Embryonic Onset of Sexually Dimorphic Heart Rates in the Viviparous Fish, Gambusia holbrooki
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Collection, Maintenance, and Sample Preparation
2.2. Heart Rate Determination by Direct Visual Count
2.3. Acquisition of Heartbeats for Digital Motion Analysis
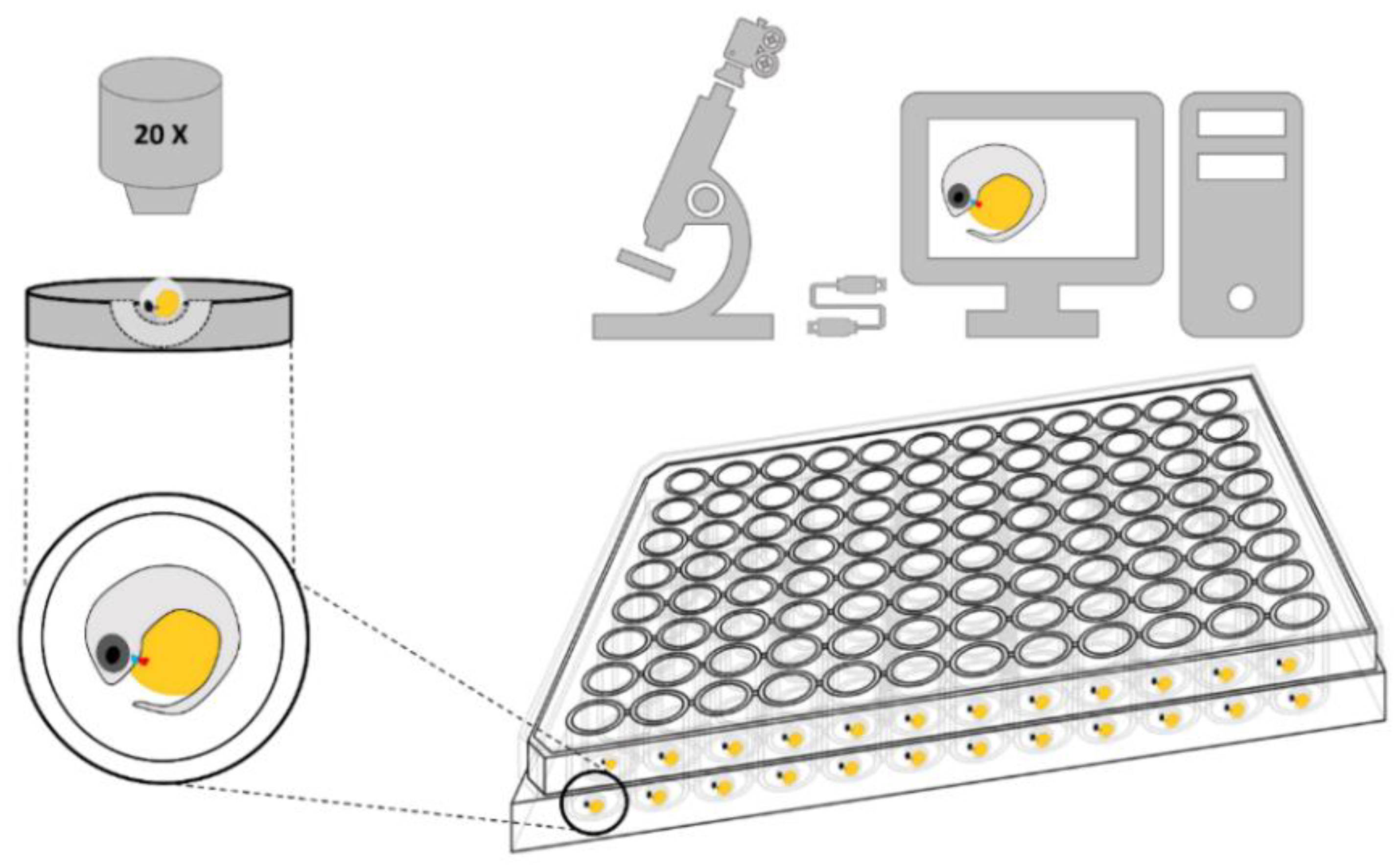
2.3.1. Video Acquisition System
2.3.2. Video Processing
2.3.3. Beat Interval, Beats Per Minute (BPM), and Heart Rate Frequency (Hz)
2.4. Validation of Digital Heartbeat Counts
2.5. Genetic Sexing of Gambusia Embryos at Different Stages
2.5.1. DNA Extraction and PCR Condition
2.6. HR and FD Determination in Sexually Mature Fish
2.7. Size Comparison of Adult Male and Female Explanted Hearts
2.8. Statistical Analysis
3. Results
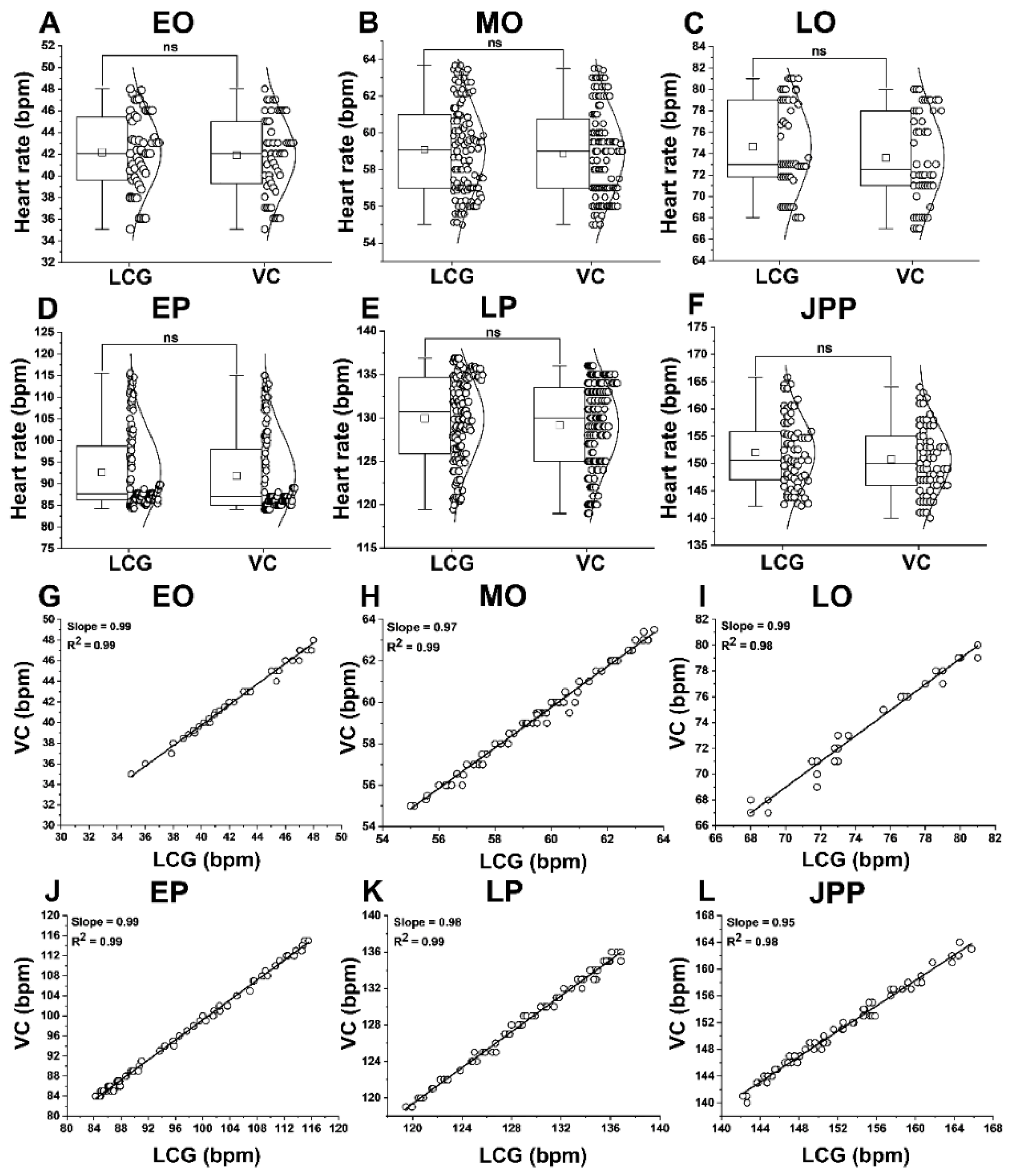
3.1. Comparison and Validation of Heart Rates: Automated vs. Manual Assessment
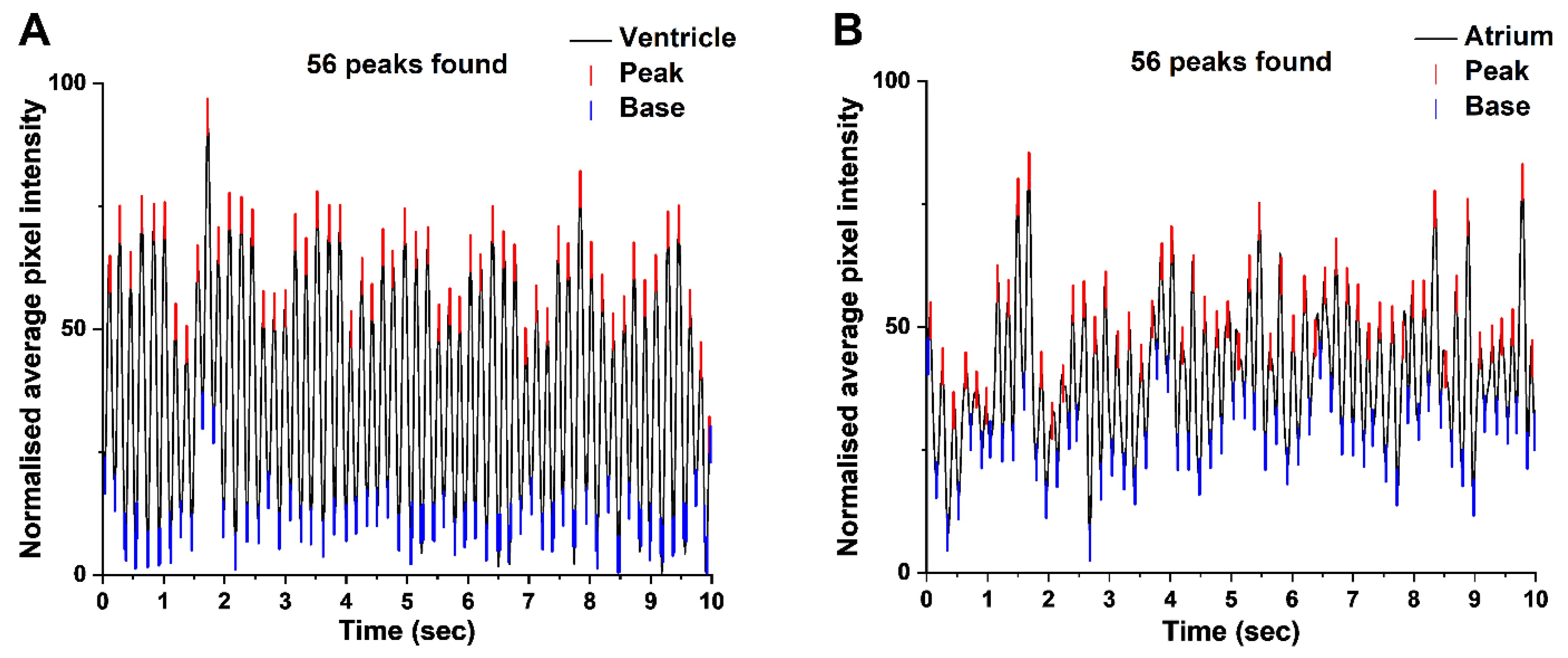
3.2. Light Cardiograms of Ventricle and Atrium Mutually Validate
3.3. Synchronicity in Ventricular and Atrial Contraction
3.4. FFT Transformed Heart Rate Frequency (FD) Validate HR Counts and Their Increase with Advancing Developmental Stages
3.5. Cardiac Rate Was Influenced by the Genetic Sex of the Embryos
3.6. Sex-Biased Cardiac Frequency in Developing Embryos
3.7. Sexual Dimorphism in HR and FD of Adults
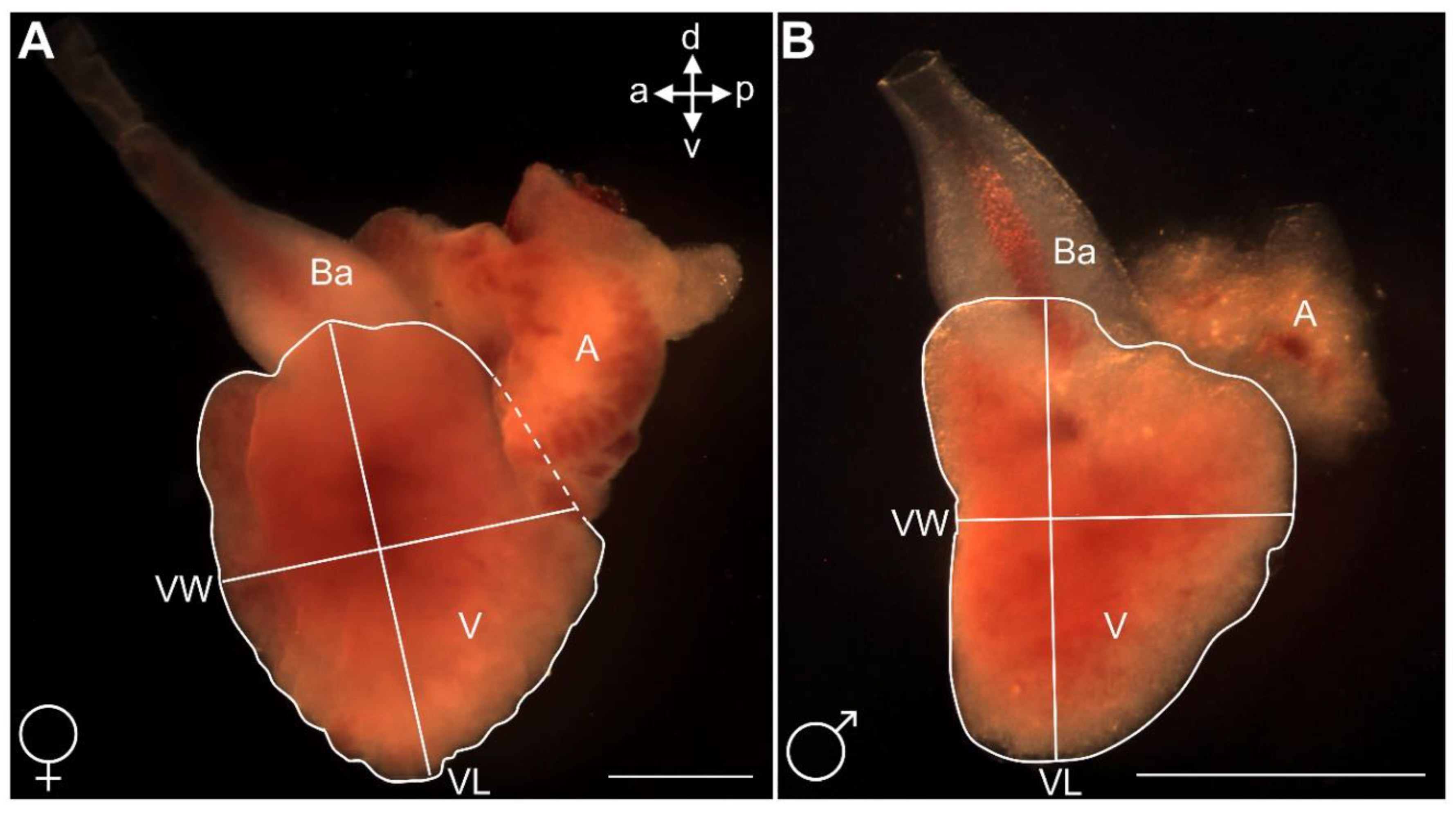
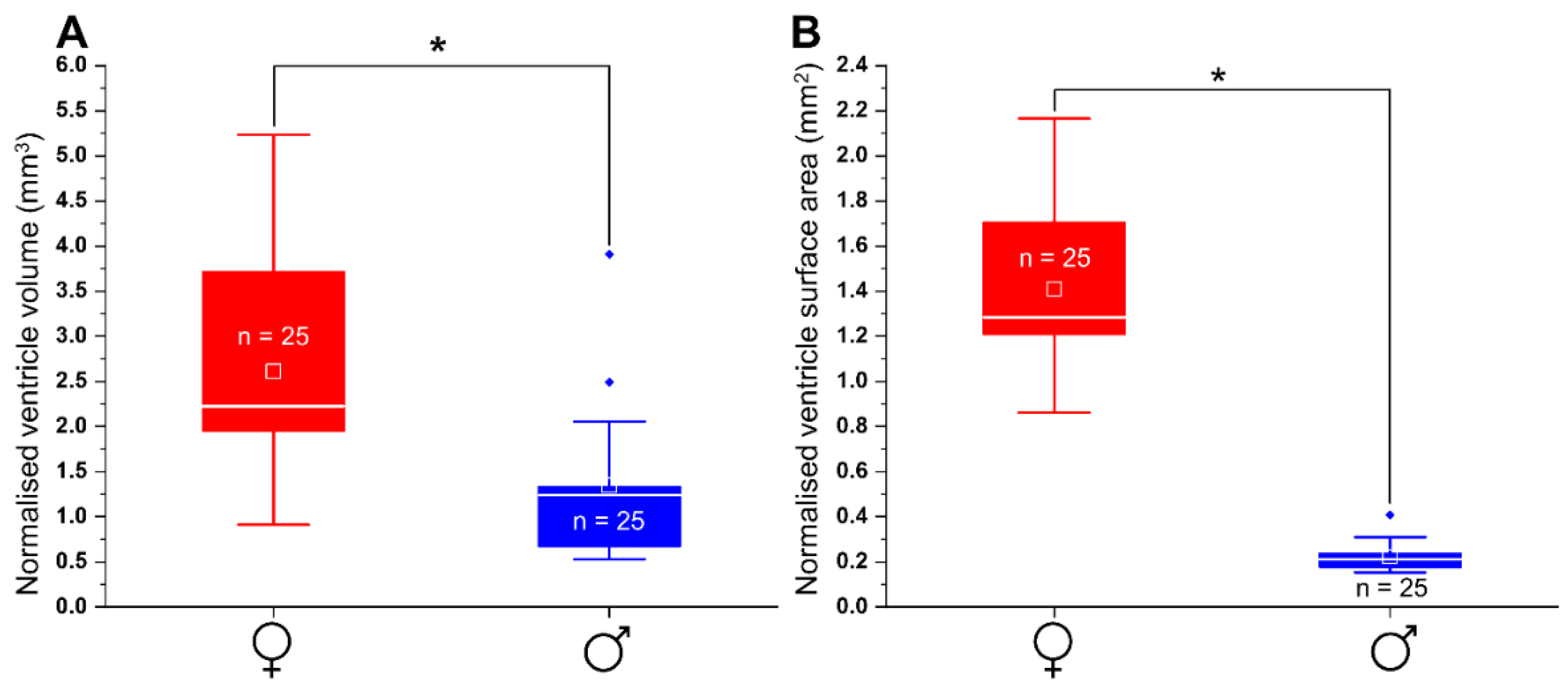
3.8. Morphology and Size of Adult Male and Female Heart
4. Discussion
4.1. The LCG Mimics ECG Morphology and Enables Reliable Determination of the Heart Rate (HR) and Frequency (HF) in Embryonic and Sexually Mature G. holbrooki
4.2. Cardiac Rate Increases with the Progression of Embryonic Development
4.3. Cardiac Rate of G. holbrooki is Influenced by the Sex of the Embryos and Adults
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, Q.; Le Manach, S.; Sotton, B.; Huet, H.; Duvernois-Berthet, E.; Paris, A.; Duval, C.; Ponger, L.; Marie, A.; Blond, A.; et al. Deep sexual dimorphism in adult medaka fish liver highlighted by multi-omic approach. Sci. Rep. 2016, 6, 32459. [Google Scholar] [CrossRef]
- Arnold, A.P. Rethinking sex determination of non-gonadal tissues. Curr. Top. Dev. Biol. 2019, 134, 289–315. [Google Scholar] [CrossRef] [PubMed]
- Herczeg, G.; Gonda, A.; Balázs, G.; Noreikiene, K.; Merilä, J. Experimental evidence for sex-specific plasticity in adult brain. Front. Zool. 2015, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Cummings, M.E. Sexual conflict and sexually dimorphic cognition—Reviewing their relationship in poeciliid fishes. Behav. Ecol. Sociobiol. 2018, 72, 73. [Google Scholar] [CrossRef]
- Head, M.L.; Vega-Trejo, R.; Jacomb, F.; Jennions, M.D. Predictors of male insemination success in the mosquitofish (Gambusia holbrooki). Ecol. Evol. 2015, 5, 4999–5006. [Google Scholar] [CrossRef]
- Dimitriadi, A.; Beis, D.; Arvanitidis, C.; Adriaens, D.; Koumoundouros, G. Developmental temperature has persistent, sexually dimorphic effects on zebrafish cardiac anatomy. Sci. Rep. 2018, 8, 8125. [Google Scholar] [CrossRef]
- Mousavi, S.E.; Patil, J.G. Light-Cardiogram, a simple technique for heart rate determination in adult zebrafish, Danio rerio. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 246, 110705. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadi, A.; Geladakis, G.; Koumoundouros, G. 3D heart morphological changes in response to developmental temperature in zebrafish: More than ventricle roundness. J. Morphol. 2020, 282. [Google Scholar] [CrossRef]
- Conway, K.W.; Britz, R.; Siegel, D.S. Different on the inside: Extreme swimbladder sexual dimorphism in the South Asian torrent minnows. Biol. Lett. 2014, 10, 20140348. [Google Scholar] [CrossRef] [PubMed]
- Conforto, T.L.; Waxman, D.J. Sex-Specific mouse liver gene expression: Genome-Wide analysis of developmental changes from pre-pubertal period to young adulthood. Biol. Sex Differ. 2012, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Kwan, T.N.; Patil, J.G. Sex biased expression of anti-Mullerian hormone (amh) gene in a live bearing fish, Gambusia holbrooki: Evolutionary implications and potential role in sex differentiation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 231, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xie, F.; Tian, L.; Fallah, S.; Babaei, F.; Manno, S.H.C.; Manno, F.A.M.; Zhu, L.; Wong, K.F.; Liang, Y.; et al. Estrogen accelerates heart regeneration by promoting the inflammatory response in zebrafish. J. Endocrinol. 2020, 245, 39–51. [Google Scholar] [CrossRef]
- Hudry, B.; Khadayate, S.; Miguel-Aliaga, I. The sexual identity of adult intestinal stem cells controls organ size and plasticity. Nature 2016, 530, 344. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P.; Cassis Lisa, A.; Eghbali, M.; Reue, K.; Sandberg, K. Sex Hormones and Sex Chromosomes Cause Sex Differences in the Development of Cardiovascular Diseases. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Sedmera, D.; Yost, H.J.; Clark, E.B. Structure and function of the developing zebrafish heart. Anat. Rec 2000, 260, 148–157. [Google Scholar] [CrossRef]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef]
- Woodhead, A.D. Aging changes in the heart of a poeciliid fish, the guppy Poecilia reticulatus. Exp. Gerontol. 1984, 19, 383–391. [Google Scholar] [CrossRef]
- Staudt, D.; Stainier, D. Uncovering the Molecular and Cellular Mechanisms of Heart Development Using the Zebrafish. Annu. Rev. Genet. 2012, 46, 397–418. [Google Scholar] [CrossRef]
- Guernsey, M.W.; van Kruistum, H.; Reznick, D.N.; Pollux, B.J.A.; Baker, J.C. Molecular Signatures of Placentation and Secretion Uncovered in Poeciliopsis Maternal Follicles. Mol. Biol. Evol. 2020, 37, 2679–2690. [Google Scholar] [CrossRef]
- Braasch, I.; Peterson, S.M.; Desvignes, T.; McCluskey, B.M.; Batzel, P.; Postlethwait, J.H. A new model army: Emerging fish models to study the genomics of vertebrate Evo-Devo. J. Exp. Zool. B Mol. Dev. Evol. 2015, 324, 316–341. [Google Scholar] [CrossRef]
- Pyke, G.H. Plague Minnow or Mosquito Fish? A Review of the Biology and Impacts of Introduced Gambusia species. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 171–191. [Google Scholar] [CrossRef]
- Norazmi-Lokman, N.H.; Purser, G.J.; Patil, J.G. Gravid Spot Predicts Developmental Progress and Reproductive Output in a Livebearing Fish, Gambusia holbrooki. PLoS ONE 2016, 11, e0147711. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Maeno, A.; Shiroishi, T.; Sakai, N. Development and growth of organs in living whole embryo and larval grafts in zebrafish. Sci. Rep. 2017, 7, 16508. [Google Scholar] [CrossRef] [PubMed]
- Woodhead, A.D.; Pond, V.; Dailey, K. Aging changes in the kidneys of two poeciliid fishes, the guppy Poecilia reticulatus and the Amazon molly P. formosa. Exp. Gerontol. 1983, 18, 211–221. [Google Scholar] [CrossRef]
- Patton, E.E.; Mitchell, D.L.; Nairn, R.S. Genetic and environmental melanoma models in fish. Pigment Cell Melanoma Res. 2010, 23, 314–337. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V. Sex and Gender Differences in Heart Failure. Int. J. Heart Fail. 2020, 2, 157–181. [Google Scholar] [CrossRef]
- Taneda, Y.; Konno, S.; Makino, S.; Morioka, M.; Fukuda, K.; Imai, Y.; Kudo, A.; Kawakami, A. Epigenetic control of cardiomyocyte production in response to a stress during the medaka heart development. Dev. Biol. 2010, 340, 30–40. [Google Scholar] [CrossRef]
- Watanabe-Asaka, T.; Sekiya, Y.; Wada, H.; Yasuda, T.; Okubo, I.; Oda, S.; Mitani, H. Regular heartbeat rhythm at the heartbeat initiation stage is essential for normal cardiogenesis at low temperature. BMC Dev. Biol. 2014, 14, 12. [Google Scholar] [CrossRef][Green Version]
- Mittal, N.; Yoon, S.H.; Enomoto, H.; Hiroshi, M.; Shimizu, A.; Kawakami, A.; Fujita, M.; Watanabe, H.; Fukuda, K.; Makino, S. Versican is crucial for the initiation of cardiovascular lumen development in medaka (Oryzias latipes). Sci. Rep. 2019, 9, 9475. [Google Scholar] [CrossRef]
- Wang, L.W.; Huttner, I.G.; Santiago, C.F.; Kesteven, S.H.; Yu, Z.-Y.; Feneley, M.P.; Fatkin, D. Standardized echocardiographic assessment of cardiac function in normal adult zebrafish and heart disease models. Dis. Model. Mech. 2017, 10, 63–76. [Google Scholar] [CrossRef]
- Sandblom, E.; Clark, T.D.; Hinch, S.G.; Farrell, A.P. Sex-Specific differences in cardiac control and hematology of sockeye salmon (Oncorhynchus nerka) approaching their spawning grounds. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1136–R1143. [Google Scholar] [CrossRef]
- Battiprolu, P.K.; Harmon, K.J.; Rodnick, K.J. Sex differences in energy metabolism and performance of teleost cardiac tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R827–R836. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.-J.; Li, T.; Mu, Y.; McGlashan, J.K.; Georges, A.; Shine, R.; Du, W.-G. Thyroid hormone modulates offspring sex ratio in a turtle with temperature-dependent sex determination. Proc. R. Soc. Lond. B Biol. Sci. 2016, 283. [Google Scholar] [CrossRef]
- Alvine, T.; Rhen, T.; Crossley, D.A. Temperature-Dependent sex determination modulates cardiovascular maturation in embryonic snapping turtles Chelydra serpentina. J. Exp. Biol. 2013, 216, 751. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Chambolle, P.; Cambar, R.; Tran, D. Table chronologique du developement embryonnaire de Gambusia sp. (Poisson teleosteen). Biol. Bull. Fr. Belg. 1970, 104, 443–452. [Google Scholar]
- De Luca, E.; Zaccaria, G.M.; Hadhoud, M.; Rizzo, G.; Ponzini, R.; Morbiducci, U.; Santoro, M.M. ZebraBeat: A flexible platform for the analysis of the cardiac rate in zebrafish embryos. Sci. Rep. 2014, 4, 4898. [Google Scholar] [CrossRef]
- Patil, J.G.; Norazmi-Lokman, N.H.; Kwan, T.N. Reproductive viability of paradoxically masculinised Gambusia holbrooki generated following diethylstilbestrol (DES) treatment. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2020, 248–249, 110468. [Google Scholar] [CrossRef]
- Singleman, C.; Holtzman, N.G. Analysis of postembryonic heart development and maturation in the zebrafish, Danio rerio. Dev. Dyn. 2012, 241, 1993–2004. [Google Scholar] [CrossRef]
- Perrichon, P.; Grosell, M.; Burggren, W.W. Heart Performance Determination by Visualization in Larval Fishes: Influence of Alternative Models for Heart Shape and Volume. Front. Physiol. 2017, 8, 464. [Google Scholar] [CrossRef]
- Chan, P.K.; Lin, C.C.; Cheng, S.H. Noninvasive technique for measurement of heartbeat regularity in zebrafish (Danio rerio) embryos. BMC Biotechnol. 2009, 9, 11. [Google Scholar] [CrossRef]
- Vornanen, M.; Hassinen, M. Zebrafish heart as a model for human cardiac electrophysiology. Channels 2016, 10, 101–110. [Google Scholar] [CrossRef]
- Chan, D.D.; Wu, K.C.; Loring, Z.; Galeotti, L.; Gerstenblith, G.; Tomaselli, G.; Weiss, R.G.; Wagner, G.S.; Strauss, D.G. Comparison of the relation between left ventricular anatomy and QRS duration in patients with cardiomyopathy with versus without left bundle branch block. Am. J. Cardiol. 2014, 113, 1717–1722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Farrell, A.P. Design and physiology of the heart | Physiology of cardiac pumping. In Encyclopedia of Fish Physiology; Farrell, A.P., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 1030–1037. [Google Scholar] [CrossRef]
- Dhillon, S.S.; Dóró, É.; Magyary, I.; Egginton, S.; Sík, A.; Müller, F. Optimisation of Embryonic and Larval ECG Measurement in Zebrafish for Quantifying the Effect of QT Prolonging Drugs. PLoS ONE 2013, 8, e60552. [Google Scholar] [CrossRef] [PubMed]
- van Weerd, J.H.; Christoffels, V.M. The formation and function of the cardiac conduction system. Development 2016, 143, 197. [Google Scholar] [CrossRef]
- Martin, W.K.; Tennant, A.H.; Conolly, R.B.; Prince, K.; Stevens, J.S.; DeMarini, D.M.; Martin, B.L.; Thompson, L.C.; Gilmour, M.I.; Cascio, W.E.; et al. High-Throughput Video Processing of Heart Rate Responses in Multiple Wild-type Embryonic Zebrafish per Imaging Field. Sci. Rep. 2019, 9, 145. [Google Scholar] [CrossRef]
- Pildner von Steinburg, S.; Boulesteix, A.-L.; Lederer, C.; Grunow, S.; Schiermeier, S.; Hatzmann, W.; Schneider, K.-T.M.; Daumer, M. What is the “normal” fetal heart rate? PeerJ 2013, 1, e82. [Google Scholar] [CrossRef]
- Quer, G.; Gouda, P.; Galarnyk, M.; Topol, E.J.; Steinhubl, S.R. Inter- and intraindividual variability in daily resting heart rate and its associations with age, sex, sleep, BMI, and time of year: Retrospective, longitudinal cohort study of 92,457 adults. PLoS ONE 2020, 15, e0227709. [Google Scholar] [CrossRef]
- Moriyama, Y.; Ito, F.; Takeda, H.; Yano, T.; Okabe, M.; Kuraku, S.; Keeley, F.W.; Koshiba-Takeuchi, K. Evolution of the fish heart by sub/neofunctionalization of an elastin gene. Nat. Commun. 2016, 7, 10397. [Google Scholar] [CrossRef] [PubMed]
- de Pater, E.; Clijsters, L.; Marques, S.R.; Lin, Y.-F.; Garavito-Aguilar, Z.V.; Yelon, D.; Bakkers, J. Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development 2009, 136, 1633. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.M.J.; Lewandowski, A.J. The Transitional Heart: From Early Embryonic and Fetal Development to Neonatal Life. Fetal Diagn. Ther. 2020, 47, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Goenezen, S.; Haller, S.; Hinds, M.T.; Thornburg, K.L.; Rugonyi, S. Alterations in pulse wave propagation reflect the degree of outflow tract banding in HH18 chicken embryos. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H386–H396. [Google Scholar] [CrossRef]
- Jensen, B.; Boukens, B.J.; Crossley, D.A., 2nd; Conner, J.; Mohan, R.A.; van Duijvenboden, K.; Postma, A.V.; Gloschat, C.R.; Elsey, R.M.; Sedmera, D.; et al. Specialized impulse conduction pathway in the alligator heart. eLife 2018, 7, e32120. [Google Scholar] [CrossRef]
- Burggren, W.W. Cardiovascular Development and Angiogenesis in the Early Vertebrate Embryo. Cardiovasc. Eng. Technol. 2013, 4, 234–245. [Google Scholar] [CrossRef]
- Arbel, E.R.; Liberthson, R.; Langendorf, R.; Pick, A.; Lev, M.; Fishman, A.P. Electrophysiological and anatomical observations on the heart of the African lungfish. Am. J. Physiol. Heart Circ. Physiol. 1977, 232, H24–H34. [Google Scholar] [CrossRef] [PubMed]
- Dzialowski, E.M.; Crossley, D.A. Chapter 11—The cardiovascular system. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 193–283. [Google Scholar] [CrossRef]
- Goodyer, W.R.; Beyersdorf Benjamin, M.; Paik David, T.; Tian, L.; Li, G.; Buikema Jan, W.; Chirikian, O.; Choi, S.; Venkatraman, S.; Adams Eliza, L.; et al. Transcriptomic Profiling of the Developing Cardiac Conduction System at Single-Cell Resolution. Circ. Res. 2019, 125, 379–397. [Google Scholar] [CrossRef]
- Tessadori, F.; van Weerd, J.H.; Burkhard, S.B.; Verkerk, A.O.; de Pater, E.; Boukens, B.J.; Vink, A.; Christoffels, V.M.; Bakkers, J. Identification and Functional Characterization of Cardiac Pacemaker Cells in Zebrafish. PLoS ONE 2012, 7, e47644. [Google Scholar] [CrossRef]
- Rentschler, S.; Harris, B.S.; Kuznekoff, L.; Jain, R.; Manderfield, L.; Lu, M.M.; Morley, G.E.; Patel, V.V.; Epstein, J.A. Notch signaling regulates murine atrioventricular conduction and the formation of accessory pathways. J. Clin. Investig. 2011, 121, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Chi, N.C.; Shaw, R.M.; Jungblut, B.; Huisken, J.; Ferrer, T.; Arnaout, R.; Scott, I.; Beis, D.; Xiao, T.; Baier, H.; et al. Genetic and Physiologic Dissection of the Vertebrate Cardiac Conduction System. PLoS Biol. 2008, 6, e109. [Google Scholar] [CrossRef]
- Milan, D.J.; Giokas, A.C.; Serluca, F.C.; Peterson, R.T.; MacRae, C.A. Notch1b and neuregulin are required for specification of central cardiac conduction tissue. Development 2006, 133, 1125. [Google Scholar] [CrossRef]
- Sedmera, D.; Reckova, M.; deAlmeida, A.; Sedmerova, M.; Biermann, M.; Volejnik, J.; Sarre, A.; Raddatz, E.; McCarthy, R.A.; Gourdie, R.G.; et al. Functional and morphological evidence for a ventricular conduction system in zebrafish and Xenopus hearts. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1152–H1160. [Google Scholar] [CrossRef] [PubMed]
- Chuck, E.T.; Watanabe, M. Differential expression of PSA-NCAM and HNK-1 epitopes in the developing cardiac conduction system of the chick. Dev. Dyn. 1997, 209, 182–195. [Google Scholar] [CrossRef]
- Chuck, E.T.; Freeman, D.M.; Watanabe, M.; Rosenbaum, D.S. Changing activation sequence in the embryonic chick heart. Implications for the development of the His-Purkinje system. Circ. Res. 1997, 81, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Crossley Ii, D.A.; Burggren, W.W. Development of cardiac form and function in ectothermic sauropsids. J. Morphol. 2009, 270, 1400–1412. [Google Scholar] [CrossRef]
- Eme, J.; Altimiras, J.; Hicks, J.W.; Crossley, D.A. Hypoxic alligator embryos: Chronic hypoxia, catecholamine levels and autonomic responses of in ovo alligators. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 160, 412–420. [Google Scholar] [CrossRef]
- Crossley, D.A.; Bagatto, B.P.; Dzialowski, E.M.; Burggren, W.W. Maturation of cardiovascular control mechanisms in the embryonic emu (Dromiceius novaehollandiae). J. Exp. Biol. 2003, 206, 2703–2710. [Google Scholar] [CrossRef]
- Acharya, G.; Gui, Y.; Cnota, W.; Huhta, J.; Wloch, A. Human embryonic cardiovascular function. Acta Obstet. Gynecol. Scand. 2016, 95, 621–628. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, J.A.; Voegtline, K.M. The gestational foundation of sex differences in development and vulnerability. Neuroscience 2017, 342, 4–20. [Google Scholar] [CrossRef]
- Glahn, R.P.; Mitsos, W.J.; Wideman, J.R.F. Evaluation of Sex Differences in Embryonic Heart Rates. Poult. Sci. 1987, 66, 1398–1401. [Google Scholar] [CrossRef]
- Hildebrand, S.F. Sex Ratio in Gambusia. Biol. Bull. 1927, 53, 390–404. [Google Scholar] [CrossRef]
- Metzger, D.C.H.; Mank, J.E. Conserved sex-biased DNA methylation patterns target key developmental genes and non-recombining region of the guppy sex chromosome. bioRxiv 2020, 261792. [Google Scholar] [CrossRef]
- Arnold, A.P. The end of gonad-centric sex determination in mammals. Trends Genet. 2011, 28. [Google Scholar] [CrossRef] [PubMed]
- White, S.J.; Houslay, T.M.; Wilson, A.J. Evolutionary genetics of personality in the Trinidadian guppy II: Sexual dimorphism and genotype-by-sex interactions. Heredity 2019, 122, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Boulton, K.; Rosenthal, G.G.; Grimmer, A.J.; Walling, C.A.; Wilson, A.J. Sex-Specific plasticity and genotype × sex interactions for age and size of maturity in the sheepshead swordtail, Xiphophorus birchmanni. J. Evol. Biol. 2016, 29, 645–656. [Google Scholar] [CrossRef]
- Arnold, A.P.; Chen, X.; Link, J.C.; Itoh, Y.; Reue, K. Cell-Autonomous sex determination outside of the gonad. Dev. Dyn. 2013, 242, 371–379. [Google Scholar] [CrossRef]
- Keen, A.N.; Fenna, A.J.; McConnell, J.C.; Sherratt, M.J.; Gardner, P.; Shiels, H.A. Macro- and micromechanical remodelling in the fish atrium is associated with regulation of collagen 1 alpha 3 chain expression. Pflug. Arch. Eur. J. Physiol. 2018, 470, 1205–1219. [Google Scholar] [CrossRef] [PubMed]
- Summerhill, V.I.; Moschetta, D.; Orekhov, A.N.; Poggio, P.; Myasoedova, V.A. Sex-Specific Features of Calcific Aortic Valve Disease. Int. J. Mol. Sci. 2020, 21, 5620. [Google Scholar] [CrossRef] [PubMed]
- Nijenkamp, L.L.A.M.; Bollen, I.A.E.; Niessen, H.W.M.; dos Remedios, C.G.; Michels, M.; Poggesi, C.; Ho, C.Y.; Kuster, D.W.D.; van der Velden, J. Sex-Specific cardiac remodeling in early and advanced stages of hypertrophic cardiomyopathy. PLoS ONE 2020, 15, e0232427. [Google Scholar] [CrossRef]
- Rovira, M.; Borràs, D.M.; Marques, I.J.; Puig, C.; Planas, J.V. Physiological Responses to Swimming-Induced Exercise in the Adult Zebrafish Regenerating Heart. Front. Physiol. 2018, 9, 1362. [Google Scholar] [CrossRef]
- Romano, S.N.; Edwards, H.E.; Souder, J.P.; Ryan, K.J.; Cui, X.; Gorelick, D.A. G protein-coupled estrogen receptor regulates embryonic heart rate in zebrafish. PLoS Genet. 2017, 13, e1007069. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi, S.E.; Purser, G.J.; Patil, J.G. Embryonic Onset of Sexually Dimorphic Heart Rates in the Viviparous Fish, Gambusia holbrooki. Biomedicines 2021, 9, 165. https://doi.org/10.3390/biomedicines9020165
Mousavi SE, Purser GJ, Patil JG. Embryonic Onset of Sexually Dimorphic Heart Rates in the Viviparous Fish, Gambusia holbrooki. Biomedicines. 2021; 9(2):165. https://doi.org/10.3390/biomedicines9020165
Chicago/Turabian StyleMousavi, Seyed Ehsan, G. John Purser, and Jawahar G. Patil. 2021. "Embryonic Onset of Sexually Dimorphic Heart Rates in the Viviparous Fish, Gambusia holbrooki" Biomedicines 9, no. 2: 165. https://doi.org/10.3390/biomedicines9020165
APA StyleMousavi, S. E., Purser, G. J., & Patil, J. G. (2021). Embryonic Onset of Sexually Dimorphic Heart Rates in the Viviparous Fish, Gambusia holbrooki. Biomedicines, 9(2), 165. https://doi.org/10.3390/biomedicines9020165






