MicroRNA Signatures Associated with Bronchopulmonary Dysplasia Severity in Tracheal Aspirates of Preterm Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Tracheal Aspirate Collection
2.3. MicroRNA Purification
2.4. MicroRNA Arrays
2.5. Ingenuity Pathway Analysis
2.6. MicroRNA Expression Validation
2.7. Statistical Analysis and Code Availability
3. Results
3.1. Patient Demographics
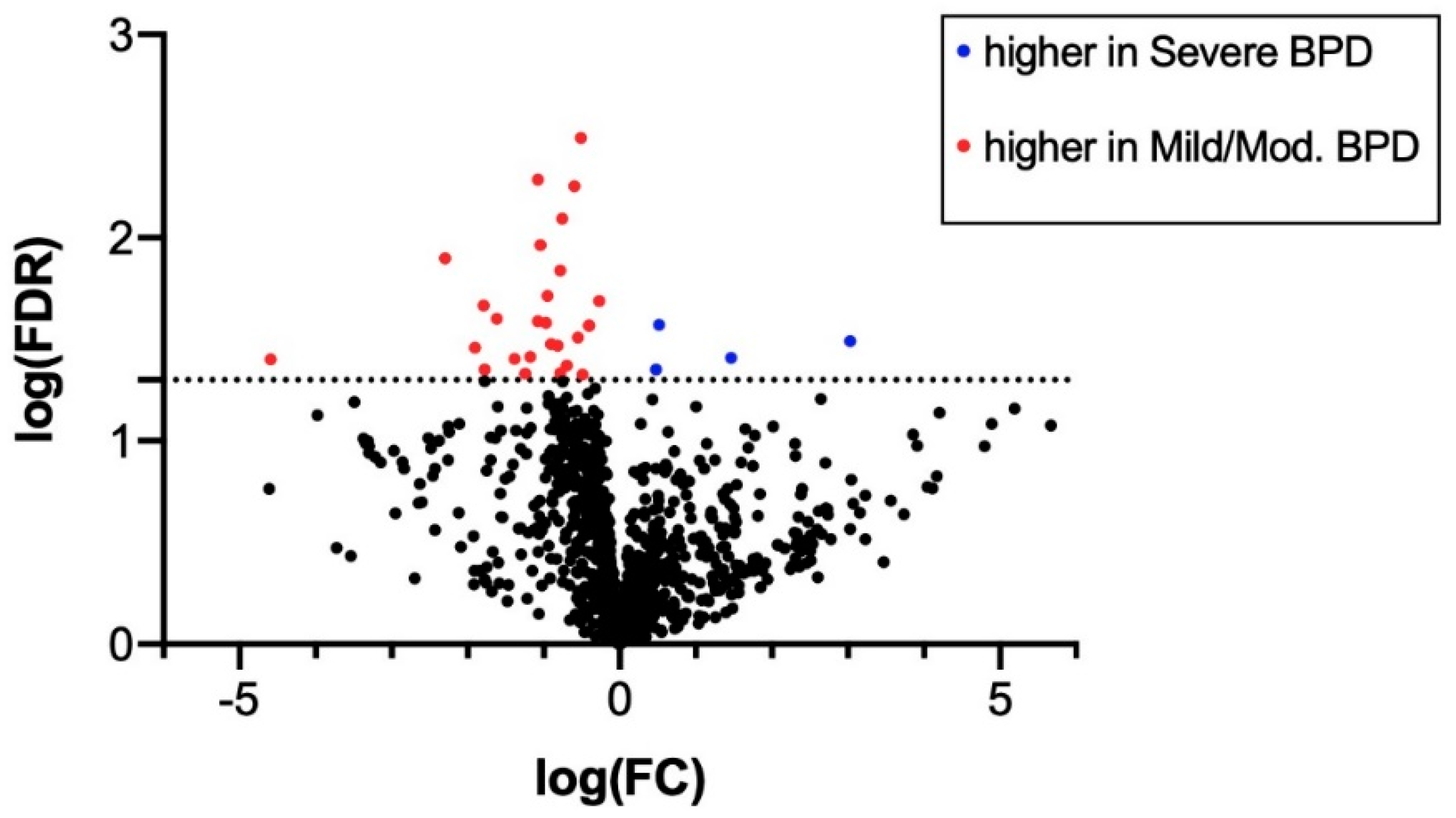
3.2. MiRNA Expression in TAs
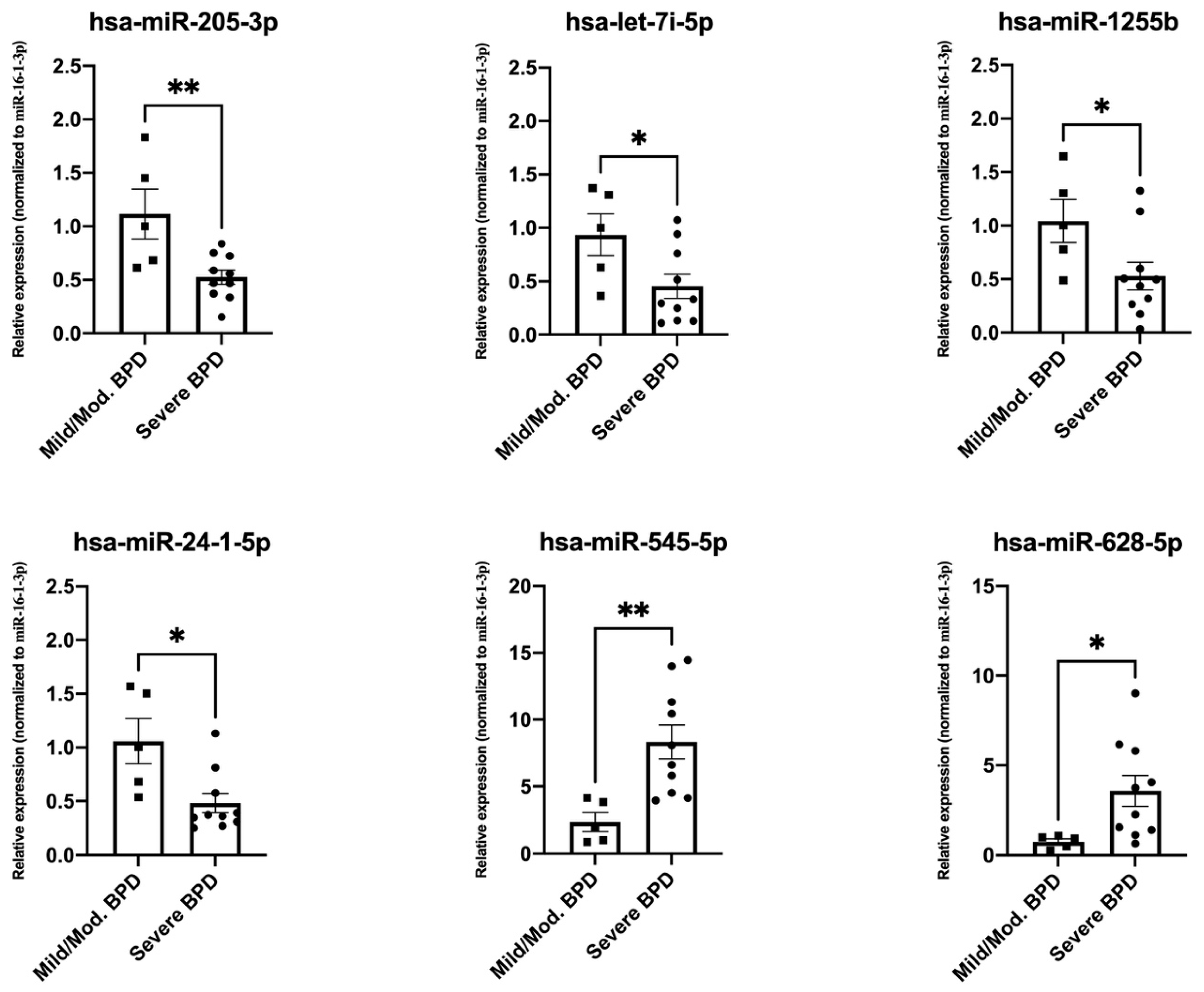
3.3. Validation of Top Differentially Expressed miRNAs
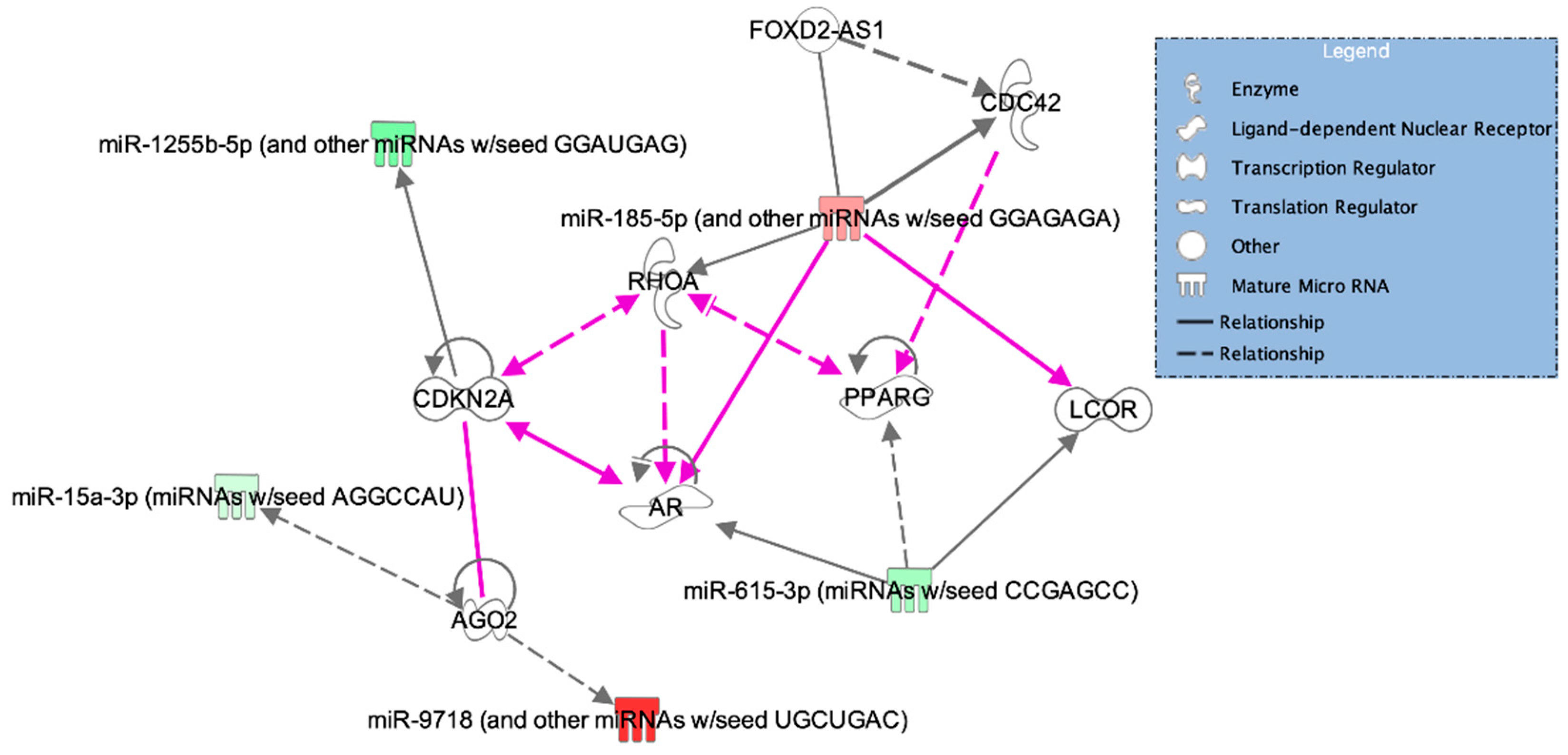
3.4. Pathway Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jobe, A.J. The new BPD: An arrest of lung development. Pediatr. Res. 1999, 46, 641–643. [Google Scholar] [CrossRef]
- Siffel, C.; Kistler, K.D.; Lewis, J.F.; Sarda, S.P. Global incidence of bronchopulmonary dysplasia among extremely preterm infants: A systematic literature review. J. Mater. Fetal Neonat. Med. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Narang, I. Review series: What goes around, comes around: Childhood influences on later lung health? Long-term follow-up of infants with lung disease of prematurity. Chron. Respir. Dis. 2010, 7, 259–269. [Google Scholar] [CrossRef]
- Northway, W.H., Jr.; Rosan, R.C.; Porter, D.Y. Pulmonary disease following respirator therapy of hyaline-membrane disease: Bronchopulmonary dysplasia. N. Engl. J. Med. 1967, 276, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Sahni, M.; Bhandari, V. Recent advances in understanding and management of bronchopulmonary dysplasia. F1000Research 2020, 9, F1000 Faculty Rev-703. [Google Scholar] [CrossRef] [PubMed]
- Schmatz, M.; Madan, J.; Marino, T.; Davis, J. Maternal obesity: The interplay between inflammation, mother and fetus. J. Perinatol. 2010, 30, 441–446. [Google Scholar] [CrossRef]
- Pelzer, E.; Gomez-Arango, L.F.; Barrett, H.L.; Nitert, M.D. Review: Maternal health and the placental microbiome. Placenta 2017, 54, 30–37. [Google Scholar] [CrossRef]
- Viscardi, R.M. Perinatal inflammation and lung injury. Semin. Fetal Neonat. Med. 2012, 17, 30–35. [Google Scholar] [CrossRef]
- Check, J.; Gotteiner, N.; Liu, X.; Su, E.; Porta, N.; Steinhorn, R.; Mestan, K.K. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J. Perinatol. 2013, 33, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Sillers, L.; Alexiou, S.; Jensen, E.A. Lifelong pulmonary sequelae of bronchopulmonary dysplasia. Curr. Op. Pediatr. 2020, 32, 252–260. [Google Scholar] [CrossRef]
- Doyle, L.W.; Cheong, J.L.; Ehrenkranz, R.A.; Halliday, H.L. Late (>7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Coch. Database Syst. Rev. 2017, 10, CD001145. [Google Scholar] [CrossRef]
- Onland, W.; De Jaegere, A.P.; Offringa, M.; van Kaam, A. Systemic corticosteroid regimens for prevention of bronchopulmonary dysplasia in preterm infants. Coch. Database Syst. Rev. 2017, 1, CD010941. [Google Scholar] [CrossRef]
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, R.A.; Walsh, M.C.; Vohr, B.R.; Jobe, A.H.; Wright, L.L.; Fanaroff, A.A.; Wrage, L.A.; Poole, K. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005, 116, 1353–1360. [Google Scholar] [CrossRef]
- Rivera, L.; Siddaiah, R.; Oji-Mmuo, C.; Silveyra, G.R.; Silveyra, P. Biomarkers for bronchopulmonary dysplasia in the preterm infant. Front. Pediatr. 2016, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Been, J.V.; Debeer, A.; van Iwaarden, J.F.; Kloosterboer, N.; Passos, V.L.; Naulaers, G.; Zimmermann, L.J. Early alterations of growth factor patterns in bronchoalveolar lavage fluid from preterm infants developing bronchopulmonary dysplasia. Pediatr. Res. 2010, 67, 83–89. [Google Scholar] [CrossRef]
- Piersigilli, F.; Lam, T.T.; Vernocchi, P.; Quagliariello, A.; Putignani, L.; Aghai, Z.H.; Bhandari, V. Identification of new biomarkers of bronchopulmonary dysplasia using metabolomics. Metabolomics 2019, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Oji-Mmuo, C.N.; Siddaiah, R.; Montes, D.T.; Pham, M.A.; Spear, D.; Donnelly, A.; Fuentes, N.; Imamura-Kawasawa, Y.; Howrylak, J.A.; Thomas, N.J.; et al. Tracheal aspirate transcriptomic and miRNA signatures of extreme premature birth with bronchopulmonary dysplasia. J. Perinatol. 2020. [Google Scholar] [CrossRef]
- Choo-Wing, R.; Syed, M.A.; Harijith, A.; Bowen, B.; Pryhuber, G.; Janér, C.; Andersson, S.; Homer, R.J.; Bhandari, V. Hyperoxia and interferon-γ-induced injury in developing lungs occur via cyclooxygenase-2 and the endoplasmic reticulum stress-dependent pathway. Am. J. Respir. Cell Mol. Biol. 2013, 48, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Britt, R.D.; Velten, M.; Tipple, T.E.; Nelin, L.D.; Rogers, L.K. Cyclooxygenase-2 in newborn hyperoxic lung injury. Free Radic. Biol. Med. 2013, 61, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Dong, W. SIRT1-Related Signaling Pathways and Their Association With Bronchopulmonary Dysplasia. Front. Med. 2021, 8, 181. [Google Scholar] [CrossRef]
- Sucre, J.M.S.; Vickers, K.C.; Benjamin, J.T.; Plosa, E.J.; Jetter, C.S.; Cutrone, A.; Ransom, M.; Anderson, Z.; Sheng, Q.; Fensterheim, B.A.; et al. Hyperoxia injury in the developing lung is mediated by mesenchymal expression of Wnt5A. Am. J. Respir. Crit. Care Med. 2020, 201, 1249–1262. [Google Scholar] [CrossRef]
- Ameis, D.; Khoshgoo, N.; Iwasiow, B.M.; Snarr, P.; Keijzer, R. MicroRNAs in lung development and disease. Paediatr. Respir. Rev. 2017, 22, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.; Qiao, L.; Olave, N.; Rezonzew, G.; Gentle, S.; Halloran, B.; Pryhuber, G.S.; Gaggar, A.; Tipple, T.E.; Ambalavanan, N.; et al. MicroRNA 219-5p inhibits alveolarization by reducing platelet derived growth factor receptor-alpha. Respir. Res. 2021, 22, 57. [Google Scholar] [CrossRef]
- Pavlek, L.R.; Vudatala, S.; Bartlett, C.W.; Buhimschi, I.A.; Buhimschi, C.S.; Rogers, L.K. MiR-29b is associated with perinatal inflammation in extremely preterm infants. Pediatr. Res. 2020. [Google Scholar] [CrossRef]
- Go, H.; Maeda, H.; Miyazaki, K.; Maeda, R.; Kume, Y.; Namba, F.; Momoi, N.; Hashimoto, K.; Otsuru, S.; Kawasaki, Y. Extracellular vesicle miRNA-21 is a potential biomarker for predicting chronic lung disease in premature infants. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L845–L851. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.V.; Olave, N.; Travers, C.; Rezonzew, G.; Dolma, K.; Simpson, A.; Halloran, B.; Aghai, Z.; Das, P.; Sharma, N. Exosomal microRNA predicts and protects against severe bronchopulmonary dysplasia in extremely premature infants. JCI Insight 2018, 3, e93994. [Google Scholar] [CrossRef] [PubMed]
- Wei, W. Research progress of relationship between miRNA and neonatal lung injury. Int. J. Pediatr. 2018, 45, 520–523. [Google Scholar]
- Sun, Y.-F.; Ma, L.; Gong, X.-H.; Hong, W.-C.; Cai, C. Expression of microRNA-495-5p in preterm infants with bronchopulmonary dysplasia: A bioinformatics analysis. Zhongguo Dang Dai Er Ke Za Zhi 2020, 22, 24–30. [Google Scholar]
- McEvoy, C.T.; Jain, L.; Schmidt, B.; Abman, S.; Bancalari, E.; Aschner, J.L. Bronchopulmonary dysplasia: NHLBI Workshop on the primary prevention of chronic lung diseases. Annu. Am. Thorac. Soc. 2014, 11, S146–S153. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Roy, A.; Mishra, V.; Cabello, N.; Silveyra, P. Sex-specific microRNA expression networks in an acute mouse model of ozone-induced lung inflammation. Biol. Sex Differ. 2018, 9, 18. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; da Silva, A.M.; Calin, G.; Pantel, K. Data normalization strategies for MicroRNA quantification. Clin. Chem. 2015, 61, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux, R.; Seoighe, C. A flexible R package for nonnegative matrix factorization. BMC Bioinform. 2010, 11, 1–9. [Google Scholar] [CrossRef]
- Abman, S.H.; Collaco, J.M.; Shepherd, E.G.; Keszler, M.; Cuevas-Guaman, M.; Welty, S.E.; Truog, W.E.; McGrath-Morrow, S.A.; Moore, P.E.; Rhein, L.M.; et al. Interdisciplinary care of children with severe bronchopulmonary dysplasia. J. Pediatr. 2017, 181, 12–28. [Google Scholar] [CrossRef]
- McKinney, R.L.; Shukla, K.; Daigle, K.; Zeigler, J.; Muller, M.; Keszler, M. The BIT:S (Bronchopulmonary Dysplasia Interdisciplinary Team: Severe) initiative at women and infants hospital of rhode island. R. I. Med. J. 2019, 102, 22–25. [Google Scholar]
- Londhe, V.A.; Sundar, I.K.; Lopez, B.; Maisonet, T.M.; Yu, Y.; Aghai, Z.H.; Rahman, I. Hyperoxia impairs alveolar formation and induces senescence through decreased histone deacetylase activity and up-regulation of p21 in neonatal mouse lung. Pediatr. Res. 2011, 69, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Kroon, A.A.; Wang, J.; Kavanagh, B.P.; Kavanagh, B.; Huang, Z.; Kuliszewski, M.; van Goudoever, J.B.; Post, M. Prolonged mechanical ventilation induces cell cycle arrest in newborn rat lung. PLoS ONE 2011, 6, e16910. [Google Scholar] [CrossRef]
- Du, B.; Wang, Z.; Zhang, X.; Feng, S.; Wang, G.; He, J.; Zhang, B. MicroRNA-545 suppresses cell proliferation by targeting cyclin D1 and CDK4 in lung cancer cells. PLoS ONE 2014, 9, e88022. [Google Scholar] [CrossRef]
- Takahashi, Y.; Forrest, A.R.; Maeno, E.; Hashimoto, T.; Daub, C.O.; Yasuda, J. MiR-107 and MiR-185 can induce cell cycle arrest in human non small cell lung cancer cell lines. PLoS ONE 2009, 4, e6677. [Google Scholar] [CrossRef] [PubMed]
- Carnino, J.M.; Lee, H.; He, X.; Groot, M.; Jin, Y. Extracellular vesicle-cargo miR-185-5p reflects type II alveolar cell death after oxidative stress. Cell Death Discov. 2020, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lee, H.; Cao, Y.; Dela Cruz, C.S.; Jin, Y. miR-185 mediates lung epithelial cell death after oxidative stress. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L700–L710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, Y.; Liu, K. miR-185 inhibits cell migration and invasion of hepatocellular carcinoma through CDC42. Oncol. Lett. 2018, 16, 3101–3107. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Liu, J.; Yin, W.; Jin, D.; Wang, D.; Zhang, W. miR-185 Inhibits the proliferation and invasion of non-small cell lung cancer by targeting KLF7. Oncol. Res. 2019, 27, 1015–1023. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Z.; Su, L.; Wang, F.; Jiang, Y.; Yu, D.; Zhang, F.; Liang, W. miRNA-185 serves as a prognostic factor and suppresses migration and invasion through Wnt1 in colon cancer. Eur. J. Pharmacol. 2018, 825, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lang, N.; Chen, X.; Tang, Q.; Liu, S.; Huang, J.; Zheng, Y.; Bi, F. miR-185 targets RhoA and Cdc42 expression and inhibits the proliferation potential of human colorectal cells. Cancer Lett. 2011, 301, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Chang, H.; Gao, G.; Zhang, B.; Li, X.; Jin, B. Long noncoding RNA FOXD2-AS1 promotes glioma malignancy and tumorigenesis via targeting miR-185-5p/CCND2 axis. J. Cell Biochem. 2019, 120, 9324–9336. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Li, Y.; Li, X.; Ma, J.; An, C.; Ma, Z. miR-185 affected the EMT, cell viability, and proliferation via DNMT1/MEG3 pathway in TGF-β1-induced renal fibrosis. Cell Biol. Int. 2019, 43, 1152–1162. [Google Scholar] [CrossRef]
- Lei, G.S.; Kline, H.L.; Lee, C.H.; Wilkes, D.S.; Zhang, C. Regulation of collagen V expression and epithelial-mesenchymal transition by mir-185 and mir-186 during idiopathic pulmonary fibrosis. Am. J. Pathol. 2016, 186, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fu, J.; Xue, X.; Yao, L.; Qiao, L.; Hou, A.; Jin, L.; Xing, Y. Epithelial-mesenchymal transitions in bronchopulmonary dysplasia of newborn rats. Pediatr. Pulmonol. 2014, 49, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Bartis, D.; Mise, N.; Mahida, R.Y.; Eickelberg, O.; Thickett, D.R. Epithelial-mesenchymal transition in lung development and disease: Does it exist and is it important? Thorax 2014, 69, 760–765. [Google Scholar] [CrossRef]
- Lei, Z.; Shi, H.; Li, W.; Yu, D.; Shen, F.; Yu, X.; Lu, D.; Sun, C.; Liao, K. miR-185 inhibits non-small cell lung cancer cell proliferation and invasion through targeting of SOX9 and regulation of Wnt signaling. Mol. Med. Rep. 2018, 17, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Pongracz, J.E.; Stockley, R.A. Wnt signalling in lung development and diseases. Respir. Res. 2006, 7, 15. [Google Scholar] [CrossRef]
- De Langhe, S.P.; Reynolds, S.D. Wnt signaling in lung organogenesis. Organogenesis 2008, 4, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.S.; Noor, S.M.; Nagoor, N.H. mir-378 and mir-1827 regulate tumor invasion, migration and angiogenesis in human lung adenocarcinoma by targeting. J. Cancer 2018, 9, 331–345. [Google Scholar] [CrossRef]
- Machado, I.F.; Teodoro, J.S.; Palmeira, C.M.; Rolo, A.P. miR-378a: A new emerging microRNA in metabolism. Cell Mol. Life Sci. 2020, 77, 1947–1958. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H. microRNA-378a regulates the reactive oxygen species (ROS)/phosphatidylinositol 3-kinases (PI3K)/AKT signaling pathway in human lens epithelial cells and cataract. Med. Sci. Monit. 2019, 25, 4314–4321. [Google Scholar] [CrossRef]
- Revel, A.; Achache, H.; Stevens, J.; Smith, Y.; Reich, R. MicroRNAs are associated with human embryo implantation defects. Hum. Reprod. 2011, 26, 2830–2840. [Google Scholar] [CrossRef]
- Li, D.; Chen, S.; Zhang, W.; Zhang, C.; Sun, T.; Du, Y.; Ding, R.; Gao, Y.; Jin, Y.; Duan, G. MicroRNA-628-5p facilitates enterovirus 71 infection by suppressing TRAF3 signaling. Cell Mol. Immunol. 2020. [Google Scholar] [CrossRef]
- Xie, P.; Wang, Y.; Liao, Y.; Han, Q.; Qiu, Z.; Chen, Y.; Zuo, X. MicroRNA-628-5p inhibits cell proliferation in glioma by targeting DDX59. J. Cell Biochem. 2019, 120, 17293–17302. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qian, Z.; Ma, X.; Lin, X.; You, Y.; Li, Y.; Chen, T.; Jiang, H. MiR-628-5p decreases the tumorigenicity of epithelial ovarian cancer cells by targeting at FGFR2. Biochem. Biophys. Res. Commun. 2018, 495, 2085–2091. [Google Scholar] [CrossRef]
- Arman, E.; Haffner-Krausz, R.; Gorivodsky, M.; Lonai, P. Fgfr2 is required for limb outgrowth and lung-branching morphogenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 11895–11899. [Google Scholar] [CrossRef]
- Shimbori, C.; El Agha, E. Good things come in 2s: Type 2 alveolar epithelial cells and fibroblast growth factor receptor 2. Am. J. Respir. Cell Mol. Biol. 2020, 62, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Rios-Colon, L.; Deep, G.; Kumar, D. Emerging role of microRNA 628-5p as a novel biomarker for cancer and other diseases. Tumour Biol. 2019, 41. [Google Scholar] [CrossRef]
- Hao, R.; Hu, X.; Wu, C.; Li, N. Hypoxia-induced miR-15a promotes mesenchymal ablation and adaptation to hypoxia during lung development in chicken. PLoS ONE 2014, 9, e98868. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.J.; Di Fiore, J.M.; Walsh, M.C. Hypoxic episodes in bronchopulmonary dysplasia. Clin. Perinatol. 2015, 42, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, X.; Sun, J.; Su, W.; Zhang, L.; Li, Y.; Liu, Y.; Lu, Y.; Shan, H.; Liang, H. YAP1/Twist promotes fibroblast activation and lung fibrosis that conferred by miR-15a loss in IPF. Cell Death Differ. 2019, 26, 1832–1844. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Mereness, J.; Baran, A.; Misra, R.; Peterson, D.; Ryan, R.; Reynolds, A.; Pryhuber, G.; Mariani, T. Lymphocyte-specific biomarkers associated with preterm birth and bronchopulmonary dysplasia. Front. Immunol. 2021. [Google Scholar] [CrossRef]
- Mahoney, J.E.; Mori, M.; Szymaniak, A.D.; Varelas, X.; Cardoso, W.V. The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev. Cell 2014, 30, 137–150. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.; Castanhole-Nunes, M.M.U.; Biselli-Chicote, P.M.; Pavarino, E.C.; da Silva, R.; da Silva, R.F.; Goloni-Bertollo, E.M. Differential expression of angiogenesis-related miRNAs and VEGFA in cirrhosis and hepatocellular carcinoma. Arch. Med. Sci. 2020, 16, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.J.; Pryhuber, G.S.; Huyck, H.; Watkins, R.H.; Metlay, L.A.; Maniscalco, W.M. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 164, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Deng, L.; Su, D.; Xiao, J.; Ge, D.; Bao, Y.; Jing, H. Identification of crucial microRNAs and genes in hypoxia-induced human lung adenocarcinoma cells. Onco. Targets Ther. 2016, 9, 4605–4616. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Gu, C.; Draga, D.; Zhou, C.; Lhamo, T.; Zheng, Z.; Qiu, Q. Integrative analysis of miRNA-mRNA network in high altitude retinopathy by bioinformatics analysis. Biosci. Rep. 2021, 41. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, J.; Yin, H.; Long, L.; Zheng, Z.; Wang, Q.; Chen, F.; Yu, X.; Zhou, Y. Exosomal miR-1255b-5p targets human telomerase reverse transcriptase in colorectal cancer cells to suppress epithelial-to-mesenchymal transition. Mol. Oncol. 2020, 14, 2589–2608. [Google Scholar] [CrossRef]
- Ratner, V.; Slinko, S.; Utkina-Sosunova, I.; Starkov, A.; Polin, R.A.; Ten, V.S. Hypoxic stress exacerbates hyperoxia-induced lung injury in a neonatal mouse model of bronchopulmonary dysplasia. Neonatology 2009, 95, 299–305. [Google Scholar] [CrossRef]
- Boucher, E.; Provost, P.R.; Devillers, A.; Tremblay, Y. Levels of dihydrotestosterone, testosterone, androstenedione, and estradiol in canalicular, saccular, and alveolar mouse lungs. Lung 2010, 188, 229–233. [Google Scholar] [CrossRef]
- Silveyra, P. Chapter 9: Developmental lung disease. In Gender, Sex Hormones and Respiratory Disease. A Comprehensive Guide; Hemnes, A.R., Ed.; Sprenger: Berlin, Germany, 2016; p. 243. [Google Scholar] [CrossRef]
- Dammann, C.E.; Ramadurai, S.M.; McCants, D.D.; Pham, L.D.; Nielsen, H.C. Androgen regulation of signaling pathways in late fetal mouse lung development. Endocrinology 2000, 141, 2923–2929. [Google Scholar] [CrossRef][Green Version]
- Kimura, Y.; Suzuki, T.; Kaneko, C.; Darnel, A.D.; Akahira, J.; Ebina, M.; Nukiwa, T.; Sasano, H. Expression of androgen receptor and 5alpha-reductase types 1 and 2 in early gestation fetal lung: A possible correlation with branching morphogenesis. Clin. Sci. Lond. 2003, 105, 709–713. [Google Scholar] [CrossRef]
- Volpe, M.V.; Ramadurai, S.M.; Mujahid, S.; Vong, T.; Brandao, M.; Wang, K.T.; Pham, L.D.; Nielsen, H.C. Regulatory interactions between androgens, Hoxb5, and TGF β signaling in murine lung development. Biomed. Res. Int. 2013, 2013, 320249. [Google Scholar] [CrossRef] [PubMed]
- Bresson, E.; Seaborn, T.; Côté, M.; Cormier, G.; Provost, P.R.; Piedboeuf, B.; Tremblay, Y. Gene expression profile of androgen modulated genes in the murine fetal developing lung. Reprod. Biol. Endocrinol. 2010, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Rehan, V.K.; Torday, J.S. PPARγ signaling mediates the evolution, development, homeostasis, and repair of the lung. PPAR Res. 2012, 2012, 289867. [Google Scholar] [CrossRef]
- Rehan, V.K.; Torday, J.S. The lung alveolar lipofibroblast: An evolutionary strategy against neonatal hyperoxic lung injury. Antioxid. Redox Signal 2014, 21, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Lecarpentier, Y.; Gourrier, E.; Gobert, V.; Vallée, A. Bronchopulmonary dysplasia: Crosstalk between PPARγ, WNT/β-catenin and TGF-β pathways; The potential therapeutic role of PPARγ agonists. Front. Pediatr. 2019, 7, 176. [Google Scholar] [CrossRef]
- Jiang, A.; Zhang, S.; Li, Z.; Liang, R.; Ren, S.; Li, J.; Pu, Y.; Yang, J. miR-615-3p promotes the phagocytic capacity of splenic macrophages by targeting ligand-dependent nuclear receptor corepressor in cirrhosis-related portal hypertension. Exp. Biol. Med. Maywood 2011, 236, 672–680. [Google Scholar] [CrossRef]
- Pagel, J.; Twisselmann, N.; Rausch, T.K.; Waschina, S.; Hartz, A.; Steinbeis, M.; Olbertz, J.; Nagel, K.; Steinmetz, A.; Faust, K.; et al. Increased regulatory T cells precede the development of bronchopulmonary dysplasia in preterm infants. Front. Immunol. 2020, 11, 565257. [Google Scholar] [CrossRef]
- Eldredge, L.C.; Creasy, R.S.; Presnell, S.; Debley, J.S.; Juul, S.E.; Mayock, D.E.; Ziegler, S.F. Infants with evolving bronchopulmonary dysplasia demonstrate monocyte-specific expression of IL-1 in tracheal aspirates. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, L49–L56. [Google Scholar] [CrossRef]
- Permall, D.L.; Pasha, A.B.; Chen, X.Q.; Lu, H.Y. The lung microbiome in neonates. Turk. J. Pediatr. 2019, 61, 821–830. [Google Scholar] [CrossRef]
- Tirone, C.; Pezza, L.; Paladini, A.; Tana, M.; Aurilia, C.; Lio, A.; D’Ippolito, S.; Tersigni, C.; Posteraro, B.; Sanguinetti, M.; et al. Gut and lung microbiota in preterm infants: Immunological modulation and implication in neonatal outcomes. Front. Immunol. 2019, 10, 2910. [Google Scholar] [CrossRef]
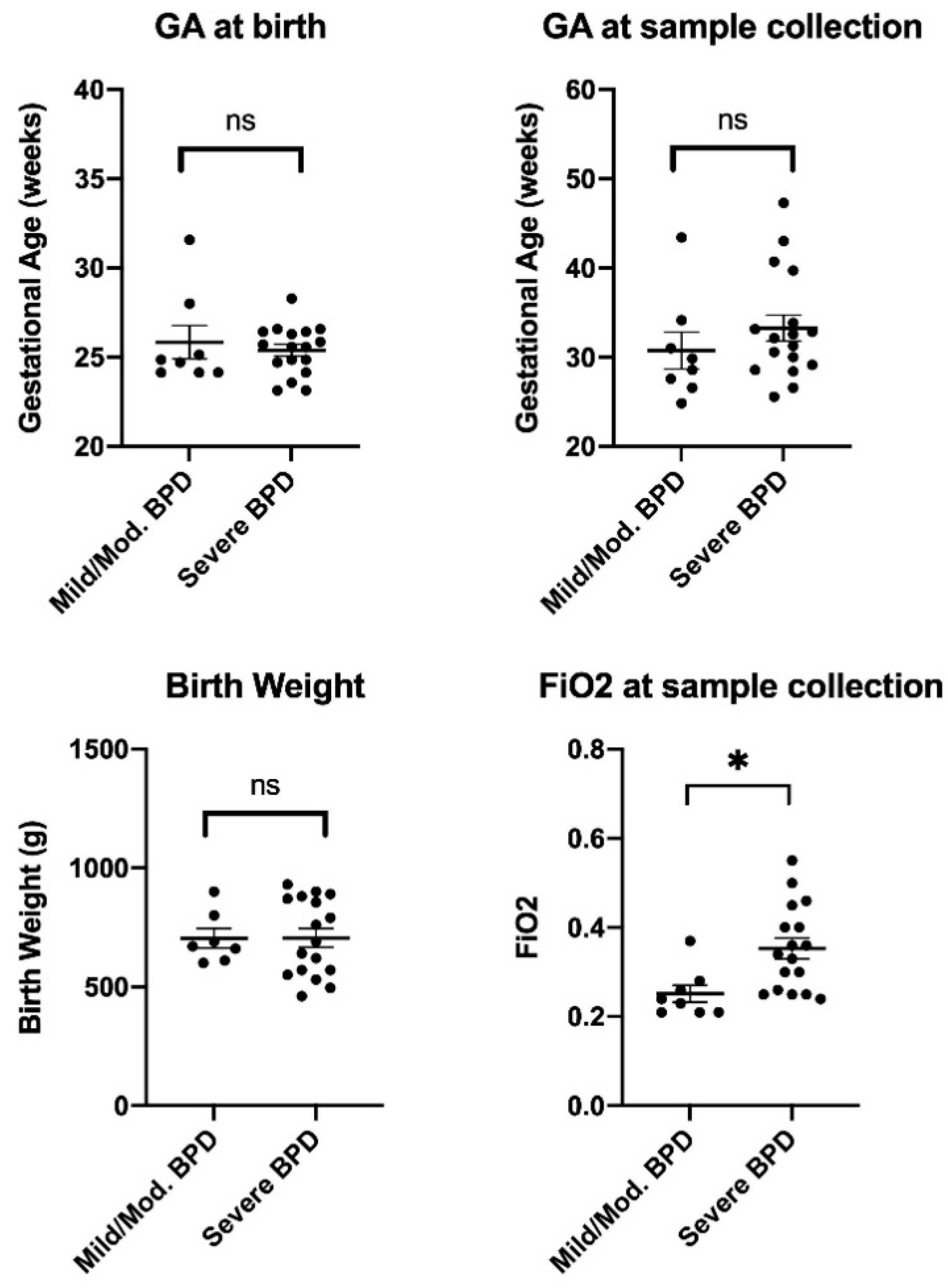
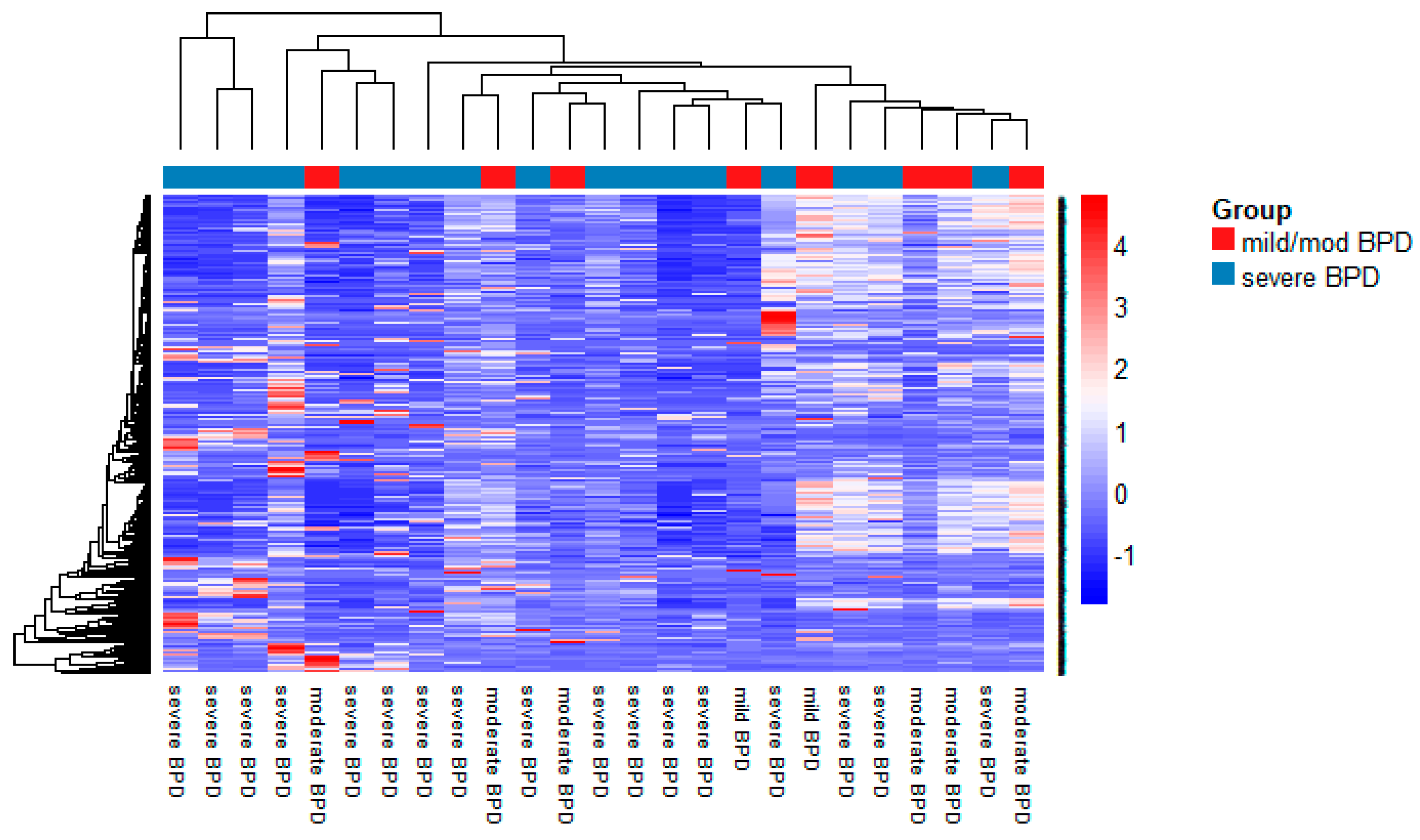
| Characteristic | Mild/Moderate BPD (n = 8) | Severe BPD (n = 17) | p-Value |
|---|---|---|---|
| Gestational age (GA) at birth, weeks (mean ± SD) | 25.84 ± 2.64 | 25.39 ± 1.38 | 0.584 |
| GA at sample collection, weeks (mean ± SD) | 30.75 ± 5.86 | 33.26 ± 6.02 | 0.337 |
| Birth weight, grams (mean ± SD) | 704 ± 108 | 706 ± 162 | 0.981 |
| Day of life (mean ± SD) | 37 ± 31 | 60 ± 40 | 0.182 |
| Male sex, % (n) | 50 (4) | 47 (8) | >0.999 |
| FiO2 at sample collection (mean ± SD) | 0.25 ± 0.05 | 0.35 ± 0.10 | 0.010 |
| Antenatal steroids exposure, % (n) 1 | 75 (6) | 76 (13) | 0.289 |
| Delivered via C-section, % (n) | 75 (6) | 88 (15) | 0.289 |
| Racial/Ethnic Group, % (n) | |||
| Non-Hispanic White | 63 (5) | 53 (9) | 0.508 |
| Non-Hispanic Black | 25 (2) | 6 (1) | 0.289 |
| Hispanic | 0 (0) | 18 (3) | 0.008 |
| Asian | 13 (1) | 18 (3) | 0.070 |
| More than one race | 0 (0) | 6 (1) | 0.008 |
| miRNA ID | log(Fold Change) | Average Expression | FDR |
|---|---|---|---|
| hsa-miR-628-5p | 3.031 | 2.823 | 0.032 |
| hsa-miR-185 | 1.463 | 1.591 | 0.039 |
| hsa-miR-545* | 0.516 | 0.361 | 0.027 |
| hsa-miR-378 | 0.479 | 0.607 | 0.044 |
| hsa-miR-3713 | −0.270 | 0.152 | 0.020 |
| hsa-miR-3151 | −0.404 | 0.414 | 0.027 |
| hsa-miR-1295 | −0.404 | 0.495 | 0.027 |
| hsa-miR-1286 | −0.488 | 0.487 | 0.047 |
| hsa-miR-380 | −0.509 | 0.318 | 0.003 |
| hsa-miR-15a* | −0.550 | 0.851 | 0.031 |
| hsa-miR-3175 | −0.599 | 0.427 | 0.006 |
| hsa-miR-493 | −0.698 | 1.030 | 0.042 |
| hsa-miR-3193 | −0.759 | 0.537 | 0.008 |
| hsa-miR-105* | −0.781 | 0.879 | 0.014 |
| hsa-miR-4300 | −0.784 | 1.019 | 0.046 |
| hsa-miR-631 | −0.815 | 0.756 | 0.034 |
| hsa-miR-2116* | −0.902 | 0.954 | 0.033 |
| hsa-miR-4304 | −0.951 | 0.986 | 0.019 |
| hsa-miR-3125 | −0.971 | 1.316 | 0.026 |
| hsa-miR-4303 | −1.046 | 1.158 | 0.011 |
| hsa-miR-1908 | −1.078 | 0.960 | 0.005 |
| hsa-miR-205* | −1.079 | 1.376 | 0.026 |
| hsa-miR-3674 | −1.176 | 1.101 | 0.039 |
| hsa-miR-615-3p | −1.243 | 1.649 | 0.047 |
| hsa-miR-4305 | −1.383 | 1.613 | 0.040 |
| hsa-let-7i* | −1.615 | 1.969 | 0.025 |
| hsa-miR-4330 | −1.776 | 1.918 | 0.045 |
| hsa-miR-1255b | −1.787 | 1.733 | 0.022 |
| hsa-miR-125b-1* | −1.905 | 2.199 | 0.035 |
| hsa-miR-24-1* | −2.300 | 1.153 | 0.013 |
| hsa-miR-646 | −4.590 | 5.529 | 0.040 |
| Top Pathways | p-Value |
|---|---|
| Top molecular and cellular functions | |
| Cell-to-cell signaling and interaction | 9.63 × 10−4–9.63 × 10−4 |
| Cellular function and maintenance | 9.63 × 10−4–9.63 × 10−4 |
| DNA replication, recombination, and repair | 1.39 × 10−2–1.39 × 10−2 |
| Cell death and survival | 1.86 × 10−2–1.86 × 10−2 |
| Cell cycle | 2.57 × 10−2–2.57 × 10−2 |
| Top diseases and disorders | |
| Inflammatory disease | 2.08 × 10−5–2.08 × 10−5 |
| Inflammatory response | 1.89 × 10−3–2.08 × 10−5 |
| Organismal injury and abnormalities | 3.97 × 10−2–2.08 × 10−5 |
| Renal and urological disease | 2.08 × 10−5–2.08 × 10−5 |
| Reproductive system disease | 3.97 × 10−2–2.01 × 10−4 |
| Top Associated Networks | Molecules | Score |
|---|---|---|
| Cell cycle, gene expression, RNA post-transcriptional modification | AGO2, miR-15a-3p, miR-9718 | 6 |
| Cell-to-cell signaling and interaction, cellular assembly and organization, small molecule biochemistry | AR, LCOR, miR-615-3p, PPARG | 3 |
| Cell cycle, cellular function and maintenance, cell signaling | CDC42, FOXD2, RHOA, miR-185-5p | 3 |
| Cardiovascular disease, cardiovascular system development and function, cancer | CDKN2A, miR-1255b-5p | 3 |
| Top Associated Networks | Score |
|---|---|
| Respiratory disease, cancer, organismal injury and abnormalities | 37 |
| Cellular movement, immune cell trafficking, immunological disease | 35 |
| Cell-to-cell signaling and interaction, cellular assembly and organization, tissue development | 33 |
| Cell-to-cell signaling and interaction, cellular development, cellular growth and proliferation | 29 |
| Cellular movement, hematological system development and function, immune cell trafficking | 27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddaiah, R.; Oji-Mmuo, C.N.; Montes, D.T.; Fuentes, N.; Spear, D.; Donnelly, A.; Silveyra, P. MicroRNA Signatures Associated with Bronchopulmonary Dysplasia Severity in Tracheal Aspirates of Preterm Infants. Biomedicines 2021, 9, 257. https://doi.org/10.3390/biomedicines9030257
Siddaiah R, Oji-Mmuo CN, Montes DT, Fuentes N, Spear D, Donnelly A, Silveyra P. MicroRNA Signatures Associated with Bronchopulmonary Dysplasia Severity in Tracheal Aspirates of Preterm Infants. Biomedicines. 2021; 9(3):257. https://doi.org/10.3390/biomedicines9030257
Chicago/Turabian StyleSiddaiah, Roopa, Christiana N. Oji-Mmuo, Deborah T. Montes, Nathalie Fuentes, Debra Spear, Ann Donnelly, and Patricia Silveyra. 2021. "MicroRNA Signatures Associated with Bronchopulmonary Dysplasia Severity in Tracheal Aspirates of Preterm Infants" Biomedicines 9, no. 3: 257. https://doi.org/10.3390/biomedicines9030257
APA StyleSiddaiah, R., Oji-Mmuo, C. N., Montes, D. T., Fuentes, N., Spear, D., Donnelly, A., & Silveyra, P. (2021). MicroRNA Signatures Associated with Bronchopulmonary Dysplasia Severity in Tracheal Aspirates of Preterm Infants. Biomedicines, 9(3), 257. https://doi.org/10.3390/biomedicines9030257







