Neuropeptide Substance P Enhances Skin Wound Healing In Vitro and In Vivo under Hypoxia
Abstract
1. Introduction
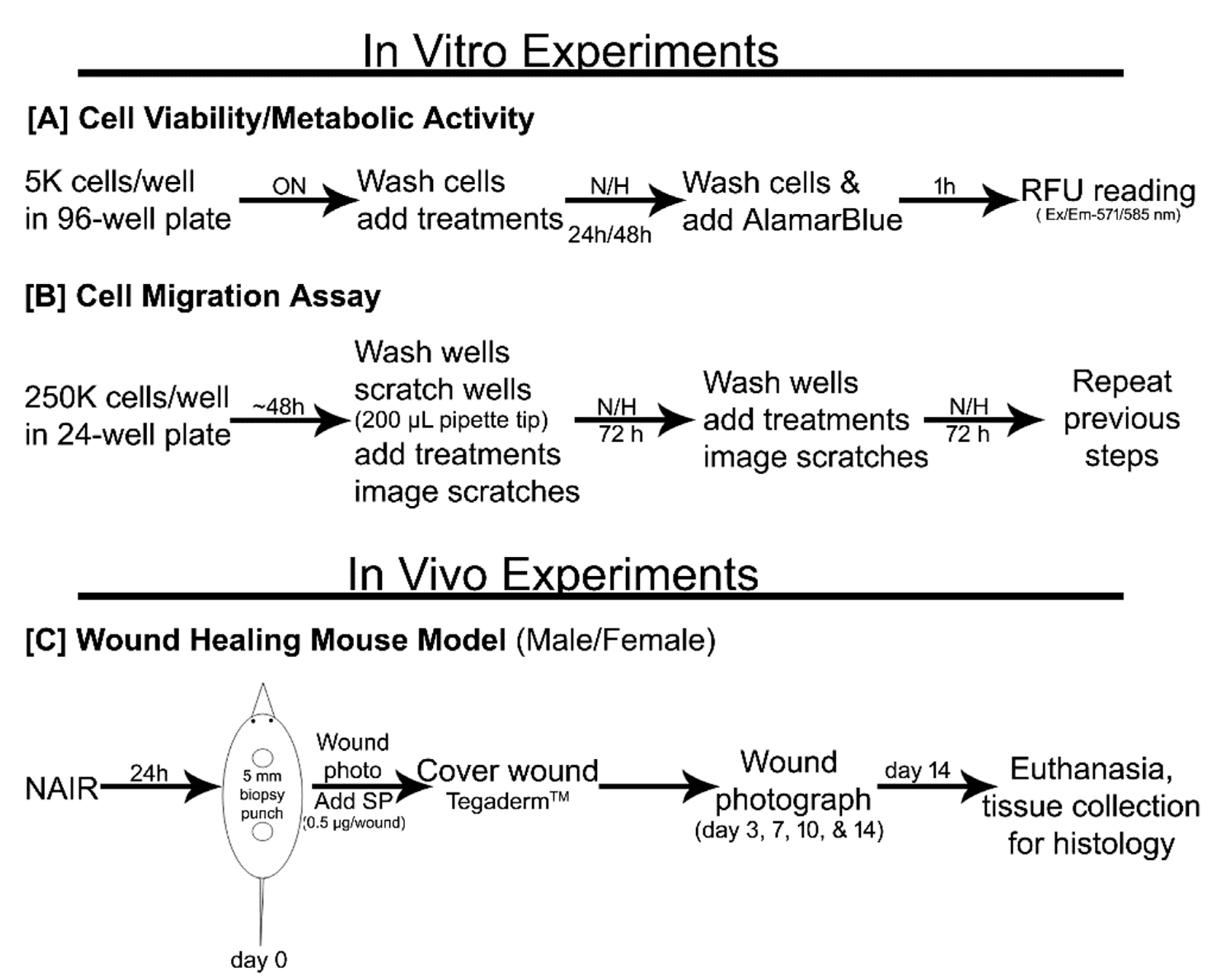
2. Experimental Section
2.1. Cell Culture Chemicals and Reagents
2.2. Cell Culture and Maintenance
2.3. Cell Proliferation/Metabolic Activity
2.4. Cell Migration (Scratch Assay)
2.5. Skin Wound Healing Animal Study
2.5.1. Skin Full-Thickness Wound Model
2.5.2. Skin Wound Histology
2.6. Statistical Analysis
3. Results
3.1. Effect of SP and Hypoxia on Cell Proliferation In Vitro
3.2. Effect of SP on Wound Closure In Vitro
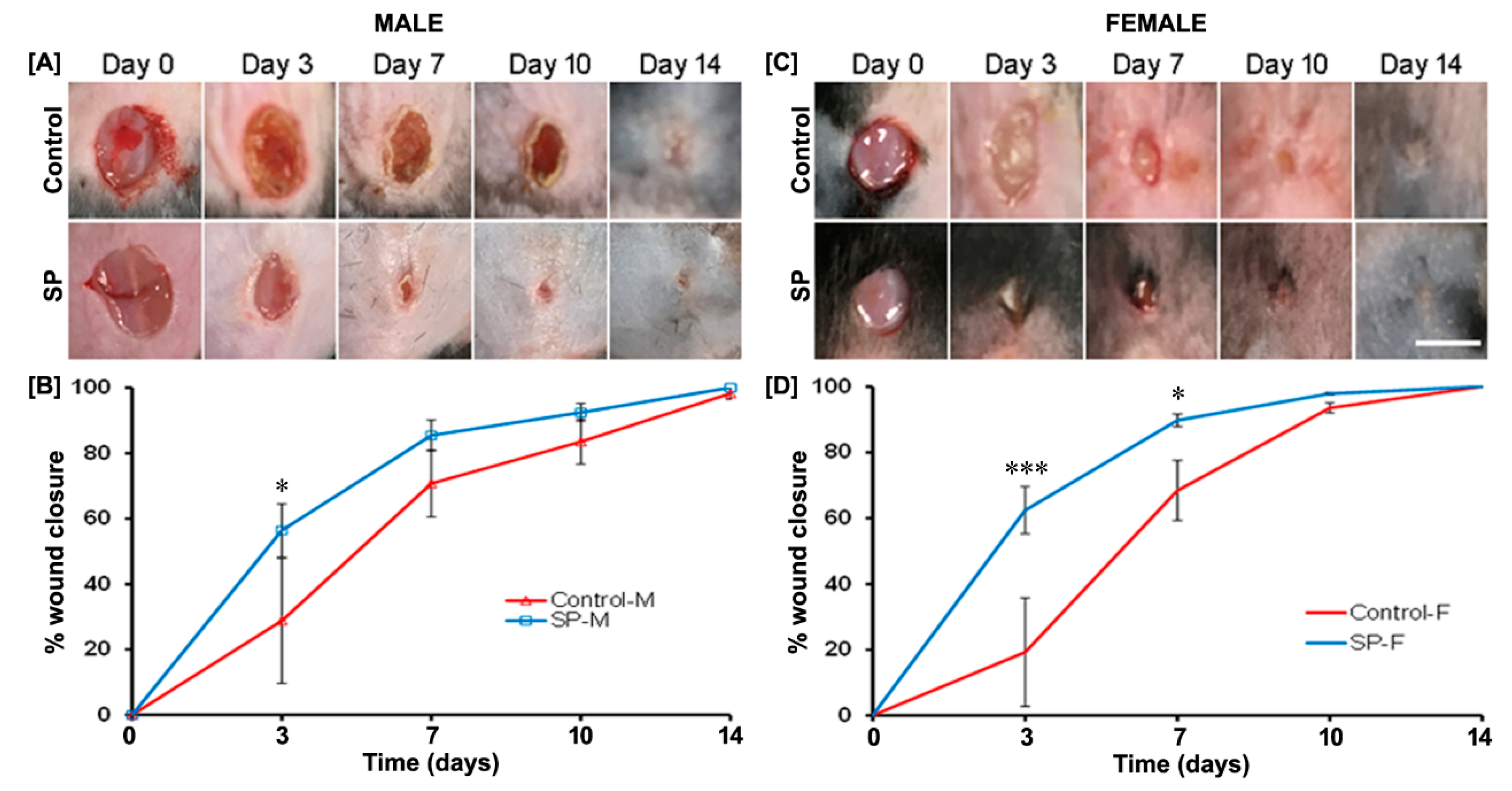
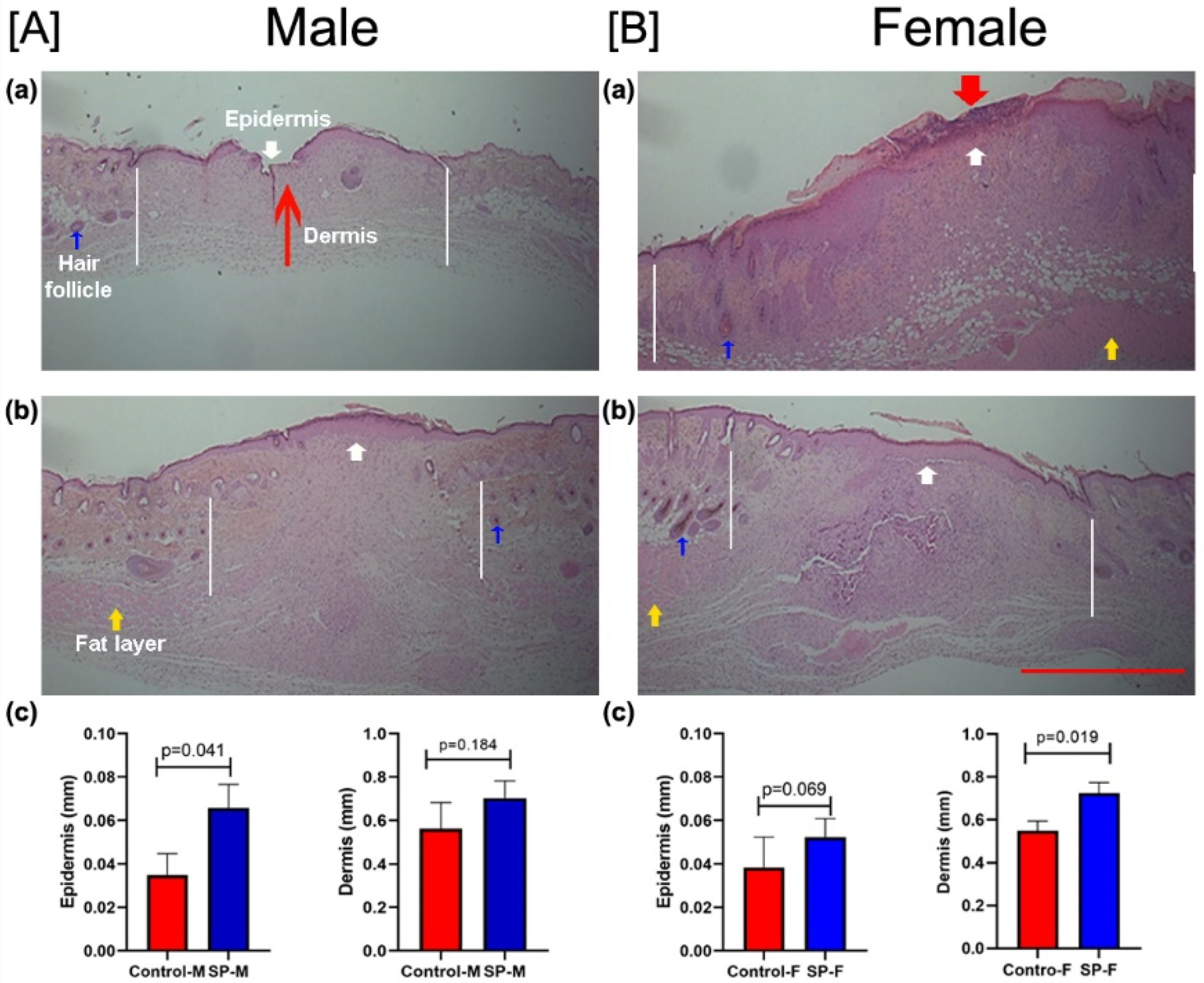
3.3. Effect of SP on Wound Closure In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef]
- Mustoe, T.A.; O’Shaughnessy, K.; Kloeters, O. Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. Plast. Reconstr. Surg. 2006, 117 (Suppl. 7), 35S–41S. [Google Scholar] [CrossRef]
- Khalil, Z.; Helme, R. Sensory peptides as neuromodulators of wound healing in aged rats. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1996, 51, B354–B361. [Google Scholar] [CrossRef]
- Rappl, L.M. Physiological changes in tissues denervated by spinal cord injury tissues and possible effects on wound healing. Int. Wound J. 2008, 5, 435–444. [Google Scholar] [CrossRef]
- Redelings, M.D.; Lee, N.E.; Sorvillo, F. Pressure ulcers: More lethal than we thought? Adv. Skin Wound Care 2005, 18, 367–372. [Google Scholar] [CrossRef]
- Brienza, D.; Krishnan, S.; Karg, P.; Sowa, G.; Allegretti, A.L. Predictors of pressure ulcer incidence following traumatic spinal cord injury: A secondary analysis of a prospective longitudinal study. Spinal Cord 2018, 56, 28–34. [Google Scholar] [CrossRef]
- Kruger, E.A.; Pires, M.; Ngann, Y.; Sterling, M.; Rubayi, S. Comprehensive management of pressure ulcers in spinal cord injury: Current concepts and future trends. J. Spinal Cord Med. 2013, 36, 572–585. [Google Scholar] [CrossRef]
- Blais, M.; Mottier, L.; Germain, M.-A.; Bellenfant, S.; Cadau, S.; Berthod, F. Sensory neurons accelerate skin reepithelialization via substance P in an innervated tissue-engineered wound healing model. Tissue Eng. Part A 2014, 20, 2180–2188. [Google Scholar] [CrossRef]
- Chéret, J.; Lebonvallet, N.; Buhé, V.; Carre, J.L.; Misery, L.; Le Gall-Ianotto, C. Influence of sensory neuropeptides on human cutaneous wound healing process. J. Dermatol. Sci. 2014, 74, 193–203. [Google Scholar] [CrossRef]
- Theocharidis, G.; Veves, A. Autonomic nerve dysfunction and impaired diabetic wound healing: The role of neuropeptides. Auton. Neurosci. Basic Clin. 2020, 223, 102610. [Google Scholar] [CrossRef]
- Leal, E.C.; Carvalho, E.; Tellechea, A.; Kafanas, A.; Tecilazich, F.; Kearney, C.; Kuchibhotla, S.; Auster, M.E.; Kokkotou, E.; Mooney, D.J.; et al. Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am. J. Pathol. 2015, 185, 1638–1648. [Google Scholar] [CrossRef]
- Suvas, S. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J. Immunol. 2017, 199, 1543–1552. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Kumar, D.; Bag, S.; Kurade, N.P.; Kumar, A.; Tandan, S.K.; Kumar, D. Topically applied substance P enhanced healing of open excision wound in rats. Eur. J. Pharmacol. 2013, 715, 345–353. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.; Hong, H.S.; Son, Y. Substance P promotes diabetic wound healing by modulating inflammation and restoring cellular activity of mesenchymal stem cells. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2016, 24, 337–348. [Google Scholar] [CrossRef]
- Benrath, J.; Zimmermann, M.; Gillardon, F. Substance P and nitric oxide mediate would healing of ultraviolet photodamaged rat skin: Evidence for an effect of nitric oxide on keratinocyte proliferation. Neurosci. Lett. 1995, 200, 17–20. [Google Scholar] [CrossRef]
- Jonsson, C.E.; Brodin, E.; Dalsgaard, C.J.; Haegerstrand, A. Release of substance-P-like immunoreactivity in dog paw lymph after scalding injury. Acta Physiol. Scand. 1986, 126, 21–24. [Google Scholar] [CrossRef]
- Kang, M.-H.; Kim, D.-Y.; Yi, J.Y.; Son, Y. Substance P accelerates intestinal tissue regeneration after gamma-irradiation-induced damage. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2009, 17, 216–223. [Google Scholar] [CrossRef]
- Kishimoto, S. The regeneration of substance P-containing nerve fibers in the process of burn wound healing in the guinea pig skin. J. Investig. Dermatol. 1984, 83, 219–223. [Google Scholar] [CrossRef]
- Xinan, L.; Suguang, L.; Liangchao, Z.; Lei, C. Change in substance P in a firearm wound and its significance. Peptides 1998, 19, 1209–1212. [Google Scholar] [CrossRef]
- Faden, A.I.; Jacobs, T.P.; Helke, C.J. Changes in substance P and somatostatin in the spinal cord after traumatic spinal injury in the rat. Neuropeptides 1985, 6, 215–225. [Google Scholar] [CrossRef]
- Leonard, A.V.; Manavis, J.; Blumbergs, P.C.; Vink, R. Changes in substance P and NK1 receptor immunohistochemistry following human spinal cord injury. Spinal Cord 2014, 52, 17–23. [Google Scholar] [CrossRef][Green Version]
- Khalil, Z.; LeVasseur, S.; Merhi, M.; Helme, R.D. Sympathetic modulation of sensory nerve activity with age: Human and rodent skin models. Clin. Exp. Pharmacol. Physiol. 1997, 24, 883–886. [Google Scholar] [CrossRef]
- Eckert, R.L.; Rorke, E.A. Molecular biology of keratinocyte differentiation. Environ. Health Perspect. 1989, 80, 109–116. [Google Scholar]
- Anderson, K.; Hamm, R.L. Factors That Impair Wound Healing. J. Am. Coll. Clin. Wound Spec. 2014, 4, 84–91. [Google Scholar] [CrossRef]
- Marin, J.; Nixon, J.; Gorecki, C. A systematic review of risk factors for the development and recurrence of pressure ulcers in people with spinal cord injuries. Spinal Cord 2013, 51, 522–527. [Google Scholar] [CrossRef]
- Sgonc, R.; Gruber, J. Age-related aspects of cutaneous wound healing: A mini-review. Gerontology 2013, 59, 159–164. [Google Scholar] [CrossRef]
- Khodr, B.; Khalil, Z. Modulation of inflammation by reactive oxygen species: Implications for aging and tissue repair. Free Radic. Biol. Med. 2001, 30, 1–8. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Kosol, W.; Kumar, S.; Marrero-BerrÍos, I.; Berthiaume, F. Medium conditioned by human mesenchymal stromal cells reverses low serum and hypoxia-induced inhibition of wound closure. Biochem. Biophys. Res. Commun. 2020, 522, 335–341. [Google Scholar] [CrossRef]
- Bae, S.; Matsunaga, Y.; Tanaka, Y.; Katayama, I. Autocrine induction of substance P mRNA and peptide in cultured normal human keratinocytes. Biochem. Biophys. Res. Commun. 1999, 263, 327–333. [Google Scholar] [CrossRef]
- Viac, J.; Gueniche, A.; Doutremepuich, J.D.; Reichert, U.; Claudy, A.; Schmitt, D. Substance P and keratinocyte activation markers: An in vitro approach. Arch. Dermatol. Res. 1996, 288, 85–90. [Google Scholar] [CrossRef]
- Haroon, Z.A.; Raleigh, J.A.; Greenberg, C.S.; Dewhirst, M.W. Early wound healing exhibits cytokine surge without evidence of hypoxia. Ann. Surg. 2000, 231, 137–147. [Google Scholar] [CrossRef]
- Hong, W.X.; Hu, M.S.; Esquivel, M.; Liang, G.Y.; Rennert, R.C.; McArdle, A.; Paik, K.J.; Duscher, D.; Gurtner, G.C.; Lorenz, H.P.; et al. The Role of Hypoxia-Inducible Factor in Wound Healing. Adv. Wound Care 2014, 3, 390–399. [Google Scholar] [CrossRef]
- Bishop, A. Role of oxygen in wound healing. J. Wound Care 2008, 17, 399–402. [Google Scholar] [CrossRef]
- Kopcewicz, M.; Walendzik, K.; Bukowska, J.; Kur-Piotrowska, A.; Machcinska, S.; Gimble, J.M.; Gawronska-Kozak, B. Cutaneous wound healing in aged, high fat diet-induced obese female or male C57BL/6 mice. Aging 2020, 12, 7066–7111. [Google Scholar] [CrossRef]
- Khalil, Z.; Ralevic, V.; Bassirat, M.; Dusting, G.J.; Helme, R.D. Effects of ageing on sensory nerve function in rat skin. Brain Res. 1994, 641, 265–272. [Google Scholar] [CrossRef]
- Muangman, P.; Tamura, R.N.; Muffley, L.A.; Isik, F.F.; Scott, J.R.; Xie, C.; Kegel, G.; Sullivan, S.R.; Liang, Z.; Gibran, N.S. Substance P enhances wound closure in nitric oxide synthase knockout mice. J. Surg. Res. 2009, 153, 201–209. [Google Scholar] [CrossRef]
- Ni, T.; Liu, Y.; Peng, Y.; Li, M.; Fang, Y.; Yao, M. Substance P induces inflammatory responses involving NF-κB in genetically diabetic mice skin fibroblasts co-cultured with macrophages. Am. J. Transl. Res. 2016, 8, 2179–2188. [Google Scholar]
- Scott, J.R.; Tamura, R.N.; Muangman, P.; Isik, F.F.; Xie, C.; Gibran, N.S. Topical substance P increases inflammatory cell density in genetically diabetic murine wounds. Wound Repair Regen. Off. Publ. Wound Healing Soc. Eur. Tissue Repair Soc. 2008, 16, 529–533. [Google Scholar] [CrossRef]
- Kohara, H.; Tajima, S.; Yamamoto, M.; Tabata, Y. Angiogenesis induced by controlled release of neuropeptide substance P. Biomaterials 2010, 31, 8617–8625. [Google Scholar] [CrossRef]
- Um, J.; Jung, N.; Chin, S.; Cho, Y.; Choi, S.; Park, K.-S. Substance P enhances EPC mobilization for accelerated wound healing. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2016, 24, 402–410. [Google Scholar] [CrossRef]
- Um, J.; Yu, J.; Park, K.-S. Substance P accelerates wound healing in type 2 diabetic mice through endothelial progenitor cell mobilization and Yes-associated protein activation. Mol. Med. Rep. 2017, 15, 3035–3040. [Google Scholar] [CrossRef]
- Felderbauer, P.; Bulut, K.; Hoeck, K.; Deters, S.; Schmidt, W.E.; Hoffmann, P. Substance P induces intestinal wound healing via fibroblasts—Evidence for a TGF-beta-dependent effect. Int. J. Colorectal Dis. 2007, 22, 1475–1480. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, W.S.; Kim, W.; Kim, H.K.; Bae, T.H.; Park, J.A. Wound contraction decreases with intravenously injected substance P in rabbits. Burn. J. Int. Soc. Burn Inj. 2014, 40, 127–134. [Google Scholar] [CrossRef]
- Chung, B.Y.; Kim, H.B.; Jung, M.J.; Kang, S.Y.; Kwak, I.-S.; Park, C.W.; Kim, H.O. Post-Burn Pruritus. Int. J. Mol. Sci. 2020, 21, 3880. [Google Scholar] [CrossRef]
- Yu, J.; Nam, D.; Park, K.-S. Substance P enhances cellular migration and inhibits senescence in human dermal fibroblasts under hyperglycemic conditions. Biochem. Biophys. Res. Commun. 2020, 522, 917–923. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Tan, Y.; Berthiaume, F. Neuropeptide Substance P Enhances Skin Wound Healing In Vitro and In Vivo under Hypoxia. Biomedicines 2021, 9, 222. https://doi.org/10.3390/biomedicines9020222
Kumar S, Tan Y, Berthiaume F. Neuropeptide Substance P Enhances Skin Wound Healing In Vitro and In Vivo under Hypoxia. Biomedicines. 2021; 9(2):222. https://doi.org/10.3390/biomedicines9020222
Chicago/Turabian StyleKumar, Suneel, Yuying Tan, and Francois Berthiaume. 2021. "Neuropeptide Substance P Enhances Skin Wound Healing In Vitro and In Vivo under Hypoxia" Biomedicines 9, no. 2: 222. https://doi.org/10.3390/biomedicines9020222
APA StyleKumar, S., Tan, Y., & Berthiaume, F. (2021). Neuropeptide Substance P Enhances Skin Wound Healing In Vitro and In Vivo under Hypoxia. Biomedicines, 9(2), 222. https://doi.org/10.3390/biomedicines9020222






