Prostaglandin F2 and EP2 Agonists Exert Different Effects on 3D 3T3-L1 Spheroids during Their Culture Phase
Abstract
1. Introduction
2. Materials and Methods
2.1. Two- or Three-Dimensional Cell Cultures of 3T3-L1 Cells under Conditions where Bimatoprost Acid (BIM-A) or EP2 Agonists Are Substituted for EP2 Agonists or BIM-A, Respectively
2.2. Lipid Staining of 2D- or 3D-Cultured 3T3-L1 Cells
2.3. Quantitative PCR and Micro-Indentation Force Analysis
2.4. Statistical Analysis
3. Results
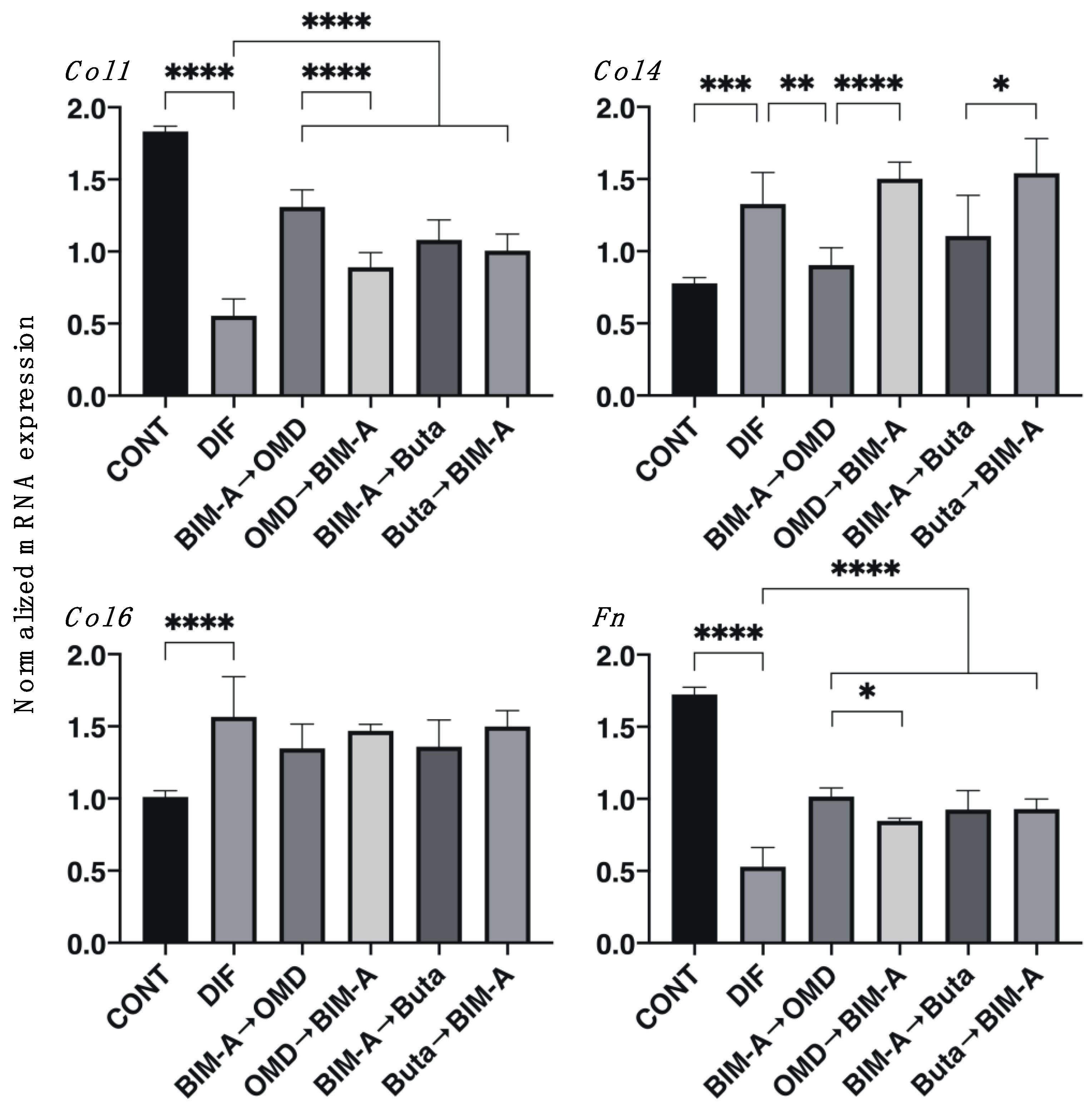
3.1. Effects of Switching the BIM-A and EP2 Agonists (OMD or Buta) on Adipogenesis and ECM Expression in 2D-Cultured 3T3-L1 Cells
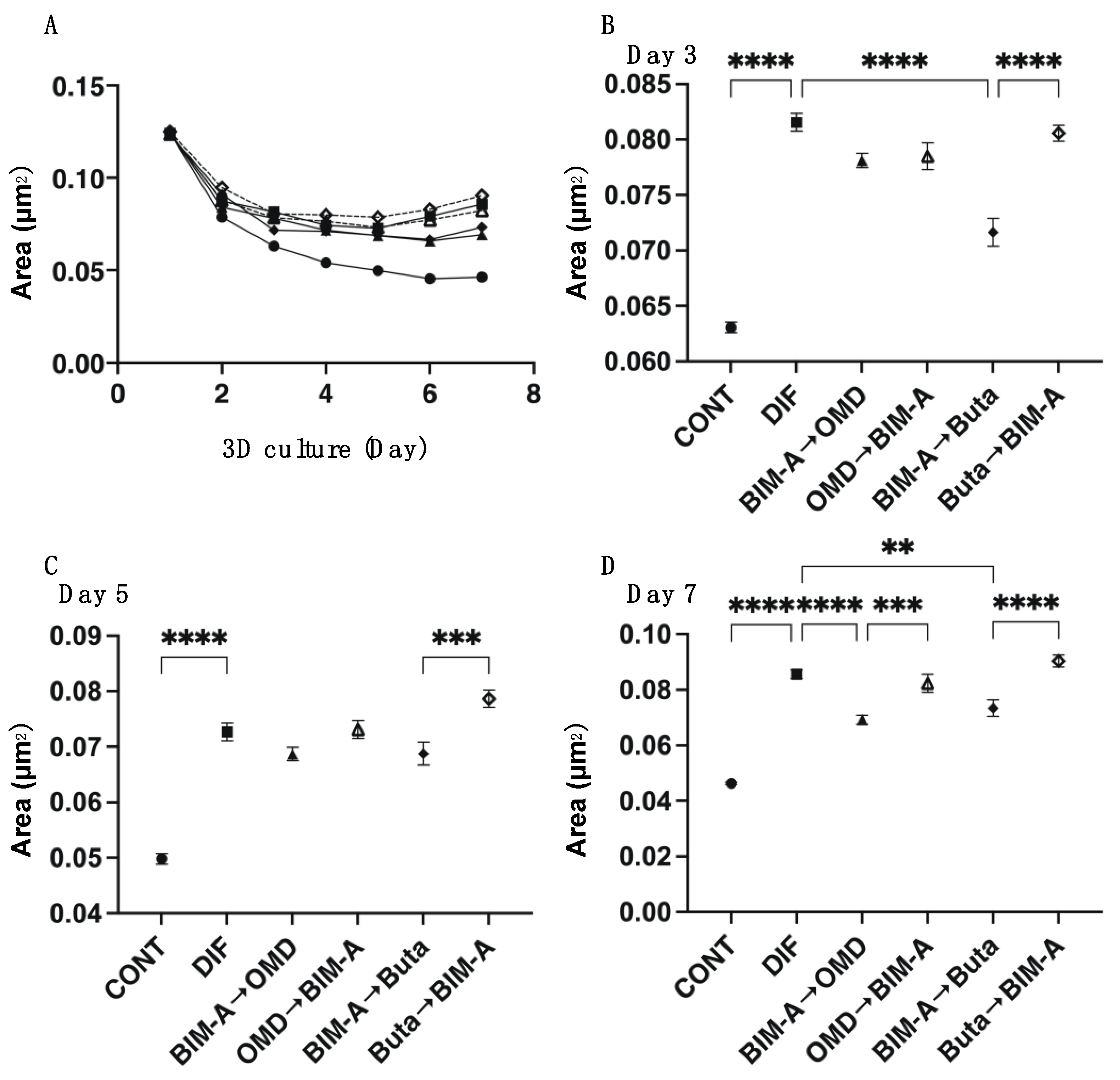
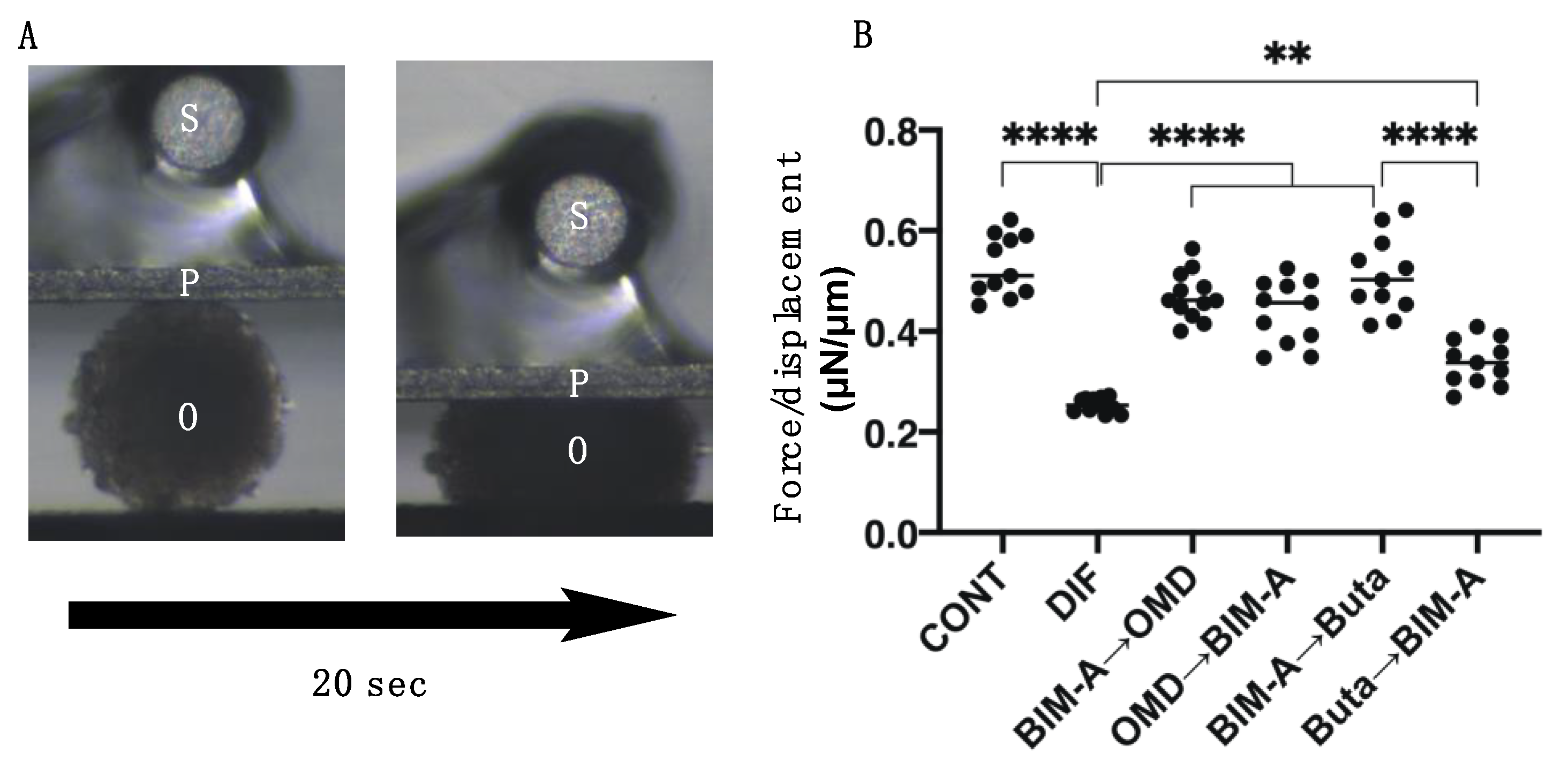
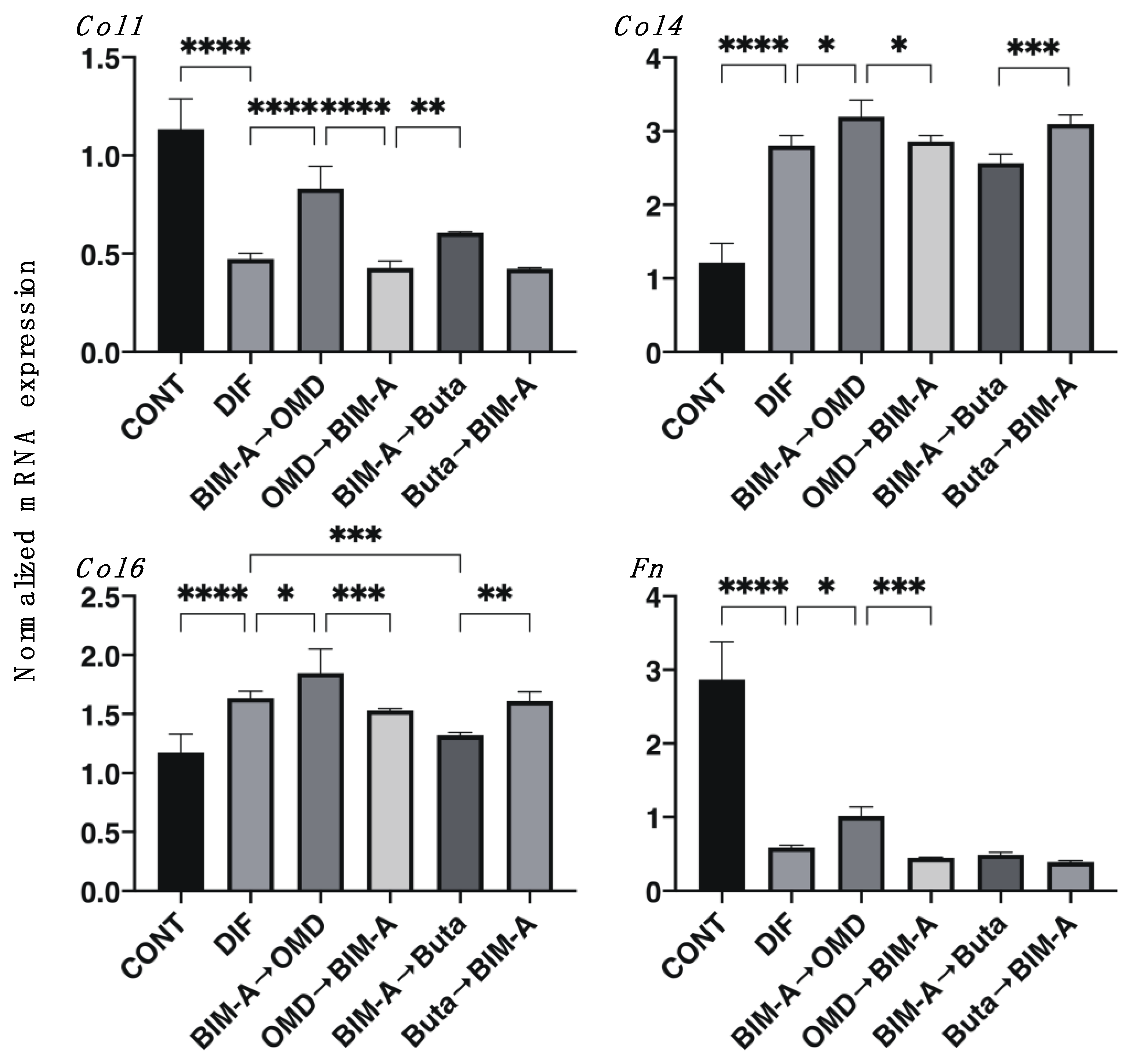
3.2. Effects of Switching BIM-A and EP2 Agonists (OMD or Buta) on Physical Properties, Size, Stiffness, Adipogenesis, and ECM Expression in 3D 3T3-L1 Spheroids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Danias, J.; Podos, S.M. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am. J. Ophthalmol. 1999, 127, 623–625. [Google Scholar] [PubMed]
- Cheng, J.-W.; Cai, J.-P.; Wei, R.-L. Meta-analysis of Medical Intervention for Normal Tension Glaucoma. Ophthalmology 2009, 116, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lindsley, K.; Rouse, B.; Hong, H.; Shi, Q.; Friedman, D.S.; Wormald, R.; Dickersin, K. Comparative Effectiveness of First-Line Medications for Primary Open-Angle Glaucoma: A Systematic Review and Network Meta-analysis. Ophthalmology 2016, 123, 129–140. [Google Scholar] [CrossRef]
- Filippopoulos, T.; Paula, J.; Torun, N.; Hatton, M.P.; Pasquale, L.R.; Grosskreutz, C.L. Periorbital Changes Associated With Topical Bimatoprost. Ophthalmic Plast. Reconstr. Surg. 2008, 24, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasam, A.; Ghazi-Nouri, S. Periorbital Fat Atrophy—An Unfamiliar Side Effect of Prostaglandin Analogues. Orbit 2010, 29, 357–359. [Google Scholar] [CrossRef]
- Miller, C.W.; Casimir, D.A.; Ntambi, J.M. The mechanism of inhibition of 3T3-L1 preadipocyte differentiation by prostaglandin F2alpha. Endocrinology 1996, 137, 5641–5650. [Google Scholar] [CrossRef] [PubMed]
- Ida, Y.; Hikage, F.; Itoh, K.; Ida, H.; Ohguro, H. Prostaglandin F2α agonist-induced suppression of 3T3-L1 cell adipogenesis affects spatial formation of extra-cellular matrix. Sci. Rep. 2020, 10, 7958. [Google Scholar] [CrossRef]
- Itoh, K.; Hikage, F.; Ida, Y.; Ohguro, H. Prostaglandin F2α Agonists Negatively Modulate the Size of 3D Organoids from Primary Human Orbital Fibroblasts. Investig. Opthalmol. Vis. Sci. 2020, 61, 13. [Google Scholar] [CrossRef] [PubMed]
- Aihara, M.; Shirato, S.; Sakata, R. Incidence of deepening of the upper eyelid sulcus after switching from latanoprost to bimatoprost. Jpn. J. Ophthalmol. 2011, 55, 600–604. [Google Scholar] [CrossRef]
- Maruyama, K.; Tsuchisaka, A.; Sakamoto, J.; Shirato, S.; Goto, H. Incidence of deepening of upper eyelid sulcus after topical use of tafluprost ophthalmic solution in Japanese patients. Clin. Ophthalmol. 2013, 7, 1441–1446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peplinski, L.S.; Smith, K.A. Deepening of Lid Sulcus from Topical Bimatoprost Therapy. Optom. Vis. Sci. 2004, 81, 574–577. [Google Scholar] [CrossRef]
- Sakata, R.; Shirato, S.; Miyata, K.; Aihara, M. Incidence of deepening of the upper eyelid sulcus in prostaglandin-associated periorbitopathy with a latanoprost ophthalmic solution. Eye 2014, 28, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, M.; Toris, C.B.; Fan, S.; Taniguchi, T.; Ichikawa, M.; Odani-Kawabata, N.; Iwamura, R.; Yoneda, K.; Matsugi, T.; Shams, N.K.; et al. Effects of a Novel Selective EP2 Receptor Agonist, Omidenepag Isopropyl, on Aqueous Humor Dynamics in Laser-Induced Ocular Hypertensive Monkeys. J. Ocul. Pharmacol. Ther. 2018, 34, 531–537. [Google Scholar] [CrossRef]
- Ida, Y.; Hikage, F.; Umetsu, A.; Ida, H.; Ohguro, H. Omidenepag, a non-prostanoid EP2 receptor agonist, induces enlargement of the 3D organoid of 3T3-L1 cells. Sci. Rep. 2020, 10, 16018. [Google Scholar] [CrossRef]
- Ida, Y.; Hikage, F.; Ohguro, H. ROCK inhibitors enhance the production of large lipid-enriched 3D organoids of 3T3-L1 cells. Sci. Rep. 2021, 11, 5479. [Google Scholar] [CrossRef]
- Shah, M.; Lee, N.; Lefebvre, D.; Kronberg, B.; Loomis, S.; Brauner, S.C.; Turalba, A.; Rhee, D.J.; Freitag, S.K.; Pasquale, L.R. A Cross-Sectional Survey of the Association between Bilateral Topical Prostaglandin Analogue Use and Ocular Adnexal Features. PLoS ONE 2013, 8, e61638. [Google Scholar] [CrossRef]
- Negishi, M.; Sugimoto, Y.; Ichikawa, A. Prostanoid receptors and their biological actions. Prog. Lipid Res. 1993, 32, 417–434. [Google Scholar] [CrossRef]
- Ungrin, M.; Carrière, M.-C.; Denis, D.; Lamontagne, S.; Sawyer, N.; Stocco, R.; Tremblay, N.; Metters, K.M.; Abramovitz, M. Key Structural Features of Prostaglandin E2 and Prostanoid Analogs Involved in Binding and Activation of the Human EP1 Prostanoid Receptor. Mol. Pharmacol. 2001, 59, 1446–1456. [Google Scholar] [CrossRef]
- Kirihara, T.; Taniguchi, T.; Yamamura, K.; Iwamura, R.; Yoneda, K.; Odani-Kawabata, N.; Shimazaki, A.; Matsugi, T.; Shams, N.; Zhang, J.-Z. Pharmacologic Characterization of Omidenepag Isopropyl, a Novel Selective EP2 Receptor Agonist, as an Ocular Hypotensive Agent. Investig. Opthalmol. Vis. Sci. 2018, 59, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Aihara, M.; Lu, F.; Kawata, H.; Iwata, A.; Odani-Kawabata, N.; Shams, N.K. Omidenepag Isopropyl Versus Latanoprost in Primary Open-angle Glaucoma and Ocular Hypertension: ThePhase 3 AYAME Study. Am. J. Ophthalmol. 2020, 220, 53–63. [Google Scholar] [CrossRef]
- Mater, M.K.; Pan, D.; Bergen, W.G.; Jump, D.B. Arachidonic acid inhibits lipogenic gene expression in 3T3-L1 adipocytes through a prostanoid pathway. J. Lipid Res. 1998, 39, 1327–1334. [Google Scholar] [CrossRef]
- Casimir, D.A.; Miller, C.W.; Ntambi, J.M. Preadipocyte differentiation blocked by prostaglandin stimulation of prostanoid FP2 receptor in murine 3T3-L1 cells. Differentiation 1996, 60, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Taketani, Y.; Yamagishi, R.; Fujishiro, T.; Igarashi, M.; Sakata, R.; Aihara, M. Activation of the Prostanoid FP Receptor Inhibits Adipogenesis Leading to Deepening of the Upper Eyelid Sulcus in Prostaglandin-Associated Periorbitopathy. Investig. Opthalmol. Vis. Sci. 2014, 55, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. Molecular Regulation of Adipogenesis. Annu. Rev. Cell Dev. Biol. 2000, 16, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Yamaguchi, K.; Kikuchi, R.; Nakamura, H.; Misaka, R.; Nagai, A.; Aoshiba, K. Improvement of metabolic disorders by an EP 2 receptor agonist via restoration of the subcutaneous adipose tissue in pulmonary emphysema. Prostaglandins Other Lipid Mediat. 2017, 130, 16–22. [Google Scholar] [CrossRef]
- Tsuji, T.; Yamaguchi, K.; Kikuchi, R.; Itoh, M.; Nakamura, H.; Nagai, A.; Aoshiba, K. Promotion of adipogenesis by an EP2 receptor agonist via stimulation of angiogenesis in pulmonary emphysema. Prostaglandins Other Lipid Mediat. 2014, 112, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Sales, K.; List, T.; Boddy, S.C.; Williams, A.R.; Anderson, R.A.; Naor, Z.; Jabbour, H.N. A Novel Angiogenic Role for Prostaglandin F2α-FP Receptor Interaction in Human Endometrial Adenocarcinomas. Cancer Res. 2005, 65, 7707–7716. [Google Scholar] [CrossRef]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid Receptors: Structures, Properties, and Functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef] [PubMed]
- Milne, S.A.; Jabbour, H.N. Prostaglandin (PG) F2αReceptor Expression and Signaling in Human Endometrium: Role of PGF2αin Epithelial Cell Proliferation. J. Clin. Endocrinol. Metab. 2003, 88, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, H.N.; Milne, S.A.; Williams, A.R.; Anderson, R.A.; Boddy, S.C. Expression of COX-2 and PGE synthase and synthesis of PGE(2)in endometrial adenocarcinoma: A possible autocrine/paracrine regulation of neoplastic cell function via EP2/EP4 receptors. Br. J. Cancer 2001, 85, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Duggan, S. Omidenepag Isopropyl Ophthalmic Solution 0.002%: First Global Approval. Drugs 2018, 78, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Holló, G.; Aung, T.; Cantor, L.B.; Aihara, M. Cystoid macular edema related to cataract surgery and topical prostaglandin analogs: Mechanism, diagnosis, and management. Surv. Ophthalmol. 2020, 65, 496–512. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Kiuchi, S.; Ouchi, A.; Hase, T.; Murase, T. Characteristic Expression of Extracellular Matrix in Subcutaneous Adipose Tissue Development and Adipogenesis; Comparison with Visceral Adipose Tissue. Int. J. Biol. Sci. 2014, 10, 825–833. [Google Scholar] [CrossRef] [PubMed]


| BIM-A * | OMD * | Buta * | B→O | O→B | B→Bu | Bu→B | ||
|---|---|---|---|---|---|---|---|---|
| size | ↓↓↓ | (−) | (−) | ↓↓↓ | (−) | ↓↓↓ | (−) | |
| stiffness | ↑↑↑ | (−) | (−) | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑ | |
| lipid stain | 2D | ↓↓ | ↓↓↓ | ↓↓↓ | ↓ | (−) | ↓↓ | ↑↑ |
| 3D | ↓↓ | ↓ | ↓ | (−) | (−) | ↓ | (−) | |
| Pparγ | 2D | ↓↓ | (−) | ↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ |
| 3D | ↓ | ↓↓ | ↓↓ | ↓↓↓ | (−) | ↓↓↓ | ↑↑↑ | |
| Ap2 | 2D | ↓↓↓ | (−) | (−) | ↓↓↓ | ↓↓ | ↓↓↓ | ↓↓↓ |
| 3D | ↓ | ↓↓ | ↓↓ | ↓↓↓ | ↑↑↑ | ↓↓↓ | ↑↑↑ | |
| Leptin | 2D | (−) | N.D | N.D | ↓↓↓ | (−) | (−) | (−) |
| 3D | (−) | N.D | N.D | (−) | (−) | (−) | ↑↑↑ | |
| Col 1 | 2D | (−) | (−) | (−) | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ |
| 3D | (−) | ↑↑ | ↑↑ | ↑↑↑ | (−) | (−) | (−) | |
| Col 4 | 2D | (−) | (−) | (−) | ↓↓ | (−) | (−) | (−) |
| 3D | (−) | ↓↓ | ↓↓ | ↑ | (−) | (−) | (−) | |
| Col 6 | 2D | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 3D | (−) | ↓↓ | ↓↓ | ↑ | (−) | ↓ | (−) | |
| Fn | 2D | (−) | (−) | (−) | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ |
| 3D | (−) | (−) | (−) | ↑ | (−) | (−) | (−) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ida, Y.; Furuhashi, M.; Watanabe, M.; Umetsu, A.; Hikage, F.; Ohguro, H. Prostaglandin F2 and EP2 Agonists Exert Different Effects on 3D 3T3-L1 Spheroids during Their Culture Phase. Biomedicines 2021, 9, 1821. https://doi.org/10.3390/biomedicines9121821
Ida Y, Furuhashi M, Watanabe M, Umetsu A, Hikage F, Ohguro H. Prostaglandin F2 and EP2 Agonists Exert Different Effects on 3D 3T3-L1 Spheroids during Their Culture Phase. Biomedicines. 2021; 9(12):1821. https://doi.org/10.3390/biomedicines9121821
Chicago/Turabian StyleIda, Yosuke, Masato Furuhashi, Megumi Watanabe, Araya Umetsu, Fumihito Hikage, and Hiroshi Ohguro. 2021. "Prostaglandin F2 and EP2 Agonists Exert Different Effects on 3D 3T3-L1 Spheroids during Their Culture Phase" Biomedicines 9, no. 12: 1821. https://doi.org/10.3390/biomedicines9121821
APA StyleIda, Y., Furuhashi, M., Watanabe, M., Umetsu, A., Hikage, F., & Ohguro, H. (2021). Prostaglandin F2 and EP2 Agonists Exert Different Effects on 3D 3T3-L1 Spheroids during Their Culture Phase. Biomedicines, 9(12), 1821. https://doi.org/10.3390/biomedicines9121821







