Factors Influencing the In Vitro Maturation (IVM) of Human Oocyte
Abstract
1. Introduction
2. Follicular Priming Methods and Collected Eggs
3. Culture Conditions
3.1. Culture Medium
3.2. Protein Sources in Culture Medium
3.3. Hormones in Culture Medium
3.4. Carbohydrates in Culture Medium
3.5. Other Supplementation in Culture Medium
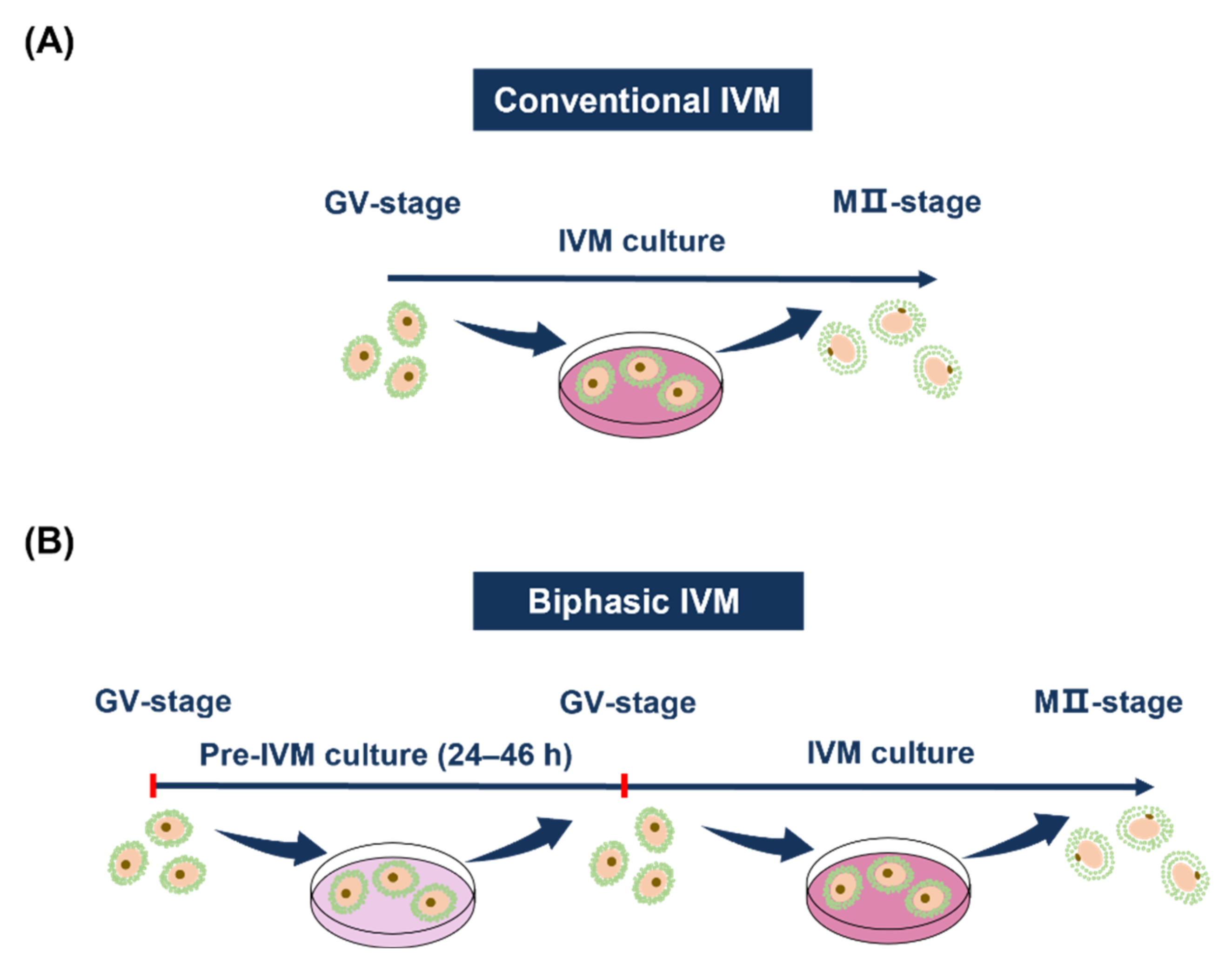
4. Two Kinds of Culturing Methods
5. Time of Oocyte Retrieval
6. Time of In Vitro Culture
7. Woman’s Age
8. Cryopreservation and Other Physical Factors
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pincus, G.; Saunders, B. Unfertilized human tubal ova. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1937, 69, 163–169. [Google Scholar] [CrossRef]
- Pincus, G.; Enzmann, E.V. The comparative behavior of mammalian eggs in vivo and in vitro. II. The activation of tubal eggs of the rabbit. J. Exp. Zool. 1936, 73, 195–208. [Google Scholar] [CrossRef]
- Rock, J.; Menkin, M.F. In Vitro Fertilization and Cleavage of Human Ovarian Eggs. Science 1944, 100, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Menkin, M.F.; Rock, J. In Vitro Fertilization and Cleavage of Human Ovarian Eggs. Am. J. Obstet. Gynecol. 1948, 55, 440–452. [Google Scholar] [CrossRef]
- Edwards, R.G. Maturation in vitro of Mouse, Sheep, Cow, Pig, Rhesus Monkey and Human Ovarian Oocytes. Nat. Cell Biol. 1965, 208, 349–351. [Google Scholar] [CrossRef]
- Edwards, R. Maturation in vitro of human ovarian oocytes. Lancet 1965, 286, 926–929. [Google Scholar] [CrossRef]
- Cha, K.Y.; Koo, J.J.; Ko, J.J.; Choi, D.H.; Han, S.Y.; Yoon, T.K. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. Fertil. Steril. 1991, 55, 109–113. [Google Scholar] [CrossRef]
- Chian, R.-C.; Uzelac, P.S.; Nargund, G. In vitro maturation of human immature oocytes for fertility preservation. Fertil. Steril. 2013, 99, 1173–1181. [Google Scholar] [CrossRef]
- Cekleniak, N.A.; Combelles, C.M.; Ganz, D.A.; Fung, J.; Albertini, D.F.; Racowsky, C. A novel system for in vitro maturation of human oocytes. Fertil. Steril. 2001, 75, 1185–1193. [Google Scholar] [CrossRef]
- Mostinckx, L.; Segers, I.; Belva, F.; Buyl, R.; Santos-Ribeiro, S.; Blockeel, C.; Smitz, J.; Anckaert, E.; Tournaye, H.; De Vos, M. Obstetric and neonatal outcome of ART in patients with polycystic ovary syndrome: IVM of oocytes versus controlled ovarian stimulation. Hum. Reprod. 2019, 34, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Belva, F.; Roelants, M.; Vermaning, S.; Desmyttere, S.; De Schepper, J.; Bonduelle, M.; Tournaye, H.; Hes, F.; De Vos, M. Growth and other health outcomes of 2-year-old singletons born after IVM versus controlled ovarian stimulation in mothers with polycystic ovary syndrome. Hum. Reprod. Open 2020, 2020, hoz043. [Google Scholar] [CrossRef] [PubMed]
- Söderström-Anttila, V.; Salokorpi, T.; Pihlaja, M.; Serenius-Sirve, S.; Suikkari, A.-M. Obstetric and perinatal outcome and preliminary results of development of children born after in vitro maturation of oocytes. Hum. Reprod. 2006, 21, 1508–1513. [Google Scholar] [CrossRef][Green Version]
- Buckett, W.M.; Chian, R.C.; Holzer, H.; Dean, N.; Usher, R.; Tan, S.L. Obstetric Outcomes and Congenital Abnormalities After In Vitro Maturation, In Vitro Fertilization, and Intracytoplasmic Sperm Injection. Obstet. Gynecol. 2007, 110, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Roesner, S.; von Wolff, M.; Elsaesser, M.; Roesner, K.; Reuner, G.; Pietz, J.; Bruckner, T.; Strowitzki, T. Two-year development of children conceived by IVM: A prospective controlled single-blinded study. Hum. Reprod. 2017, 32, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.J.; Yoon, T.K.; Lee, W.S.; Park, E.A.; Heo, J.Y.; Ko, Y.K.; Kim, J. Obstetrical, neonatal, and long-term outcomes of children conceived from in vitro matured oocytes. Fertil. Steril. 2019, 112, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, P.; Yuan, P.; Qiu, Q.; Yang, D. Successful live birth in a woman with resistant ovary syndrome following in vitro maturation of oocytes. J. Ovarian Res. 2016, 9, 54. [Google Scholar] [CrossRef]
- Fesahat, F.; Kalantar, S.M.; Sheikhha, M.H.; Saeedi, H.; Montazeri, F.; Firouzabadi, R.D.; Khalili, M.A. Developmental and cytogenetic assessments of preimplantation embryos derived from in-vivo or in-vitro matured human oocytes. Eur. J. Med Genet. 2018, 61, 235–241. [Google Scholar] [CrossRef] [PubMed]
- De Vos, M.; Grynberg, M.; Ho, T.M.; Yuan, Y.; Albertini, D.F.; Gilchrist, R.B. Perspectives on the development and future of oocyte IVM in clinical practice. J. Assist. Reprod. Genet. 2021, 38, 1265–1280. [Google Scholar] [CrossRef]
- Ao, A.; Jin, S.; Rao, D.; Son, W.-Y.; Chian, R.-C.; Tan, S.L. First successful pregnancy outcome after preimplantation genetic diagnosis for aneuploidy screening in embryos generated from natural-cycle in vitro fertilization combined with an in vitro maturation procedure. Fertil. Steril. 2006, 85, 1510.e9–1510.e11. [Google Scholar] [CrossRef] [PubMed]
- Walls, M.L.; Hart, R.J. In vitro maturation. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 53, 60–72. [Google Scholar] [CrossRef]
- Practice Committees of the American Society for Reproductive Medicine, the Society of Reproductive Biologists and Technologists, and the Society for Assisted Reproductive Technology. In vitro maturation: A committee opinion. Fertil. Steril. 2021, 115, 298–304. [Google Scholar] [CrossRef]
- The Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. In vitro maturation: A committee opinion. Fertil. Steril. 2013, 99, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Coticchio, G. IVM in need of clear definitions. Hum. Reprod. 2016, 31, 1387–1389. [Google Scholar] [CrossRef][Green Version]
- De Vos, M.; Smitz, J.; Thompson, J.G.; Gilchrist, R.B. The definition of IVM is clear—Variations need defining. Hum. Reprod. 2016, 31, 2411–2415. [Google Scholar] [CrossRef] [PubMed]
- Chian, R.-C.; Buckett, W.M.; Too, L.-L.; Tan, S.-L. Pregnancies resulting from in vitro matured oocytes retrieved from patients with polycystic ovary syndrome after priming with human chorionic gonadotropin. Fertil. Steril. 1999, 72, 639–642. [Google Scholar] [CrossRef]
- Wynn, P.; Picton, H.M.; Krapez, J.A.; Rutherford, A.J.; Balen, A.H.; Gosden, R.G. Pretreatment with follicle stimulating hor-mone promotes the numbers of human oocytes reaching metaphase II by in-vitro maturation. Hum. Reprod. 1998, 13, 3132–3138. [Google Scholar] [CrossRef] [PubMed]
- Fadini, R.; Canto, M.D.; Renzini, M.M.; Brambillasca, F.; Comi, R.; Fumagalli, D.; Lain, M.; Merola, M.; Milani, R.; De Ponti, E. Effect of different gonadotrophin priming on IVM of oocytes from women with normal ovaries: A prospective randomized study. Reprod. Biomed. Online 2009, 19, 343–351. [Google Scholar] [CrossRef]
- Sacha, C.R.; Kaser, D.J.; Farland, L.; Srouji, S.; Missmer, S.A.; Racowsky, C. The effect of short-term exposure of cumulus-oocyte complexes to in vitro maturation medium on yield of mature oocytes and usable embryos in stimulated cycles. J. Assist. Reprod. Genet. 2018, 35, 841–849. [Google Scholar] [CrossRef]
- Mikkelsen, A.L.; Smith, S.D.; Lindenberg, S. In-vitro maturation of human oocytes from regularly menstruating women may be successful without follicle stimulating hormone priming. Hum. Reprod. 1999, 14, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Chian, R.; Buckett, W.; Tulandi, T.; Tan, S. Prospective randomized study of human chorionic gonadotrophin priming before immature oocyte retrieval from unstimulated women with polycystic ovarian syndrome. Hum. Reprod. 2000, 15, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hwang, J.; Huang, L.; Mu, S.; Seow, K.; Chung, J.; Hsieh, B.; Huang, S.; Chen, C.; Chen, P. Combination of FSH priming and hCG priming for in-vitro maturation of human oocytes. Hum. Reprod. 2003, 18, 1632–1636. [Google Scholar] [CrossRef]
- Mikkelsen, A.L.; Lindenberg, S. Benefit of FSH priming of women with PCOS to the in vitro maturation procedure and the outcome: A randomized prospective study. Reproduction 2001, 122, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Licht, P.; Fluhr, H.; Neuwinger, J.; Wallwiener, D.; Wildt, L. Is human chorionic gonadotropin directly involved in the regu-lation of human implantation? Mol. Cell. Endocrinol. 2007, 269, 85–92. [Google Scholar] [CrossRef] [PubMed]
- De Vos, M.; Ortega-Hrepich, C.; Albuz, F.K.; Guzman, L.; Polyzos, N.P.; Smitz, J.; Devroey, P. Clinical outcome of non–hCG-primed oocyte in vitro maturation treatment in patients with polycystic ovaries and polycystic ovary syndrome. Fertil. Steril. 2011, 96, 860–864.e1. [Google Scholar] [CrossRef]
- Brackett, B.G.; Zuelke, K.A. Analysis of factors involved in the in vitro production of bovine embryos. Theriogenology 1993, 39, 43–64. [Google Scholar] [CrossRef]
- Trounson, A.; Anderiesz, C.; Jones, G. Maturation of human oocytes in vitro and their developmental competence. Reproduction 2001, 121, 51–75. [Google Scholar] [CrossRef]
- Kim, M.; Hong, S.J.; Lee, J.H.; Min, C.K.; Hwang, K.J.; Park, R.W. Comparison of in vitro maturation media of immature oocytes: The effectiveness of blastocyst culture media. Fertil. Steril. 2011, 95, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Son, W.-Y.; Tan, S.L. Laboratory and embryological aspects of hCG-primed in vitro maturation cycles for patients with poly-cystic ovaries. Hum. Reprod. Update 2010, 16, 675–689. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, C.H.M.; Nogueira, D.; De Araujo, M.C.P.M.; Martins, W.D.P.; Ferriani, R.A.; Dos Reis, R.M. Supplemented tissue culture medium 199 is a better medium for in vitro maturation of oocytes from women with polycystic ovary syndrome women than human tubal fluid. Fertil. Steril. 2009, 91, 509–513. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Yu, Q.; Liu, H.; Huang, T.; Zhao, S.; Ma, J.; Zhao, H. Growth Hormone Promotes in vitro Maturation of Human Oocytes. Front. Endocrinol. 2019, 10, 485. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Wu, K.; Liu, H.; Huang, T.; Chen, Z.-J.; Zhao, S.; Ma, J.; Zhao, H. Melatonin promotes human oocyte maturation and early embryo development by enhancing clathrin-mediated endocytosis. J. Pineal Res. 2019, 67, e12601. [Google Scholar] [CrossRef]
- Goud, P.T.; Goud, A.P.; Qian, C.; Laverge, H.; Van Der Elst, J.; De Sutter, P.; Dhont, M. In-vitro maturation of human germinal vesicle stage oocytes: Role of cumulus cells and epidermal growth factor in the culture medium. Hum. Reprod. 1998, 13, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Bayne, R.A.; Gardner, J.; De Sousa, P.A. Brain-derived neurotrophic factor is a regulator of human oocyte maturation and early embryo development. Fertil. Steril. 2010, 93, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhang, Z.; Han, D.; Cao, Y.; Zhou, P.; Wei, Z.; Lv, M.; Chen, D. Gene expression profiling of human blastocysts from in vivo and ‘rescue IVM’ with or without melatonin treatment. Mol. Med. Rep. 2017, 16, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhao, H.; Wang, Z.; Zhang, C.; Bian, Y.; Liu, X.; Zhang, C.; Zhang, X.; Zhao, Y. Quercetin promotes in vitro maturation of oocytes from humans and aged mice. Cell Death Dis. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-J.; Sun, A.-G.; Zhao, S.-G.; Liu, H.; Ma, S.-Y.; Li, M.; Huai, Y.-X.; Zhao, H.; Liu, H. Resveratrol improves in vitro maturation of oocytes in aged mice and humans. Fertil. Steril. 2018, 109, 900–907. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, S.-B.; Song, X.-B.; Deng, T.-F.; Zhang, T.-T.; Yin, S.; Luo, S.-M.; Shen, W.; Zhang, C.-L.; Ge, Z.-J. High-glucose concentrations change DNA methylation levels in human IVM oocytes. Hum. Reprod. 2018, 33, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Vutyavanich, P.P.T. Comparison of Medicult and Sage Media for In Vitro Maturation of Immature Oocytes Obtained during Cesarean Deliveries. J. Fertil. Vitr. IVF-Worldwide Reprod. Med. Genet. Stem Cell Biol. 2014, 3, 1000136. [Google Scholar] [CrossRef]
- Filali, M.; Hesters, L.; Fanchin, R.; Tachdjian, G.; Frydman, R.; Frydman, N. Retrospective comparison of two media for invitro maturation of oocytes. Reprod. Biomed. Online 2008, 16, 250–256. [Google Scholar] [CrossRef]
- Pongsuthirak, P.; Songveeratham, S.; Vutyavanich, T. Comparison of Blastocyst and Sage Media for In Vitro Maturation of Human Immature Oocytes. Reprod. Sci. 2014, 22, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Fesahat, F.; Faramarzi, A.; Sheikhha, M.H.; Firouzabadi, R.D.; Khalili, M.A. Comparing the effects of different in vitro matu-ration media on IVM outcomes of MI oocytes. Middle East Fertil. Soc. J. 2017, 22, 174–177. [Google Scholar] [CrossRef]
- Fesahat, F.; Firouzabadi, R.D.; Faramarzi, A.; Khalili, M.A. The effects of different types of media on in vitro maturation out-comes of human germinal vesicle oocytes retrieved in intracytoplasmic sperm injection cycles. Clin. Exp. Reprod. Med. 2017, 44, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Moschini, R.M.; Chuang, L.; Poleshchuk, F.; Slifkin, R.E.; Copperman, A.B.; Barritt, J. Commercially available enhanced in vitro maturation medium does not improve maturation of germinal vesicle and metaphase I oocytes in standard in vitro fertilization cases. Fertil. Steril. 2011, 95, 2645–2647. [Google Scholar] [CrossRef] [PubMed]
- Jee, B.C.; Han, S.H.; Moon, J.H.; Suh, C.S.; Kim, S.H. Influence of well defined protein source on in vitro maturation of human oocyte: Human follicular fluid versus human serum albumin. Fertil. Steril. 2008, 89, 348–352. [Google Scholar] [CrossRef]
- Cha, K.; Do, B.; Chi, H.; Yoon, T.; Choi, D.; Koo, J.; Ko, J. Viability of Human Follicular Oocytes Collected from Unstimulated Ovaries and Matured and Fertilized in vitro. Reprod. Fertil. Dev. 1992, 4, 695–701. [Google Scholar] [CrossRef]
- Chatroudi, M.H.; Khalili, M.A.; Ashourzadeh, S.; Anbari, F.; Shahedi, A.; Safari, S. Growth differentiation factor 9 and cumulus cell supplementation in in vitro maturation culture media enhances the viability of human blastocysts. Clin. Exp. Reprod. Med. 2019, 46, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, I.; Komsky, A.; Bern, O.; Kasterstein, E.; Komarovsky, D.; Ron-El, R. In vitro maturation of human germinal vesicle-stage oocytes: Role of epidermal growth factor-like growth factors in the culture medium. Hum. Reprod. 2011, 26, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, A.; Høst, E.; Blaabjerg, J.; Lindenberg, S. Maternal serum supplementation in culture medium benefits maturation of immature human oocytes. Reprod. Biomed. Online 2001, 3, 112–116. [Google Scholar] [CrossRef]
- Ge, H.-S.; Huang, X.-F.; Zhang, W.; Zhao, J.-Z.; Lin, J.-J.; Zhou, W. Exposure to human chorionic gonadotropin during in vitro maturation does not improve the maturation rate and developmental potential of immature oocytes from patients with poly-cystic ovary syndrome. Fertil. Steril. 2008, 89, 98–103. [Google Scholar] [CrossRef]
- Zhao, P.; Qiao, J.; Huang, S.; Zhang, Y.; Liu, S.; Yan, L.-Y.; Hsueh, A.J.; Duan, E.-K. Gonadotrophin-induced paracrine regu-lation of human oocyte maturation by BDNF and GDNF secreted by granulosa cells. Hum. Reprod. 2011, 26, 695–702. [Google Scholar] [CrossRef]
- Ye, J.; Campbell, K.; Craigon, J.; Luck, M. Dynamic Changes in Meiotic Progression and Improvement of Developmental Competence of Pig Oocytes in Vitro by Follicle-Stimulating Hormone and Cycloheximide1. Biol. Reprod. 2005, 72, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, A.; Smith, S.; Lindenberg, S. Impact of oestradiol and inhibin A concentrations on pregnancy rate in in-vitro oocyte maturation. Hum. Reprod. 2000, 15, 1685–1690. [Google Scholar] [CrossRef]
- Junk, S.M.; Yeap, D. Improved implantation and ongoing pregnancy rates after single-embryo transfer with an optimized protocol for in vitro oocyte maturation in women with polycystic ovaries and polycystic ovary syndrome. Fertil. Steril. 2012, 98, 888–892. [Google Scholar] [CrossRef]
- Le Du, A.; Kadoch, I.; Bourcigaux, N.; Doumerc, S.; Bourrier, M.-C.; Chevalier, N.; Fanchin, R.; Chian, R.-C.; Tachdjian, G.; Frydman, N.A. In vitro oocyte maturation for the treatment of infertility associated with polycystic ovarian syndrome: The French experience. Hum. Reprod. 2005, 20, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, J.; Li, M.; Yan, L.; Zhao, Y.; Lian, Y.; Li, R.; Liu, P.; Qiao, J. Effects of combined epidermal growth factor, brain-derived neurotrophic factor and insulin-like growth factor-1 on human oocyte maturation and early fertilized and cloned embryo development. Hum. Reprod. 2012, 27, 2146–2159. [Google Scholar] [CrossRef] [PubMed]
- Ashourzadeh, S.; Khalili, M.A.; Omidi, M.; A Nottola, S.; Faramarzi, A. Supplementation of IVM culture media with GDF-9 enhanced oocyte quality, fertilization and embyo development in ICSI program. Cent. Asian J. Med Pharm. Sci. Innov. 2021, 1, 44–54. [Google Scholar]
- Virant-Klun, I.; Bauer, C.; Ståhlberg, A.; Kubista, M.; Skutella, T. Human oocyte maturation in vitro is improved by co-culture with cumulus cells from mature oocytes. Reprod. Biomed. Online 2018, 36, 508–523. [Google Scholar] [CrossRef]
- Hreinsson, J.; Rosenlund, B.; Fridén, B.; Levkov, L.; Ek, I.; Suikkari, A.-M.; Hovatta, O.; Fridström, M. Recombinant LH is equally effective as recombinant hCG in promoting oocyte maturation in a clinical in-vitro maturation programme: A randomized study. Hum. Reprod. 2003, 18, 2131–2136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dinopoulou, V.; Drakakis, P.; Kefala, S.; Kiapekou, E.; Bletsa, R.; Anagnostou, E.; Kallianidis, K.; Loutradis, D. Effect of re-combinant-LH and hCG in the absence of FSH on in vitro maturation (IVM) fertilization and early embryonic development of mouse germinal vesicle (GV)-stage oocytes. Reprod. Biol. 2016, 16, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.M. Regulation of the G2/M transition in rodent oocytes. Mol. Reprod. Dev. 2010, 77, 566–585. [Google Scholar] [CrossRef] [PubMed]
- Albertini, D.F.; Sanfins, A.; Combelles, C.M. Origins and manifestations of oocyte maturation competencies. Reprod. Biomed. Online 2003, 6, 410–415. [Google Scholar] [CrossRef]
- Gilchrist, R.B. Recent insights into oocyte—Follicle cell interactions provide opportunities for the development of new ap-proaches to in vitro maturation. Reprod. Fertil. Dev. 2011, 23, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Maman, E.; Yung, Y.; Kedem, A.; Yerushalmi, G.M.; Konopnicki, S.; Cohen, B.; Dor, J.; Hourvitz, A. High expression of lute-inizing hormone receptors messenger RNA by human cumulus granulosa cells is in correlation with decreased fertilization. Fertil. Steril. 2012, 97, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, L.A.; Norris, R.P. Initiation of the Meiotic Prophase-to-Metaphase Transition in Mammalian Oocytes. In Oogenesis; Wiley: Hoboken, NJ, USA, 2010; pp. 179–197. [Google Scholar]
- Lim, J.M.; Lee, B.C.; Lee, E.S.; Chung, H.M.; Ko, J.J.; Park, S.E.; Cha, K.Y.; Hwang, W.S. In vitro maturation and in vitro fertilization of bovine oocytes cultured in a chemically defined, protein-free medium: Effects of carbohydrates and amino acids. Reprod. Fertil. Dev. 1999, 11, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Cetica, P.; Pintos, L.; Dalvit, G.; Beconi, M. Effect of lactate dehydrogenase activity and isoenzyme localization in bovine oocytes and utilization of oxidative substrates on in vitro maturation. Theriogenology 1999, 51, 541–550. [Google Scholar] [CrossRef]
- Nandi, S.; Kumar, V.G.; Manjunatha, B.; Ramesh, H.; Gupta, P. Follicular fluid concentrations of glucose, lactate and pyruvate in buffalo and sheep, and their effects on cultured oocytes, granulosa and cumulus cells. Theriogenology 2008, 69, 186–196. [Google Scholar] [CrossRef]
- Leese, H.; Lenton, E. Glucose and lactate in human follicular fluid: Concentrations and interrelationships. Hum. Reprod. 1990, 5, 915–919. [Google Scholar] [CrossRef]
- Eppig, J.J.; Pendola, F.L.; Wigglesworth, K.; Pendola, J.K. Mouse Oocytes Regulate Metabolic Cooperativity Between Granulosa Cells and Oocytes: Amino Acid Transport. Biol. Reprod. 2005, 73, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Cai, L.; Hu, M.; Wang, J.; Xie, J.; Xing, Y.; Shen, J.; Cui, Y.; Liu, X.J.; Liu, J. Coenzyme Q10 supplementation of human oocyte in vitro maturation reduces postmeiotic aneuploidies. Fertil. Steril. 2020, 114, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kuhn, C.; Kolben, T.; Ma, Z.; Lin, P.; Mahner, S.; Jeschke, U.; Von Schönfeldt, V. Early Life Oxidative Stress and Long-Lasting Cardiovascular Effects on Offspring Conceived by Assisted Reproductive Technologies: A Review. Int. J. Mol. Sci. 2020, 21, 5175. [Google Scholar] [CrossRef]
- Nyga, A.; Loizidou, M.; Emberton, M.; Cheema, U. A novel tissue engineered three-dimensional in vitro colorectal cancer model. Acta Biomater. 2013, 9, 7917–7926. [Google Scholar] [CrossRef]
- Torre, M.L.; Munari, E.; Albani, E.; Levi-Setti, P.E.; Villani, S.; Faustini, M.; Conte, U.; Vigo, D. In vitro maturation of human oocytes in a follicle-mimicking three-dimensional coculture. Fertil. Steril. 2006, 86, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Combelles, C.M.; Fissore, R.A.; Albertini, D.F.; Racowsky, C. In vitro maturation of human oocytes and cumulus cells using a co-culture three-dimensional collagen gel system. Hum. Reprod. 2005, 20, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, L.; Nogueira, D.; Dumortier, F.; De Sutter, P. Assessment of a new in vitro maturation system for mouse and human cumulus-enclosed oocytes: Three-dimensional prematuration culture in the presence of a phosphodiesterase 3-inhibitor. Hum. Reprod. 2009, 24, 1946–1959. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, L.; Nogueira, D.; De Sutter, P. Prematuration of human denuded oocytes in a three-dimensional co-culture system: Effects on meiosis progression and developmental competence. Hum. Reprod. 2008, 24, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadeh, M.; Tabibnejad, N.; Vatanparast, M.; Anbari, F.; Khalili, M.A.; Karimi-Zarchi, M. Vitrification has detrimental effects on maturation, viability, and subcellular quality of oocytes post IVM in cancerous women: An experimental study. Int. J. Reprod. Biomed. 2019, 17, 167–176. [Google Scholar] [CrossRef]
- Cui, L.; Fang, L.; Mao, X.; Chang, H.-M.; Leung, P.C.K.; Ye, Y. GDNF-Induced Downregulation of miR-145-5p Enhances Human Oocyte Maturation and Cumulus Cell Viability. J. Clin. Endocrinol. Metab. 2018, 103, 2510–2521. [Google Scholar] [CrossRef]
- Sánchez, F.; Lolicato, F.; Romero, S.; De Vos, M.; Van Ranst, H.; Verheyen, G.; Anckaert, E.; Smitz, J. An improved IVM method for cumulus-oocyte complexes from small follicles in polycystic ovary syndrome patients enhances oocyte competence and embryo yield. Hum. Reprod. 2017, 32, 2056–2068. [Google Scholar] [CrossRef]
- Xie, Q.; Xing, Y.; Zhou, J.; Wang, L.; Wu, J.; Chian, R.-C. The effect of lysophosphatidic acid-supplemented culture medium on human immature oocytes matured in vitro. Reprod. Biol. Endocrinol. 2021, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Madkour, A.; Bouamoud, N.; Kaarouch, I.; Louanjli, N.; Saadani, B.; Assou, S.; Aboulmaouahib, S.; Sefrioui, O.; Amzazi, S.; Copin, H.; et al. Follicular fluid and supernatant from cultured cumulus-granulosa cells improve in vitro maturation in patients with polycystic ovarian syndrome. Fertil. Steril. 2018, 110, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.-T.; Ren, Z.; Guzman, L.; Wang, X.; Sutton-McDowall, M.L.; Ritter, L.J.; De Vos, M.; Smitz, J.; Thompson, J.G.; Gilchrist, R.B. Heparin and cAMP modulators interact during pre-in vitro maturation to affect mouse and human oocyte meiosis and developmental competence. Hum. Reprod. 2013, 28, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Machtinger, R.; Combelles, C.M.; Missmer, S.A.; Correia, K.F.; Williams, P.; Hauser, R.; Racowsky, C. Bisphenol-A and human oocyte maturation in vitro. Hum. Reprod. 2013, 28, 2735–2745. [Google Scholar] [CrossRef] [PubMed]
- Leon, P.; Campos, V.; Kaefer, C.; Begnini, K.; McBride, A.; Dellagostin, O.; Seixas, F.; Deschamps, J.; Collares, T. Expression of apoptotic genes in immature and in vitro matured equine oocytes and cumulus cells. Zygote 2013, 21, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Coticchio, G.; Canto, M.D.; Renzini, M.M.; Guglielmo, M.C.; Brambillasca, F.; Turchi, D.; Novara, P.V.; Fadini, R. Oocyte maturation: Gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Reprod. Update 2015, 21, 427–454. [Google Scholar] [CrossRef]
- Brown, H.M.; Dunning, K.R.; Sutton-McDowall, M.; Gilchrist, R.B.; Thompson, J.G.; Russell, D.L.; Gilchrist, R.B. Failure to launch: Aberrant cumulus gene expression during oocyte in vitro maturation. Reproduction 2017, 153, R109–R120. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Luciano, A.M.; Richani, D.; Zeng, H.T.; Wang, X.; De Vos, M.; Sugimura, S.; Smitz, J.; Richard, F.J.; Thompson, J.G. Oocyte maturation and quality: Role of cyclic nucleotides. Reproduction 2016, 152, R143–R157. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, H.; Cantley, T.C.; Day, B.N. Synchronization of Meiosis in Porcine Oocytes by Exposure to Dibutyryl Cyclic Adenosine Monophosphate Improves Developmental Competence Following in Vitro Fertilization1. Biol. Reprod. 1997, 57, 49–53. [Google Scholar] [CrossRef]
- Luciano, A.M.; Pocar, P.; Milanesi, E.; Modina, S.; Rieger, D.; Lauria, A.; Gandolfi, F. Effect of different levels of intracellular cAMP on the in vitro maturation of cattle oocytes and their subsequent development following in vitro fertilization. Mol. Reprod. Dev. 1999, 54, 86–91. [Google Scholar] [CrossRef]
- Huang, W.; Nagano, M.; Kang, S.-S.; Yanagawa, Y.; Takahashi, Y. Effects of in vitro growth culture duration and prematuration culture on maturational and developmental competences of bovine oocytes derived from early antral follicles. Theriogenology 2013, 80, 793–799. [Google Scholar] [CrossRef]
- Franciosi, F.; Coticchio, G.; Lodde, V.; Tessaro, I.; Modina, S.; Fadini, R.; Canto, M.D.; Renzini, M.M.; Albertini, D.F.; Luciano, A.M. Natriuretic Peptide Precursor C Delays Meiotic Resumption and Sustains Gap Junction-Mediated Communication in Bovine Cumulus-Enclosed Oocytes1. Biol. Reprod. 2014, 91, 61. [Google Scholar] [CrossRef]
- Romero, S.; Sánchez, F.; Lolicato, F.; Van Ranst, H.; Smitz, J. Immature Oocytes from Unprimed Juvenile Mice Become a Valuable Source for Embryo Production When Using C-Type Natriuretic Peptide as Essential Component of Culture Medium. Biol. Reprod. 2016, 95, 64. [Google Scholar] [CrossRef]
- Zhao, Y.; Liao, X.; Krysta, A.; Bertoldo, M.; Richani, D.; Gilchrist, R. Capacitation IVM improves cumulus function and oocyte quality in minimally stimulated mice. J. Assist. Reprod. Genet. 2020, 37, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wei, Q.; Cai, J.; Zhao, X.; Ma, B. Effect of C-Type Natriuretic Peptide on Maturation and Developmental Competence of Goat Oocytes Matured In Vitro. PLoS ONE 2015, 10, e0132318. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, C.; Fan, X.; Li, R.; Zhang, J. Effect of C-type natriuretic peptide pretreatment on in vitro bovine oocyte maturation. Vitr. Cell. Dev. Biol. Anim. 2017, 53, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, F.; Le, A.H.; Ho, V.N.A.; Romero, S.; Van Ranst, H.; De Vos, M.; Gilchrist, R.B.; Ho, T.M.; Vuong, L.N.; Smitz, J. Biphasic in vitro maturation (CAPA-IVM) specifically improves the developmental capacity of oocytes from small antral follicles. J. Assist. Reprod. Genet. 2019, 36, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Vuong, L.N.; Le, A.H.; Ho, V.N.A.; Pham, T.D.; Sánchez, F.; Romero, S.; De Vos, M.; Ho, T.M.; Gilchrist, R.B.; Smitz, J. Live births after oocyte in vitro maturation with a prematuration step in women with polycystic ovary syndrome. J. Assist. Reprod. Genet. 2020, 37, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Bunyaeva, E.; Van Ranst, H.; Khabas, G.; Farmakovskaya, M.; Kamaletdinov, N.; Nazarenko, T.; Abubakirov, A.; Sukhikh, G.; Smitz, J.E.J. Improved maturation competence of ovarian tissue oocytes using a biphasic in vitro maturation system for patients with gynecological malignancy: A study on sibling oocytes. J. Assist. Reprod. Genet. 2021, 38, 1331–1340. [Google Scholar] [CrossRef]
- Zhang, M.; Su, Y.-Q.; Sugiura, K.; Xia, G.; Eppig, J.J. Granulosa Cell Ligand NPPC and Its Receptor NPR2 Maintain Meiotic Arrest in Mouse Oocytes. Science 2010, 330, 366–369. [Google Scholar] [CrossRef]
- Jaffe, L.A.; Egbert, J.R. Regulation of Mammalian Oocyte Meiosis by Intercellular Communication Within the Ovarian Follicle. Annu. Rev. Physiol. 2017, 79, 237–260. [Google Scholar] [CrossRef]
- Geister, K.A.; Brinkmeier, M.L.; Hsieh, M.; Faust, S.M.; Karolyi, I.J.; Perosky, J.E.; Kozloff, K.M.; Conti, M.; Camper, S.A. A novel loss-of-function mutation in Npr2 clarifies primary role in female reproduction and reveals a potential therapy for ac-romesomelic dysplasia, Maroteaux type. Hum. Mol. Genet. 2013, 22, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Kiyosu, C.; Akiyama, K.; Kunieda, T. CNP/NPR2 signaling maintains oocyte meiotic arrest in early antral follicles and is suppressed by EGFR-mediated signaling in preovulatory follicles. Mol. Reprod. Dev. 2012, 79, 795–802. [Google Scholar] [CrossRef]
- Hiradate, Y.; Hoshino, Y.; Tanemura, K.; Sato, E. C-type natriuretic peptide inhibits porcine oocyte meiotic resumption. Zygote 2014, 22, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, Y.; Liu, W.; Chen, Q.; Wang, H.; Wang, X.; Zhang, Y.; Zhang, M.; Xia, G. Brain Natriuretic Peptide and C-Type Natriuretic Peptide Maintain Porcine Oocyte Meiotic Arrest. J. Cell. Physiol. 2015, 230, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Lin, J.; Liu, X.; Hou, J.; Zhang, Y.; Zhao, X. C-Type natriuretic peptide maintains domestic cat oocytes in meiotic arrest. Reprod. Fertil. Dev. 2016, 28, 1553. [Google Scholar] [CrossRef] [PubMed]
- Cavilla, J.; Byskov, A.; Hartshorne, G.; Kennedy, C. Human immature oocytes grow during culture for IVM. Hum. Reprod. 2007, 23, 37–45. [Google Scholar] [CrossRef]
- Sánchez, F.; Romero, S.; De Vos, M.; Verheyen, G.; Smitz, J. Human cumulus-enclosed germinal vesicle oocytes from early antral follicles reveal heterogeneous cellular and molecular features associated with in vitro maturation capacity. Hum. Reprod. 2015, 30, 1396–1409. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.; Pella, R.; Escudero, F.; Pérez, Y.; García, M.; Orihuela, P. Occurrence of ovarian follicular dominance during stimulation for IVM impacts usable blastocyst yield. JBRA Assist. Reprod. 2018, 22, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Shalom-Paz, E.; Holzer, H.E.G.; Son, W.-Y.; Levin, I.; Tan, S.L.; Almog, B. PCOS patients can benefit from in vitro maturation (IVM) of oocytes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 165, 53–56. [Google Scholar] [CrossRef]
- Hatırnaz, Ş.; Ata, B.; Hatırnaz, E.S.; Dahan, M.; Tannus, S.; Tan, J.; Tan, S.L. Oocyte in vitro maturation: A sytematic review. J. Turk. Soc. Obstet. Gynecol. 2018, 15, 112–125. [Google Scholar] [CrossRef]
- Son, W.-Y.; Chung, J.-T.; Herrero, B.; Dean, N.; Demirtas, E.; Holzer, H.E.G.; Elizur, S.; Chian, R.-C.; Tan, S.L. Selection of the optimal day for oocyte retrieval based on the diameter of the dominant follicle in hCG-primed in vitro maturation cycles. Hum. Reprod. 2008, 23, 2680–2685. [Google Scholar] [CrossRef][Green Version]
- Cobo, A.C.; Requena, A.; Neuspiller, F.; Aragonés, M.; Mercader, A.; Navarro, J.; Simón, C.; Remohí, J.; Pellicer, A. Maturation in vitro of human oocytes from unstimulated cycles: Selection of the optimal day for ovum retrieval based on follicular size. Hum. Reprod. 1999, 14, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Son, W.-Y.; Yoon, S.-H.; Lim, J.-H. Effect of gonadotrophin priming on in-vitro maturation of oocytes collected from women at risk of OHSS. Reprod. Biomed. Online 2006, 13, 340–348. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Y.; Zheng, X.; Song, X.; Yang, R.; Yan, J.; Feng, H.; Qiao, J. Current perspectives on in vitro maturation and its effects on oocyte genetic and epigenetic profiles. Sci. China Life Sci. 2018, 61, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Cao, C.; Zhang, M.; Fang, F.; Liu, Y.; Zhang, Y.; Ding, J.; Zhang, X. Effects of cumulus cells on rabbit oocyte in vitro-maturation. J. Anim. Physiol. Anim. Nutr. 2008, 92, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Heinzmann, J.; Mattern, F.; Aldag, P.; Bernal-Ulloa, S.M.; Schneider, T.; Haaf, T.; Niemann, H. Extended in vitro maturation affects gene expression and DNA methylation in bovine oocytes. Mol. Hum. Reprod. 2015, 21, 770–782. [Google Scholar] [CrossRef]
- Segers, I.; Mateizel, I.; Van Moer, E.; Smitz, J.; Tournaye, H.; Verheyen, G.; De Vos, M. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: A promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J. Assist. Reprod. Genet. 2015, 32, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Combelles, C.; Cekleniak, N.; Racowsky, C.; Albertini, D. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum. Reprod. 2002, 17, 1006–1016. [Google Scholar] [CrossRef]
- Navot, D.; Bergh, R.; Williams, M.; Garrisi, G.; Guzman, I.; Sandler, B.; Grunfeld, L. Poor oocyte quality rather than implan-tation failure as a cause of age-related decline in female fertility. Lancet 1991, 337, 1375–1377. [Google Scholar] [CrossRef]
- Marangos, P.; Stevense, M.; Niaka, K.; Lagoudaki, M.; Nabti, I.; Jessberger, R.; Carroll, J. DNA damage-induced metaphase I arrest is mediated by the spindle assembly checkpoint and maternal age. Nat. Commun. 2015, 6, 8706. [Google Scholar] [CrossRef] [PubMed]
- Selesniemi, K.; Lee, H.-J.; Muhlhauser, A.; Tilly, J.L. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc. Natl. Acad. Sci. USA 2011, 108, 12319–12324. [Google Scholar] [CrossRef] [PubMed]
- Valeri, C.; Pappalardo, S.; De Felici, M.; Manna, C. Correlation of oocyte morphometry parameters with woman’s age. J. Assist. Reprod. Genet. 2011, 28, 545–552. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Omidi, M.; Khalili, M.A.; Nahangi, H.; Ashourzadeh, S.; Rahimipour, M. Does women’s age influence zona pellucida bire-fringence of metaphase II oocytes in in-vitro maturation program? Int. J. Reprod. Biomed. 2013, 11, 823–828. [Google Scholar]
- Karavani, G.; Wasserzug-Pash, P.; Mordechai-Daniel, T.; Bauman, D.; Klutstein, M.; Imbar, T. Age-Dependent in vitro Maturation Efficacy of Human Oocytes—Is There an Optimal Age? Front. Cell Dev. Biol. 2021, 9, 1638. [Google Scholar] [CrossRef] [PubMed]
- Wasserzug-Pash, P.; Klutstein, M. Epigenetic changes in mammalian gametes throughout their lifetime: The four seasons metaphor. Chromosoma 2019, 128, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.M.; Silva, E.; Chitwood, J.L.; Schoolcraft, W.B.; Krisher, R.L.; Ross, P.J. Differing molecular response of young and advanced maternal age human oocytes to IVM. Hum. Reprod. 2017, 32, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Llonch, S.; Barragán, M.; Nieto, P.; Mallol, A.; Elosua-Bayes, M.; Lorden, P.; Ruiz, S.; Zambelli, F.; Heyn, H.; Vassena, R.; et al. Single human oocyte transcriptome analysis reveals distinct maturation stage-dependent pathways impacted by age. Aging Cell 2021, 20, e13360. [Google Scholar] [CrossRef]
- Anderson, R.A.; McLaughlin, M.; Wallace, W.H.B.; Albertini, D.F.; Telfer, E. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum. Reprod. 2014, 29, 97–106. [Google Scholar] [CrossRef]
- Tucker, M.J.; Wright, G.; Morton, P.C.; Massey, J.B. Birth after cryopreservation of immature oocytes with subsequent in vitro maturation. Fertil. Steril. 1998, 70, 578–579. [Google Scholar] [CrossRef]
- Park, S.-E.; Son, W.-Y.; Lee, S.-H.; Lee, K.-A.; Ko, J.-J.; Cha, K.-Y. Chromosome and spindle configurations of human oocytes matured in vitro after cryopreservation at the germinal vesicle stage. Fertil. Steril. 1997, 68, 920–926. [Google Scholar] [CrossRef]
- Mohsenzadeh, M.; Salehi-Abargouei, A.; Tabibnejad, N.; Karimi-Zarchi, M.; Khalili, M.A. Effect of vitrification on human oocyte maturation rate during in vitro maturation procedure: A systematic review and meta-analysis. Cryobiology 2018, 83, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Blanco, I.; Soto-Heras, S.; Catalá, M.G.; Piras, A.-R.; Izquierdo, D.; Paramio, M.-T. Effect of vitrification of in vitro matured prepubertal goat oocytes on embryo development after parthenogenic activation and intracytoplasmic sperm injection. Cryobiology 2020, 93, 56–61. [Google Scholar] [CrossRef]
- Khazaei, M.; Aghaz, F. Reactive Oxygen Species Generation and Use of Antioxidants during In Vitro Maturation of Oocytes. Int. J. Fertil. Steril. 2017, 11, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, H.; Sakurai, N.; Muto, N. Protection of Porcine Oocytes Against Apoptotic Cell Death Caused by Oxidative Stress During In Vitro Maturation: Role of Cumulus Cells. Biol. Reprod. 2000, 63, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-M.; Hao, H.-S.; Du, W.-H.; Zhao, S.-J.; Wang, H.-Y.; Wang, N.; Wang, D.; Liu, Y.; Qin, T.; Zhu, H.-B. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal Res. 2016, 60, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Fukuda, A.; Murata, Y.; Kikkawa, M.; Oku, H.; Kanaya, H.; Sonoda, M.; Sugihara, K.; Murata, T.; Nagata, F.; et al. Effect of aspiration vacuum on the developmental competence of immature human oocytes retrieved using a 20-gauge needle. Reprod. Biomed. Online 2007, 14, 444–449. [Google Scholar] [CrossRef]
- Yoon, H.-G.; Yoon, S.-H.; Son, W.-Y.; Lee, S.-W.; Park, S.-P.; Im, K.-S.; Lim, J.-H. Clinical Assisted Reproduction: Pregnancies Resulting from In Vitro Matured Oocytes Collected from Women with Regular Menstrual Cycle. J. Assist. Reprod. Genet. 2001, 18, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Minami, N.; Takakura, R.; Yamada, M.; Imai, H.; Kashima, N. Low oxygen tension during in vitro maturation is beneficial for supporting the subsequent development of bovine cumulus-oocyte complexes. Mol. Reprod. Dev. 2000, 57, 353–360. [Google Scholar] [CrossRef]
- Stamperna, K.; Giannoulis, T.; Nanas, I.; Kalemkeridou, M.; Dadouli, K.; Moutou, K.; Amiridis, G.S.; Dovolou, E. Short term temperature elevation during IVM affects embryo yield and alters gene expression pattern in oocytes, cumulus cells and blas-tocysts in cattle. Theriogenology 2020, 156, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-H.; Yoon, S.-H.; Jung, J.-H.; Lim, J.-H.; Ko, Y. Improvement of embryonic development and clinical outcomes of germinal vesicle stage oocytes using a microvibration culture system. Syst. Biol. Reprod. Med. 2019, 65, 333–341. [Google Scholar] [CrossRef]
- Yang, H.; Ma, Z.; Peng, L.; Kuhn, C.; Rahmeh, M.; Mahner, S.; Jeschke, U.; von Schönfeldt, V. Comparison of Histone H3K4me3 between IVF and ICSI Technologies and between Boy and Girl Offspring. Int. J. Mol. Sci. 2021, 22, 8574. [Google Scholar] [CrossRef]

| Study | Follicular Priming Methods | Maturation Rate | Fertilization Rate | Clinical Pregnancy Rate | Participants (Samle Size) | Collected Oocytes (Sampe Size) |
|---|---|---|---|---|---|---|
| Mikkelsen et al., 1999 [29] | No stimulation | 76% | 62% | 3/10 | Normal cycling women (n = 10) | Oocytes for IVM (n = 37) |
| Three days FSH stimulation | 85% | 65% | 2/10 | Normal cycling women (n = 10) | Oocytes for IVM (n = 40) | |
| Fadini et al., 2009 [27] | No stimulation | 48.4% | 77.6% | 15.3% | Normo-ovulatory women (n = 100) | Immature COCs (n = 477) |
| hCG | 57.9% | 71.5% | 7.6% | Normo-ovulatory women (n = 100) | Immature COCs (n = 442) | |
| In vivo matured MII (n = 28) | ||||||
| FSH | 50.8% | 73.0% | 17.3% | Normo-ovulatory women (n = 100) | Immature COCs (n = 461) | |
| hCG+FSH | 77.4%↑ | 73.0% | 29.9%↑ | Normo-ovulatory women (n = 100) | Immature COCs (n = 416) | |
| In vivo matured MII (n = 109) | ||||||
| Mikkelsen et al., 2001 [32] | No stimulation | 44% | 69% | 0 | Women with PCOS (n = 9) | Immature COCs (n = 81) |
| Three days FSH stimulation | 59%↑ | 70% | 29%↑ | Women with PCOS (n = 20) | Immature COCs (n = 156) | |
| Wynn et al., 1998 [26] | No stimulation | 43.5% | N/A | N/A | Women with healthy ovaries (n = 9) | Immature COCs (n = 46) |
| Three days FSH stimulation | 71.1%↑ | N/A | N/A | Women with healthy ovaries (n = 17) | Immature COCs (n = 114) | |
| Lin et al., 2003 [31] | 10,000 IU hCG injection | 71.9% | 69.5% | 36.4% | Women with PCOS (n = 30) | Immature COCs (n = 762) |
| Six days FSH stimulation+ 10,000 IU hCG injection | 76.5% | 75.8% | 31.4% | Women with PCOS (n = 30) | Immature COCs (n = 766) | |
| Chian et al., 2000 [30] | No stimulation | 69.1% | 83.9% | 27.3% | Women with PCOS (n = 17) | Immature COCs (n = 81) |
| 10,000 IU hCG injection | 84.3%↑ | 90.7% | 38.5% | Immature COCs (n = 102) |
| Study | Medium | Maturation Rate | Fertilization Rate | Clinical Pregnancy Rate | Participants | Age (Years) | Total Sample Size |
|---|---|---|---|---|---|---|---|
| Cekleniak et al., 2001 [9] | Glucose-free medium (P1) | 24 h: 59.7% | N/A | N/A | 108 women in ICSI | Mean age: 35.2 | 369 GV/MI oocytes |
| 48 h: 71.7% | |||||||
| TCM-199 | 24 h: 44.9% | N/A | N/A | ||||
| 48 h: 61.0% | |||||||
| de Araujo et al., 2009 [39] | TCM-199 | 82.0%↑ | 70.0%↑ | N/A | 13 infertile women with a previous diagnosis of PCOS | 26–36 | 119 immature oocytes |
| HTF medium | 56.9% | 39.4% | N/A | ||||
| Pongsuthirak et al., 2014 [48] | IVM-MediCult | 65.0% | 69.9% | N/A | 93 pregnancy women during cesarean deliveries | 18–42 | 1015 immature oocytes |
| IVM-SAGE | 64.2% | 65.2% | N/A | ||||
| Filali et al., 2008 [49] | TCM-199 | 61.0% | 61.5% | 25% | 93 PCOS patients | Mean age: 32.1 | 1585 immature oocytes |
| IVM-MediCult | 60.6% | 56.8% | 28.6% | ||||
| Pongsuthirak et al., 2014 [50] | IVM-SAGE | 65.0% | 66.9% | N/A | 89 pregnant women during cesarean deliveries | 18-40 | 1032 immature oocytes |
| Blastocyst medium | 68.7% | 66.4% | N/A | ||||
| Fesahat et al., 2017 [51] | Homemade IVM medium | 73.3% | 54.5% | N/A | 220 infertile women | 23–37 | 114 MI oocytes |
| Cleavage medium | 55.8% | 52.6% | N/A | ||||
| Blastocyst medium | 72.2% | 65.3% | N/A | ||||
| IVM-SAGE | 65.5% | 63.1% | N/A | ||||
| Fesahat et al., 2017 [52] | Homemade IVM medium | 55.0% | 52.7% | N/A | 320 infertile women | Mean age: 31 | 400 GV oocytes |
| Cleavage medium | 53.0% | 56.6% | N/A | ||||
| Blastocyst medium | 78.0%↑ | 69.0% | N/A | ||||
| IVM-SAGE | 68.0% | 54.7% | N/A | ||||
| Moschini et al., 2011 [53] | Standard culture medium (cleavage medium) | GV→MII: 50.5% | N/A | N/A | 28 women in IVF | N/A | 127 GV/MI oocytes |
| MI→MII: 80.6% | |||||||
| IVM-MediCult | GV→MII: 41.2% | N/A | N/A | ||||
| MI→MII: 66.7% |
| Study | Supplements | Sort | Maturation Rate | Fertilization Rate | Clinical Pregnancy Rate | Culture Medium | Participants | Age (Years) | Total Sample Size |
|---|---|---|---|---|---|---|---|---|---|
| Ashourzadeh et al., 2021 [66] | CCs | Others | ns | ↑ | N/A | IVM-SAGE | 270 women in ICSI | ≤35 | 328 denuded GV oocytes |
| GDF9 | Cytokine | ns | ns | N/A | |||||
| CCs + GDF9 | Cytokine | ns | ns | N/A | |||||
| Mohsenzadeh et al., 2019 [87] | GDF9 | Cytokine | ns | N/A | N/A | IVM-MediCult | women with cervix and uterine malignancy | 21–39 | 59 denuded frozen-thawed GV/MI oocytes |
| Chatroudi et al., 2019 [56] | CCs | Others | ns | ↑ | N/A | IVM medium | women in ICSI | N/A | 80 denuded GV oocytes |
| GDF9 | Cytokine | ns | ns | N/A | |||||
| CCs + GDF9 | Cytokine | ns | ns | N/A | |||||
| Zhao et al., 2011 [60] | BDNF | Cytokine | ↑ | N/A | N/A | HTF medium | 167 women in ICSI | 31 ± 0.3 | 366 denuded GV/MI oocytes |
| GDNF | Cytokine | ↑ | N/A | N/A | |||||
| Cui et al., 2018 [88] | GDNF | Cytokine | ↑ | ns | N/A | G-IVF medium | 82 women in IVF | N/A | 200 GV COCs |
| Ben-Ami et al., 2011 [57] | AREG + EREG | Cytokine | ↑ | ns | N/A | N/A | 30 women in ICSI | 20–40 | 105 GV oocytes with partly remaining cumulus oophorus |
| Yu et al., 2012 [65] | EGF + BDNF + IGF-1 | Cytokine | ↑ | ns | N/A | IVM medium | women in ICSI | N/A | GV oocytes |
| EGF + BDNF + IGF-1 | Cytokine | ns | ns | N/A | IVM medium | women in ICSI | N/A | MI oocytes | |
| Goud et al., 1998 [42] | EGF | Cytokine | ↑ | ns | N/A | Medium 199 | 38 women in ICSI | mean age 31.9 | 112 cumulus-denuded GV oocytes |
| EGF | Cytokine | ns | ↑ | N/A | Medium 199 | 54 women in ICSI | mean age 31.8 | 177 cumulus-intact GV oocytes | |
| Sánchez et al., 2017 [89] | PMC with CNP+IVM with FSH and AREG | Cytokine | ↑ | ns | N/A | IVM-MediCult | 15 PCOS patients in IVM | N/A | 381 immature COCs |
| Ma et al., 2020 [80] | Coenzyme Q10 | Antioxidant | ↑ | N/A | N/A | IVM medium | 45 women in IVF | 38-46 | 92 GV oocytes enclosed by CCs |
| Coenzyme Q10 | Antioxidant | ns | N/A | N/A | IVM medium | 18 women in IVF | ≤ 30 | 74 GV oocytes enclosed by CCs | |
| Li et al., 2019 [41] | Melatonin | Antioxidant | ↑ | ↑ | N/A | Medium 199 | women in ICSI | N/A | 197 denuded GV oocytes |
| Cao et al., 2020 [45] | Quercetin | Antioxidant | ↑ | ns | N/A | Medium 199 | 57 women in IVF | 22–42 | 105 denuded GV oocytes |
| ns | ns | N/A | 37 denuded GVBD oocytes | ||||||
| Liu et al., 2018 [46] | Resveratrol | Antioxidant | ↑ | N/A | N/A | Medium 199 | 64 women in ICSI | 38–45 | 75 denuded GV oocytes |
| Xie et al., 2021 [90] | Lysophosphatidic acid | Others | ↑ | N/A | N/A | N/A | 43 healthy women with cesarean sections | 18–35 | 155 denuded GV/MI oocytes |
| Madkour et al., 2018 [91] | Autologous FF | Others | ns | ns | N/A | S-IVM medium (ATL) | 47 PCOS patients in IVM | < 40 | 159 denuded GV/MI oocytes |
| Heterologous FF | Others | ns | ns | N/A | |||||
| Heterologous FF + heterologous CGC supernatant | Others | ↑ | ns | N/A | |||||
| Li et al., 2019 [40] | GH | Others | ↑ | ns | N/A | Medium 199 | women in ICSI | N/A | 252 denuded GV oocytes |
| Virant-Klun et al., 2018 [67] | CCs from autologous mature oocytes | Others | ↑ | N/A | N/A | IVM-MediCult | women in IVF | N/A | 174 denuded GV oocytes |
| Wang et al., 2018 [47] | High-glucose | Others | ↓ | N/A | N/A | TCM-199 | 68 women in ICSI | < 35 | 109 denuded MI oocytes |
| Anderson et al., 2010 [43] | Blocking antibodies to BDNF | Others | ↑ | N/A | N/A | T-199 medium | 67 women requesting laparoscopic sterilization | 21.2–42.9 | Immature COCs |
| Zeng et al., 2013 [92] | Pre-IVM with heparin + cAMP modulators | Others | ↓ | N/A | N/A | pre-IVM media | 42 PCO/PCOS patients in IVM | N/A | Immature COCs |
| Machtinger et al., 2013 [93] | BPA | Others | ↓ | N/A | N/A | IVM-SAGE | 121 women in IVF/ICSI | 23.9–43.9 | 352 GV oocytes enclosed by some residual cumulus/corona radiata cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Kolben, T.; Meister, S.; Paul, C.; van Dorp, J.; Eren, S.; Kuhn, C.; Rahmeh, M.; Mahner, S.; Jeschke, U.; et al. Factors Influencing the In Vitro Maturation (IVM) of Human Oocyte. Biomedicines 2021, 9, 1904. https://doi.org/10.3390/biomedicines9121904
Yang H, Kolben T, Meister S, Paul C, van Dorp J, Eren S, Kuhn C, Rahmeh M, Mahner S, Jeschke U, et al. Factors Influencing the In Vitro Maturation (IVM) of Human Oocyte. Biomedicines. 2021; 9(12):1904. https://doi.org/10.3390/biomedicines9121904
Chicago/Turabian StyleYang, Huixia, Thomas Kolben, Sarah Meister, Corinna Paul, Julia van Dorp, Sibel Eren, Christina Kuhn, Martina Rahmeh, Sven Mahner, Udo Jeschke, and et al. 2021. "Factors Influencing the In Vitro Maturation (IVM) of Human Oocyte" Biomedicines 9, no. 12: 1904. https://doi.org/10.3390/biomedicines9121904
APA StyleYang, H., Kolben, T., Meister, S., Paul, C., van Dorp, J., Eren, S., Kuhn, C., Rahmeh, M., Mahner, S., Jeschke, U., & von Schönfeldt, V. (2021). Factors Influencing the In Vitro Maturation (IVM) of Human Oocyte. Biomedicines, 9(12), 1904. https://doi.org/10.3390/biomedicines9121904







