Intractable Itch in Atopic Dermatitis: Causes and Treatments
Abstract
1. Introduction
2. Methods
3. Transduction of Itch
4. Substances and Receptors Inducing Itch in AD
4.1. Histamine
4.2. Proteases
4.3. Cytokines
4.4. Neuropeptides
4.5. Lipids
4.6. Opioids
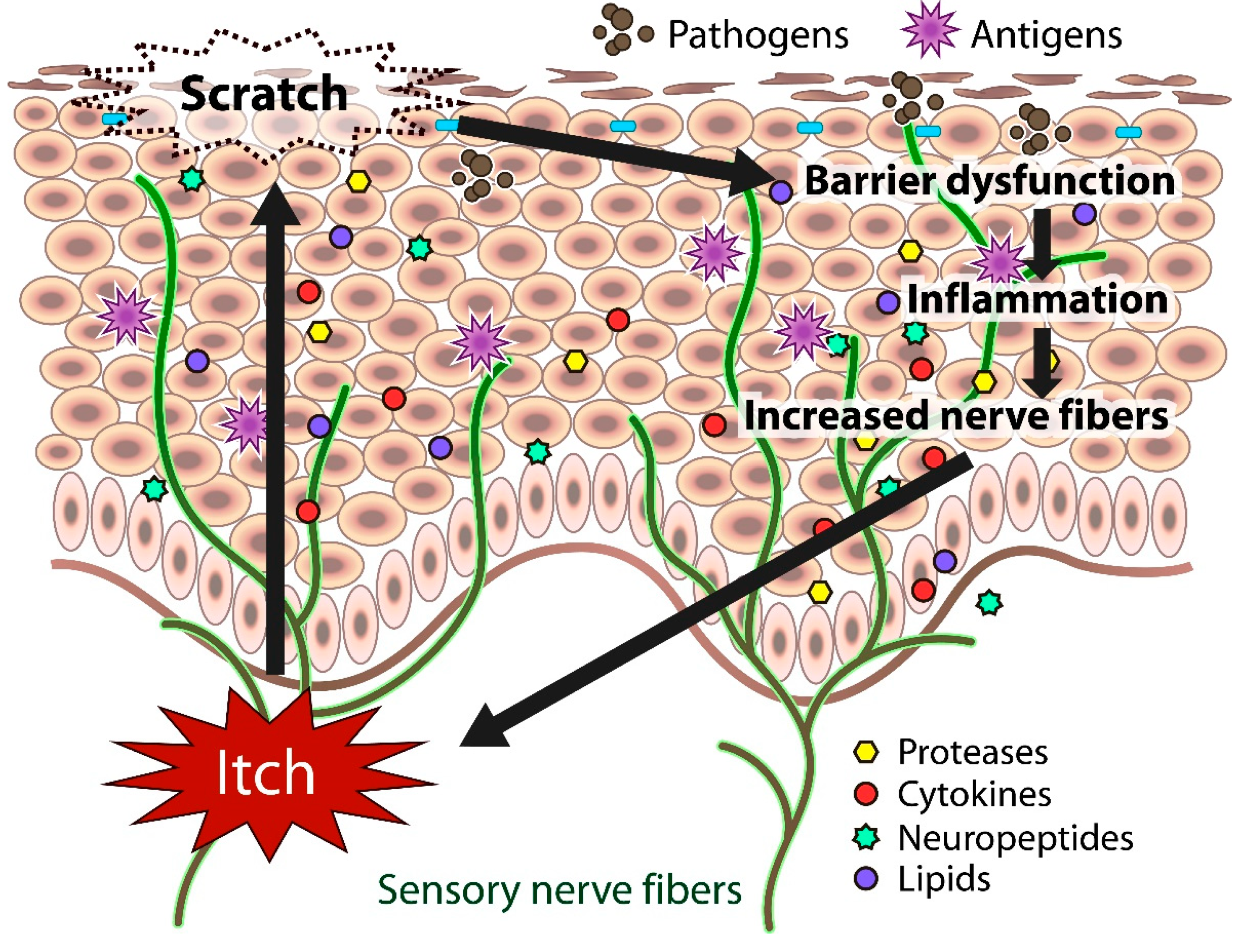
5. Cutaneous Nerve Fibers
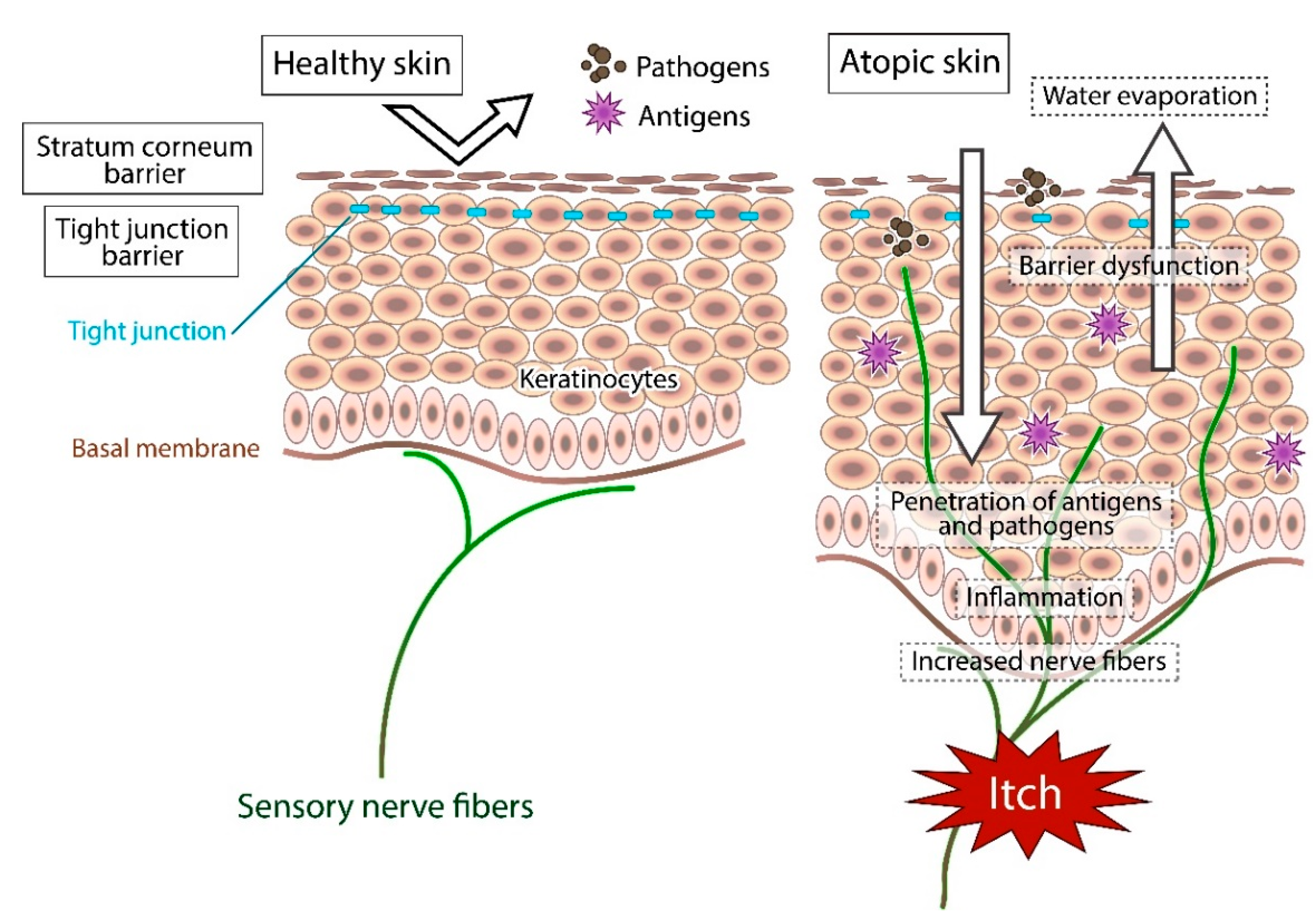
6. Skin Dryness
7. Dysregulation of the Expression of Antimicrobial Peptides
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nettis, E.; Ortoncelli, M.; Pellacani, G.; Foti, C.; Di Leo, E.; Patruno, C.; Rongioletti, F.; Argenziano, G.; Ferrucci, S.M.; Macchia, L.; et al. A multicenter study on the prevalence of clinical patterns and clinical phenotypes in adult atopic dermatitis. J. Investig. Allergol. Clin. Immunol. 2020, 30, 448–450. [Google Scholar] [CrossRef]
- Girolomoni, G.; Luger, T.; Nosbaum, A.; Gruben, D.; Romero, W.; Llamado, L.J.; DiBonaventura, M. The economic and psychosocial comorbidity burden among adults with moderate-to-severe atopic dermatitis in Europe: Analysis of a cross-sectional survey. Dermatol. Ther. 2021, 11, 117–130. [Google Scholar] [CrossRef]
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 2017, 1027, 21–37. [Google Scholar] [CrossRef]
- Murota, H.; Katayama, I. Exacerbating factors of itch in atopic dermatitis. Allergol. Int. 2017, 66, 8–13. [Google Scholar] [CrossRef]
- Suárez, A.L.; Feramisco, J.D.; Koo, J.; Steinhoff, M. Psychoneuroimmunology of psychological stress and atopic dermatitis: Pathophysiologic and therapeutic updates. Acta Derm. Venereol. 2012, 92, 7–15. [Google Scholar] [CrossRef]
- Bonamonte, D.; Filoni, A.; Vestita, M.; Romita, P.; Foti, C.; Angelini, G. The role of the environmental risk factors in the pathogenesis and clinical outcome of atopic dermatitis. Biomed. Res. Int. 2019, 2019, 2450605. [Google Scholar] [CrossRef]
- Klein, P.A.; Clark, R.A.F. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch. Dermatol. 1999, 135, 1522–1525. [Google Scholar] [CrossRef]
- Ikoma, A.; Steinhoff, M.; Ständer, S.; Yosipovitch, G.; Schmelz, M. The neurobiology of itch. Nat. Rev. Neurosci. 2006, 7, 535–547. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Kittaka, H.; Tominaga, M. The molecular and cellular mechanisms of itch and the involvement of TRP channels in the peripheral sensory nervous system and skin. Allergol. Int. 2017, 66, 22–30. [Google Scholar] [CrossRef]
- Tóth, B.I.; Oláh, A.; Szöllősi, A.G.; Bíró, T. TRP channels in the skin. Br. J. Pharmacol. 2014, 171, 2568–2581. [Google Scholar] [CrossRef]
- Sousa-Valente, J.; Andreou, A.P.; Urban, L.; Nagy, I. Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics. Brit. J. Pharmacol. 2014, 171, 2508–2527. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Greaves, M.W.; Schmelz, M. Itch. Lancet 2003, 361, 690–694. [Google Scholar] [CrossRef]
- Ikoma, A.; Fartasch, M.; Heyer, G.; Miyachi, Y.; Handwerker, H.; Schmelz, M. Painful stimuli evoke itch in patients with chronic pruritus: Central sensitization for itch. Neurology 2004, 62, 212–217. [Google Scholar] [CrossRef]
- Thurmond, R.L.; Kazerouni, K.; Chaplan, S.R.; Greenspan, A.J. Peripheral Neuronal Mechanism of Itch: Histamine and Itch. In Itch: Mechanisms and Treatment; Carstens, E., Akiyama, T., Eds.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Ohsawa, Y.; Hirasawa, N. The role of histamine H1 and H4 receptors in atopic dermatitis: From basic research to clinical study. Allergol. Int. 2014, 63, 533–542. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Takano, N.; Nakamura, A.; Nakaike, S.; Yu, Z.; Endo, Y.; Arai, I. Scratching behavior in NC/Nga mice with dermatitis: Involvement of histamine-induced itching. Allergol. Int. 2004, 53, 349–358. [Google Scholar]
- Kamo, A.; Negi, O.; Tengara, S.; Kamata, Y.; Noguchi, A.; Ogawa, H.; Tominaga, M.; Takamori, K. Histamine H(4) receptor antagonists ineffective against itch and skin inflammation in atopic dermatitis mouse model. J. Investig. Dermatol. 2014, 134, 546–548. [Google Scholar] [CrossRef]
- Akiyama, T.; Lerner, E.A.; Carstens, E. Protease-Activated Receptors and Itch. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2015; Volume 226, pp. 219–235. [Google Scholar] [CrossRef]
- Järvikallio, A.; Naukkarinen, A.; Harvima, I.T.; Aalto, M.L.; Horsmanheimo, M. Quantitative analysis of tryptase- and chymase-containing mast cells in atopic dermatitis and nummular eczema. Br. J. Dermatol. 1997, 136, 871–877. [Google Scholar]
- Komatsu, N.; Saijoh, K.; Kuk, C.; Liu, A.C.; Khan, S.; Shirasaki, F.; Takehara, K.; Diamandis, E.P. Human tissue kallikrein expression in the stratum corneum and serum of atopic dermatitis patients. Exp. Dermatol. 2007, 16, 513–519. [Google Scholar] [CrossRef]
- Tsujii, K.; Andoh, T.; Lee, J.B.; Kuraishi, Y. Activation of proteinase-activated receptors induces itch-associated response through histamine-dependent and -independent pathways in mice. J. Pharmacol. Sci. 2008, 108, 385–388. [Google Scholar] [CrossRef]
- Tsujii, K.; Andoh, T.; Ui, H.; Lee, J.B.; Kuraishi, Y. Involvement of tryptase and proteinase-activated receptor-2 in spontaneous itch-associated response in mice with atopy-like dermatitis. J. Pharmacol. Sci. 2009, 109, 388–395. [Google Scholar] [CrossRef]
- Reddy, V.B.; Iuga, A.O.; Shimada, S.G.; LaMotte, R.H.; Lerner, E.A. Cowhage-evoked itch is mediated by a novel cysteine protease: A ligand of protease-activated receptors. J. Neurosci. 2008, 28, 4331–4335. [Google Scholar] [CrossRef]
- Reddy, V.B.; Lerner, E.A. Plant cysteine proteases that evoke itch activate protease-activated receptors. Br. J. Dermatol. 2010, 163, 532–535. [Google Scholar] [CrossRef]
- Costa, R.; Marotta, D.M.; Manjavachi, M.N.; Fernandes, E.S.; Lima-Garcia, J.F.; Paszcuk, A.F.; Quintao, N.L.; Juliano, L.; Brain, S.D.; Calixto, J.B. Evidence for the role of neurogenic inflammation components in trypsin-elicited scratching behaviour in mice. Br. J. Pharmacol. 2008, 154, 1094–1103. [Google Scholar] [CrossRef]
- Liu, Q.; Weng, H.J.; Patel, K.N.; Tang, Z.; Bai, H.; Steinhoff, M.; Dong, X. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci. Signal. 2011, 4, ra45. [Google Scholar] [CrossRef]
- Ui, H.; Andoh, T.; Lee, J.B.; Nojima, H.; Kuraishi, Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur. J. Pharmacol. 2006, 530, 172–178. [Google Scholar] [CrossRef]
- Nakano, T.; Andoh, T.; Tayama, M.; Kosaka, M.; Lee, J.B.; Kuraishi, Y. Effects of topical application of tacrolimus on acute itch-associated responses in mice. Biol. Pharm. Bull. 2008, 31, 752–754. [Google Scholar] [CrossRef]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemeny, L.; Alenius, H.; Dieu-Nosjean, M.C.; Meller, S.; Rieker, J.; et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 117, 411–417. [Google Scholar] [CrossRef]
- Neis, M.M.; Peters, B.; Dreuw, A.; Wenzel, J.; Bieber, T.; Mauch, C.; Krieg, T.; Stanzel, S.; Heinrich, P.C.; Merk, H.F.; et al. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J. Allergy Clin. Immunol. 2006, 118, 930–937. [Google Scholar] [CrossRef]
- Raap, U.; Wichmann, K.; Bruder, M.; Ständer, S.; Wedi, B.; Kapp, A.; Werfel, T. Correlation of IL-31 serum levels with severity of atopic dermatitis. J. Allergy Clin. Immunol. 2008, 122, 421–423. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Patsinakidis, N.; Raap, U. Role of the pruritic cytokine IL-31 in autoimmune skin diseases. Front. Immunol. 2019, 10, 1383. [Google Scholar] [CrossRef]
- Arai, I.; Tsuji, M.; Takeda, H.; Akiyama, N.; Saito, S. A single dose of interleukin-31 (IL-31) causes continuous itch-associated scratching behaviour in mice. Exp. Dermatol. 2013, 22, 669–671. [Google Scholar] [CrossRef]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef]
- Arita, K.; South, A.P.; Hans-Filho, G.; Sakuma, T.H.; Lai-Cheong, J.; Clements, S.; Odashiro, M.; Odashiro, D.N.; Hans-Neto, G.; Hans, N.R.; et al. Oncostatin M receptor-beta mutations underlie familial primary localized cutaneous amyloidosis. Am. J. Hum. Genet. 2008, 82, 73–80. [Google Scholar] [CrossRef]
- Nemoto, O.; Furue, M.; Nakagawa, H.; Shiramoto, M.; Hanada, R.; Matsuki, S.; Imayama, S.; Kato, M.; Hasebe, I.; Taira, K.; et al. The first trial of CIM331, a humanized antihuman interleukin-31 receptor A antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double-blind, placebo-controlled study. Br. J. Dermatol. 2016, 174, 296–304. [Google Scholar] [CrossRef]
- Ruzicka, T.; Hanifin, J.M.; Furue, M.; Pulka, G.; Mlynarczyk, I.; Wollenberg, A.; Galus, R.; Etoh, T.; Mihara, R.; Yoshida, H.; et al. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N. Engl. J. Med. 2017, 376, 826–835. [Google Scholar] [CrossRef]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef]
- Kashyap, M.; Rochman, Y.; Spolski, R.; Samsel, L.; Leonard, W.J. Thymic stromal lymphopoietin is produced by dendritic cells. J. Immunol. 2011, 187, 1207–1211. [Google Scholar] [CrossRef]
- Moniaga, C.S.; Jeong, S.K.; Egawa, G.; Nakajima, S.; Hara-Chikuma, M.; Jeon, J.E.; Lee, S.H.; Hibino, T.; Miyachi, Y.; Kabashima, K. Protease activity enhances production of thymic stromal lymphopoietin and basophil accumulation in flaky tail mice. Am. J. Pathol. 2013, 182, 841–851. [Google Scholar] [CrossRef]
- Wilson, S.R.; The, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef]
- Pandey, A.; Ozaki, K.; Baumann, H.; Levin, S.D.; Puel, A.; Farr, A.G.; Ziegler, S.F.; Leonard, W.J.; Lodish, H.F. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat. Immunol. 2000, 1, 59–64. [Google Scholar] [CrossRef]
- Ziegler, S.F.; Roan, F.; Bell, B.D.; Stoklasek, T.A.; Kitajima, M.; Han, H. The biology of thymic stromal lymphopoietin (TSLP). Adv. Pharmacol. 2013, 66, 129–155. [Google Scholar] [CrossRef]
- Oyoshi, M.K.; Larson, R.P.; Ziegler, S.F.; Geha, R.S. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J. Allergy Clin. Immunol. 2010, 126, 976–984.e5. [Google Scholar] [CrossRef]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 2017, 171, 217–228.e13. [Google Scholar] [CrossRef]
- Beck, L.A.; Thaçi, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E.; et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef]
- Thaçi, D.; Simpson, E.L.; Beck, L.A.; Bieber, T.; Blauvelt, A.; Papp, K.; Soong, W.; Worm, M.; Szepietowski, J.C.; Sofen, H.; et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: A randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet 2016, 387, 40–52. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Silverberg, J.I.; Nemoto, O.; Forman, S.B.; Wilke, A.; Prescilla, R.; de la Peña, A.; Nunes, F.P.; Janes, J.; Gamalo, M.; et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: A phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J. Am. Acad. Dermatol. 2019, 80, 913–921.e9. [Google Scholar] [CrossRef]
- Simpson, E.L.; Flohr, C.; Eichenfield, L.F.; Bieber, T.; Sofen, H.; Taïeb, A.; Owen, R.; Putnam, W.; Castro, M.; DeBusk, K.; et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J. Am. Acad. Dermatol. 2018, 78, 863–871.e11. [Google Scholar] [CrossRef]
- Wollenberg, A.; Howell, M.D.; Guttman-Yassky, E.; Silverberg, J.I.; Kell, C.; Ranade, K.; Moate, R.; van der Merwe, R. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J. Allergy Clin. Immunol. 2019, 143, 135–141. [Google Scholar] [CrossRef]
- Napolitano, M.; Marasca, C.; Fabbrocini, G.; Patruno, C. Adult atopic dermatitis: New and emerging therapies. Expert Rev. Clin. Pharmacol. 2018, 11, 867–878. [Google Scholar] [CrossRef]
- Fabbrocini, G.; Napolitano, M.; Megna, M.; Balato, N.; Patruno, C. Treatment of atopic dermatitis with biologic drugs. Dermatol. Ther. 2018, 8, 527–538. [Google Scholar] [CrossRef]
- Dattola, A.; Bennardo, L.; Silvestri, M.; Nisticò, S.P. What’s new in the treatment of atopic dermatitis? Dermatol. Ther. 2019, 32, e12787. [Google Scholar] [CrossRef]
- Andoh, T.; Kuraishi, Y. Substance P and itch. In Itch: Basic Mechanisms and Therapy; Yosipovitch, G., Ed.; Dekker: New York, NY, USA, 2004; pp. 87–95. [Google Scholar]
- Järvikallio, A.; Harvima, I.T.; Naukkarinen, A. Mast cells, nerves and neuropeptides in atopic dermatitis and nummular eczema. Arch. Dermatol. Res. 2003, 295, 2–7. [Google Scholar] [CrossRef]
- Ständer, S.; Siepmann, D.; Herrgott, I.; Sunderkötter, C.; Luger, T.A. Targeting the neurokinin receptor 1 with aprepitant: A novel antipruritic strategy. PLoS ONE 2010, 5, e10968. [Google Scholar] [CrossRef]
- Steinhoff, M.; Ständer, S.; Seeliger, S.; Ansel, J.C.; Schmelz, M.; Luger, T. Modern aspects of cutaneous neurogenic inflammation. Arch. Dermatol. 2003, 139, 1479–1488. [Google Scholar] [CrossRef]
- Schmelz, M.; Petersen, L.J. Neurogenic inflammation in human and rodent skin. News Physiol. Sci. 2001, 16, 33–37. [Google Scholar] [CrossRef]
- Rosa, A.C.; Fantozzi, R. The role of histamine in neurogenic inflammation. Br. J. Pharmacol. 2013, 170, 38–45. [Google Scholar] [CrossRef]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid receptors: Structures, properties, and functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef]
- Töröcsik, D.; Weise, C.; Gericke, J.; Szegedi, A.; Lucas, R.; Mihaly, J.; Worm, M.; Rühl, R. Transcriptomic and lipidomic profiling of eicosanoid/docosanoid signalling in affected and non-affected skin of human atopic dermatitis patients. Exp. Dermatol. 2019, 28, 177–189. [Google Scholar] [CrossRef]
- Neisius, U.; Olsson, R.; Rukwied, R.; Lischetzki, G.; Schmelz, M. Prostaglandin E2 induces vasodilation and pruritus, but no protein extravasation in atopic dermatitis and controls. J. Am. Acad. Dermatol. 2002, 47, 28–32. [Google Scholar] [CrossRef]
- Andoh, T.; Haza, S.; Saito, A.; Kuraishi, Y. Involvement of leukotriene B4 in spontaneous itch-related behaviour in NC mice with atopic dermatitis-like skin lesions. Exp. Dermatol. 2011, 20, 894–898. [Google Scholar] [CrossRef]
- Andoh, T.; Nishikawa, Y.; Yamaguchi-Miyamoto, T.; Nojima, H.; Narumiya, S.; Kuraishi, Y. Thromboxane A2 induces itch-associated responses through TP receptors in the skin in mice. J. Investig. Dermatol. 2007, 127, 2042–2047. [Google Scholar] [CrossRef]
- Arai, I.; Takano, N.; Hashimoto, Y.; Futaki, N.; Sugimoto, M.; Takahashi, N.; Inoue, T.; Nakaike, S. Prostanoid DP1 receptor agonist inhibits the pruritic activity in NC/Nga mice with atopic dermatitis. Eur. J. Pharmacol. 2004, 505, 229–235. [Google Scholar] [CrossRef]
- Goldstein, A.; Naidu, A. Multiple opioid receptors: Ligand selectivity profiles and binding site signatures. Mol. Pharmacol. 1989, 36, 265–272. [Google Scholar]
- Bigliardi, P.L.; Tobin, D.J.; Gaveriaux-Ruff, C.; Bigliardi-Qi, M. Opioids and the skin--where do we stand? Exp. Dermatol. 2009, 18, 424–430. [Google Scholar] [CrossRef]
- Bigliardi-Qi, M.; Bigliardi, P.L.; Eberle, A.N.; Buchner, S.; Rufli, T. beta-endorphin stimulates cytokeratin 16 expression and downregulates mu-opiate receptor expression in human epidermis. J. Investig. Dermatol. 2000, 114, 527–532. [Google Scholar] [CrossRef]
- Ständer, S.; Gunzer, M.; Metze, D.; Luger, T.; Steinhoff, M. Localization of mu-opioid receptor 1A on sensory nerve fibers in human skin. Regul. Pept. 2002, 110, 75–83. [Google Scholar] [CrossRef]
- Lee, C.H.; Chuang, H.Y.; Shih, C.C.; Jong, S.B.; Chang, C.H.; Yu, H.S. Transepidermal water loss, serum IgE and beta-endorphin as important and independent biological markers for development of itch intensity in atopic dermatitis. Br. J. Dermatol. 2006, 154, 1100–1107. [Google Scholar] [CrossRef]
- Bigliardi, P.L.; Stammer, H.; Jost, G.; Rufli, T.; Buchner, S.; Bigliardi-Qi, M. Treatment of pruritus with topically applied opiate receptor antagonist. J. Am. Acad. Dermatol. 2007, 56, 979–988. [Google Scholar] [CrossRef]
- Rivard, J.; Lim, H.W. Ultraviolet phototherapy for pruritus. Dermatol. Ther. 2005, 18, 344–354. [Google Scholar] [CrossRef]
- Legat, F.J. Is there still a role for UV therapy in itch treatment? Exp. Dermatol. 2019, 28, 1432–1438. [Google Scholar] [CrossRef]
- Tominaga, M.; Ogawa, H.; Takamori, K. Possible roles of epidermal opioid systems in pruritus of atopic dermatitis. J. Investig. Dermatol. 2007, 127, 2228–2235. [Google Scholar] [CrossRef]
- Fujii, M.; Akita, K.; Mizutani, N.; Nabe, T.; Kohno, S. Development of numerous nerve fibers in the epidermis of hairless mice with atopic dermatitis-like pruritic skin inflammation. J. Pharmacol. Sci. 2007, 104, 243–251. [Google Scholar] [CrossRef]
- Tominaga, M.; Ozawa, S.; Ogawa, H.; Takamori, K. A hypothetical mechanism of intraepidermal neurite formation in NC/Nga mice with atopic dermatitis. J. Dermatol. Sci. 2007, 46, 199–210. [Google Scholar] [CrossRef]
- Tominaga, M.; Takamori, K. Recent advances in pathophysiological mechanisms of itch. Expert Rev. Dermatol. 2014, 5, 197–212. [Google Scholar] [CrossRef]
- Tominaga, M.; Takamori, K. Itch and nerve fibers with special reference to atopic dermatitis: Therapeutic implications. J. Dermatol. 2014, 41, 205–212. [Google Scholar] [CrossRef]
- Toyoda, M.; Nakamura, M.; Makino, T.; Hino, T.; Kagoura, M.; Morohashi, M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br. J. Dermatol. 2002, 147, 71–79. [Google Scholar] [CrossRef]
- Salomon, J.; Baran, E. The role of selected neuropeptides in pathogenesis of atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 223–228. [Google Scholar] [CrossRef]
- Murota, H.; Izumi, M.; Abd El-Latif, M.I.; Nishioka, M.; Terao, M.; Tani, M.; Matsui, S.; Sano, S.; Katayama, I. Artemin causes hypersensitivity to warm sensation, mimicking warmth-provoked pruritus in atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 671–682.e4. [Google Scholar] [CrossRef]
- Hidaka, T.; Ogawa, E.; Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Fujimura, T.; Aiba, S.; Nakayama, K.; Okuyama, R.; et al. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat. Immunol. 2017, 18, 64–73. [Google Scholar] [CrossRef]
- Tominaga, M.; Ogawa, H.; Takamori, K. Decreased production of semaphorin 3A in the lesional skin of atopic dermatitis. Br. J. Dermatol. 2008, 158, 842–844. [Google Scholar] [CrossRef]
- Ko, K.C.; Tominaga, M.; Kamata, Y.; Umehara, Y.; Matsuda, H.; Takahashi, N.; Kina, K.; Ogawa, M.; Ogawa, H.; Takamori, K. Possible antipruritic mechanism of cyclosporine A in atopic dermatitis. Acta Derm. Venereol. 2016, 96, 624–629. [Google Scholar] [CrossRef]
- Wallengren, J.; Sundler, F. Phototherapy reduces the number of epidermal and CGRP-positive dermal nerve fibres. Acta Derm. Venereol. 2004, 84, 111–115. [Google Scholar] [CrossRef]
- Tominaga, M.; Tengara, S.; Kamo, A.; Ogawa, H.; Takamori, K. Psoralen-ultraviolet A therapy alters epidermal Sema3A and NGF levels and modulates epidermal innervation in atopic dermatitis. J. Dermatol. Sci. 2009, 55, 40–46. [Google Scholar] [CrossRef]
- Kamo, A.; Tominaga, M.; Kamata, Y.; Kaneda, K.; Ko, K.C.; Matsuda, H.; Kimura, U.; Ogawa, H.; Takamori, K. The excimer lamp induces cutaneous nerve degeneration and reduces scratching in a dry-skin mouse model. J. Investig. Dermatol. 2014, 134, 2977–2984. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Misery, L.; Proksch, E.; Metz, M.; Stander, S.; Schmelz, M. Skin barrier damage and itch: Review of mechanisms, topical management and future directions. Acta Derm. Venereol. 2019, 99, 1201–1209. [Google Scholar] [CrossRef]
- Angelova-Fischer, I.; Fernandez, I.M.; Donnadieu, M.H.; Bulfone-Paus, S.; Zillikens, D.; Fischer, T.W.; Soumelis, V. Injury to the stratum corneum induces in vivo expression of human thymic stromal lymphopoietin in the epidermis. J. Investig. Dermatol. 2010, 130, 2505–2507. [Google Scholar] [CrossRef]
- Denda, M.; Sato, J.; Tsuchiya, T.; Elias, P.M.; Feingold, K.R. Low humidity stimulates epidermal DNA synthesis and amplifies the hyperproliferative response to barrier disruption: Implication for seasonal exacerbations of inflammatory dermatoses. J. Investig. Dermatol. 1998, 111, 873–878. [Google Scholar] [CrossRef]
- Miyamoto, T.; Nojima, H.; Shinkado, T.; Nakahashi, T.; Kuraishi, Y. Itch-associated response induced by experimental dry skin in mice. Jpn. J. Pharmacol. 2002, 88, 285–292. [Google Scholar] [CrossRef]
- Inami, Y.; Sasaki, A.; Andoh, T.; Kuraishi, Y. Surfactant-induced chronic pruritus: Role of L-histidine decarboxylase expression and histamine production in epidermis. Acta Derm. Venereol. 2014, 94, 645–650. [Google Scholar] [CrossRef]
- Akiyama, T.; Carstens, M.I.; Carstens, E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain 2010, 151, 378–383. [Google Scholar] [CrossRef]
- Akiyama, T.; Nagamine, M.; Carstens, M.I.; Carstens, E. Behavioral model of itch, alloknesis, pain and allodynia in the lower hindlimb and correlative responses of lumbar dorsal horn neurons in the mouse. Neuroscience 2014, 266, 38–46. [Google Scholar] [CrossRef]
- Liu, T.; Han, Q.; Chen, G.; Huang, Y.; Zhao, L.X.; Berta, T.; Gao, Y.J.; Ji, R.R. Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice. Pain 2016, 157, 806–817. [Google Scholar] [CrossRef]
- Beutler, B. Innate immunity: An overview. Mol. Immunol. 2004, 40, 845–859. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Kiatsurayanon, C.; Chieosilapatham, P.; Ogawa, H. Friends or Foes? Host defense (antimicrobial) peptides and proteins in human skin diseases. Exp. Dermatol. 2017, 26, 989–998. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Kiatsurayanon, C.; Ogawa, H. The role of human beta-defensins in allergic diseases. Clin. Exp. Allergy 2016, 46, 1522–1530. [Google Scholar] [CrossRef]
- Ong, P.Y.; Ohtake, T.; Brandt, C.; Strickland, I.; Boguniewicz, M.; Ganz, T.; Gallo, R.L.; Leung, D.Y. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 2002, 347, 1151–1160. [Google Scholar] [CrossRef]
- de Jongh, G.J.; Zeeuwen, P.L.; Kucharekova, M.; Pfundt, R.; van der Valk, P.G.; Blokx, W.; Dogan, A.; Hiemstra, P.S.; van de Kerkhof, P.C.; Schalkwijk, J. High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J. Investig. Dermatol. 2005, 125, 1163–1173. [Google Scholar] [CrossRef]
- Nomura, I.; Goleva, E.; Howell, M.D.; Hamid, Q.A.; Ong, P.Y.; Hall, C.F.; Darst, M.A.; Gao, B.; Boguniewicz, M.; Travers, J.B.; et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J. Immunol. 2003, 171, 3262–3269. [Google Scholar] [CrossRef]
- Howell, M.D.; Novak, N.; Bieber, T.; Pastore, S.; Girolomoni, G.; Boguniewicz, M.; Streib, J.; Wong, C.; Gallo, R.L.; Leung, D.Y. Interleukin-10 downregulates anti-microbial peptide expression in atopic dermatitis. J. Investig. Dermatol. 2005, 125, 738–745. [Google Scholar] [CrossRef]
- Liang, S.C.; Tan, X.Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef]
- Kiatsurayanon, C.; Niyonsaba, F.; Smithrithee, R.; Akiyama, T.; Ushio, H.; Hara, M.; Okumura, K.; Ikeda, S.; Ogawa, H. Host defense (Antimicrobial) peptide, human beta-defensin-3, improves the function of the epithelial tight-junction barrier in human keratinocytes. J. Investig. Dermatol. 2014, 134, 2163–2173. [Google Scholar] [CrossRef]
- Akiyama, T.; Niyonsaba, F.; Kiatsurayanon, C.; Nguyen, T.T.; Ushio, H.; Fujimura, T.; Ueno, T.; Okumura, K.; Ogawa, H.; Ikeda, S. The human cathelicidin LL-37 host defense peptide upregulates tight junction-related proteins and increases human epidermal keratinocyte barrier function. J. Innate Immun. 2014, 6, 739–753. [Google Scholar] [CrossRef]
- Hattori, F.; Kiatsurayanon, C.; Okumura, K.; Ogawa, H.; Ikeda, S.; Okamoto, K.; Niyonsaba, F. The antimicrobial protein S100A7/psoriasin enhances the expression of keratinocyte differentiation markers and strengthens the skin’s tight junction barrier. Br. J. Dermatol. 2014, 171, 742–753. [Google Scholar] [CrossRef]
- Umehara, Y.; Kamata, Y.; Tominaga, M.; Niyonsaba, F.; Ogawa, H.; Takamori, K. Cathelicidin LL-37 induces semaphorin 3A expression in human epidermal keratinocytes: Implications for possible application to pruritus. J. Investig. Dermatol. 2015, 135, 2887–2890. [Google Scholar] [CrossRef]
- Umehara, Y.; Kamata, Y.; Tominaga, M.; Niyonsaba, F.; Ogawa, H.; Takamori, K. Antimicrobial peptides human LL-37 and beta-defensin-3 modulate the expression of nerve elongation factors in human epidermal keratinocytes. J. Dermatol. Sci. 2017, 88, 365–367. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Someya, A.; Hirata, M.; Ogawa, H.; Nagaoka, I. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur. J. Immunol. 2001, 31, 1066–1075. [Google Scholar] [CrossRef]
- Chen, X.; Niyonsaba, F.; Ushio, H.; Hara, M.; Yokoi, H.; Matsumoto, K.; Saito, H.; Nagaoka, I.; Ikeda, S.; Okumura, K.; et al. Antimicrobial peptides human beta-defensin (hBD)-3 and hBD-4 activate mast cells and increase skin vascular permeability. Eur. J. Immunol. 2007, 37, 434–444. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Hara, M.; Yokoi, H.; Tominaga, M.; Takamori, K.; Kajiwara, N.; Saito, H.; Nagaoka, I.; Ogawa, H.; et al. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J. Immunol. 2010, 184, 3526–3534. [Google Scholar] [CrossRef]
- Kanda, N.; Watanabe, S. Increased serum human beta-defensin-2 levels in atopic dermatitis: Relationship to IL-22 and oncostatin M. Immunobiology 2012, 217, 436–445. [Google Scholar] [CrossRef]
- Clausen, M.L.; Jungersted, J.M.; Andersen, P.S.; Slotved, H.C.; Krogfelt, K.A.; Agner, T. Human beta-defensin-2 as a marker for disease severity and skin barrier properties in atopic dermatitis. Br. J. Dermatol. 2013, 169, 587–593. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umehara, Y.; Kiatsurayanon, C.; Trujillo-Paez, J.V.; Chieosilapatham, P.; Peng, G.; Yue, H.; Nguyen, H.L.T.; Song, P.; Okumura, K.; Ogawa, H.; et al. Intractable Itch in Atopic Dermatitis: Causes and Treatments. Biomedicines 2021, 9, 229. https://doi.org/10.3390/biomedicines9030229
Umehara Y, Kiatsurayanon C, Trujillo-Paez JV, Chieosilapatham P, Peng G, Yue H, Nguyen HLT, Song P, Okumura K, Ogawa H, et al. Intractable Itch in Atopic Dermatitis: Causes and Treatments. Biomedicines. 2021; 9(3):229. https://doi.org/10.3390/biomedicines9030229
Chicago/Turabian StyleUmehara, Yoshie, Chanisa Kiatsurayanon, Juan Valentin Trujillo-Paez, Panjit Chieosilapatham, Ge Peng, Hainan Yue, Hai Le Thanh Nguyen, Pu Song, Ko Okumura, Hideoki Ogawa, and et al. 2021. "Intractable Itch in Atopic Dermatitis: Causes and Treatments" Biomedicines 9, no. 3: 229. https://doi.org/10.3390/biomedicines9030229
APA StyleUmehara, Y., Kiatsurayanon, C., Trujillo-Paez, J. V., Chieosilapatham, P., Peng, G., Yue, H., Nguyen, H. L. T., Song, P., Okumura, K., Ogawa, H., & Niyonsaba, F. (2021). Intractable Itch in Atopic Dermatitis: Causes and Treatments. Biomedicines, 9(3), 229. https://doi.org/10.3390/biomedicines9030229








