Statins Directly Influence the Polarization of Adipose Tissue Macrophages: A Role in Chronic Inflammation
Abstract
1. Introduction
2. Methods
2.1. Adipose Tissue Specimen
2.2. Flow Cytometry
2.3. Lipoprotein Analysis
2.4. Animal Experimental Model with Prague Hereditary Hypercholesterolemic Rat
2.5. Flow Cytometry of Animal Adipose Tissue Samples
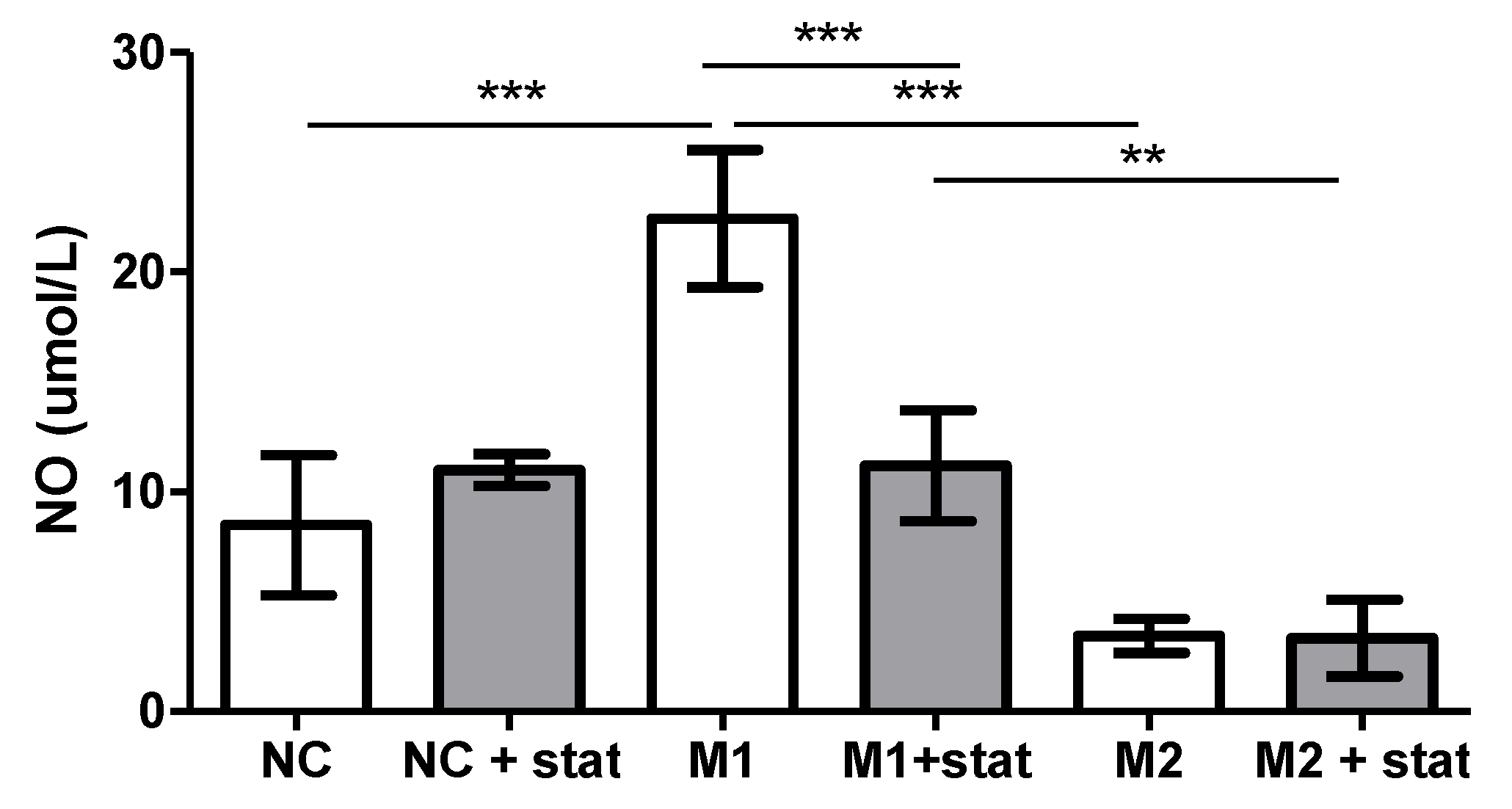
2.6. Nitric Oxide Production by Macrophages In Vitro
2.7. Statistical Analysis
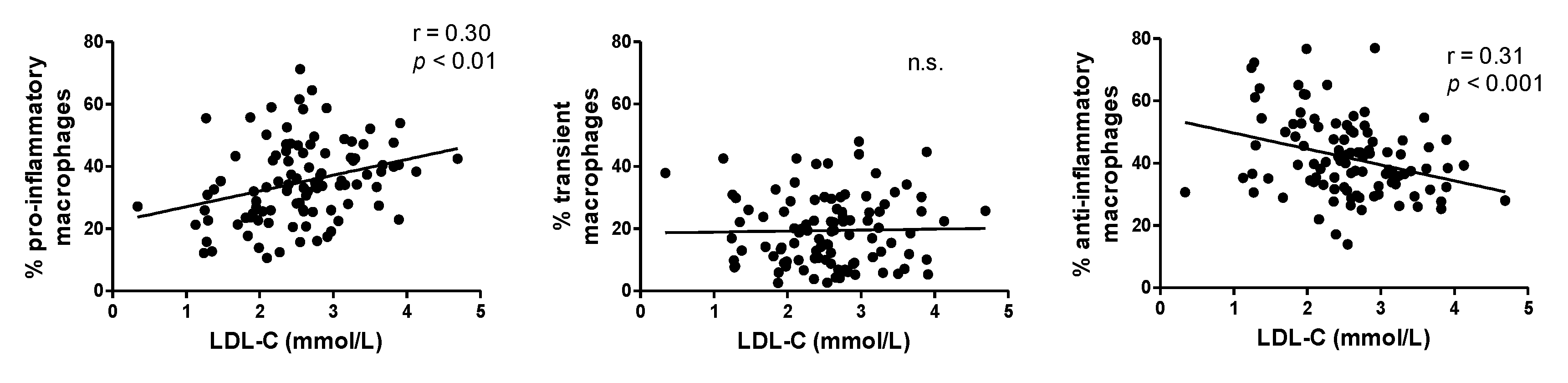
3. Results
3.1. Animal Experiments
3.2. In Vitro Experiment
4. Discussion
5. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kannel, W.B.; Thom, T.J. Declining cardiovascular mortality. Circulation 1984, 70, 331–336. [Google Scholar] [CrossRef]
- Ford, E.S.; Ajani, U.A.; Croft, J.B.; Critchley, J.A.; Labarthe, D.R.; Kottke, T.E.; Giles, W.H.; Capewell, S. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N. Engl. J. Med. 2007, 356, 2388–2398. [Google Scholar] [CrossRef] [PubMed]
- Bjorck, L.; Rosengren, A.; Bennett, K.; Lappas, G.; Capewell, S. Modelling the decreasing coronary heart disease mortality in Sweden between 1986 and 2002. Eur. Heart J. 2009, 30, 1046–1056. [Google Scholar] [CrossRef]
- Boren, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Rose, L.; Buring, J.E.; Cook, N.R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 2002, 347, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; MacFadyen, J.; Libby, P.; Glynn, R.J. Relation of Baseline High-Sensitivity C-Reactive Protein Level to Cardiovascular Outcomes with Rosuvastatin in the Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER). Am. J. Cardiol. 2010, 106, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Berg, A.H.; Scherer, P.E. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef]
- Kralova Lesna, I.; Petras, M.; Cejkova, S.; Kralova, A.; Fronek, J.; Janousek, L.; Thieme, F.; Tyll, T.; Poledne, R. Cardiovascular disease predictors and adipose tissue macrophage polarization: Is there a link? Eur. J. Prev. Cardiol. 2018, 25, 328–334. [Google Scholar] [CrossRef]
- Zhou, R.B.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.T.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.H.; Brickey, W.J.; Ting, J.P.Y. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; Akhtari, M.; Tolani, S.; Pagler, T.; Bijl, N.; Kuo, C.L.; Wang, M.; Sanson, M.; Abramowicz, S.; Welch, C.; et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Invest. 2011, 121, 4138–4149. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. High-sensitivity C-reactive protein as a predictor of all-cause mortality: Implications for research and patient care. Clin. Chem. 2008, 54, 234–237. [Google Scholar] [CrossRef]
- Lee, M.K.S.; Moore, X.L.; Fu, Y.; Al-Sharea, A.; Dragoljevic, D.; Fernandez-Rojo, M.A.; Parton, R.; Sviridov, D.; Murphy, A.J.; Chin-Dusting, J.P.F. High-density lipoprotein inhibits human M1 macrophage polarization through redistribution of caveolin-1. Br. J. Pharm. 2016, 173, 741–751. [Google Scholar] [CrossRef]
- Fu, Y.; Moore, S.; Chin-Dusting, J.P.F. Caveolin-1 plays a critical role in the differentiation of monocytes into macrophages. Vasc. Pharm. 2012, 56, 373. [Google Scholar] [CrossRef]
- Zeiser, R. Immune modulatory effects of statins. Immunology 2018, 154, 69–75. [Google Scholar] [CrossRef]
- Hechinger, A.K.; Maas, K.; Durr, C.; Leonhardt, F.; Prinz, G.; Marks, R.; Gerlach, U.; Hofmann, M.; Fisch, P.; Finke, J.; et al. Inhibition of protein geranylgeranylation and farnesylation protects against graft-versus-host disease via effects on CD4 effector T cells. Haematologica 2013, 98, 31–40. [Google Scholar] [CrossRef][Green Version]
- Kobashigawa, J.A.; Katznelson, S.; Laks, H.; Johnson, J.A.; Yeatman, L.; Wang, X.M.; Chia, D.; Terasaki, P.I.; Sabad, A.; Cogert, G.A.; et al. Effect of Pravastatin on Outcomes after Cardiac Transplantation. N. Engl. J. Med. 1995, 333, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K.; Laufs, U. Pleiotropic effects of statins. Annu. Rev. Pharm. 2005, 45, 89–118. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cannon, C.P.; Braunwald, E. C-reactive protein levels and outcomes after statin therapy—Reply. N. Engl. J. Med. 2005, 352, 1604–1605. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cannon, C.P.; Morrow, D.; Rifai, N.; Rose, L.M.; McCabe, C.H.; Pfeffer, M.A.; Braunwald, E.; Investigators, P.I.-T. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 2005, 352, 20–28. [Google Scholar] [CrossRef]
- Sacks, F.M.; Pfeffer, M.A.; Moye, L.A.; Rouleau, J.L.; Rutherford, J.D.; Cole, T.G.; Brown, L.; Warnica, J.W.; Arnold, J.M.; Wun, C.C.; et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 1996, 335, 1001–1009. [Google Scholar] [CrossRef]
- Albert, M.A.; Danielson, E.; Rifai, N.; Ridker, P.M.; Investigators, P. Effect of statin therapy on C-reactive protein levels: The pravastatin inflammation/CRP evaluation (PRINCE): A randomized trial and cohort study. JAMA 2001, 286, 64–70. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; Macfadyen, J.G.; et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the JUPITER trial. Lancet 2009, 373, 1175–1182. [Google Scholar] [CrossRef]
- Patel, J.M.; Snaith, C.; Thickett, D.R.; Linhartova, L.; Melody, T.; Hawkey, P.; Barnett, A.H.; Jones, A.; Hong, T.; Cooke, M.W.; et al. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS Trial). Crit Care 2012, 16, R231. [Google Scholar] [CrossRef] [PubMed]
- Hohensinner, P.J.; Baumgartner, J.; Ebenbauer, B.; Thaler, B.; Fischer, M.B.; Huber, K.; Speidl, W.S.; Wojta, J. Statin treatment reduces matrix degradation capacity of proinflammatory polarized macrophages. Vasc. Pharm. 2018, 110, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Nagashima, S.; Wakabayashi, T.; Tumenbayar, B.; Hayakawa, H.; Hayakawa, M.; Karasawa, T.; Ohashi, K.; Yamazaki, H.; Takei, A.; et al. Myeloid HMG-CoA (3-Hydroxy-3-Methylglutaryl-Coenzyme A) Reductase Determines Atherosclerosis by Modulating Migration of Macrophages. Arter. Throm. Vas. Biol. 2018, 38, 2590–2600. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gao, W.; Cheng, S.; Yin, D.; Li, F.; Wu, Y.; Sun, D.; Zhou, S.; Wang, D.; Zhang, Y.; et al. Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury. J. Neuroinflamm. 2017, 14, 167. [Google Scholar] [CrossRef]
- Abe, M.; Matsuda, M.; Kobayashi, H.; Miyata, Y.; Nakayama, Y.; Komuro, R.; Fukuhara, A.; Shimomura, I. Effects of statins on adipose tissue inflammation their inhibitory effect on MyD88-independent IRF3/IFN-beta pathway in macrophages. Arter. Throm. Vas. Biol. 2008, 28, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Kralova Lesna, I.; Kralova, A.; Cejkova, S.; Fronek, J.; Petras, M.; Sekerkova, A.; Thieme, F.; Janousek, L.; Poledne, R. Characterisation and comparison of adipose tissue macrophages from human subcutaneous, visceral and perivascular adipose tissue. J. Transl. Med. 2016, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Kovar, J.; Tonar, Z.; Heczkova, M.; Poledne, R. Prague hereditary hypercholesterolemic (PHHC) rat—A model of polygenic hypercholesterolemia. Physiol. Res. 2009, 58 (Suppl. 2), S95–S99. [Google Scholar] [PubMed]
- Poledne, R.; Malinska, H.; Kubatova, H.; Fronek, J.; Thieme, F.; Kauerova, S.; Lesna, I.K. Polarization of Macrophages in Human Adipose Tissue is Related to the Fatty Acid Spectrum in Membrane Phospholipids. Nutrients 2019, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, R.; Low, H.; Mukhamedova, N.; Fu, Y.; Lai, S.J.; Sasaoka, M.; Hara, A.; Yamazaki, A.; Kameda, T.; Horiuchi, Y.; et al. Cholesterol transport between red blood cells and lipoproteins contributes to cholesterol metabolism in blood. J. Lipid Res. 2020, 61, 1577–1588. [Google Scholar] [CrossRef]
- Poledne, R.; Kralova Lesna, I.; Kralova, A.; Fronek, J.; Cejkova, S. The relationship between non-HDL cholesterol and macrophage phenotypes in human adipose tissue. J. Lipid Res. 2016, 57, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Bonacina, F.; Norata, G.D.; Catapano, A.L. The Interplay of Lipids, Lipoproteins, and Immunity in Atherosclerosis. Curr. Atheroscler. Rep. 2018, 20, 1–9. [Google Scholar] [CrossRef]
- Lemaire-Ewing, S.; Lagrost, L.; Neel, D. Lipid rafts: A signalling platform linking lipoprotein metabolism to atherogenesis. Atherosclerosis 2012, 221, 303–310. [Google Scholar] [CrossRef]
- Ortegren, U.; Karlsson, M.; Blazic, N.; Blomqvist, M.; Nystrom, F.H.; Gustavsson, J.; Fredman, P.; Stralfors, P. Lipids and glycosphingolipids in caveolae and surrounding plasma membrane of primary rat adipocytes. Eur. J. Biochem. 2004, 271, 2028–2036. [Google Scholar] [CrossRef]
- Rothberg, K.G.; Heuser, J.E.; Donzell, W.C.; Ying, Y.S.; Glenney, J.R.; Anderson, R.G.W. Caveolin, a Protein-Component of Caveolae Membrane Coats. Cell 1992, 68, 673–682. [Google Scholar] [CrossRef]
- Galkina, E.; Ley, K. Vascular adhesion molecules in atherosclerosis. Arter. Throm. Vas. Biol. 2007, 27, 2292–2301. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zhang, A.L.; Li, N.J.; Li, P.L.; Zhang, F. Concentration-Dependent Diversification Effects of Free Cholesterol Loading on Macrophage Viability and Polarization. Cell. Physiol. Biochem. 2015, 37, 419–431. [Google Scholar] [CrossRef]
- Liu, S.L.; Sheng, R.; Jung, J.H.; Wang, L.; Stec, E.; O’Connor, M.J.; Song, S.; Bikkavilli, R.K.; Winn, R.A.; Lee, D.; et al. Orthogonal lipid sensors identify transbilayer asymmetry of plasma membrane cholesterol. Nat. Chem. Biol. 2017, 13, 268–274. [Google Scholar] [CrossRef]
- Montecucco, F.; Burger, F.; Pelli, G.; Poku, N.K.; Berlier, C.; Steffens, S.; Mach, F. Statins inhibit C-reactive protein-induced chemokine secretion, ICAM-1 upregulation and chemotaxis in adherent human monocytes. Rheumatology 2009, 48, 233–242. [Google Scholar] [CrossRef]
- Komukai, K.; Kubo, T.; Kitabata, H.; Matsuo, Y.; Ozaki, Y.; Takarada, S.; Okumoto, Y.; Shiono, Y.; Orii, M.; Shimamura, K.; et al. Effect of Atorvastatin Therapy on Fibrous Cap Thickness in Coronary Atherosclerotic Plaque as Assessed by Optical Coherence Tomography. J. Am. Coll. Cardiol. 2014, 64, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Navarro, A.; Guerrero-Hue, M.; Martn-Fernandez, B.; Cortegano, I.; Olivares-Alvaro, E.; Heras, N.D.; Alia, M.; de Andres, B.; Gaspar, M.L.; Egido, J.; et al. Phenotypic Characterization of Macrophages from Rat Kidney by Flow Cytometry. JoVE J. Vis. Exp. 2016, e54599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Xiao, S.J.; Li, Q.Z. Pravastatin polarizes the phenotype of macrophages toward M2 and elevates serum cholesterol levels in apolipoprotein E knockout mice. J. Int Med. Res. 2018, 46, 3365–3373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Q.; Tan, Q.; Feng, Z.; He, Z.; Tang, J.; Feng, H.; Zhu, G.; Chen, Z. Simvastatin accelerates hematoma resolution after intracerebral hemorrhage in a PPARgamma-dependent manner. Neuropharmacology 2018, 128, 244–254. [Google Scholar] [CrossRef] [PubMed]




| Clinical and Biochemical Characteristics | N = 118 | Subjects with Statins Treatment (n = 11) | Subjects without Statins Treatment (n = 107) | p with/without Statins Treatment |
|---|---|---|---|---|
| Age (years) | 50.27 ± 12.3 | 60.03 ± 8.9 | 49.36 ± 11.26 | p < 0.01 |
| BMI (kg/m2) | 25.59 ± 4.74 | 25.59 ± 2.85 | 25.55 ± 4.26 | n.s. |
| Sex, male% (n) | 30.5% (36) | 18.2% (2) | 31.8% (34) | n.s. |
| Hypertension% (n) | 22.9% (27) | 36.4% (4) | 21.5% (23) | n.s. |
| Smoking% (n) | 26.3% (31) | 18.2% (2) | 27.1% (29) | n.s. |
| Total cholesterol (mmol/L) | 4.61 ± 0.99 | 3.95 ± 0.92 | 4.66 ± 0.87 | p < 0.05 |
| Total triglycerides (mmol/L) | 1.53 ± 0.84 | 1.30 ± 0.65 | 1.55 ± 0.85 | n.s. |
| HDL cholesterol (mmol/L) | 1.31 ± 0.42 | 1.26 ± 0.46 | 1.32 ± 0.39 | n.s. |
| LDL cholesterol (calculated) (mmol/L) | 2.55 ± 0.79 | 2.10 ± 0.75 | 2.54 ± 0.94 | n.s. |
| hs-CRP (mg/L) | 1.40 ± 2.47 | 0.85 ± 0.60 | 1.46 ± 2.59 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kauerova, S.; Bartuskova, H.; Muffova, B.; Janousek, L.; Fronek, J.; Petras, M.; Poledne, R.; Kralova Lesna, I. Statins Directly Influence the Polarization of Adipose Tissue Macrophages: A Role in Chronic Inflammation. Biomedicines 2021, 9, 211. https://doi.org/10.3390/biomedicines9020211
Kauerova S, Bartuskova H, Muffova B, Janousek L, Fronek J, Petras M, Poledne R, Kralova Lesna I. Statins Directly Influence the Polarization of Adipose Tissue Macrophages: A Role in Chronic Inflammation. Biomedicines. 2021; 9(2):211. https://doi.org/10.3390/biomedicines9020211
Chicago/Turabian StyleKauerova, Sona, Hana Bartuskova, Barbora Muffova, Libor Janousek, Jiri Fronek, Marek Petras, Rudolf Poledne, and Ivana Kralova Lesna. 2021. "Statins Directly Influence the Polarization of Adipose Tissue Macrophages: A Role in Chronic Inflammation" Biomedicines 9, no. 2: 211. https://doi.org/10.3390/biomedicines9020211
APA StyleKauerova, S., Bartuskova, H., Muffova, B., Janousek, L., Fronek, J., Petras, M., Poledne, R., & Kralova Lesna, I. (2021). Statins Directly Influence the Polarization of Adipose Tissue Macrophages: A Role in Chronic Inflammation. Biomedicines, 9(2), 211. https://doi.org/10.3390/biomedicines9020211







