The Lectin-Like Domain of Thrombomodulin Inhibits β1 Integrin-Dependent Binding of Human Breast Cancer-Derived Cell Lines to Fibronectin
Abstract
1. Introduction
2. Materials and Methods
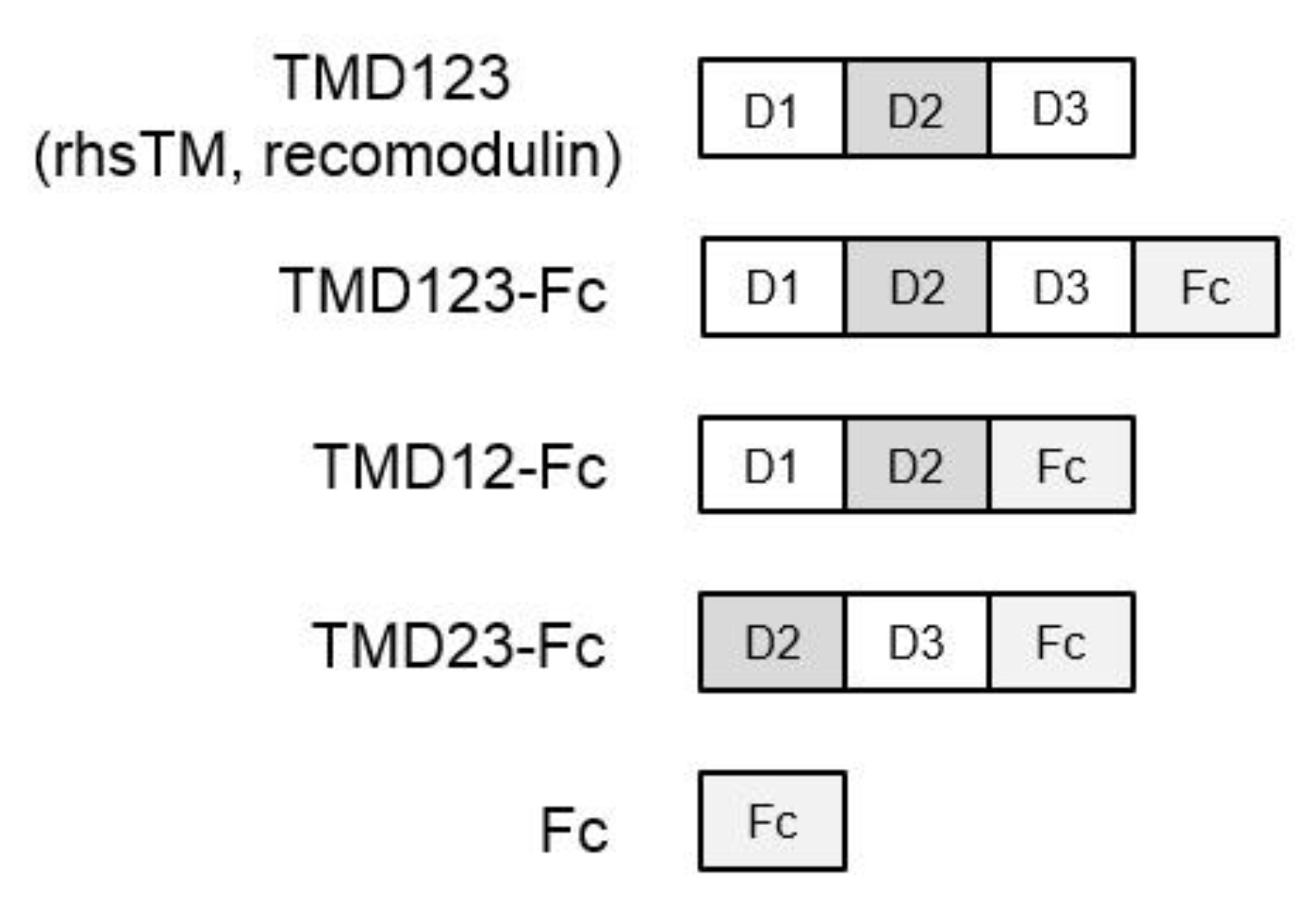
2.1. Construction of a Recombinant Human TM (Domains 1, 2, and 3)-Immunoglobulin Fc Fusion Protein (TMD123-Fc) Expression Vector
2.2. Expression and Purification of TM Domain-Fc Fusion Proteins
2.3. V-Well Cell Adhesion Assay
2.4. Cell Culture
2.5. β1. Integrin KO Cell with CRISPR/CAS9 System
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
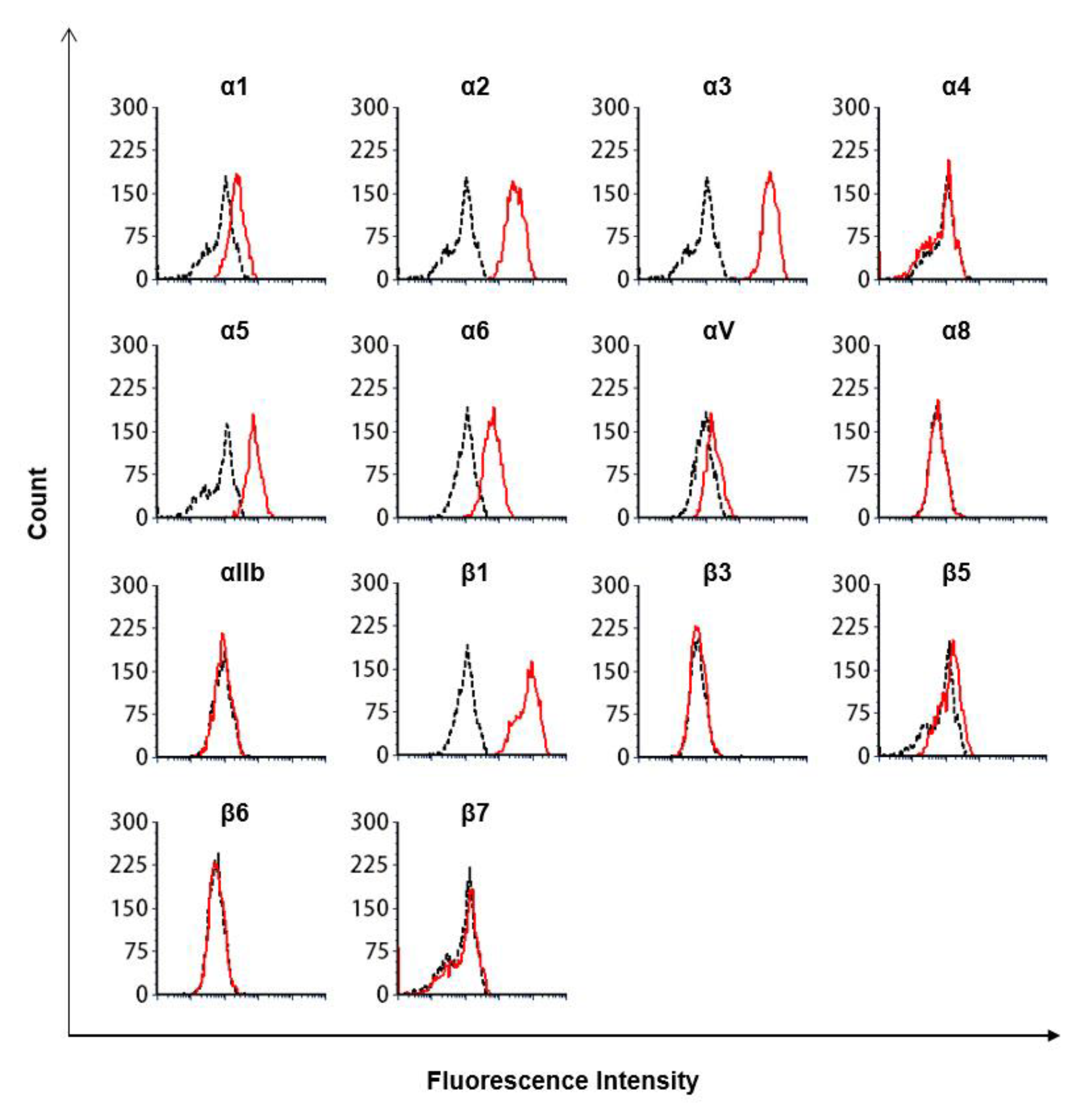
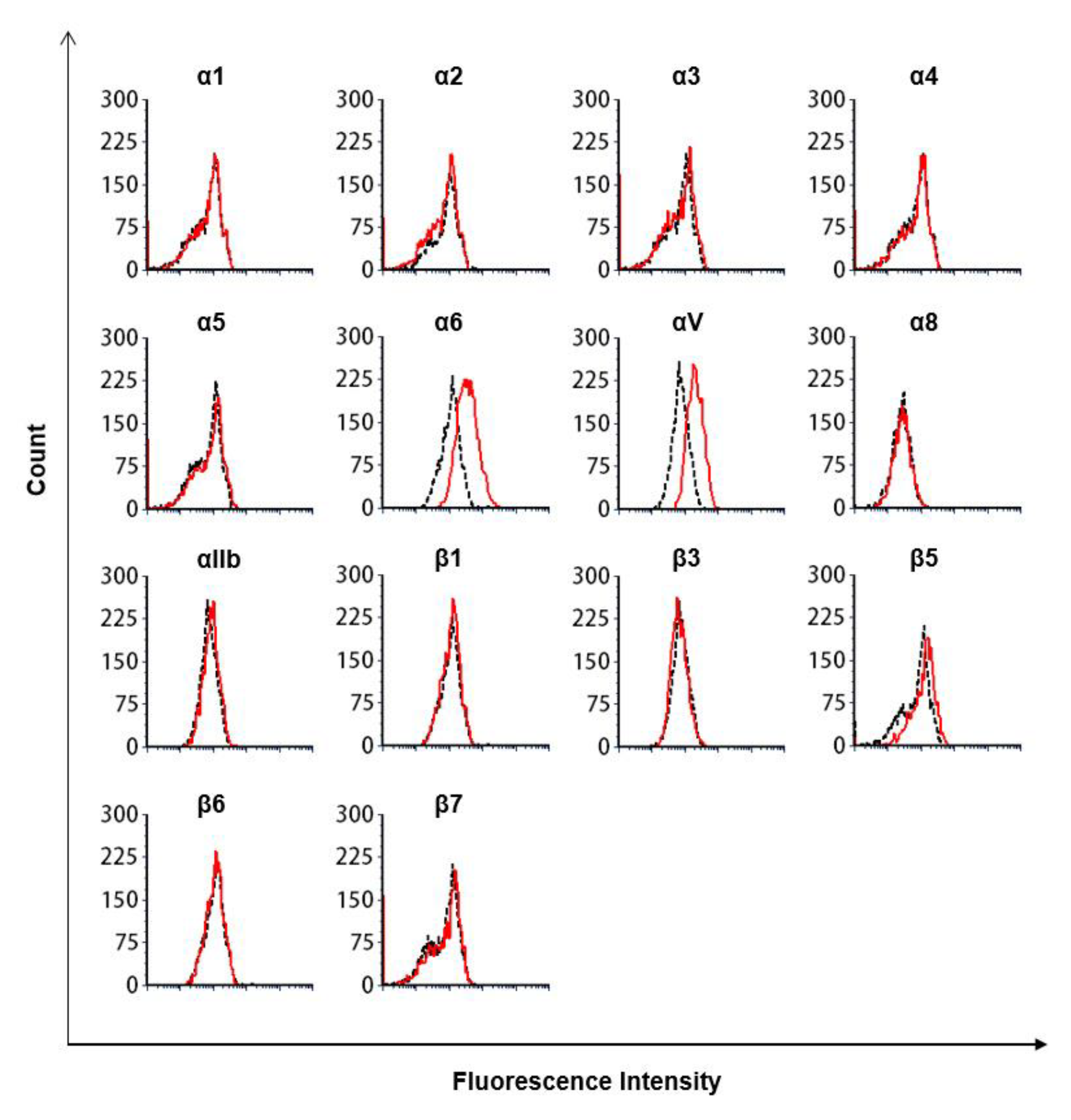
2.7. Flow Cytometry Analysis
2.8. Antibodies Targeting Integrins
2.9. Cell Adhesion Experiments Using Fibronectin-Coated Fluorescent Beads
2.10. Statistical Analysis
3. Result
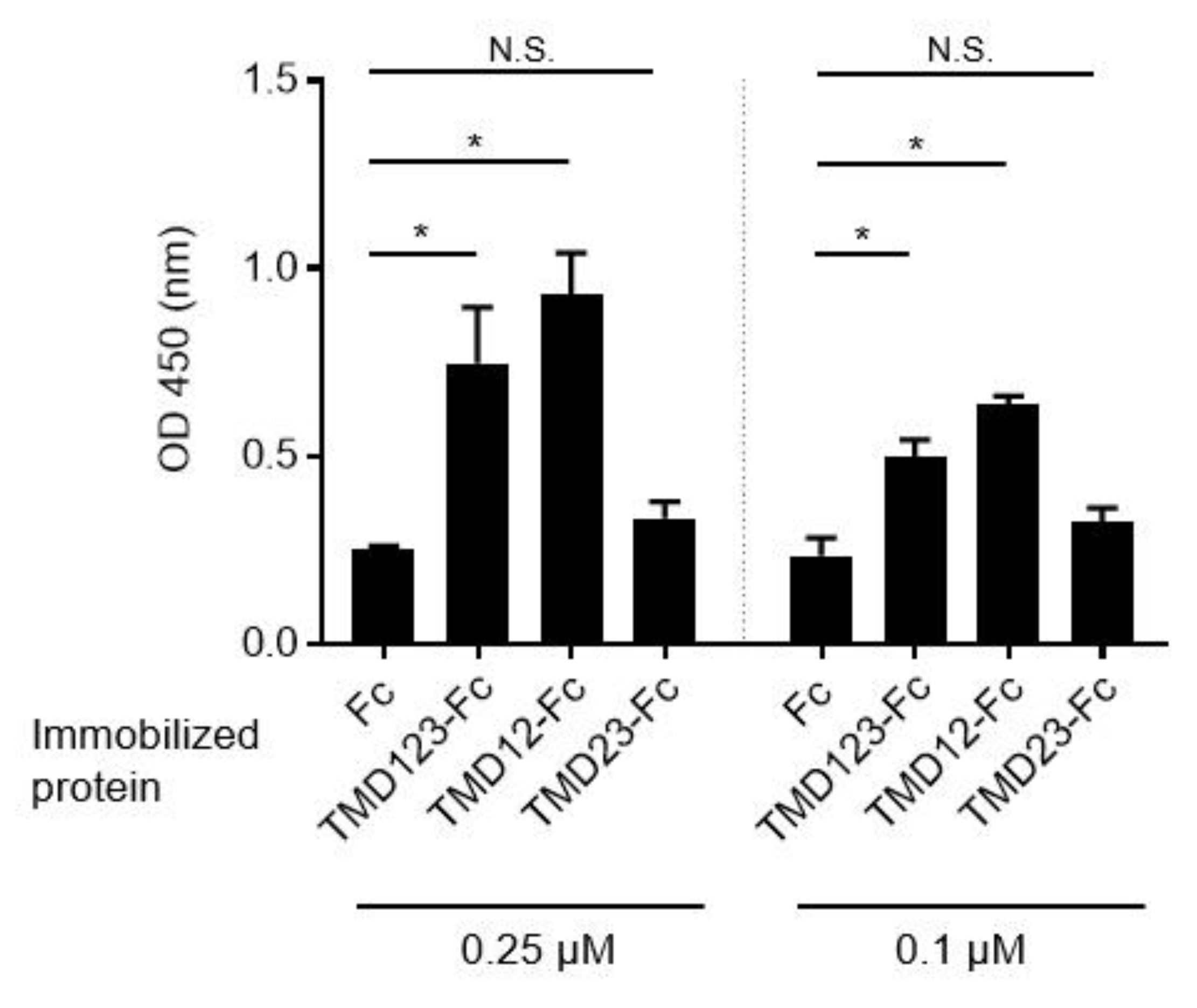
3.1. The Lectin-Like Extracellular Domain of TM Binds Fibronectin
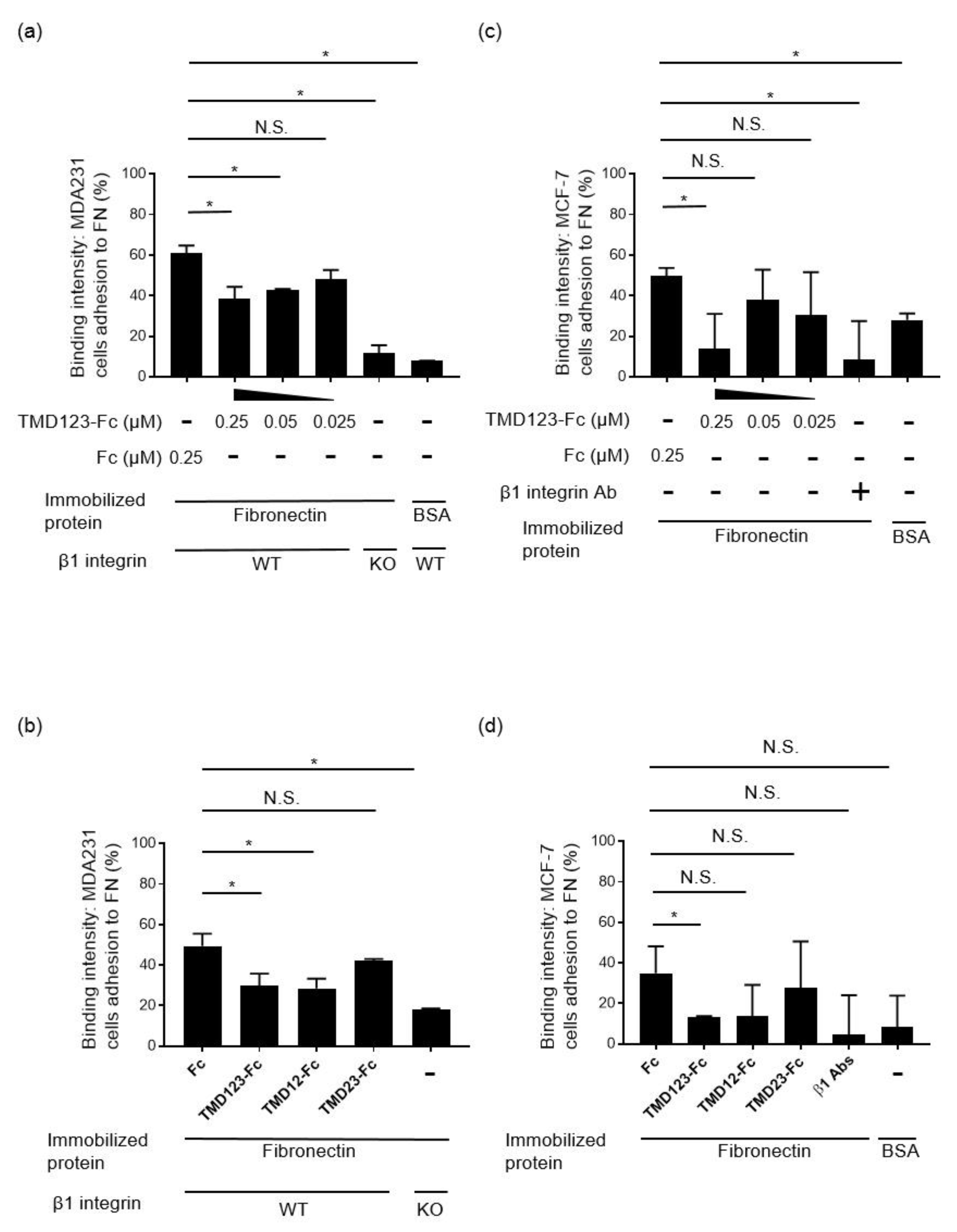
3.2. Binding of Human Breast Cancer-Derived Cell Lines to Fibronectin Is Inhibited by TMD123-Fc in a Concentration-Dependent Manner
3.3. The Lectin-Like Domain of TM Inhibits the Binding of β1 Integrin from Human Breast Cancer-Derived Cell Lines to Fibronectin
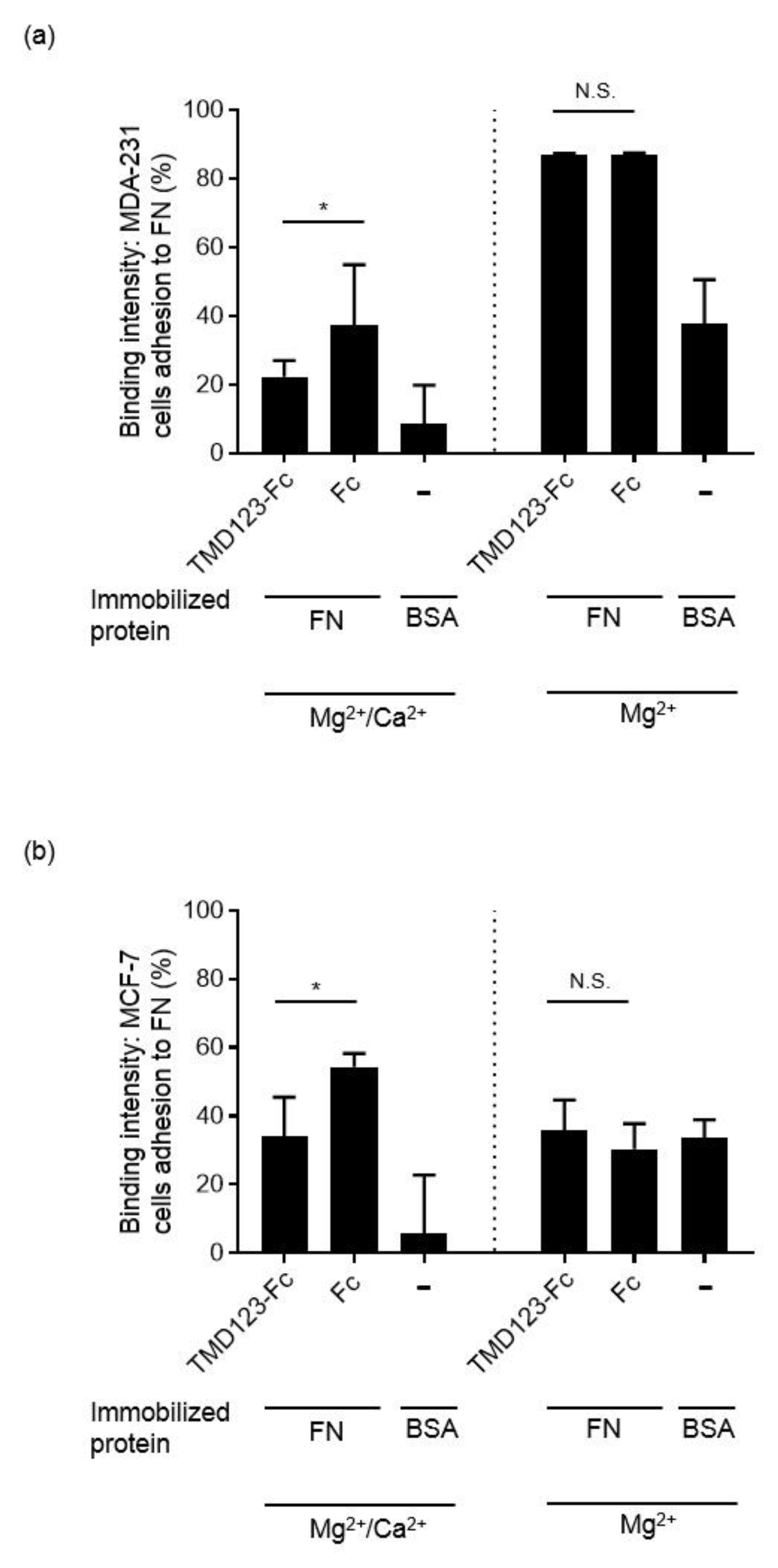
3.4. TM Inhibits the Integrin-Dependent Binding of MDA-MB-231 to Fibronectin in the Presence of Ca2+
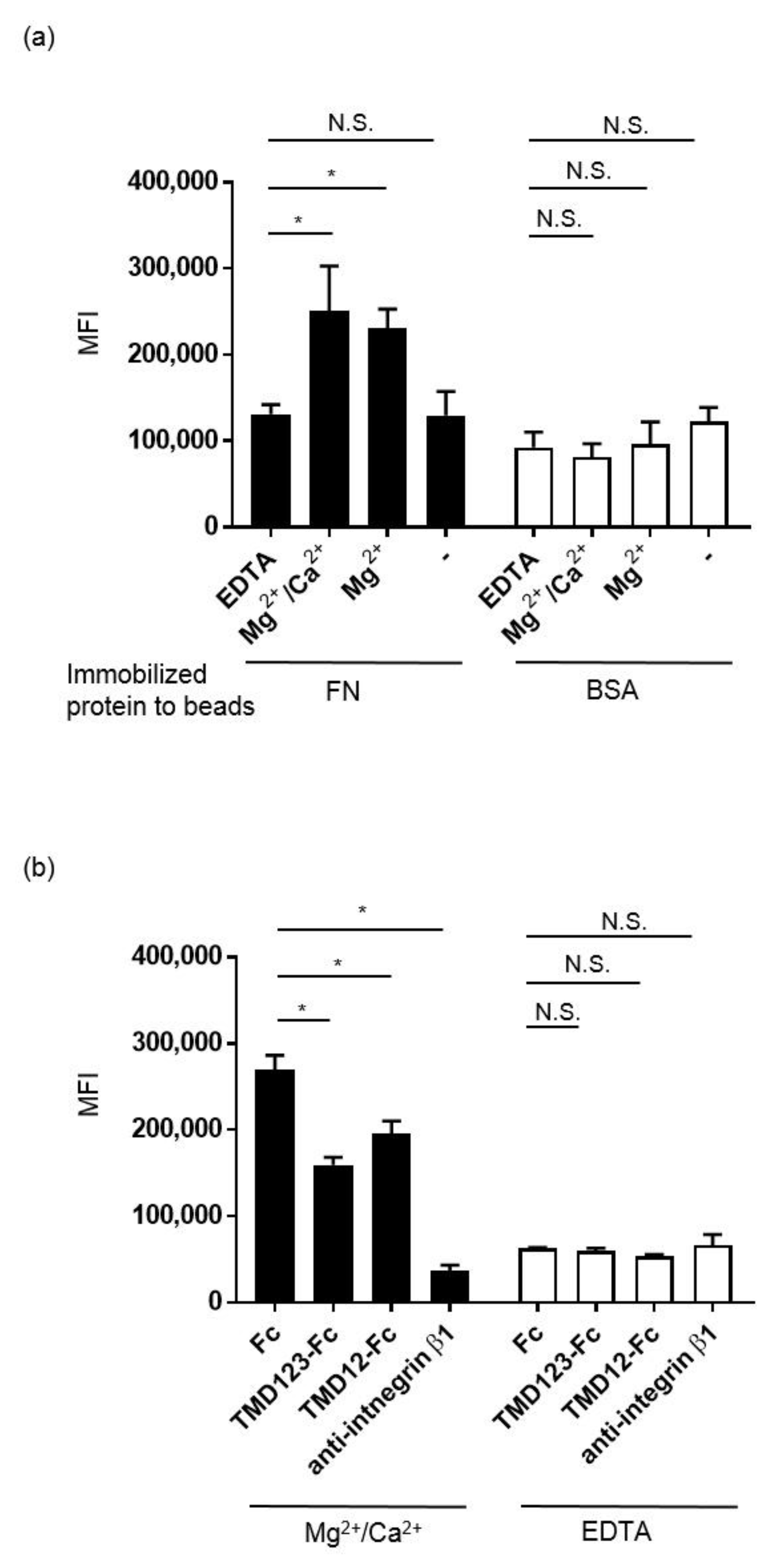
3.5. Binding of MDA-231 Cells to Fibronectin Is Inhibited by TMD123-Fc
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esmon, C.T. The protein C pathway. Chest 2003, 124, 26S–32S. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Tanigami, H.; Suzuki, K.; Shimaoka, M. Thrombomodulin: A bifunctional modulator of inflammation and coagulation in sepsis. Crit. Care Res. Pr. 2012, 2012, 614545. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, Y.; Yonezawa, S.; Maruyama, I.; Matsushita, Y.; Shimizu, T.; Obama, H.; Sagara, M.; Shirao, K.; Kusano, C.; Natsugoe, S.; et al. Expression of thrombomodulin in esophageal squamous cell carcinoma and its relationship to lymph node metastasis. Cancer Res. 1995, 55, 4196–4200. [Google Scholar]
- Kim, S.J.; Shiba, E.; Ishii, H.; Inoue, T.; Taguchi, T.; Tanji, Y.; Kimoto, Y.; Izukura, M.; Takai, S. Thrombomodulin is a new biological and prognostic marker for breast cancer: An immunohistochemical study. Anticancer Res. 1997, 17, 2319–2323. [Google Scholar] [PubMed]
- Hsu, Y.Y.; Shi, G.Y.; Wang, K.C.; Ma, C.Y.; Cheng, T.L.; Wu, H.L. Thrombomodulin promotes focal adhesion kinase activation and contributes to angiogenesis by binding to fibronectin. Oncotarget 2016, 7, 68122–68139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xiao, T.; Takagi, J.; Coller, B.S.; Wang, J.H.; Springer, T.A. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature 2004, 432, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, F.; Ray, A.M.; Dontenwill, M. Integrin alpha5beta1, the fibronectin receptor, as a pertinent therapeutic target in solid tumors. Cancers 2013, 5, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.B.; Zanetti, J.S.; Ribeiro-Silva, A.; Beltrao, E.I. Beta 1 integrin predicts survival in breast cancer: A clinicopathological and immunohistochemical study. Diagn. Pathol. 2012, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Yao, E.S.; Zhang, H.; Chen, Y.Y.; Lee, B.; Chew, K.; Moore, D.; Park, C. Increased beta1 integrin is associated with decreased survival in invasive breast cancer. Cancer Res. 2007, 67, 659–664. [Google Scholar] [CrossRef]
- Dingemans, A.M.; van den Boogaart, V.; Vosse, B.A.; van Suylen, R.J.; Griffioen, A.W.; Thijssen, V.L. Integrin expression profiling identifies integrin alpha5 and beta1 as prognostic factors in early stage non-small cell lung cancer. Mol. Cancer 2010, 9, 152. [Google Scholar] [CrossRef]
- White, D.E.; Kurpios, N.A.; Zuo, D.; Hassell, J.A.; Blaess, S.; Mueller, U.; Muller, W.J. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell 2004, 6, 159–170. [Google Scholar] [CrossRef]
- Huck, L.; Pontier, S.M.; Zuo, D.M.; Muller, W.J. beta1-integrin is dispensable for the induction of ErbB2 mammary tumors but plays a critical role in the metastatic phase of tumor progression. Proc. Natl. Acad. Sci. USA 2010, 107, 15559–15564. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kusumoto, H.; Deyashiki, Y.; Nishioka, J.; Maruyama, I.; Zushi, M.; Kawahara, S.; Honda, G.; Yamamoto, S.; Horiguchi, S. Structure and expression of human thrombomodulin, a thrombin receptor on endothelium acting as a cofactor for protein C activation. EMBO J. 1987, 6, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, E.; Okamoto, T.; Takagi, Y.; Honda, G.; Suzuki, K.; Imai, H.; Shimaoka, M. LFA-1 and Mac-1 integrins bind to the serine/threonine-rich domain of thrombomodulin. Biochem. Biophys. Res. Commun. 2016, 473, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Weetall, M.; Hugo, R.; Friedman, C.; Maida, S.; West, S.; Wattanasin, S.; Bouhel, R.; Weitz-Schmidt, G.; Lake, P. A homogeneous fluorometric assay for measuring cell adhesion to immobilized ligand using V-well microtiter plates. Anal. Biochem. 2001, 293, 277–287. [Google Scholar] [CrossRef]
- Hou, S.; Isaji, T.; Hang, Q.; Im, S.; Fukuda, T.; Gu, J. Distinct effects of beta1 integrin on cell proliferation and cellular signaling in MDA-MB-231 breast cancer cells. Sci. Rep. 2016, 6, 18430. [Google Scholar] [CrossRef] [PubMed]
- Danen, E.H.; Sonneveld, P.; Brakebusch, C.; Fassler, R.; Sonnenberg, A. The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J. Cell Biol. 2002, 159, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef]
- Li, Y.H.; Kuo, C.H.; Shi, G.Y.; Wu, H.L. The role of thrombomodulin lectin-like domain in inflammation. J. Biomed. Sci. 2012, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Light, D.R.; Glaser, C.B.; Betts, M.; Blasko, E.; Campbell, E.; Clarke, J.H.; McCaman, M.; McLean, K.; Nagashima, M.; Parkinson, J.F.; et al. The interaction of thrombomodulin with Ca2+. Eur. J. Biochem. 1999, 262, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.R.; Esmon, C.T. The function of calcium in protein C activation by thrombin and the thrombin-thrombomodulin complex can be distinguished by mutational analysis of protein C derivatives. J. Biol. Chem. 1992, 267, 26104–26109. [Google Scholar] [CrossRef]
- Gingras, M.C.; Roussel, E.; Bruner, J.M.; Branch, C.D.; Moser, R.P. Comparison of cell adhesion molecule expression between glioblastoma multiforme and autologous normal brain tissue. J. Neuroimmunol. 1995, 57, 143–153. [Google Scholar] [CrossRef]
- Strohl, W.R. Current progress in innovative engineered antibodies. Protein Cell 2018, 9, 86–120. [Google Scholar] [CrossRef] [PubMed]
- Moll, S.; Lindley, C.; Pescatore, S.; Morrison, D.; Tsuruta, K.; Mohri, M.; Serada, M.; Sata, M.; Shimizu, H.; Yamada, K.; et al. Phase I study of a novel recombinant human soluble thrombomodulin, ART-123. JTH 2004, 2, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Kontermann, R.E. Strategies for extended serum half-life of protein therapeutics. Curr. Opin. Biotechnol. 2011, 22, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, P.H.; Elliott, J.F.; Kubes, P. Neutrophils can adhere via alpha4beta1-integrin under flow conditions. Blood 1997, 89, 3837–3846. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, E.; Nago, N.; Okamoto, T.; Gaowa, A.; Masui-Ito, A.; Sakakura, Y.; Akama, Y.; Soe, Z.Y.; Prajuabjinda, O.; Darkwah, S.; et al. Anti-adhesive effects of human soluble thrombomodulin and its domains. Biochem. Biophys. Res. Commun. 2019, 511, 312–317. [Google Scholar] [CrossRef]
- Iba, T.; Aihara, K.; Watanabe, S.; Yanagawa, Y.; Takemoto, M.; Yamada, A.; Yang, D. Recombinant thrombomodulin improves the visceral microcirculation by attenuating the leukocyte-endothelial interaction in a rat LPS model. Thromb. Res. 2013, 131, 295–299. [Google Scholar] [CrossRef]
- Tzima, E.; del Pozo, M.A.; Shattil, S.J.; Chien, S.; Schwartz, M.A. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001, 20, 4639–4647. [Google Scholar] [CrossRef]
- Davies, P.F.; Barbee, K.A.; Volin, M.V.; Robotewskyj, A.; Chen, J.; Joseph, L.; Griem, M.L.; Wernick, M.N.; Jacobs, E.; Polacek, D.C.; et al. Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Ann. Rev. Physiol. 1997, 59, 527–549. [Google Scholar] [CrossRef]
- Banerjee, A.K.; Angulo, A.F.; Polak-Vogelzang, A.A.; Kershof, A.M. Naturally occurring genital mycoplasmosis in mice. Lab. Anim. 1985, 19, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Segre, J.A.; Bauer, C.; Fuchs, E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat. Genet. 1999, 22, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Karaman, S.; Detmar, M. Mechanisms of lymphatic metastasis. J. Clin. Investig. 2014, 124, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.E. The incidence of renal artery stenosis and hypertension in patients on chronic intermittent haemodialysis. Dan. Med. Bull. 1983, 30, 343–345. [Google Scholar] [PubMed]
- Schmidt-Kittler, O.; Ragg, T.; Daskalakis, A.; Granzow, M.; Ahr, A.; Blankenstein, T.J.; Kaufmann, M.; Diebold, J.; Arnholdt, H.; Muller, P.; et al. From latent disseminated cells to overt metastasis: Genetic analysis of systemic breast cancer progression. Proc. Natl. Acad. Sci. USA 2003, 100, 7737–7742. [Google Scholar] [CrossRef] [PubMed]
- Murtomaki, A.; Uh, M.K.; Kitajewski, C.; Zhao, J.; Nagasaki, T.; Shawber, C.J.; Kitajewski, J. Notch signaling functions in lymphatic valve formation. Development 2014, 141, 2446–2451. [Google Scholar] [CrossRef]
- Sobocinski, G.P.; Toy, K.; Bobrowski, W.F.; Shaw, S.; Anderson, A.O.; Kaldjian, E.P. Ultrastructural localization of extracellular matrix proteins of the lymph node cortex: Evidence supporting the reticular network as a pathway for lymphocyte migration. BMC Immunol. 2010, 11, 42. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawamoto, E.; Nago, N.; Okamoto, T.; Gaowa, A.; Masui-Ito, A.; Akama, Y.; Darkwah, S.; Appiah, M.G.; Myint, P.K.; Obeng, G.; et al. The Lectin-Like Domain of Thrombomodulin Inhibits β1 Integrin-Dependent Binding of Human Breast Cancer-Derived Cell Lines to Fibronectin. Biomedicines 2021, 9, 162. https://doi.org/10.3390/biomedicines9020162
Kawamoto E, Nago N, Okamoto T, Gaowa A, Masui-Ito A, Akama Y, Darkwah S, Appiah MG, Myint PK, Obeng G, et al. The Lectin-Like Domain of Thrombomodulin Inhibits β1 Integrin-Dependent Binding of Human Breast Cancer-Derived Cell Lines to Fibronectin. Biomedicines. 2021; 9(2):162. https://doi.org/10.3390/biomedicines9020162
Chicago/Turabian StyleKawamoto, Eiji, Nodoka Nago, Takayuki Okamoto, Arong Gaowa, Asami Masui-Ito, Yuichi Akama, Samuel Darkwah, Michael Gyasi Appiah, Phyoe Kyawe Myint, Gideon Obeng, and et al. 2021. "The Lectin-Like Domain of Thrombomodulin Inhibits β1 Integrin-Dependent Binding of Human Breast Cancer-Derived Cell Lines to Fibronectin" Biomedicines 9, no. 2: 162. https://doi.org/10.3390/biomedicines9020162
APA StyleKawamoto, E., Nago, N., Okamoto, T., Gaowa, A., Masui-Ito, A., Akama, Y., Darkwah, S., Appiah, M. G., Myint, P. K., Obeng, G., Ito, A., Caidengbate, S., Esumi, R., Yamaguchi, T., Park, E. J., Imai, H., & Shimaoka, M. (2021). The Lectin-Like Domain of Thrombomodulin Inhibits β1 Integrin-Dependent Binding of Human Breast Cancer-Derived Cell Lines to Fibronectin. Biomedicines, 9(2), 162. https://doi.org/10.3390/biomedicines9020162







