Trifarotene: A Current Review and Perspectives in Dermatology
Abstract
1. Introduction
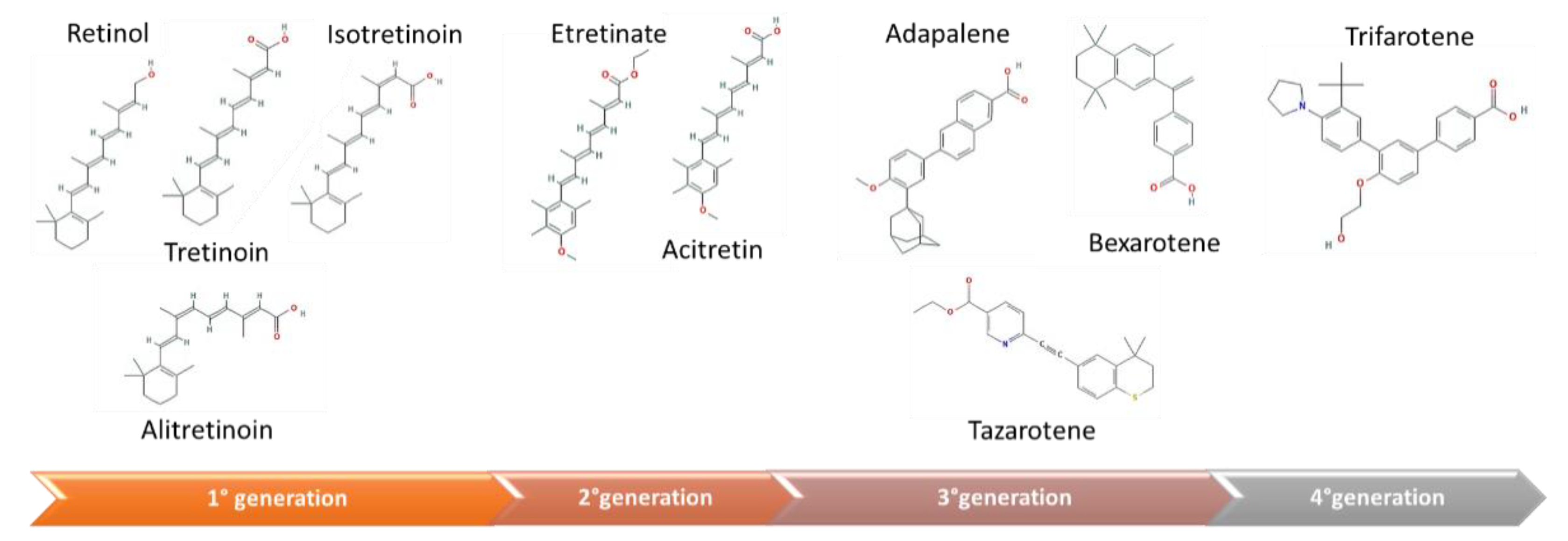
2. Mechanism of Action of Vitamin A and Its Analogues
- The first generation is composed by natural retinoids, obtained modifying polar groups of vitamin A, which do not act selectively: retinol and its metabolites, such as retinal, tretinoin, isotretinoin, and alitretinoin;
- The second generation is constituted by monoaromatic retinoids, synthetic compounds where a benzene ring replaces the cyclohexene ring: etretinate, and acitretin;
- The third generation is made up by polyaromatic retinoids, resulting from cyclization of the side chain and characterized by a selective activity towards receptor: adapalene, tazarotene, and bexarotene [16].
- 1)
- Skin hydration: trifarotene induces skin peptidyl arginine deiminase 1 and aquaporin-3 channels, and, therefore, influences skin barrier functions;
- 2)
- Cell adhesion: trifarotene weakens hemidesmosomes, reducing intercellular adhesion. The minor cohesion among keratinocytes explains its comedolytic properties;
- 3)
3. Methods and Study Design
3.1. Search Strategy
3.2. Inclusion Criteria
3.3. Exclusion Criteria
3.4. Search Results
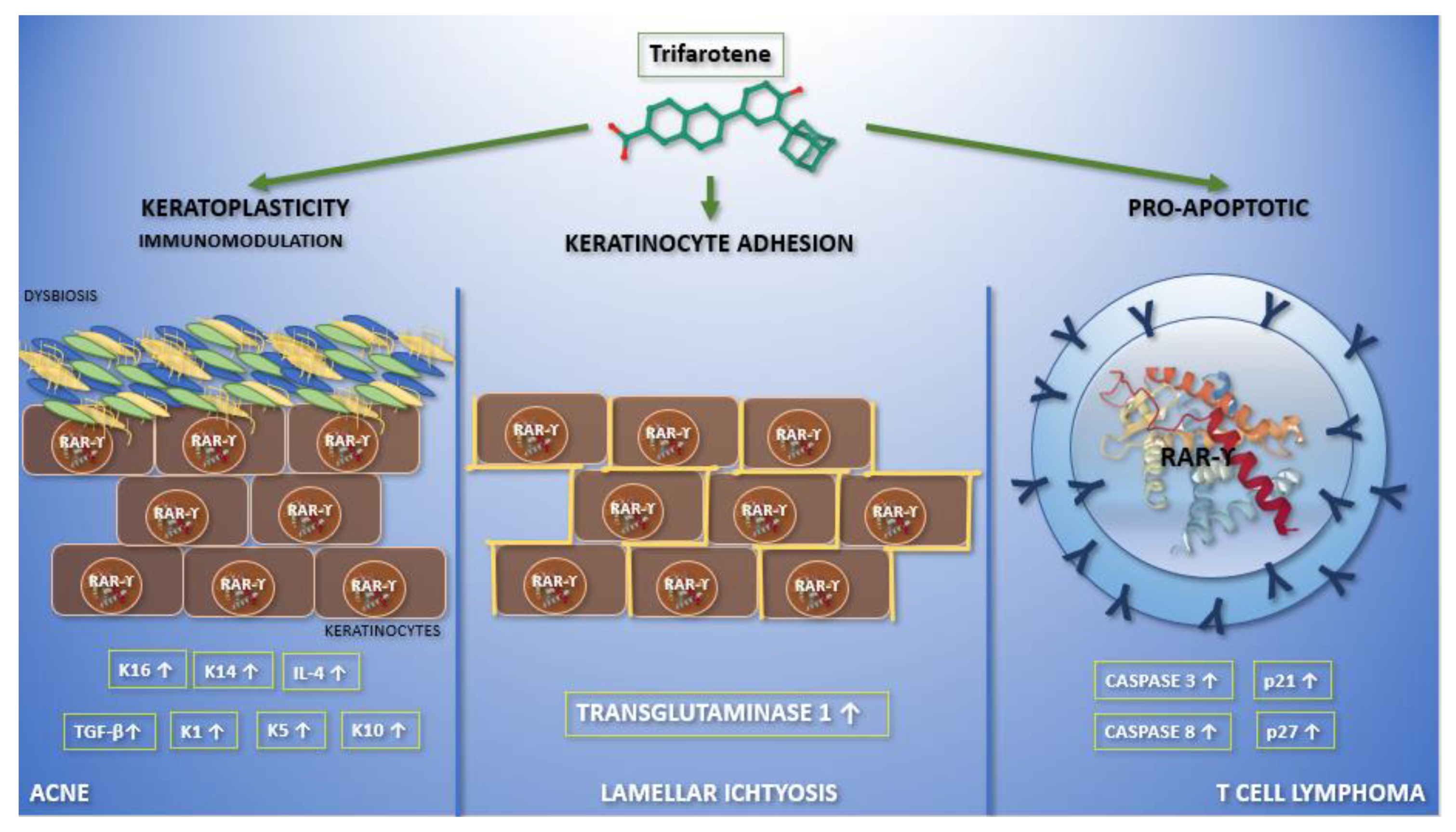
4. Trifarotene Properties and Current Applications in Dermatology
4.1. RAR-γ Selectivity
4.2. Trifarotene Safety and Tolerability
4.3. Current Applications in Dermatology
4.3.1. Acne Vulgaris
4.3.2. Autosomal Recessive Congenital Ichthyosis
4.3.3. Primary Cutaneous T-Cell Lymphoma
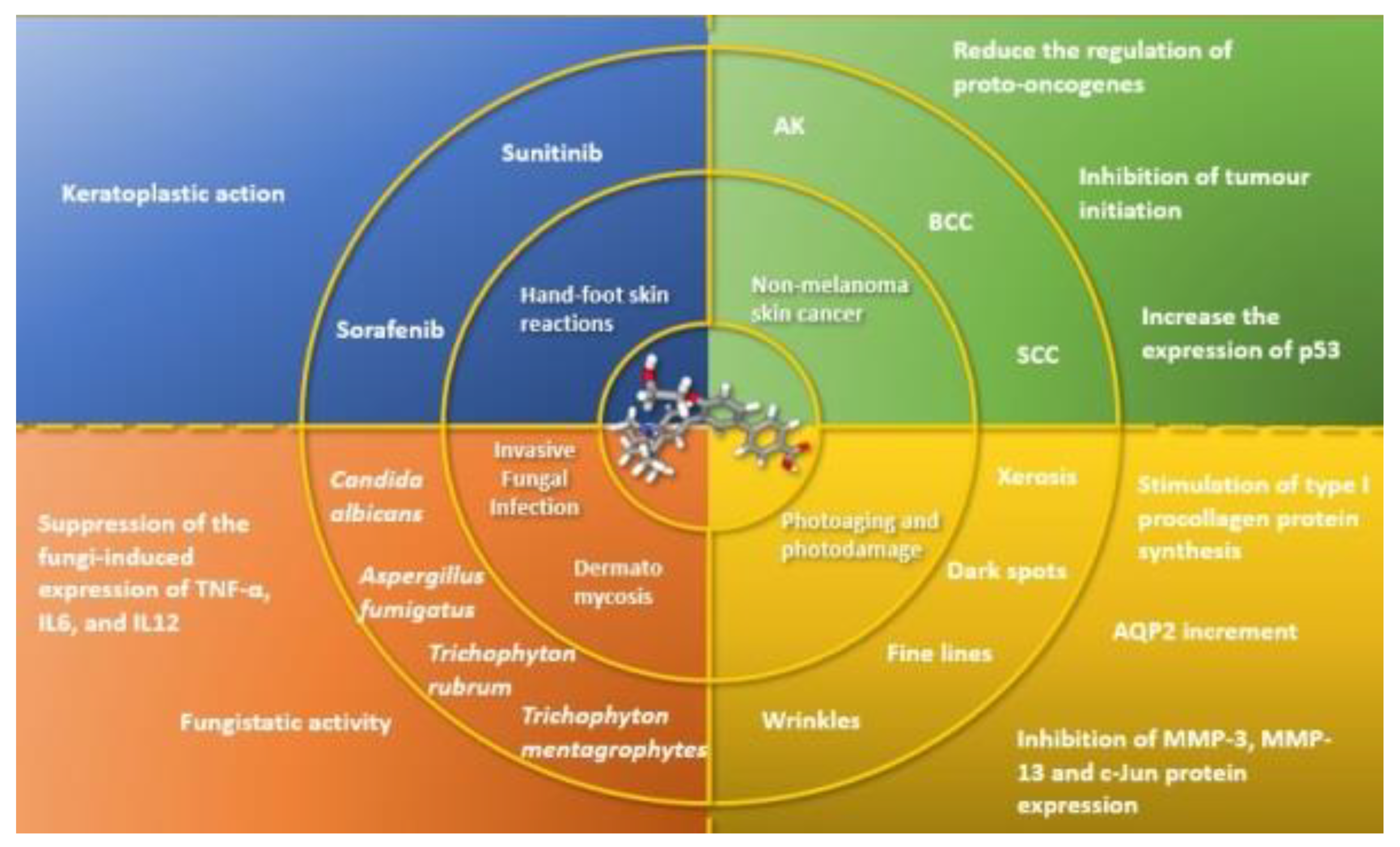
5. Perspectives
5.1. Non-Melanoma Skin Cancer
5.2. Invasive Fungal Infection (IFI)
5.3. Skin and Nail Mycosis
5.4. Photoaging
5.5. Hand-Foot Skin Reaction
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nomenclature of retinoids. Recommendations. Eur. J. Biochem. 1982, 129, 1–5.
- Campione, E.; Cosio, T.; Lanna, C.; Mazzilli, S.; Ventura, A.; Dika, E.; Gaziano, R.; Dattola, A.; Candi, E.; Bianchi, L. Predictive role of vitamin A serum concentration in psoriatic patients treated with IL-17 inhibitors to prevent skin and systemic fungal infections. J. Pharmacol. Sci. 2020, 144, 52–56. [Google Scholar] [CrossRef]
- Kawaguchi, R.; Yu, J.; Honda, J.; Hu, J.; Whitelegge, J.; Ping, P.; Wiita, P.; Bok, D.; Sun, H. A Membrane Receptor for Retinol Binding Protein Mediates Cellular Uptake of Vitamin A. Science 2007, 315, 820–825. [Google Scholar] [CrossRef]
- Donovan, M.; Olofsson, B.; Gustafson, A.-L.; Dencker, L.; Eriksson, U. The cellular retinoic acid binding proteins. J. Steroid Biochem. Mol. Biol. 1995, 53, 459–465. [Google Scholar] [CrossRef]
- Gronemeyer, H.; Gustafsson, J.-Å.; Laudet, V. Principles for modulation of the nuclear receptor superfamily. Nat. Rev. Drug Discov. 2004, 3, 950–964. [Google Scholar] [CrossRef] [PubMed]
- Allenby, G.; Bocquel, M.T.; Saunders, M.; Kazmer, S.; Speck, J.; Rosenberger, M.; Lovey, A.; Kastner, P.; Grippo, J.F.; Chambon, P. Retinoic acid receptors and retinoid X receptors: Interactions with endogenous retinoic acids. Proc. Natl. Acad. Sci. USA 1993, 90, 30–34. [Google Scholar] [CrossRef]
- Balmer, J.; Blomhoff, R. A robust characterization of retinoic acid response elements based on a comparison of sites in three species. J. Steroid Biochem. Mol. Biol. 2005, 96, 347–354. [Google Scholar] [CrossRef]
- Chambon, P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996, 10, 940–954. [Google Scholar] [CrossRef]
- Samarut, E.; Rochette-Egly, C. Nuclear retinoic acid receptors: Conductors of the retinoic acid symphony during development. Mol. Cell. Endocrinol. 2012, 348, 348–360. [Google Scholar] [CrossRef]
- Dilworth, F.J.; Chambon, P. Nuclear receptors coordinate the activities of chromatin remodeling complexes and coactivators to facilitate initiation of transcription. Oncogene 2001, 20, 3047–3054. [Google Scholar] [CrossRef]
- Farboud, B.; Hauksdottir, H.; Wu, Y.; Privalsky, M.L. Isotype-restricted corepressor recruitment: A constitutively closed helix 12 conformation in retinoic acid receptors beta and gamma interferes with corepressor recruitment and prevents transcriptional repression. Mol. Cell. Biol. 2003, 23, 2844–2858. [Google Scholar] [CrossRef]
- Hauksdottir, H.; Farboud, B.; Privalsky, M.L. Retinoic acid receptors beta and gamma do not repress, but instead activate target gene transcription in both the absence and presence of hormone ligand. Mol. Endocrinol. 2003, 17, 373–385. [Google Scholar] [CrossRef][Green Version]
- Asson-Batres, M.A.; Rochette-Egly, C. (Eds.) The Biochemistry of Retinoic Acid Receptors I: Structure, Activation, and Function at the Molecular Level; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Ferry, C.; Gianni, M.; Lalevée, S.; Bruck, N.; Plassat, J.-L.; Raska, I.; Garattini, E.; Rochette-Egly, C. SUG-1 Plays Proteolytic and Non-proteolytic Roles in the Control of Retinoic Acid Target Genes via Its Interaction with SRC. J. Biol. Chem. 2009, 284, 8127–8135. [Google Scholar] [CrossRef] [PubMed]
- Giannì, M.; Bauer, A.; Garattini, E.; Chambon, P.; Rochette-Egly, C. Phosphorylation by p38MAPK and recruitment of SUG-1 are required for RA-induced RAR gamma degradation and transactivation. EMBO J. 2002, 21, 3760–3769. [Google Scholar] [CrossRef]
- Khalil, S.; Bardawil, T.; Stephan, C.; Darwiche, N.; Abbas, O.; Kibbi, A.G.; Nemer, G.; Kurban, M. Retinoids: A journey from the molecular structures and mechanisms of action to clinical uses in dermatology and adverse effects. J. Dermatol. Treat. 2017, 28, 684–696. [Google Scholar] [CrossRef]
- Redfern, C.P.; Todd, C. Retinoic acid receptor expression in human skin keratinocytes and dermal fibroblasts in vitro. J. Cell Sci. 1992, 102 Pt 1, 113–121. [Google Scholar]
- Thoreau, E.; Arlabosse, J.-M.; Bouix-Peter, C.; Chambon, S.; Chantalat, L.; Daver, S.; Dumais, L.; Duvert, G.; Feret, A.; Ouvry, G.; et al. Structure-based design of Trifarotene (CD5789), a potent and selective RARγ agonist for the treatment of acne. Bioorg. Med. Chem. Lett. 2018, 28, 1736–1741. [Google Scholar] [CrossRef]
- Aubert, J.; Piwnica, D.; Bertino, B.; Blanchet-Réthoré, S.; Carlavan, I.; Déret, S.; Dreno, B.; Gamboa, B.; Jomard, A.; Luzy, A.; et al. Nonclinical and human pharmacology of the potent and selective topical retinoic acid receptor-γ agonist trifarotene. Br. J. Dermatol. 2018, 179, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Novel Drug Approvals for 2019. Available online: http://www.fda.gov (accessed on 6 August 2020).
- Galderma. Aklief® (Trifarotene): US Prescribing Information. Available online: http://www.galderma.com/ (accessed on 6 August 2020).
- Chien, A. Retinoids in Acne Management: Review of Current Understanding, Future Considerations, and Focus on Topical Treatments. J. Drugs Dermatol. 2018, 17, s51–s55. [Google Scholar] [PubMed]
- Charton, J.; Deprez-Poulain, R.; Hennuyer, N.; Tailleux, A.; Staels, B.; Deprez, B. Novel non-carboxylic acid retinoids: 1,2,4-Oxadiazol-5-one derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, B.; Bernardon, J.-M.; Eustache, J.; Millois, C.; Martin, B.; Michel, S.; Shroot, B. Synthesis, Structure-Affinity Relationships, and Biological Activities of Ligands Binding to Retinoic Acid Receptor Subtypes. J. Med. Chem. 1995, 38, 4993–5006. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human Tissue-specific Expression by Genome-wide Integration of Transcriptomics and Antibody-based Proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Maynepharma. Available online: https://www.maynepharma.com/innovation/novel-pipeline/ (accessed on 6 August 2020).
- Ms, N.W.; Benkali, K.; Sáenz, A.A.; Poncet, M.; Graeber, M. Clinical Pharmacology and Safety of Trifarotene, a First-in-Class RARγ-Selective Topical Retinoid. J. Clin. Pharmacol. 2020, 60, 660–668. [Google Scholar] [CrossRef]
- Drugs.com. Gleolan (Aminolevulinic Acid) Drug Interactions from Drugs.com; c1996–2018 [Updated: 20 November 2018]. Available online: https://www.drugs.com/mtm/gleolan.html (accessed on 6 August 2020).
- Basak, S.A.; Zaenglein, A.L. Acne and Its Management. Pediatr. Rev. 2013, 34, 479–497. [Google Scholar] [CrossRef][Green Version]
- Lasek, R.J.; Chren, M.-M. Acne vulgaris and the quality of life of adult dermatology patients. Arch. Dermatol. 1998, 134, 454–458. [Google Scholar] [CrossRef]
- Newton, J.N.; Mallon, E.; Klassen, A.; Ryan, T.J.; Finlay, A.Y. The effectiveness of acne treatment: An assessment by patients of the outcome of therapy. Br. J. Dermatol. 1997, 137, 563–567. [Google Scholar] [CrossRef]
- Nast, A.; Dréno, B.; Bettoli, V.; Degitz, K.; Erdmann, R.; Finlay, A.Y.; Ganceviciene, R.; Haedersdal, M.; Layton, A.; López-Estebaranz, J.; et al. European Evidence-based (S3) Guidelines for the Treatment of Acne. J. Eur. Acad. Dermatol. Venereol. 2012, 26 (Suppl. S1), 1–29. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e33. [Google Scholar] [CrossRef] [PubMed]
- Czernielewski, J.; Michel, S.; Bouclier, M.; Baker, M.; Hensby, C. Adapalene biochemistry and the evolution of a new topical retinoid for treatment of acne. J. Eur. Acad. Dermatol. Venereol. 2001, 15 (Suppl. S3), 5–12. [Google Scholar] [CrossRef]
- Michel, S.; Jomard, A.; Démarchez, M. Pharmacology of adapalene. Br. J. Dermatol. 1998, 139 (Suppl. S52), 3–7. [Google Scholar] [CrossRef]
- Tan, J.; Thiboutot, D.; Popp, G.; Gooderham, M.; Lynde, C.; Del Rosso, J.; Weiss, J.; Blume-Peytavi, U.; Weglovska, J.; Johnson, S.; et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J. Am. Acad. Dermatol. 2020, 80, 1691–1699. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Fowler, J.; Kemény, L.; Draelos, Z.; Cook-Bolden, F.; Dirschka, T.; Eichenfield, L.; Graeber, M.; Ahmad, F.; Saenz, A.A.; et al. Long-term safety and efficacy of trifarotene 50 μg/g cream, a first-in-class RAR-γ selective topical retinoid, in patients with moderate facial and truncal acne. J. Eur. Acad. Dermatol. Venereol. 2019, 34, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Chavda, R.; Dubois, J.C. Subject Satisfaction with Trifarotene 50 μg/g Cream in the Treatment of Facial and Truncal Acne Vulgaris: A Case Series. Dermatol. Ther. 2020, 10, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential Role of the Microbiome in Acne: A Comprehensive Review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef]
- McCoy, W.H.; Otchere, E.; Rosa, B.A.; Martin, J.; Mann, C.M.; Mitreva, M. Skin Ecology during Sebaceous Drought—How Skin Microbes Respond to Isotretinoin. J. Investig. Dermatol. 2019, 139, 732–735. [Google Scholar] [CrossRef]
- Baron, J.M.; Heise, R.; Blaner, W.S.; Neis, M.; Joussen, S.; Dreuw, A.; Marquardt, Y.; Saurat, J.-H.; Merk, H.F.; Bickers, D.R.; et al. Retinoic Acid and its 4-Oxo Metabolites are Functionally Active in Human Skin Cells In Vitro. J. Investig. Dermatol. 2005, 125, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Duell, E.A.; Astrom, A.; Griffiths, C.E.; Chambon, P.; Voorhees, J.J. Human skin levels of retinoic acid and cytochrome P-450-derived 4-hydroxyretinoic acid after topical application of retinoic acid in vivo compared to concentrations required to stimulate retinoic acid receptor-mediated transcription in vitro. J. Clin. Investig. 1992, 4, 1269–1274. [Google Scholar] [CrossRef]
- Everts, H.B. Endogenous retinoids in the hair follicle and sebaceous gland. Biochim. Biophys. Acta 2012, 1821, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Finzi, E.; Blake, M.J.; Celano, P.; Skouge, J.; Diwan, R. Cellular localization of retinoic acid receptor-gamma expression in normal and neoplastic skin. Am. J. Pathol. 1992, 140, 1463–1471. [Google Scholar]
- Jiang, Y.J.; Lu, B.; Kim, P.; Paragh, G.; Schmitz, G.; Elias, P.M.; Feingold, K.R. PPAR and LXR Activators Regulate ABCA12 Expression in Human Keratinocytes. J. Investig. Dermatol. 2008, 128, 104–109. [Google Scholar] [CrossRef]
- Marulli, G.C.; Campione, E.; Chimenti, M.S.; Terrinoni, A.; Melino, G.; Bianchi, L. Type I lamellar ichthyosis improved by tazarotene 0.1% gel. Clin. Exp. Dermatol. 2003, 28, 391–393. [Google Scholar] [CrossRef]
- Virtanen, M.; Gedde-Dahl, T., Jr.; Mörk, N.J.; Leigh, I.; Bowden, P.E.; Vahlquist, A. Phenotypic/Genotypic Correlations in Patients with Epidermolytic Hyperkeratosis and the Effects of Retinoid Therapy on Keratin Expression. Acta Derm. Venereol. 2001, 81, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Dooren-Greebe, R.; Van De Kerkhof, P.; Happle, R. Acitretin monotherapy in Darier’s disease. Br. J. Dermatol. 1989, 121, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Marukian, N.V.; Hu, R.-H.; Craiglow, B.G.; Milstone, L.M.; Zhou, J.; Theos, A.; Kaymakcalan, H.; Akkaya, D.A.; Uitto, J.J.; Vahidnezhad, H.; et al. Expanding the Genotypic Spectrum of Bathing Suit Ichthyosis. JAMA Dermatol. 2017, 153, 537–543. [Google Scholar] [CrossRef]
- Whittaker, S.J.; MacKie, R.M. Cutaneous lymphomas and lymphocytic infiltrates. In Rook’s Textbook of Dermatology, 7th ed.; Burns, T., Breathnach, S., Cox, N., Griffiths, C., Eds.; Blackwell Science Ltd.: Oxford, UK, 2004; p. 54.1. [Google Scholar]
- Siegel, R.S.; Pandolfino, T.; Guitart, J.; Rosen, S.; Kuzel, T.M. Primary Cutaneous T-Cell Lymphoma: Review and Current Concepts. J. Clin. Oncol. 2000, 18, 2908–2925. [Google Scholar] [CrossRef] [PubMed]
- Diamandidou, E.; Cohen, P.R.; Kurzrock, R. Mycosis fungoides and Sezary syndrome. Blood 1996, 88, 2385–2409. [Google Scholar] [CrossRef]
- Vonderheid, E.C.; Bernengo, M.G.; Burg, G.; Duvic, M.; Heald, P.; Laroche, L.; Olsen, E.; Pittelkow, M.; Russell-Jones, R.; Takigawa, M.; et al. Update on erythrodermic cutaneous T-cell lymphoma: Report of the international society for cutaneous lymphomas. J. Am. Acad. Dermatol. 2002, 46, 95–106. [Google Scholar] [CrossRef]
- Paulli, M.; Berti, E. Cutaneous T-cell lymphomas (including rare subtypes). Current concepts. II. Haematologica 2004, 89, 1372–1388. [Google Scholar]
- Jenerowicz, D.; Silny, W.; Dańczak-Pazdrowska, A.; Polańska, A.; Osmola-Mańkowska, A.; Olek-Hrab, K. Environmental factors and allergic diseases. Ann. Agric. Environ. Med. 2012, 19, 475–481. [Google Scholar]
- Joks, M.; Myśliwiec, K.; Lewandowski, K. Primary breast lymphoma—A review of the literature and report of three cases. Arch. Med. Sci. 2011, 1, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Su, I.J.; Chen, C.C.; Tien, H.F.; Lay, J.D.; Chen, B.R.; Pu, Y.S.; Hong, R.L.; Shen, M.C.; Wang, C.H. Use of retinoic acids in the treatment of peripheral T-cell lymphoma: A pilot study. J. Clin. Oncol. 1994, 12, 1185–1192. [Google Scholar] [CrossRef]
- Sidell, N.; Chang, B.; Bhatti, L. Upregulation by Retinoic Acid of Interleukin-2-Receptor mRNA in Human T Lymphocytes. Cell. Immunol. 1993, 146, 28–37. [Google Scholar] [CrossRef]
- Gorgun, G.; Foss, F. Immunomodulatory effects of RXR rexinoids: Modulation of high-affinity IL-2R expression enhances susceptibility to denileukin diftitox. Blood 2002, 100, 1399–1403. [Google Scholar] [CrossRef][Green Version]
- Schadt, C.R. Topical and oral bexarotene. Dermatol. Ther. 2013, 26, 400–403. [Google Scholar] [CrossRef]
- Tarabadkar, E.S.; Shinohara, M.M. Skin Directed Therapy in Cutaneous T-Cell Lymphoma. Front. Oncol. 2019, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Besner Morin, C.; Roberge, D.; Turchin, I.; Petrogiannis-Haliotis, T.; Popradi, G.; Pehr, K. Tazarotene 0.1% Cream as Monotherapy for Early-Stage Cutaneous T-Cell Lymphoma. J. Cutan. Med. Surg. 2016, 20, 244–248. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Identifier NCT03738800, A Safety, Efficacy and Systemic Exposure Study of CD5789 Cream in Adults and Adolescents with Lamellar Ichthyosis; 2019 May 1 [about 4 Screens]. 29 February. Available online: https://ichgcp.net/clinical-trials-registry/NCT03738800/ (accessed on 6 August 2020).
- Campbell, R.M.; DiGiovanna, J.J. Skin cancer chemoprevention with systemic retinoids: An adjunct in the management of selected high-risk patients. Dermatol. Ther. 2006, 19, 306–314. [Google Scholar] [CrossRef]
- Lotan, R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim. Biophys. Acta 1980, 605, 33–91. [Google Scholar] [CrossRef]
- Lens, M.; Medenica, L. Systemic retinoids in chemoprevention of non-melanoma skin cancer. Expert Opin. Pharmacother. 2008, 9, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Cheepala, S.B.; Yin, W.; Syed, Z.; Gill, J.N.; Mcmillian, A.; Kleiner, H.E.; Lynch, M.; Loganantharaj, R.; Trutschl, M.; Cvek, U.; et al. Identification of the B-Raf/Mek/Erk MAP kinase pathway as a target for all-trans retinoic acid during skin cancer promotion. Mol. Cancer 2009, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Sorg, O.; Tran, C.; Saurat, J.-H. Cutaneous Vitamins A and E in the Context of Ultraviolet- or Chemically-Induced Oxidative Stress. Skin Pharmacol. Appl. Skin Physiol. 2001, 14, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, M.; Dunn, J.; Darragh, A.; Lambe, R.; Brick, I. Etretinate in treatment of actinic keratosis. A double-blind crossover study. Lancet 1982, 319, 364–365. [Google Scholar] [CrossRef]
- Bavinck, J.N.; Tieben, L.M.; Van Der Woude, F.J.; Tegzess, A.M.; Hermans, J.; Ter Schegget, J.; Vermeer, B.J. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: A double-blind, placebo-controlled study. J. Clin. Oncol. 1995, 13, 1933–1938. [Google Scholar] [CrossRef] [PubMed]
- Bollag, W.; Ott, F. Retinoic acid: Topical treatment of senile or actinic keratoses and basal cell carcinomas. Agents Actions 1970, 1, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, A.; Bianchi, L.; Costanzo, A.; Campione, E.; Spagnoli, L.G.; Chimenti, S. Evidence of Increased Apoptosis and Reduced Proliferation in Basal Cell Carcinomas Treated with Tazarotene. J. Investig. Dermatol. 2004, 122, 1037–1041. [Google Scholar] [CrossRef]
- Bianchi, L.; Orlandi, A.; Campione, E.; Angeloni, C.; Costanzo, A.; Spagnoli, L.G.; Chimenti, S. Topical treatment of basal cell carcinoma with tazarotene: A clinicopathological study on a large series of cases. Br. J. Dermatol. 2004, 151, 148–156. [Google Scholar] [CrossRef]
- Nijsten, T.E.C.; Stern, R.S. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: A nested cohort study. J. Am. Acad. Dermatol. 2003, 49, 644–650. [Google Scholar] [CrossRef]
- Kim, J.; Park, M.K.; Li, W.-Q.; Qureshi, A.A.; Cho, E. Association of Vitamin A Intake With Cutaneous Squamous Cell Carcinoma Risk in the United States. JAMA Dermatol. 2019, 155, 1260–1268. [Google Scholar] [CrossRef]
- Cosio, T.; Di Prete, M.; Campione, E. Arsenic Trioxide, Itraconazole, All-Trans Retinoic Acid and Nicotinamide: A Proof of Concept for Combined Treatments with Hedgehog Inhibitors in Advanced Basal Cell Carcinoma. Biomedicines 2020, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Babino, G.; Diluvio, L.; Bianchi, L.; Orlandi, A.; Di Prete, M.; Chimenti, S.; Milani, M.; Campione, E. Long-term use of a new topical formulation containing piroxicam 0.8% and sunscreen: Efficacy and tolerability on actinic keratosis. A proof of concept study. Curr. Med. Res. Opin. 2016, 32, 1345–1349. [Google Scholar] [CrossRef]
- Girmenia, C.; Coco, F.L.; Breccia, M.; Latagliata, R.; Spadea, A.; D’Andrea, M.; Gentile, G.; Micozzi, A.; Alimena, G.; Martino, P.; et al. Infectious complications in patients with acute promyelocytic leukaemia treated with the AIDA regimen. Leukemia 2003, 17, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Paterno, E.J.; Diluvio, L.; Costanza, G.; Bianchi, L.; Carboni, I.; Chimenti, S.; Orlandi, A.; Marino, D.; Favalli, C. Tazarotene as alternative topical treatment for onychomycosis. Drug Des. Dev. Ther. 2015, 9, 879–886. [Google Scholar] [CrossRef]
- Campione, E.; Gaziano, R.; Marino, D.; Orlandi, A. Fungistatic activity of all-trans retinoic acid against Aspergillus fumigatus and Candida albicans. Drug Des. Dev. Ther. 2016, 10, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.-S.; Zhang, C.; Shao, S.; Jung, H.-W.; Durant, P.J.; Lee, C.-H. All-Trans Retinoic Acid in Combination with Primaquine Clears Pneumocystis Infection. PLoS ONE 2013, 8, e53479. [Google Scholar] [CrossRef] [PubMed]
- Klassert, T.E.; Hanisch, A.; Bräuer, J.; Klaile, E.; Heyl, K.A.; Mansour, M.M.; Tam, J.M.; Vyas, J.M.; Slevogt, H. Modulatory role of vitamin A on the Candida albicans-induced immune response in human monocytes. Med. Microbiol. Immunol. 2014, 203, 415–424. [Google Scholar] [CrossRef]
- Campione, E.; Gaziano, R.; Doldo, E.; Marino, D.; Falconi, M.; Iacovelli, F.; Tagliaferri, D.; Pacello, L.; Bianchi, L.; Lanna, C.; et al. Antifungal Effect of All-trans Retinoic Acid against Aspergillus fumigatus In Vitro and in a Pulmonary Aspergillosis In Vivo Model. Antimicrob. Agents Chemother. 2020, 65. [Google Scholar] [CrossRef]
- Lipner, S.R.; Scher, R.K. Onychomycosis: Treatment and prevention of recurrence. J. Am. Acad. Dermatol. 2019, 80, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E. Onychomycosis: Pathogenesis, Diagnosis, and Management. Clin. Microbiol. Rev. 1998, 11, 415–429. [Google Scholar] [CrossRef]
- Carratù, M.R.; Marasco, C.; Mangialardi, G.; Vacca, A. Retinoids: Novel immunomodulators and tumour-suppressive agents? Br. J. Pharmacol. 2012, 167, 483–492. [Google Scholar] [CrossRef]
- Kligman, A.M.; Grove, G.L.; Hirose, R.; Leyden, J.J. Topical tretinoin for photoaged skin. J. Am. Acad. Dermatol. 1986, 15 (Pt 2), 836–859. [Google Scholar] [CrossRef]
- Li, Z.; Niu, X.; Xiao, S.; Ma, H. Retinoic acid ameliorates photoaged skin through RAR-mediated pathway in mice. Mol. Med. Rep. 2017, 16, 6240–6247. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rusu, A.; Tanase, C.; Pascu, G.-A.; Todoran, N. Recent Advances Regarding the Therapeutic Potential of Adapalene. Pharmaceuticals 2020, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Cosio, T.; Lanna, C.; Mazzilli, S.; Dika, E.; Bianchi, L. Clinical efficacy and reflectance confocal microscopy monitoring in moderate-severe skin aging treated with a polyvinyl gel containing retinoic and glycolic acid: An assessor-blinded 1-month study proof-of-concept trial. J. Cosmet. Dermatol. 2020, 20, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Grandinetti, C.A.; Goldspiel, B.R. Sorafenib and Sunitinib: Novel Targeted Therapies for Renal Cell Cancer. Pharmacotherapy 2007, 27, 1125–1144. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Reilly, L.M.; Gerami, P.; Guitart, J. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann. Oncol. 2008, 19, 1955–1961. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Identifier NCT00667589, Four-Arm Study to Evaluate Urea 40% Cream, Fluocinonide 0.05% Cream, Tazarotene 0.1% Cream, and an Emollient Cream for the Treatment of Hand-Foot Skin Reaction Related to the Use of Multi-Targeted Tyrosine Kinase Inhibitor Sorafenib. 5 June 2013; [about 4 Screens]. 29 February. Available online: https://clinicaltrials.gov/ct2/show/study/NCT00667589?cond=hand-foot+skin+reaction&draw=2&rank=5 (accessed on 3 September 2020).
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Identifier NCT04071756, A Phase II Randomized Double-Blind Trial of Topical Tazarotene 0.1% Gel Versus Placebo Gel for the Prevention of Regoraf-Enib-Induced Hand-Foot-Skin Reaction; 12 December 2019; [about 4 Screens]. 29 February. Available online: https://clinicaltrials.gov/ct2/show/NCT04071756?term=tazarotene&draw=2&rank=1 (accessed on 3 September 2020).
| Receptor | RAR-α | RAR-β | RAR-γ | |
|---|---|---|---|---|
| Tissue Expression | Lung, Spleen, Gallbladder | Placenta, Prostate, Urinary Bladder, Kidney, Heart | Skin | |
| Drug | Tazarotene | ☑ | ☑ | ☑ |
| Tretinoin | ☑ | ☑ | ☑ | |
| Trifarotene | X | X | ☑ | |
| Adapalene | X | ☑ | ☑ | |
| Alitetrinoin | ☑ | ☑ | ☑ | |
| Tamibarotene | ☑ | X | X | |
| Palovarotene | X | X | ☑ | |
| Official Title on ClinicalTrials.gov or Publication Title (NCT Number and Status) | Phase; Evaluation Time; Sample Size | Endpoints and Results |
|---|---|---|
| A Multi-Centre, Randomized, Double-Blind, Parallel-Group Vehicle Controlled Study to Compare The Efficacy And Safety Of CD5789 (Trifarotene) 50 μg/g Cream Versus Vehicle Cream In Subjects With Acne Vulgaris (NCT02566369; Completed) [36] | III; 12 weeks; 1208 patients, randomized, parallel assignment | IGA: trifarotene arm 42.6%; placebo arm 25.8% |
| A Multi-Center, Randomized, Double-Blind, Parallel-Group Vehicle Controlled Study To Compare The Efficacy And Safety Of CD5789 (Trifarotene) 50 µg/g Cream Versus Vehicle Cream In Subjects With Acne Vulgaris (NCT02556788; Completed) [36] | III; 12 weeks; 1212 patients, randomized, parallel assignment | IGA: trifarotene arm 29.4%; placebo arm 19.5% |
| A long-term safety and efficacy study of cd5789 (trifarotene) 50 µg/g cream in subjects with acne vulgaris (NCT02189629; Completed) [37] | III; 52 weeks; 453 patients, single group assignment | - Primary: IGA 65.1% - Secondary: PGA 66.9% |
| Official Title on ClinicalTrials.gov or Publication Title (NCT Number and Status) | Phase; Sample Size | Drugs Evaluated | Endpoints and Results |
|---|---|---|---|
| A Randomized, Multi-centre, Investigator-blind, Vehicle- and Active-controlled, Phase 2 Study to Assess the Efficacy and Safety of Different Concentrations of CD5789 Cream Applied Once Daily in Subjects With Moderate to Severe Acne Vulgaris (NCT01616654;Completed) | II; 304 patients, randomized, parallel assignment | CD5789 25 µg/g cream; CD5789 50 µg/g cream; CD5789 100 µg/g cream; tazarotene 0.1% gel; vehicle cream | - Endpoints: (1) success rate (IGA); (2) absolute change in total lesion counts; (3) percentage ghange in total lesion counts - Results: not yet reported |
| A Multi-Centre Study to Evaluate Subject Reported Outcomes with Use of Trifarotene 50 μg/g Cream in the Treatment of Moderate Facial and Truncal Acne Vulgaris (NCT03915860 Active, non-recruiting) | III; 40 patients, single group assignment, open label | Trifarotene 50 μg/g cream | - Endpoint: success rate (IGA) score of 1 or 0 and at least a 2-grade improvement - Resuls: not yet reported |
| A Multi-Centre, Randomized, Double-Blind, Placebo Controlled Study to Compare Efficacy and Safety of Trifarotene (CD5789) Cream When Used with an Oral Antibiotic for the Treatment of Severe Acne Vulgaris (NCT04451330; Not recruiting) | IV; 198 patients, randomized, parallel assignment | Trifarotene cream; Doxycycline hyclate; Trifarotene Vehicle; Doxycycline Placebo | - Primary endpoint: change in facial total lesion counts (inflammatory and non-inflammatory) - Secondary endpoints: (1) change in facial inflammatory lesions counts; (2) change in facial non-inflammatory lesions count - Results: not yet reported |
| Official Title on ClinicalTrials.gov or Publication Title (NCT Number and Status) | Phase; Sample Size | Endpoints and Results |
|---|---|---|
| A Phase 2 Randomized, Multicenter, Doubleblind, Vehicle Controlled, 12 Week, Safety, Efficacy & Systemic Exposure Study Followed by a 12 Week Open-label Extension of CD5789 in Adults and Adolescents With Autosomal Recessive Ichthyosis With Lamellar Scale (NCT03738800; Recruiting) [49] | II; 120 patients, randomized, parallel assignment | - Primary endpoint: successful resolution of lamellar ichtyosis - Secondary endpoints: (1) difference in mean scores using Individual score for roughness; (2) difference in mean scores using Palm Sole Assessment; (3) difference in proportion of subjects with fissures between the active and vehicle groups; (4) Dermatology Life Quality Index; (5) 5-point Visual Index for Ichthyosis Severity - Results: not yet reported |
| Official Title on ClinicalTrials.gov or Publication Title (NCT Number and Status) | Phase; Sample Size | Endpoints and Results |
|---|---|---|
| Exploratory Study to Evaluate the Safety and Efficacy of CD5789 in Subjects with Early Stage Cutaneous T-Cell Lymphoma (NCT01804335; Completed) [63] | I; 11 patients, single group assignment, open label | - Endpoint: tolerance score of CD5789 0.01% cream - Results: not yet reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosio, T.; Di Prete, M.; Gaziano, R.; Lanna, C.; Orlandi, A.; Di Francesco, P.; Bianchi, L.; Campione, E. Trifarotene: A Current Review and Perspectives in Dermatology. Biomedicines 2021, 9, 237. https://doi.org/10.3390/biomedicines9030237
Cosio T, Di Prete M, Gaziano R, Lanna C, Orlandi A, Di Francesco P, Bianchi L, Campione E. Trifarotene: A Current Review and Perspectives in Dermatology. Biomedicines. 2021; 9(3):237. https://doi.org/10.3390/biomedicines9030237
Chicago/Turabian StyleCosio, Terenzio, Monia Di Prete, Roberta Gaziano, Caterina Lanna, Augusto Orlandi, Paolo Di Francesco, Luca Bianchi, and Elena Campione. 2021. "Trifarotene: A Current Review and Perspectives in Dermatology" Biomedicines 9, no. 3: 237. https://doi.org/10.3390/biomedicines9030237
APA StyleCosio, T., Di Prete, M., Gaziano, R., Lanna, C., Orlandi, A., Di Francesco, P., Bianchi, L., & Campione, E. (2021). Trifarotene: A Current Review and Perspectives in Dermatology. Biomedicines, 9(3), 237. https://doi.org/10.3390/biomedicines9030237









