalmol. 2001, 131, 751–755. [Google Scholar] [CrossRef]
- Grunwald, J.E.; Jacob, S.S.; Siu, K.; Piltz, J.; Dupont, J. Acute effects of sildenafil citrate (Viagra®) on intraocular pressure in open-angle glaucoma. Am. J. Ophthalmol. 2001, 132, 872–874. [Google Scholar] [CrossRef]
- Luu, J.K.; Chappelow, A.V.; Mcculley, T.J.; Marmor, M.F. Acute effects of sildenafil on the electroretinogram and multifocal electroretinogram. Am. J. Ophthalmol. 2001, 132, 388–394. [Google Scholar] [CrossRef]
- Friedman, M.; Applegate, R.; Sponsel, W.E.; McKinnon, S.J.; Gross, H.; Trigo, Y.; Pena, M. Effects of Sildenafil Citrate on Optical Properties of the Eye. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3250. [Google Scholar]
- Grunwald, J.E.; Metelitsina, T.; Grunwald, L. Effect of sildenafil citrate (Viagra) on retinal blood vessel diameter. Am. J. Ophthalmol. 2002, 133, 809–812. [Google Scholar] [CrossRef]
- McCulley, T.J.; Luu, J.K.; Marmor, M.F.; Feuer, W.J. Effects of sildenafil citrate (Viagra) on choroidal congestion. Ophthalmologica 2002, 216, 455–458. [Google Scholar] [CrossRef]
- Mollon, J.D.; Regan, B.C.; Foo, R.; Morris, B.J. Sildenafil Increases Persistence of Vision. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4097. [Google Scholar]
- Polak, K.; Wimpissinger, B.; Berisha, F.; Georgopoulos, M.; Schmetterer, L. Effects of Sildenafil on Retinal Blood Flow and Flicker-Induced Retinal Vasodilatation in Healthy Subjects. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4872–4876. [Google Scholar] [CrossRef]
- Koksal, M.; Ozdemir, H.; Kargi, S.; Yesilli, C.; Tomaç, S.; Mahmutyazicioglu, K.; Mungan, A. The effects of sildenafil on ocular blood flow. Acta Ophthalmol. Scand. 2005, 83, 355–359. [Google Scholar] [CrossRef]
- Metelitsina, T.I.; Grunwald, J.E.; DuPont, J.C.; Ying, G.S. Effect of Viagra on the foveolar choroidal circulation of AMD patients. Exp. Eye Res. 2005, 81, 159–164. [Google Scholar] [CrossRef]
- Dündar, S.O.; Topalo Gcaron Lu, A.; Dündar, M.; Koçak, I. Effects of sildenafil on blue-on-yellow and white-on-white Humphrey perimetry in 3 months regular use. Eye 2006, 20, 810–813. [Google Scholar] [CrossRef]
- Metelitsina, T.I.; Grunwald, J.E.; DuPont, J.C.; Ying, G.-s.; Liu, C. Effect of viagra on retinal vein diameter in AMD patients. Exp. Eye Res. 2006, 83, 128–132. [Google Scholar] [CrossRef]
- A Double Blind, Randomized, Placebo Controlled, Two Part, Two Session Balanced, Crossover Study to Evaluate Visual Changes in Healthy Male Subjects Aged 18–55 Years after Receiving: 1. at Least 15 Doses of 20 mg Vardenafil, Compared to Placebo and 2. Two Doses of Sildenafil, 200 mg Compared to Placebo. Available online: https://clinicaltrials.gov/ct2/show/NCT00461565 (accessed on 16 December 2020).
- Ibrahim, N.M.S.; Hashem, H.A.; Helal, A.Y. Evaluation of the acute effect of Sildenafil citrate on visual function in patients with early-stage age-related macular degeneration. Int. J. Ophthalmol. 2009, 9, 824–827. [Google Scholar] [CrossRef]
- Cordell, W.H.; Maturi, R.K.; Costigan, T.M.; Marmor, M.F.; Weleber, R.G.; Coupland, S.G.; Danis, R.P.; McGettigan, J.W.; Antoszyk, A.N.; Klise, S.; et al. Retinal effects of 6 months of daily use of tadalafil or sildenafil. Arch. Ophthalmol. 2009, 127, 367–373. [Google Scholar] [CrossRef]
- Silver, B.; McCarthy, S.; Lu, M.; Mitsias, P.; Russman, A.N.; Katramados, A.; Morris, D.C.; Lewandowski, C.A.; Chopp, M. Sildenafil treatment of subacute ischemic stroke: A safety study at 25-mg daily for 2 weeks. J. Stroke Cerebrovasc. Dis. 2009, 18, 381–383. [Google Scholar] [CrossRef]
- A Phase 3, Multi-Center, Open-Label Study To Investigate Safety, Efficacy, and Tolerability Of Sildenafil Citrate in Pediatric Patients with Pulmonary Arterial Hypertension. Available online: https://clinicaltrials.gov/ct2/show/NCT01642407 (accessed on 16 December 2020).
- Wirostko, B.M.; Tressler, C.; Hwang, L.J.; Burgess, G.; Laties, A.M. Ocular safety of sildenafil citrate when administered chronically for pulmonary arterial hypertension: results from phase III, randomised, double masked, placebo controlled trial and open label extension. BMJ 2012, 344, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Effects of Sildenafil on Choroidal Thickness in Age-Related Macular Degeneration. Available online: https://clinicaltrials.gov/ct2/show/NCT01830790 (accessed on 16 December 2020).
- A Phase 1, Open-Label, Within-Subject Dose-Escalation Study to Evaluate the Clinical Vulvar-Vaginal Safety and Pharmacokinetic Profile of SST-6006, a Topical Sildenafil Cream (5% w/w), in Healthy Postmenopausal Women. Available online: https://clinicaltrials.gov/ct2/show/NCT02364882 (accessed on 16 December 2020).
- Coleman, D.J.; Lee, W.; Chang, S.; Silverman, R.H.; Lloyd, H.O.; Daly, S.; Tsang, S.H. Treatment of Macular Degeneration with Sildenafil: Results of a Two-Year Trial. Ophthalmologica 2018, 240, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, J.A. Nitrovasodilators as a new class of ocular hypotensive agents. J. Pharmacol. Exp. Ther. 1992, 260, 956–965. [Google Scholar]
- Siu, K.; Grunwald, J.E.; Jacob, S.S.; Dupont, J. Effect of Viagra on ocular circulation. Investig. Ophthalmol. Vis. Sci. 2000, 41, 514. [Google Scholar]
- Kim, D.Y.; Silverman, R.H.; Chan, R.V.P.; Khanifar, A.A.; Rondeau, M.; Lloyd, H.; Schlegel, P.; Coleman, D.J. Measurement of choroidal perfusion and thickness following systemic sildenafil (Viagra®). Acta Ophthalmol. 2013, 91, 183–188. [Google Scholar] [CrossRef]
- Harris, A.; Kagemann, L.; Ehrlich, R.; Ehrlich, Y.; López, C.R.; Purvin, V.A. The effect of sildenafil on ocular blood flow. Br. J. Ophthalmol. 2008, 92, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Paris, G.; Sponsel, W.E.; Sandoval, S.S.; Elliott, W.R.; Trigo, Y.; Sanford, D.K.; Harrison, J.M. Sildenafil increases ocular perfusion. Int. Ophthalmol. 2001, 23, 355–358. [Google Scholar] [CrossRef]
- Sponsel, W.E.; Paris, G.; Sandoval, S.S.; Sanford, D.K.; Harrison, J.M.; Elliott, W.R.; Trigo, Y. Sildenafil and Ocular Perfusion. N. Engl. J. Med. 2000, 342, 1680. [Google Scholar] [CrossRef] [PubMed]
- Kurtulan, E.; Gulcu, A.; Secil, M.; Celebi, I.; Aslan, G.; Esen, A.A. Effects of sildenafil on ocular perfusion demonstrated by color Doppler ultrasonography. Int. J. Impot. Res. 2004, 16, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Aliferis, K.; Petropoulos, I.K.; Farpour, B.; Matter, M.A.; Safran, A.B. Should central serous chorioretinopathy be added to the list of ocular side effects of phosphodiesterase 5 inhibitors? Ophthalmologica 2012, 227, 85–89. [Google Scholar] [CrossRef]
- Damar, E.; Toklu, Y.; Tuncel, A.; Balci, M.; Aslan, Y.; Simsek, S.; Atan, A. Does Therapeutic Dose of Sildenafil Citrate Treatment Lead to Central Serous Chorioretinopathy in Patients With Erectile Dysfunction? Am. J. Mens. Health 2013, 7, 439–443. [Google Scholar] [CrossRef]
- Lipecz, A.; Miller, L.; Kovacs, I.; Czakó, C.; Csipo, T.; Baffi, J.; Csiszar, A.; Tarantini, S.; Ungvari, Z.; Yabluchanskiy, A.; et al. Microvascular contributions to age-related macular degeneration (AMD): From mechanisms of choriocapillaris aging to novel interventions. GeroScience 2019, 41, 813–845. [Google Scholar] [CrossRef] [PubMed]
- Trinh, M.; Kalloniatis, M.; Nivison-Smith, L. Vascular Changes in Intermediate Age-Related Macular Degeneration Quantified Using Optical Coherence Tomography Angiography. Transl. Vis. Sci. Technol. 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, H.D. Erectile Dysfunction Agents and Nonarteritic Anterior Ischemic Optic Neuropathy. Neurol. Clin. 2017, 35, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Laties, A.M.; Zrenner, E. Viagra® (sildenafil citrate) and ophthalmology. Prog. Retin. Eye Res. 2002, 21, 485–506. [Google Scholar] [CrossRef]
- Vobig, M.A.; Klotz, T.; Staak, M.; Bartz-Schmidt, K.U.; Engelmann, U.; Walter, P. Retinal side-effects of sildenafil. Lancet 1999, 353, 375. [Google Scholar] [CrossRef]
- Kretschmann, C.; Gockein, R.; Meschi, M.; Stief, C.G.; Winter, R. Short time influences of sildenafil on visual function. Investig. Ophthalmol. Vis. Sci. 1999, 40, S766, Association for Research in Vision and Ophthalmology Annual Meeting, abstract 4047. [Google Scholar]
- Jägle, H.; Jägle, C.; Sèrey, L.; Sharpe, L.T. Dose-dependency and time-course of electrophysiologic short-term effects of VIAGRA®: A case study. Doc. Ophthalmol. 2005, 110, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Gabrieli, C.B.; Regine, F.; Vingolo, E.M.; Rispoli, E.; Fabbri, A.; Isidori, A. Subjective visual halos after sildenafil (Viagra) administration: Electroretinographic evaluation. Ophthalmology 2001, 108, 877–881. [Google Scholar] [CrossRef]
- Zoumalan, C.I.; Zamanian, R.T.; Doyle, R.L.; Marmor, M.F. ERG evaluation of daily, high-dose sildenafil usage. Doc. Ophthalmol. 2009, 118, 225–231. [Google Scholar] [CrossRef]
- van Landingham, S.W.; Singman, E.L. A case report: Consecutive cranial neuropathies following the use of phosphodiesterase-5 inhibitors. Am. Orthopt. J. 2015, 65, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Nivison-Smith, L.; Zhu, Y.; Whatham, A.; Bui, B.V.; Fletcher, E.L.; Acosta, M.L.; Kalloniatis, M. Sildenafil alters retinal function in mouse carriers of Retinitis Pigmentosa. Exp. Eye Res. 2014, 128, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Behn, D.; Potter, M.J. Sildenafil-mediated reduction in retinal function in heterozygous mice lacking the γ-subunit of phosphodiesterase. Investig. Ophthalmol. Vis. Sci. 2001, 42, 523–527. [Google Scholar] [PubMed]
- Pierce, K.E.; Curran, P.G.; Zelinka, C.P.; Fischer, A.J.; Petersen-Jones, S.M.; Bartoe, J. Sildenafil Administration in Dogs Heterozygous for a Functional Null Mutation in Pde6a: Suppressed Rod-Mediated ERG Responses and Apparent Retinal Outer Nuclear Layer Thinning. Adv. Exp. Med. Biol. 2019, 1185, 371–376. [Google Scholar]
- Eltony, S.A.; Abdelhameed, S.Y. Effect of chronic administration of sildenafil citrate (Viagra) on the histology of the retina and optic nerve of adult male rat. Tissue Cell 2017, 49, 323–335. [Google Scholar] [CrossRef]
- Vatansever, H.; Kayikcioglu, O.; Gumus, B. Histopathologic effect of chronic use of sildenafil citrate on the choroid & retina in male rats. Indian J. Med. Res. 2003, 117, 211–215. [Google Scholar]
- Braund, R.; Ratnayake, K.; Tong, K.; Song, J.; Chai, S.; Gauld, N. Pharmacist supply of sildenafil: Pharmacists’ experiences and perceptions on training and tools for supply. Int. J. Clin. Pharm. 2018, 40, 650–658. [Google Scholar] [CrossRef]
- Kayık, G.; Tüzün, N.; Durdagi, S. Investigation of PDE5/PDE6 and PDE5/PDE11 selective potent tadalafil-like PDE5 inhibitors using combination of molecular modeling approaches, molecular fingerprint-based virtual screening protocols and structure-based pharmacophore development. J. Enzyme Inhib. Med. Chem. 2017, 32, 311–330. [Google Scholar] [CrossRef]
- Hosny, K.M.; Alhakamy, N.A.; Almodhwahi, M.A.; Kurakula, M.; Almehmady, A.M.; Elgebaly, S.S. Self-nanoemulsifying system loaded with sildenafil citrate and incorporated within oral lyophilized flash tablets: Preparation, optimization, and in vivo evaluation. Pharmaceutics 2020, 12, 1124. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).