News about the Role of Fluid and Imaging Biomarkers in Neurodegenerative Diseases
Abstract
:1. Introduction
2. Fluids
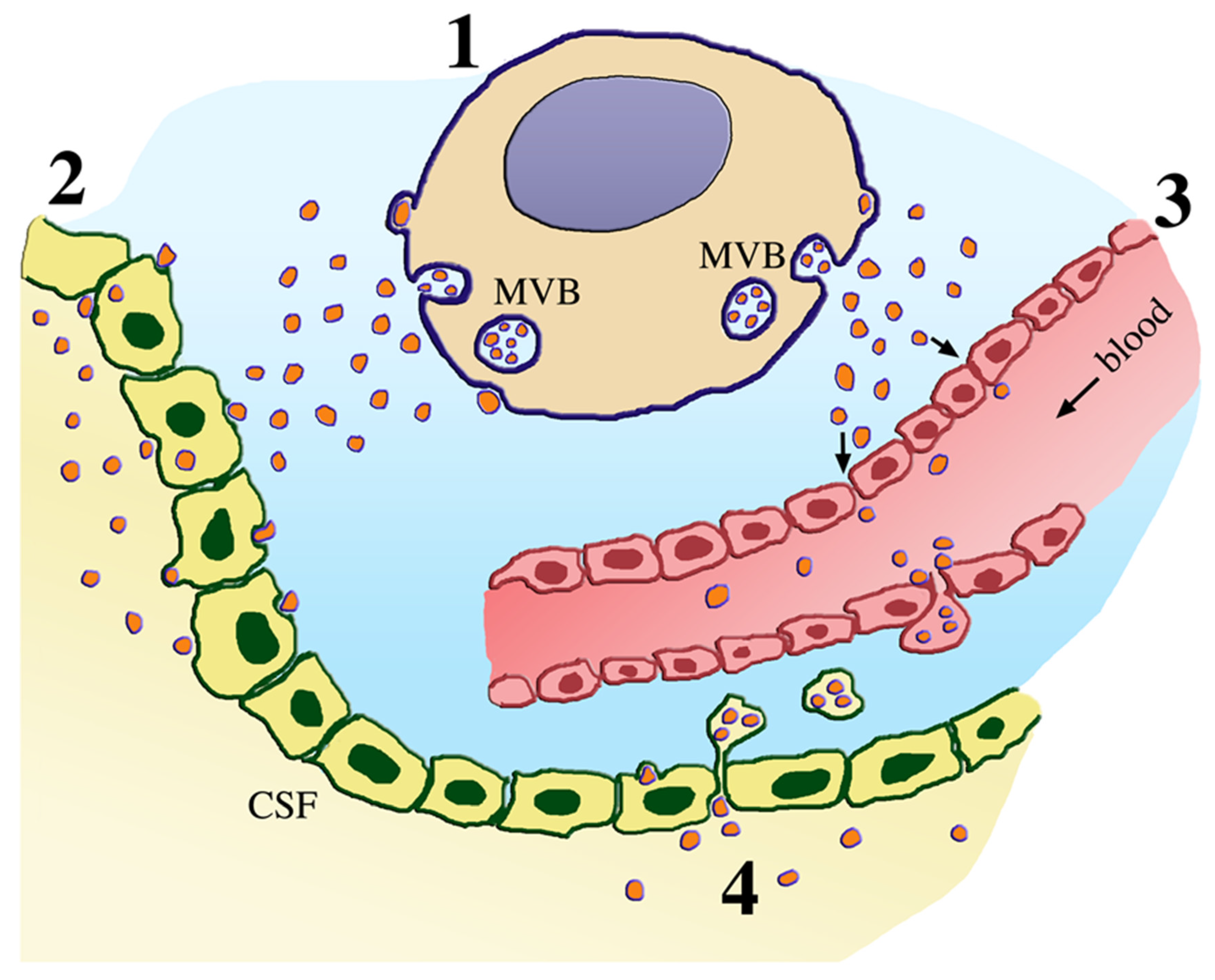
3. From Molecules to Extracellular Vesicles
4. AD and Its Multiple Fluid Biomarkers
5. Imaging Biomarkers from AD and PD
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ALS | amyotrophic lateral sclerosis |
| BBB | blood-brain barrier |
| CSF | central spinal fluid |
| EV | extracellular vesicle |
| miRNA and circRNA | micro and circular RNAs |
| MVB | multivesicular body |
| OPI | ocular imaging |
| PD | Parkinson’s disease |
| TDP-43 | transactive response DNA/RNA binding protein of 43kDa |
References
- Gromova, M.; Vaggelas, A.; Dallmann, G.; Seimtz, D. Biomarkers: Opportunities and challenges for drug development in the current regulatory landscapes. Biomark. Insights 2020, 15. [Google Scholar] [CrossRef]
- Diray-Arce, J.; Conti, M.G.; Petrova, B.; Kanarek, N.; Angelidou, A.; Levy, O. Integrative metabolomics to identify molecular signatures of responses to vaccines and infections. Metabolites 2020, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Rabbito, A.; Dulewicz, M.; Kulczynska-Przybik, A.; Moczko, B. Biochemical markers in Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 1989. [Google Scholar] [CrossRef] [Green Version]
- Casamitjana, A.; Petrone, P.; Molinuevo, J.L.; Gispert, J.D.; Vilaplana, V. Projection to latent spaces entangles pathological effects on brain morphology in the symptomatic phase of Alzheimer’s disease. Front. Neurol. 2020, 11, 648. [Google Scholar] [CrossRef]
- Laulagnier, K.; Javalet, C.; Hemming, F.J.; Sadoul, R. Purification and analysis of exosomes released by mature cortical neurons following synaptic activation. Methods Mol. Biol. 2017, 1545, 129–138. [Google Scholar] [PubMed]
- Maraoka, S.; Lin, W.; Chen, M.; Hersh, S.W.; Emil, A.; Xia, W.; Ikezu, T. Assessment of separation methods for extracellular vesicles from human and mouse brain tissues and human cerebrospinal fluids. Methods 2020, 177, 35–49. [Google Scholar] [CrossRef]
- Klunk, W.E.; Engler, H.; Nordberg, A.; Wang, Y.; Blomqvist, G.; Holt, D.P.; Bergstrom, M.; Savitcheva, I.; Huang, G.F.; Es-trada, S.; et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound. Ann. Neurol. 2004, 55, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Mattson-Calgren, N.; Palmqvist, S.; Blennow, K.; Hansson, O. Increasing the reproducibility of fluid biomarker studies in neurodegenerative studies. Nat. Commun. 2020, 11, 6252. [Google Scholar] [CrossRef] [PubMed]
- Torok, N.; Tanaka, M.; Vecsei, L. Searching for peripheral biomarkers in neurodegenerative diseases: The tryptophan-kynurenine metabolic pathway. Int. J. Mol. Sci. 2020, 21, 9338. [Google Scholar] [CrossRef] [PubMed]
- Mantzavinos, V.; Alexiou, A. Biomarkers for Alzheimer’s disease diagnosis. Curr. Alzheimer Res. 2017, 14, 1149–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boniecki, V.; Zetterberg, H.; Aarsland, D.; Vannini, P.; Kvartsberg, H.; Winblad, B.; Blennow, K.; Freud-Levi, Y. Are neuropsychiatric symptoms in dementia linked to CSF biomarkers of synaptic and axonal degeneration? Alzheimer’s Res. Ther. 2020, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- Banks, A.W.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of extracellular vesicles across the blood-brain barrier: Brain pharmacokinetics and effects of inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef]
- Badhwar, A.; Haqqani, A.S. Biomarker potential of brain-secreted extracellular vesicles in blood in Alzheimer’s disease. Alzheimer’s Dement. 2020, 12, e12001. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, L.; Paolini Paoletti, F.; Bellomo, G.; Mancini, A.; Simoni, S.; Di Filippo, M.; Parnetti, L. CSF and blood biomarkers in neuroinflammatory and neurodegenerative diseases: Implications for treatment. Trends Pharmacol. Sci. 2020, 41, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Eden, A.; Kanberg, N.; Gostner, J.; Fuchs, D.; Hagberg, L.; Andersson, L.M.; Lindh, M.; Price, R.W.; Zetterberg, H.; Gisslen, M. CSF biomarkers in patients with COVID-19 and neurological symptoms: A case series. Neurology 2021, 96, e294–e300. [Google Scholar] [PubMed]
- Lim, C.Z.J.; Natalia, A.; Sundah, N.R.; Shao, H. Biomarker organization in circulating extracellular vesicles: New applica-tions in detecting neurodegenerative diseases. Adv. Biosyst. 2020, 4, e1900309. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L. Circulating exosomal miRNA as diagnostic biomarkers of neurodegenerative diseases. Front. Mol. Neurosci. 2020, 13, 53. [Google Scholar] [CrossRef] [Green Version]
- Kapogiannis, D. Exosome biomarkers revolutionize preclinical diagnosis of neurodegenerative diseases and assessment of treatment responses in clinical trials. Adv. Exp. Med. Biol. 2020, 1195, 149. [Google Scholar] [CrossRef] [PubMed]
- Hornung, S.; Dutta, S.; Bitan, G. CNS-derived blood exosomes as a promising source of biomarkers: Opportunities and challenges. Front. Mol. Neurosci. 2020, 13, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yo, Y.K.; Lee, J.; Kim, H.; Hwang, K.S.; Yoon, D.S.; Lee, J.H. Toward exosome-based neuronal diagnostic devices. Micromachines 2018, 9, 634. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, W.; Shi, H.; Han, L.; Pan, P. Blood neurofilament light chain in Parkinson disease and atypical parkin-sonisms, A protocol for systematic review and meta-analysis. Medicine 2020, 99, e21871. [Google Scholar] [CrossRef] [PubMed]
- Yin, O.; Ji, X.; Ly, R.; Pei, J.J.; Du, Y.; Shen, C.; Hou, X. Targeting exosomes as a new biomarker and therapeutic approach for Alzheimer’s disease. Clin. Interv. Aging 2020, 15, 195–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eren, E.; Hunt, J.F.; Shardell, M.; Chawla, S.; Tran, J.; Gu, J.; Vogy, N.M.; Johnson, S.C.; Bendlin, B.B.; Kapogiannis, D. Ex-tracellular vesicle biomarkers of Azheimer’s disease associated with sub-clinical cognitive-decline in late middle age. Alzheimer’s Dement. 2020, 16, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Cantero, J.; Atienza, M.; Amos-Cejudo, J.; Fossai, S.; Wisniewski, T.; Osorio, R.S. Plasma tau predicts cerebral vulnerability in aging. Aging 2020, 12, 21004–21022. [Google Scholar] [CrossRef] [PubMed]
- Guha, D.; Lorenz, D.R.; Misra, V.; Chettimada, S.; Morgello, S.; Gabuzda, D. Proteomic analysis of cerebrospinal fluid extracellular vesicles reveals synaptic injury, inflammation, and stress response markers in HIV patients with cognitive impairment. Neuroinflammation 2019, 16, 254. [Google Scholar] [CrossRef] [Green Version]
- Mazzucchi, S.; Palermo, G.; Campese, N.; Galgani, A.; Della Vecchia, A.; Vergallo, A.; Siciliano, G.; Ceravolo, R.; Hamel, H.; Baldacci, F. The role of synaptic biomarkers in the spectrum of neurodegenerative diseases. Expert Rev. Proteom. 2020, 17, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Xin, J.; Le, W.; Yang, Y. Neurogranin, A potential biomarker of neurological and mental diseases. Front. Aging Neurosci. 2020, 12, 584743. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Li, C.H.; Yang, K.C.; Lin, F.J.; Wu, C.C.; Chieh, J.J.; Chu, M.J. Blood NfL: A biomarker for disease severity and progression in Parkinson disease. Neurology 2019, 93, e1104–e1111. [Google Scholar] [CrossRef] [PubMed]
- Gamez-Valero, A.; Beyer, K.; Borras, F.E. Extracellular vesicles, new actors in the search for biomarkers of dementia. Neurobiol. Aging 2019, 74, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Lugli, C.; Cohen, A.M.; Bennett, D.A.; Shah, R.C.; Fields, C.J.; Hernandez, A.G.; Smalheiser, N.R. Plasma exosomal miRNAs in persons with and without Alzheimer’s disease: Altered expression and prospects for biomarkers. PLoS ONE 2015, 10, e139233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosaka, T.; Yamashita, T.; Tamaoka, A.; Swak, S. Extracelluar RNAs as biomarkers of sporadic amyotrophic lateral sclerosis and other neurodegenerative disease. Int. J. Mol. Sci. 2019, 20, 3148. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Avalos, J.A.; Ferandez-Macias, J.C.; Galez-Palomo, A.K. Circulating exosomal microRNAs: New non-invasive biomarkers of non-communicable disease. Mol. Biol. Rep. 2020. [CrossRef]
- Prieto-Fernandez, E.; Lopez-Lopez, E.; Martin-Guerrero, I.; Barcen, L.; Gonzalez-Lopez, M.; Aransay, A.M.; Lozano, J.J.; Benito, J.; Falcon-Perez, J.M.; Garcia-Orad, A. Variability in cerebrospinal fluid microRNA through life. Mol. Neurobiol. 2020, 57, 4134–4142. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.H.; Wu, W.P.; Xiong, X.D. Circular RNAs: Promising biomarkers for age related diseases. Aging Dis. 2020, 11, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.J.; Hou, W.H.; Jiang, D.G.; Tian, H.J.; Wang, L.N.; Jia, F.; Zhou, C.H.; Zhu, J.J. Circular RNAs in early brain development and their influence and clinical significance in neuropsychiatric disorders. Neural Regen. Res. 2020, 15, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Compta, Y.; Revesz, T. Neuropathological and biomarker findings in Parkinson’s disease and Alzheimer’s disease from protein aggregates to synaptic dysfunction. J. Parkinsons Dis. 2020, 11, 102–121. [Google Scholar]
- Kim, J.; Kim, Y.K. Inflammatory biomarkers in AD: Implications for diagnosis. Curr. Alzheimer’s Res. 2020, 17, 962–971. [Google Scholar] [CrossRef]
- Vasileff, N.; Cheng, L.; Hill, A.F. Extracellular vesicles-propagators of neuropathology and sources of potential biomarkers and therapeutics for neurodegenerative diseases. J. Cell Sci. 2020, 133, jcs243139. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Vo, V.G. Role of body-fluid biomarkers in Alzheimer’s disease diagnosis. Diagnostics 2020, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Tarawsneh, R. Biomarkers, Our path towards a cure for Alzheimer’s disease. Biomark. Insights 2020, 15, 1177271920976367. [Google Scholar] [CrossRef]
- Horie, K.; Barthlemy, N.R.; Sato, C.; Bateman, R.J. CSF tau microtubule binding region identifies tau tangle and clinical stages of Alzheimer’s disease. Brain 2020, awaa373. [Google Scholar] [CrossRef]
- Guo, M.; Yin, Z.; Chen, F.; Ping, L. Mesenchymal stem cell-derived exosome: A promising alternative in the therapy of Alzheimer’s disease. Alzheimer’s Res. Ther. 2020, 12, 109. [Google Scholar] [CrossRef]
- Martins, T.S.; Trindade, D.; Vaz, M.; Campelo, I.; Almeida, M.; Trigo, G.; da Cruz E Silva, O.A.B.; Henriques, A.G. Diagnostic and therapeutic potential of exosomes in Alzheimer’s disease. J. Neurochem. 2020, 156, 162–181. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Haiting, L.I.; Liu, W.; Zhang, L.; Tia, O.; Li, H.; Li, M. MiR-485-3p serves as a biomarker and therapeutic target of Alzheimer’s disease via regulating neuronal cell viability and neurofiammation by target AKT3. Mol. Genet. Genom. Med. 2020, e1548. [Google Scholar] [CrossRef]
- Liu, Q.; Lei, C. Neuroprotective effects of miR-331-3p through improved cell viability and inflammatory marker expression, Correlation of serum miR-331-3p levels with diagnosis and severity of Alzheimer’s disease. Exp. Gerontol. 2020, 144, 111187. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J. Drug development for psychotropic, cognitive-enhancing and disease-modifying treatments for Alzheimer’s disease. J. Neuropsychiatry Clin. Neurosci. 2020, 33, 3–13. [Google Scholar] [CrossRef]
- Clifford, R.J.; Bennett, D.A.; Blennow, K.; Carillo, M.C.; Feldman, H.H.; Frisono, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N, An unbiased descriptive classification scheme for Alzheimer’s disease biomarkers. Neurology 2016, 87, 539–547. [Google Scholar]
- Lashley, T.; Shott, J.M.; Weston, P.; Murray, C.E.; Wellington, H.; Keshavan, A.; Foti, S.C.; Foinani, M.; Toombs, J.; Rohrer, J.D.; et al. Molecular biomarkers of Alzheimer’s disease: Progress and prospects. Dis. Model. Mech. 2018, 11, dmm031781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of the Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 229–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Insel, P.; Donohue, M.C.; Berron, D.; Hansson, O.; Mattson-Calgren, N. Time between milestone events in the Alzheimer’s disease amyloid cascade. Neuroimage 2020, 22, 117676. [Google Scholar] [CrossRef]
- Babulal, G.M.; Johnson, A.; Fagan, A.M.; Morris, J.C.; Roe, C.M. Identifying preclinical Alzheimer’s disease using everyday driving behavior: Proof of concepts. J. Alzheimer’s Dis. 2021, 79, 1009–1014. [Google Scholar] [CrossRef]
- Li, T.R.; Wu, Y.; Jiang, J.J.; Lin, H.; Han, C.L.; Jang, J.H.; Han, Y. Radiomic analysis of magnetic resonance imaging facili-tates the identification of preclinical Alzheimer’s disease: An exploratory study. Cell Dev. Biol. 2020, 8, 605734. [Google Scholar] [CrossRef]
- Saed, U.; Compagnone, J.; Aviv, R.; Strafella, A.P.; Black, S.E.; Lang, A.E.; Masellis, M. Imaging biomarkers in Parkinson’s disease and parkinsonin syndromes: Current and emergins concepts. Transl. Neurodegener. 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, U.; Lang, A.E.; Masellis, M. Neuroimaging advances in Parkinson’s disease and atypical parkinsonian syndromes. Front. Neurol. 2020, 11, 572976. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, R.; Martensson, G.; Poulakis, K.; Muelboueck, J.S.; Rodriguez-Vieitez, E.; Chotis, K.; Grothe, M.J.; Nordberg, A.; Ferreira, D.; Westman, E. Comparison of subtyping methods for neuroimaging studies in Alzheimer’s disease, A call for harmonization. Brain Commun. 2020, 2, fcaa192. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.; Politis, M.; Rabiner, E.A.; Middleton, L.T. Novel PET biomarkers to disentangle molecular pathways across age-related neurodegenerative diseases. Cells 2020, 9, 2581. [Google Scholar] [CrossRef] [PubMed]
- Munch, M.; Rotstein, B.H.; Ulrich, G. Florine-18-labeled fluorescent dyes for dual-mode molecular imaging. Molecules 2020, 25, 6042. [Google Scholar] [CrossRef] [PubMed]
- Rowley, P.A.; Samsonov, A.A.; Betthauser, T.J.; Pirasteh, A.; Johnson, S.C.; Eisenmenger, L.B. Amyloid and tau PET imaging of Alzheimer’s disease and other neurodegenerative conditions. Semin. Ultrasound CT MR 2020, 41, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Jagust, W.J. Alzheimer’s disease spectrum: Syndrome and etiology from clinical and PET imaging per-spectives. Neurology 2020. [CrossRef]
- Ehremberg, A.J.; Khatun, A.; Coomans, E.; Betts, M.J.; Capraro, F.; Thijssen, E.H.; Senkevich, K.; Bharucha, T.; Jafarpour, M.; Young, P.N.E.; et al. Relevance of biomarkers across different neurodegenerative diseases. Alzheimer’s Res. Ther. 2020, 12, 56. [Google Scholar] [CrossRef]
- Pedrero-Prieto, C.M.; Garcia-Capitero, S.; Frontinan-Rubio, J.; Llanos-Gonzalez, E.; Aguilera-Garcia, C.; Alcan, F.C.; Lind-berg, I.; Duran-Prado, M.; Peinado, J.R.; Rabanal-Luiz, Y. A comprehensive systematic review of CSF proteins and peptides to define Alzheimer’s disease. Clin. Proteom. 2020, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, W.; Su, L.; Xing, Y.; Fessen, F.; Sun, Y.; Shu, N.; Han, Y. Neuroimaging advances regarding subjective cognitive decline in preclinical Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 55. [Google Scholar] [CrossRef] [PubMed]
| Genes | Proteins/Biomarkers | Diseases |
|---|---|---|
| APP | amyloid precursor protein/Aβ * | AD |
| PSEN1 | presenilin1 * | AD |
| PSEN2 | presenilin2 | AD |
| MAPT | tau * | AD, PD, DLB, FTD |
| C9orf72 | C9orf72 * | FTD, ALS |
| GRN | progranulin * | FTD, ALS |
| VCP | valosin-containing protein * | ALS, FTD, PD |
| TARDBP | TDP-43 * | ALS |
| FUS | fused in sarcoma (FUS) | ALS |
| HTT | huntingtin * | HD |
| SNCA | α-synuclein * | PD, DLB, AD |
| GBA | β-glucocerebrosidase | PD, DLB |
| ApoE | apolipoprotein-E | AD (risk factor) |
| TREM2 | TREM2 | AD (risk factor) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meldolesi, J. News about the Role of Fluid and Imaging Biomarkers in Neurodegenerative Diseases. Biomedicines 2021, 9, 252. https://doi.org/10.3390/biomedicines9030252
Meldolesi J. News about the Role of Fluid and Imaging Biomarkers in Neurodegenerative Diseases. Biomedicines. 2021; 9(3):252. https://doi.org/10.3390/biomedicines9030252
Chicago/Turabian StyleMeldolesi, Jacopo. 2021. "News about the Role of Fluid and Imaging Biomarkers in Neurodegenerative Diseases" Biomedicines 9, no. 3: 252. https://doi.org/10.3390/biomedicines9030252
APA StyleMeldolesi, J. (2021). News about the Role of Fluid and Imaging Biomarkers in Neurodegenerative Diseases. Biomedicines, 9(3), 252. https://doi.org/10.3390/biomedicines9030252






