SYNE1 Exonic Variant rs9479297 Contributes to Concurrent Hepatocellular and Transitional Cell Carcinoma Double Primary Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Targeted Exome Sequencing (TES)
2.3. SYNE1-rs9479297 Genotyping
2.4. SYNE1-rs9479297 Genotyping
2.5. Cell Culture
2.6. Lentivirus-Mediated Knockdown of SYNE1
2.7. Cell Proliferation Assay
2.8. Cell Migration Assay
2.9. RNA Isolation and Quantitative RT-PCR
2.10. Statistical Analysis
3. Results
3.1. Identifying a Panel of Potentially Pathogenic Exonic Variants in Patients with HCC/TCC DPC
3.2. SYNE1-rs9479297 as a Potentially Pathogenic Allele for Occurrence of HCC/TCC DPC in Taiwan
3.3. SYNE1-rs9479297-TT Genotype Associated with Occurrence of HCC/TCC DPC in Taiwan
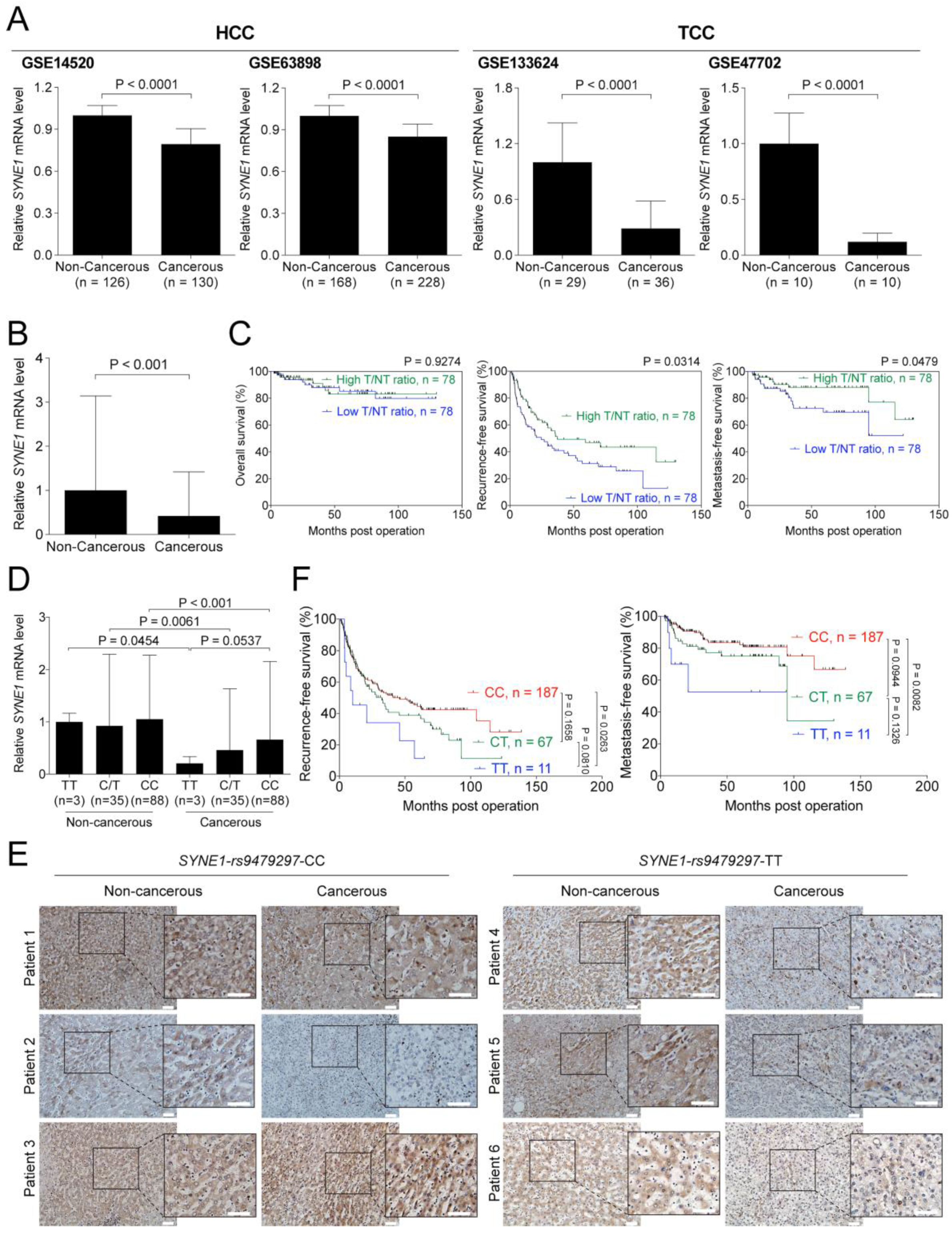
3.4. SYNE1 Expression is Downregulated in HCC and TCC and Associated with Clinical Outcomes in HCC Patients
3.5. SYNE1-rs9479297 Genotypes Predict Postoperative Prognosis in Patients with HCC
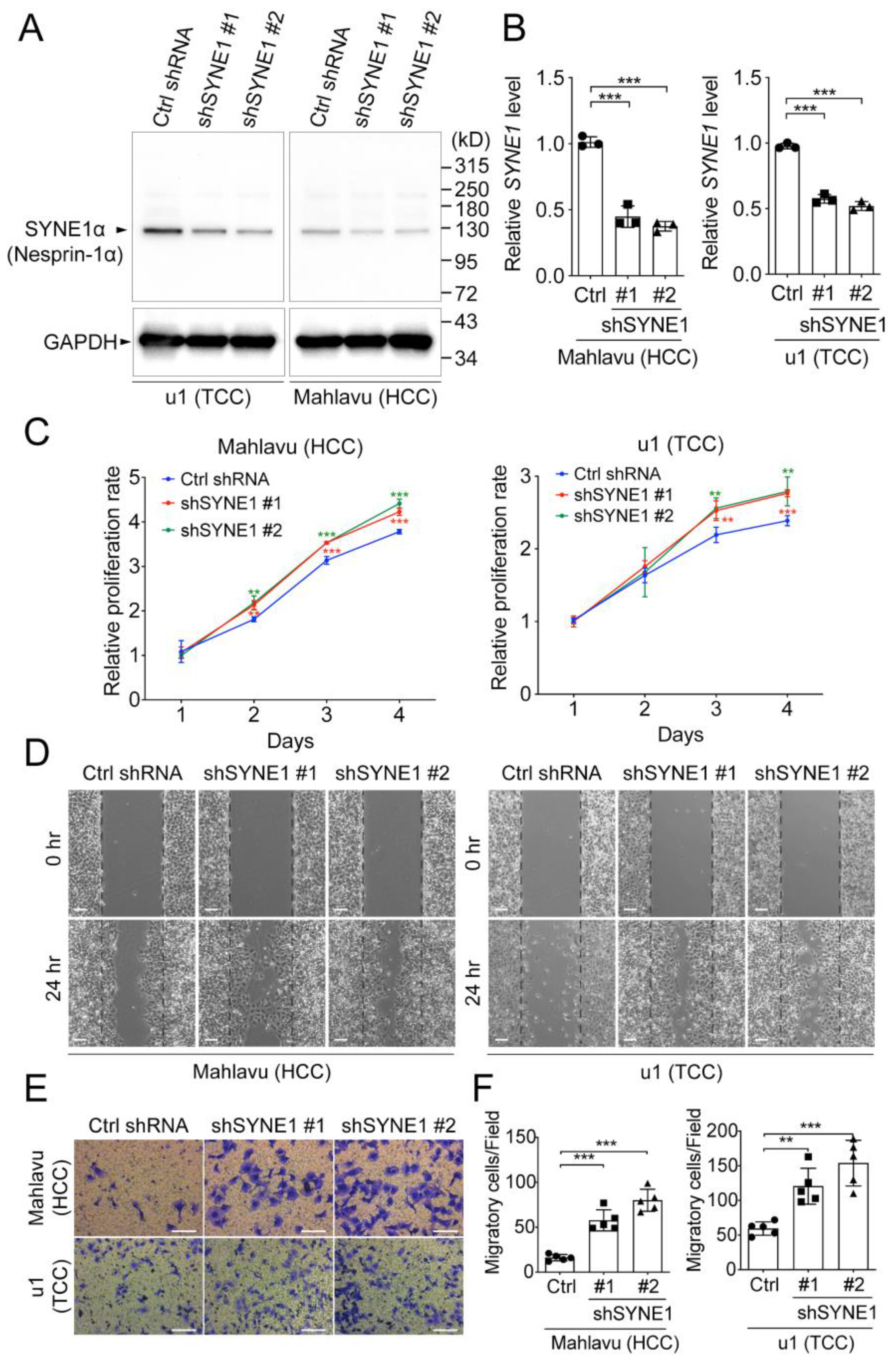
3.6. SYNE1 Silencing Promotes Cell Proliferation and Migration in HCC and TCC Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Goossens, N.; Sun, X.; Hoshida, Y. Molecular classification of hepatocellular carcinoma: Potential therapeutic implications. Hepat. Oncol. 2015, 2, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.A.; Roberts, L.R.; Cancer Genome Atlas Research Network. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017, 169, 1327–1341.e1323. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Wang, S.Y.; Lin, S.M.; Diagnosis, G.; Systemic Therapy, G. Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J. Formos. Med. Assoc. 2021, 120, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Chu, Y.D.; Yeh, C.T. Emergence of Oncogenic-Enhancing Hepatitis B Virus X Gene Mutants in Patients Receiving Suboptimal Entecavir Treatment. Hepatology 2018, 69, 2292. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.W.; Yeh, C.T. Emergence of hepatitis B virus S gene mutants in patients experiencing hepatitis B surface antigen seroconversion after peginterferon therapy. Hepatology 2011, 54, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.T.; So, M.; Ng, J.; Yang, H.W.; Chang, M.L.; Lai, M.W.; Chen, T.C.; Lin, C.Y.; Yeh, T.S.; Lee, W.C. Hepatitis B virus-DNA level and basal core promoter A1762T/G1764A mutation in liver tissue independently predict postoperative survival in hepatocellular carcinoma. Hepatology 2010, 52, 1922–1933. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.P.; Gane, E.; Rodriguez-Torres, M.; Stoehr, A.; Yeh, C.T.; Marcellin, P.; Wiedmann, R.T.; Hwang, P.M.; Caro, L.; Barnard, R.J.; et al. Vaniprevir with pegylated interferon alpha-2a and ribavirin in treatment-naive patients with chronic hepatitis C: A randomized phase II study. Hepatology 2012, 56, 884–893. [Google Scholar] [CrossRef]
- Kee, K.M.; Wang, J.H.; Wang, C.C.; Cheng, Y.F.; Lu, S.N. Hepatocellular Carcinoma associated with Extra-hepatic Primary Malignancy: Its Secular change, Clinical Manifestations and Survival. Sci. Rep. 2016, 6, 30156. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.Y.; Liaw, Y.F.; Wu, C.S.; Chang-Chien, C.S.; Chen, P.C.; Chen, T.J. Hepatocellular carcinoma associated with second primary malignancy. Liver 1987, 7, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, M.; Imaeda, T.; Yamawaki, Y.; Hirose, Y.; Inoue, A.; Goto, H.; Doi, H. Hepatocellular carcinoma with extrahepatic primary neoplasms. Gastrointest. Radiol. 1992, 17, 53–57. [Google Scholar] [CrossRef]
- Takayasu, K.; Kasugai, H.; Ikeya, S.; Muramafsu, Y.; Moriyama, N.; Makuuchi, M.; Yamazaki, S.; Hirohashi, S. A clinical and radiologic study of primary liver cancer associated with extrahepatic primary cancer. Cancer 1992, 69, 45–51. [Google Scholar] [CrossRef]
- Yeh, M.L.; Huang, C.I.; Huang, C.F.; Hsieh, M.Y.; Lin, Z.Y.; Huang, J.F.; Dai, C.Y.; Yu, M.L.; Chen, S.C.; Chuang, W.L. The impact of an additional extra-hepatic primary malignancy on the outcomes of patients with hepatocellular carcinoma. PLoS ONE 2017, 12, e0184878. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.D.; Lin, W.R.; Lin, Y.H.; Kuo, W.H.; Tseng, C.J.; Lim, S.N.; Huang, Y.L.; Huang, S.C.; Wu, T.J.; Lin, K.H.; et al. COX5B-Mediated Bioenergetic Alteration Regulates Tumor Growth and Migration by Modulating AMPK-UHMK1-ERK Cascade in Hepatoma. Cancers 2020, 12, 1646. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.D.; Lai, H.Y.; Pai, L.M.; Huang, Y.H.; Lin, Y.H.; Liang, K.H.; Yeh, C.T. The methionine salvage pathway-involving ADI1 inhibits hepatoma growth by epigenetically altering genes expression via elevating S-adenosylmethionine. Cell Death Dis. 2019, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.D.; Chen, H.K.; Huang, T.; Chan, S.P. A novel function for the DEAD-box RNA helicase DDX-23 in primary microRNA processing in Caenorhabditis elegans. Dev. Biol. 2016, 409, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Rentzsch, P.; Witten, D.; Cooper, G.M.; Shendure, J.; Kircher, M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019, 47, D886–D894. [Google Scholar] [CrossRef] [PubMed]
- Sur, I.; Neumann, S.; Noegel, A.A. Nesprin-1 role in DNA damage response. Nucleus 2014, 5, 173–191. [Google Scholar] [CrossRef][Green Version]
- Doherty, J.A.; Rossing, M.A.; Cushing-Haugen, K.L.; Chen, C.; Van Den Berg, D.J.; Wu, A.H.; Pike, M.C.; Ness, R.B.; Moysich, K.; Chenevix-Trench, G.; et al. ESR1/SYNE1 polymorphism and invasive epithelial ovarian cancer risk: An Ovarian Cancer Association Consortium study. Cancer Epidemiol. Biomark. Prev. 2010, 19, 245–250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, Y.C.; Lin, H.C.; Chiang, Y.H.; Chen, C.G.; Huang, L.; Wang, W.T.; Cheng, C.C.; Lin, J.; Chang, Y.F.; Chang, M.C.; et al. Targeted next-generation sequencing identified novel mutations in triple-negative myeloproliferative neoplasms. Med. Oncol. 2017, 34, 83. [Google Scholar] [CrossRef] [PubMed]
- Nakagaki, T.; Tamura, M.; Kobashi, K.; Koyama, R.; Fukushima, H.; Ohashi, T.; Idogawa, M.; Ogi, K.; Hiratsuka, H.; Tokino, T.; et al. Profiling cancer-related gene mutations in oral squamous cell carcinoma from Japanese patients by targeted amplicon sequencing. Oncotarget 2017, 8, 59113–59122. [Google Scholar] [CrossRef] [PubMed]
- Er, T.K.; Su, Y.F.; Wu, C.C.; Chen, C.C.; Wang, J.; Hsieh, T.H.; Herreros-Villanueva, M.; Chen, W.T.; Chen, Y.T.; Liu, T.C.; et al. Targeted next-generation sequencing for molecular diagnosis of endometriosis-associated ovarian cancer. J. Mol. Med. 2016, 94, 835–847. [Google Scholar] [CrossRef]
- Huhn, S.; Bevier, M.; Pardini, B.; Naccarati, A.; Vodickova, L.; Novotny, J.; Vodicka, P.; Hemminki, K.; Försti, A. Colorectal cancer risk and patients’ survival: Influence of polymorphisms in genes somatically mutated in colorectal tumors. Cancer Causes Control 2014, 25, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Masica, D.L.; Karchin, R. Correlation of somatic mutation and expression identifies genes important in human glioblastoma progression and survival. Cancer Res. 2011, 71, 4550–4561. [Google Scholar] [CrossRef] [PubMed]
- Chong, I.Y.; Cunningham, D.; Barber, L.J.; Campbell, J.; Chen, L.; Kozarewa, I.; Fenwick, K.; Assiotis, I.; Guettler, S.; Garcia-Murillas, I.; et al. The genomic landscape of oesophagogastric junctional adenocarcinoma. J. Pathol. 2013, 231, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Rafnar, T.; Gunnarsson, B.; Stefansson, O.A.; Sulem, P.; Ingason, A.; Frigge, M.L.; Stefansdottir, L.; Sigurdsson, J.K.; Tragante, V.; Steinthorsdottir, V.; et al. Variants associating with uterine leiomyoma highlight genetic background shared by various cancers and hormone-related traits. Nat. Commun. 2018, 9, 3636. [Google Scholar] [CrossRef]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Duong, N.T.; Morris, G.E.; Lam, L.T.; Zhang, Q.; Sewry, C.A.; Shanahan, C.M.; Holt, I. Nesprins: Tissue-specific expression of epsilon and other short isoforms. PLoS ONE 2014, 9, e94380. [Google Scholar] [CrossRef]
- Rajgor, D.; Mellad, J.A.; Autore, F.; Zhang, Q.; Shanahan, C.M. Multiple novel nesprin-1 and nesprin-2 variants act as versatile tissue-specific intracellular scaffolds. PLoS ONE 2012, 7, e40098. [Google Scholar] [CrossRef]
- Cartwright, S.; Karakesisoglou, I. Nesprins in health and disease. Semin. Cell Dev. Biol. 2014, 29, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Tessema, M.; Belinsky, S.A. Mining the epigenome for methylated genes in lung cancer. Proc. Am. Thorac. Soc. 2008, 5, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Mokarram, P.; Kumar, K.; Brim, H.; Naghibalhossaini, F.; Saberi-firoozi, M.; Nouraie, M.; Green, R.; Lee, E.; Smoot, D.T.; Ashktorab, H. Distinct high-profile methylated genes in colorectal cancer. PLoS ONE 2009, 4, e7012. [Google Scholar] [CrossRef] [PubMed]
- Tessema, M.; Willink, R.; Do, K.; Yu, Y.Y.; Yu, W.; Machida, E.O.; Brock, M.; Van Neste, L.; Stidley, C.A.; Baylin, S.B.; et al. Promoter methylation of genes in and around the candidate lung cancer susceptibility locus 6q23-25. Cancer Res. 2008, 68, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.H.; Factor, R.E.; Ullman, K.S. The nuclear envelope environment and its cancer connections. Nat. Rev. Cancer 2012, 12, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Patel, S.; Modi, B.; Shah, F.; Rawal, R. Uncovering the potential of CD44v/SYNE1/miR34a axis in salivary fluids of oral cancer patients. J. Oral. Pathol. Med. 2018, 47, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, M.L.; Witte, N.; Im, K.M.; Turan, S.; Owens, C.; Misner, K.; Tsang, S.X.; Cai, Z.; Wu, S.; Dean, M.; et al. Molecular analysis of urothelial cancer cell lines for modeling tumor biology and drug response. Oncogene 2017, 36, 35–46. [Google Scholar] [CrossRef]
- Sur-Erdem, I.; Hussain, M.S.; Asif, M.; Pinarbasi, N.; Aksu, A.C.; Noegel, A.A. Nesprin-1 impact on tumorigenic cell phenotypes. Mol. Biol. Rep. 2020, 47, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Faraj Shaglouf, L.H.; Ranjpour, M.; Wajid, S.; Jain, S.K. Elevated expression of cellular SYNE1, MMP10, and GTPase1 and their regulatory role in hepatocellular carcinoma progression. Protoplasma 2020, 257, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zheng, H.; Wang, Y.; Lian, F.; Hu, Z.; Xue, S. Nesprin-1 plays an important role in the proliferation and apoptosis of mesenchymal stem cells. Int. J. Mol. Med. 2013, 32, 805–812. [Google Scholar] [CrossRef][Green Version]
- Libotte, T.; Zaim, H.; Abraham, S.; Padmakumar, V.C.; Schneider, M.; Lu, W.; Munck, M.; Hutchison, C.; Wehnert, M.; Fahrenkrog, B.; et al. Lamin A/C–dependent Localization of Nesprin-2, a Giant Scaffolder at the Nuclear Envelope. Mol. Biol. Cell 2005, 16, 3411–3424. [Google Scholar] [CrossRef] [PubMed]

| Cohort | Cohort-1 | Cohort-1 | Cohort-2 | Cohort-3 | Cohort-3 | Cohort-3 |
|---|---|---|---|---|---|---|
| Case number | n = 15 | n = 101 | n = 216 | n = 44 | n = 265 | n = 153 |
| Disease | DPC | DPC | Healthy | TCC | HCC | Non-HCC Non-TCC |
| Experiment or assay | TES | Rs9479297 genotype comparison | Rs9479297 genotype comparison | Rs9479297 genotype comparison | 265 for Rs9479297 genotype comparison; 156 paired noncancerous and cancerous tissues for RT-qPCR; 20 paired noncancerous and cancerous tissues for IHC | Rs9479297 genotype comparison |
| Baseline characteristics | ||||||
| Gender, male, n (%) | 11 (73.3%) | 65 (64.4%) | 108 (50.0%) | 34 (77.3%) | 211 (79.6%) | 91 (59.5%) |
| Age of sampling ± SD | 59.3 ± 11.9 | 65.7 ± 9.3 | 70.6 ± 9.5 | 59.0 ± 8.9 | 54.8 ± 14.3 | 71.9 ± 6.0 |
| HCC Ages (years) ± SD | 59.3 ± 11.9 | 65.7 ± 9.3 | NA | NA | 54.8 ± 14.3 | NA |
| TCC Ages (years) ± SD | 59.5 ± 14.0 | 65.2 ± 9.6 | NA | 59.0 ± 8.9 | NA | NA |
| Sequential order score ± SD a | 2.1 ± 0.8 | 1.9 ± 0.8 | NA | NA | NA | NA |
| Body weight ± SD | 65.0 ± 15.5 | 62.5 ± 11.7 | NA | NA | NA | NA |
| Body height ± SD | 164.7 ± 9.1 | 161.2 ± 7.5 | NA | NA | NA | NA |
| Heavy smoker, n (%) | 7 (46.7%) | 41 (40.6%) | NA | NA | NA | NA |
| BCLC stage for HCC, n (%) Stage 0 Stage A Stage B Stage C Stage D | 4 (26.7%) 7 (46.7%) 4 (26.7%) 0 (0%) 0 (0%) | 15 (14.9%) 23 (22.8%) 20 (19.8%) 42 (41.5%) 1 (1.0%) | NA NA NA NA NA | NA NA NA NA NA | 0 (0.0%) 8 (3.0%) 88 (33.2%) 144 (54.3%) 25 (9.4%) | NA NA NA NA NA |
| AJCC7 stage for TCC, n (%) Stage 0 Stage I Stage II Stage III Stage IV | 0 (0%) 10 (66.7%) 2 (13.3%) 3 (20.0%) 0 (0%) | 23 (22.8%) 49 (48.5%) 15 (14.9%) 12 (11.9%) 2 (2.0%) | NA NA NA NA NA | 5 (11.4%) 12 (27.3%) 6 (13.6%) 10 (22.7%) 11 (25.0%) | NA NA NA NA NA | NA NA NA NA NA |
| HBsAg positive, n (%) | 8 (53.3%) | 30 (29.7%) | NA | NA | 210 (79.2%) | 124 (81.0%) |
| Anti-HCV positive, n (%) | 5 (33.3%) | 47 (46.5%) | NA | NA | 66 (24.9%) | 54 (35.3%) |
| Biochemistry and hemogram | ||||||
| Total bilirubin (mg/dL) ± SD | 0.7 ± 0.3 | 1.0 ± 0.9 | NA | NA | 1.3 ± 1.7 | NA |
| AST(U/L) ± SD | 44.3 ± 25.5 | 143.2 ± 55.3 | NA | NA | 76.5 ± 103.3 | NA |
| ALT(U/L) ± SD | 37.2 ± 19.0 | 108.3 ± 51.2 | NA | NA | 75.6 ± 97.5 | NA |
| Albumin (g/dL) ± SD | 4.0 ± 0.6 | 3.7 ± 0.6 | NA | NA | 3.9 ± 0.6 | NA |
| Platelet count (×103/mL) ± SD | 153.0 ± 42.4 | 163 ± 72.0 | NA | NA | NA | NA |
| AFP (ng/mL), median (range) | 5.7 (2.4–87.2) | 8.1 (2.1–20844.1) | NA | NA | 33.0 (1.0–443209.0) | NA |
| Symbol | SNP ID | Chr | Position | Ref | Var | NM_ID | cDNA Change | Codon Change | EXON | Protein Change | CADD Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DCC | rs9951523 | 18 | 49867224 | T | C | NM_005215 | c.67T > C | Ttc/Ctc | 1/29 | p.F23L | 22.3 |
| EML4 | rs6736913 | 2 | 42510018 | A | G | NM_019063 | c.847A > G | Aaa/Gaa | 8/23 | p.K283E | 22.5 |
| ERCC5 | rs9514066 | 13 | 103527849 | G | C | NM_000123 | c.3157G > C | Gga/Cga | 15/15 | p.G1053R | 21.5 |
| FN1 | rs386524617 | 2 | 216235089 | C | T | NM_002026 | c.6415G > A | Gtc/Atc | 41/46 | p.V2139I | 20.3 |
| ITGB2 | rs235330 | 21 | 46314907 | T | A | NM_000211 | c.1062A > T | caA/caT | 10/17 | p.Q354H | 21.5 |
| PKHD1 | rs2435322 | 6 | 51875250 | A | C | NM_138694 | c.5608T > G | Ttg/Gtg | 35/67 | p.L1870V | 20.3 |
| ITGA9 | rs267561 | 3 | 37574951 | G | A | NM_002207 | c.1520G > A | gGa/gAa | 14/28 | p.G507E | 24.4 |
| FN1 | rs1250259 | 2 | 216300482 | T | A | NM_002026 | c.44A > T | cAg/cTg | 1/46 | p.Q15L | 22 |
| DPYD | rs1801265 | 1 | 98348885 | G | A | NM_000110 | c.85C > T | Cgt/Tgt | 2/23 | p.R29C | 23.2 |
| PLCG1 | rs753381 | 20 | 39797465 | T | C | NM_002660 | c.2438T > C | aTc/aCc | 22/33 | p.I813T | 22.9 |
| SYNE1 | rs214976 | 6 | 152772264 | A | G | NM_033071 | c.3125T > C | gTa/gCa | 26/146 | p.V1042A | 20.1 |
| FLT3 | rs1933437 | 13 | 28624294 | G | A | NM_004119 | c.680C > T | aCg/aTg | 6/24 | p.T227M | 21.4 |
| SYNE1 | rs2306916 | 6 | 152647681 | A | T | NM_033071 | c.14830T > A | Ttg/Atg | 78/146 | p.L4944M | 21 |
| PBX1 | rs2275558 | 1 | 164529120 | G | A | NM_001204961 | c.61G > A | Ggc/Agc | 1/9 | p.G21S | 20.9 |
| CYP2D6 | rs1065852 | 22 | 42526694 | G | A | NM_001025161 | c.100C > T | Cca/Tca | 1/8 | p.P34S | 23.7 |
| FLT4 | rs448012 | 5 | 180046344 | G | C | NM_002020 | c.2670C > G | caC/caG | 19/30 | p.H890Q | 22.9 |
| SYNE1 | rs9479297 | 6 | 152658142 | T | C | NM_033071 | c.12149A > G | aAg/aGg | 75/146 | p.K4050R | 20.4 |
| PTCH1 | rs357564 | 9 | 98209594 | G | A | NM_000264 | c.3944C > T | cCc/cTc | 23/24 | p.P1315L | 24.9 |
| ERBB2 | rs1058808 | 17 | 37884037 | C | G | NM_004448 | c.3508C > G | Ccc/Gcc | 27/27 | p.P1170A | 23.5 |
| EPHA3 | rs35124509 | 3 | 89521693 | T | C | NM_005233 | c.2770T > C | Tgg/Cgg | 16/17 | p.W924R | 22.3 |
| ERCC5 | rs17655 | 13 | 103528002 | G | C | NM_000123 | c.3310G > C | Gat/Cat | 15/15 | p.D1104H | 22.9 |
| CSF1R | rs10079250 | 5 | 149450132 | T | C | NM_005211 | c.1085A > G | cAc/cGc | 8/22 | p.H362R | 20.4 |
| LRP1B | rs12990449 | 2 | 142567910 | T | C | NM_018557 | c.143A > G | cAg/cGg | 2/91 | p.Q48R | 20.4 |
| DST | rs11756977 | 6 | 56420538 | C | T | NM_001144770 | c.6872G > A | cGt/cAt | 41/84 | p.R2291H | 22.9 |
| NIN | rs2295847 | 14 | 51202311 | G | C | NM_182946 | c.5800C > G | Caa/Gaa | 28/30 | p.Q1934E | 22.5 |
| PARP1 | rs1136410 | 1 | 226555302 | A | G | NM_001618 | c.2285T > C | gTg/gCg | 17/23 | p.V762A | 28.1 |
| PIK3R1 | rs386584794 | 5 | 67588148 | G | A | NM_181523 | c.978G > A | atG/atA | 8/16 | p.M326I | 20.7 |
| SETD2 | rs76208147 | 3 | 47162886 | C | T | NM_014159 | c.3240G > A | atG/atA | 3/21 | p.M1080I | 20.7 |
| CRTC1 | rs3746266 | 19 | 18876309 | A | G | NM_015321 | c.982A > G | Acc/Gcc | 9/14 | p.T328A | 22.5 |
| SYNE1 | rs76160752 | 6 | 152629631 | C | T | NM_033071 | c.17126G > A | cGg/cAg | 90/146 | p.R5709Q | 22.4 |
| CASC5 | rs11858113 | 15 | 40914177 | T | C | NM_170589 | c.1793T > C | aTg/aCg | 11/27 | p.M598T | 20.8 |
| CDH11 | rs35195 | 16 | 65025718 | G | A | NM_001797 | c.764C > T | aCg/aTg | 6/13 | p.T255M | 25.1 |
| CDH11 | rs1130821 | 16 | 65022234 | C | T | NM_001797 | c.825G > A | atG/atA | 7/13 | p.M275I | 24.9 |
| DST | rs80260070 | 6 | 56351972 | G | C | NM_001144770 | c.13108C > G | Ctg/Gtg | 68/84 | p.L4370V | 23 |
| EPHA3 | rs17801309 | 3 | 89521664 | G | A | NM_005233 | c.2741G > A | cGc/cAc | 16/17 | p.R914H | 22.3 |
| EPHA7 | rs2278106 | 6 | 94120219 | G | A | NM_004440 | c.832C > T | Ccc/Tcc | 3/17 | p.P278S | 23.4 |
| ERBB2 | rs1136201 | 17 | 37879588 | A | G | NM_004448 | c.1963A > G | Atc/Gtc | 17/27 | p.I655V | 22.3 |
| FANCA | rs11646374 | 16 | 89857935 | G | A | NM_000135 | c.1235C > T | gCg/gTg | 14/43 | p.A412V | 21.4 |
| HNF1A | rs1169288 | 12 | 121416650 | A | C | NM_000545 | c.79A > C | Atc/Ctc | 1/10 | p.I27L | 23.4 |
| IGF2R | rs8191754 | 6 | 160448324 | C | G | NM_000876 | c.754C > G | Ctg/Gtg | 6/48 | p.L252V | 23.4 |
| IKBKB | rs2272736 | 8 | 42177163 | G | A | NM_001556 | c.1577G > A | cGg/cAg | 15/22 | p.R526Q | 24.4 |
| MTRR | rs2287780 | 5 | 7889304 | C | T | NM_002454 | c.1324C > T | Cgc/Tgc | 9/15 | p.R442C | 23.1 |
| ROS1 | rs1998206 | 6 | 117725448 | T | G | NM_002944 | c.433A > C | Act/Cct | 5/43 | p.T145P | 23.7 |
| TRIP11 | rs80200454 | 14 | 92460227 | C | T | NM_004239 | c.5086G > A | Gaa/Aaa | 15/21 | p.E1696K | 23.5 |
| Symbol | SNP ID | Genotype | Three-Group Comparison | Two-Group Comparison | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCC + TCC | Normal Population | p | HCC + TCC | Normal Population | p | ||||||||||
| Ref | Var | Var Homo | Hetero | Ref Homo | Var Homo | Hetero | Ref Homo | Var Homo | Non-Var Homo | Var Homo | Non-Var Homo | ||||
| SYNE1 | rs9479297 | T | C | 5 | 8 | 2 | 4 | 62 | 150 | 0.002 | 5 | 10 | 4 | 212 | 0.009 |
| SYNE1 | rs76160752 | A | G | 1 | 0 | 14 | 0 | 15 | 201 | <0.001 | 1 | 14 | 0 | 216 | 0.077 |
| EPHA | rs17801309 | G | C | 1 | 3 | 11 | 0 | 28 | 188 | <0.001 | 1 | 14 | 0 | 216 | 0.077 |
| TRIP11 | rs80200454 | C | T | 1 | 0 | 14 | 0 | 19 | 197 | <0.001 | 1 | 14 | 0 | 216 | 0.077 |
| ITGA9 | rs267561 | T | A | 14 | 1 | 0 | 151 | 54 | 11 | 0.148 | 14 | 1 | 151 | 65 | 0.100 |
| SETD2 | rs76208147 | A | C | 2 | 3 | 10 | 5 | 52 | 159 | 0.055 | 2 | 13 | 5 | 211 | 0.103 |
| PBX1 | rs2275558 | G | A | 8 | 5 | 2 | 82 | 101 | 33 | 0.488 | 8 | 7 | 82 | 134 | 0.238 |
| CRTC1 | rs3746266 | T | A | 2 | 6 | 7 | 8 | 64 | 144 | 0.113 | 2 | 13 | 8 | 208 | 0.264 |
| SYNE1 | rs214976 | G | A | 11 | 4 | 0 | 127 | 75 | 14 | 0.42 | 11 | 4 | 127 | 89 | 0.267 |
| EPHA3 | rs35124509 | T | C | 2 | 6 | 7 | 8 | 72 | 136 | 0.15 | 2 | 13 | 8 | 208 | 0.264 |
| CYP2D6 | rs1065852 | A | G | 7 | 3 | 5 | 71 | 99 | 46 | 0.147 | 7 | 8 | 71 | 145 | 0.275 |
| FLT3 | rs1933437 | G | A | 10 | 4 | 1 | 116 | 84 | 16 | 0.609 | 10 | 5 | 116 | 100 | 0.330 |
| FLT4 | rs448012 | A | T | 6 | 2 | 7 | 59 | 93 | 64 | 0.077 | 6 | 9 | 59 | 157 | 0.447 |
| ERCC5 | rs17655 | G | A | 2 | 6 | 7 | 55 | 94 | 67 | 0.377 | 2 | 13 | 55 | 161 | 0.457 |
| SYNE1 | rs2306916 | G | A | 9 | 5 | 1 | 148 | 63 | 5 | 0.534 | 9 | 6 | 148 | 68 | 0.467 |
| NIN | rs2295847 | G | C | 2 | 7 | 6 | 53 | 117 | 46 | 0.216 | 2 | 13 | 53 | 163 | 0.502 |
| CDH11 | rs1130821 | T | C | 1 | 8 | 6 | 35 | 108 | 73 | 0.604 | 1 | 14 | 35 | 181 | 0.537 |
| MTRR | rs2287780 | G | A | 1 | 4 | 10 | 3 | 65 | 148 | 0.314 | 1 | 14 | 3 | 213 | 0.623 |
| FN1 | rs1250259 | C | G | 14 | 1 | 0 | 184 | 30 | 2 | 0.673 | 14 | 1 | 184 | 32 | 0.624 |
| DPYD | rs1801265 | T | C | 13 | 2 | 0 | 184 | 31 | 1 | 0.959 | 13 | 2 | 184 | 32 | 0.624 |
| LRP1B | rs12990449 | G | C | 2 | 5 | 8 | 48 | 93 | 75 | 0.337 | 2 | 13 | 48 | 168 | 0.628 |
| CASC5 | rs11858113 | T | C | 1 | 5 | 9 | 12 | 61 | 143 | 0.887 | 1 | 14 | 12 | 204 | 0.690 |
| ERBB2 | rs1136201 | T | C | 1 | 3 | 11 | 4 | 48 | 164 | 0.462 | 1 | 14 | 4 | 212 | 0.748 |
| IKBKB | rs2272736 | C | T | 1 | 5 | 9 | 4 | 40 | 172 | 0.152 | 1 | 14 | 4 | 212 | 0.748 |
| IGF2R | rs8191754 | G | C | 1 | 4 | 10 | 10 | 70 | 136 | 0.862 | 1 | 14 | 10 | 206 | 0.788 |
| FANCA | rs11646374 | A | G | 1 | 6 | 8 | 10 | 75 | 131 | 0.836 | 1 | 14 | 10 | 206 | 0.788 |
| PLCG1 | rs753381 | G | A | 11 | 4 | 0 | 144 | 65 | 7 | 0.728 | 11 | 4 | 144 | 72 | 0.805 |
| CSF1R | rs10079250 | C | T | 2 | 5 | 8 | 42 | 107 | 67 | 0.203 | 2 | 13 | 42 | 174 | 0.808 |
| PARP1 | rs1136410 | A | G | 2 | 8 | 5 | 42 | 98 | 76 | 0.786 | 2 | 13 | 42 | 174 | 0.808 |
| CDH11 | rs35195 | C | T | 1 | 3 | 11 | 5 | 76 | 135 | 0.328 | 1 | 14 | 5 | 211 | 0.853 |
| ERBB2 | rs1058808 | T | C | 3 | 5 | 7 | 32 | 104 | 80 | 0.536 | 3 | 12 | 32 | 184 | 0.866 |
| HNF1A | rs1169288 | G | A | 1 | 7 | 7 | 25 | 109 | 82 | 0.735 | 1 | 14 | 25 | 191 | 0.874 |
| DST | rs11756977 | C | T | 2 | 7 | 6 | 34 | 84 | 98 | 0.836 | 2 | 13 | 34 | 182 | 0.905 |
| DST | rs80260070 | G | C | 1 | 4 | 10 | 6 | 62 | 148 | 0.695 | 1 | 14 | 6 | 210 | 0.944 |
| EPHA7 | rs2278106 | G | A | 1 | 4 | 10 | 7 | 58 | 151 | 0.78 | 1 | 14 | 7 | 209 | 0.977 |
| ROS1 | rs1998206 | G | A | 1 | 3 | 11 | 7 | 65 | 144 | 0.59 | 1 | 14 | 7 | 209 | 0.977 |
| PTCH1 | rs357564 | A | G | 4 | 8 | 3 | 66 | 104 | 46 | 0.924 | 4 | 11 | 66 | 150 | 0.979 |
| DCC | rs9951523 | G | A | 15 | 0 | 0 | 216 | 0 | 0 | NA | 15 | 0 | 216 | 0 | NA |
| EML4 | rs6736913 | A | C | 15 | 0 | 0 | 216 | 0 | 0 | NA | 15 | 0 | 216 | 0 | NA |
| ERCC5 | rs9514066 | C | G | 15 | 0 | 0 | 216 | 0 | 0 | NA | 15 | 0 | 216 | 0 | NA |
| FN1 | rs386524617 | G | A | 15 | 0 | 0 | 216 | 0 | 0 | NA | 15 | 0 | 216 | 0 | NA |
| ITGB2 | rs235330 | C | T | 15 | 0 | 0 | 216 | 0 | 0 | NA | 15 | 0 | 216 | 0 | NA |
| PKHD1 | rs2435322 | T | G | 15 | 0 | 0 | 216 | 0 | 0 | NA | 15 | 0 | 216 | 0 | NA |
| PIK3R1 | rs386584794 | C | T | 2 | 2 | 11 | NA | NA | NA | NA | 2 | 13 | NA | NA | NA |
| Population in Taiwan | SYNE1-rs9479297 Genotypes | p | SYNE1-rs9479297 Genotypes | p | |||
|---|---|---|---|---|---|---|---|
| CC | CT | TT | TT | Non-TT | |||
| HCC + TCC patients (n = 101) | 72 | 17 | 12 | 12 | 89 | ||
| TCC patients (n = 44) | 34 | 10 | 0 | 0.051 | 0 | 44 | 0.039 |
| HCC patients (n = 265) | 187 | 67 | 11 | 0.010 | 11 | 254 | 0.006 |
| Non-TCC and non-HCC patients (n = 153) | 113 | 38 | 2 | 0.001 | 2 | 146 | <0.001 |
| Normal population (n = 216) | 150 | 62 | 4 | <0.001 | 4 | 212 | <0.001 |
| Characteristics | rs9479297-TT | rs9479297-Non-TT | p |
|---|---|---|---|
| Gender, male, n (%) | 8 (58.3%) | 57 (70.4%) | 0.5005 |
| HCC diagnosed Ages (years) ± SD | 61.9 ± 8.5 | 67.5 ± 8.1 | 0.0358 |
| TCC diagnosed Ages (years) ± SD | 60.3 ± 7.1 | 67.2 ± 8.1 | 0.0067 |
| Sequential order score ± SD a | 1.8 ± 0.8 | 1.9 ± 0.8 | 0.5352 |
| Body weight ± SD | 61.1 ± 11.4 | 63.3 ± 11.3 | 0.7355 |
| Body height ± SD | 161.1 ± 7.7 | 161.2 ± 7.1 | 0.9756 |
| Heavy smoker, n (%) | 4 (33.3%) | 37 (41.6%) | 0.8161 |
| BCLC stage for HCC, n (%) Stage 0 Stage A Stage B Stage C Stage D | 0 (0.0%) 5 (41.7%) 3 (25.0%) 4 (33.3%) 0 (0.0%) | %15 (16.9%) 18 (20.2%) 17 (19.1%) 38 (42.7%) 1 (1.1%) | 0.2675 0.1950 0.9239 0.7598 0.7121 |
| AJCC7 stage for TCC, n (%) Stage 0 Stage I Stage II Stage III Stage IV | 3 (25.0%) 6 (50.0%) 2 (16.7%) 1 (8.3%) 0 (0.0%) | 20 (22.5%) 43 (48.3%) 13 (14.6%) 11 (12.4%) 2 (2.2%) | 0.8446 0.9127 0.8506 0.6857 0.5999 |
| HBsAg positive, n (%) | 2 (16.7%) | 28 (31.4%) | 0.4738 |
| Anti-HCV positive, n (%) | 7 (58.3%) | 40 (44.9%) | 0.5723 |
| Biochemistry and hemogram | |||
| Total bilirubin (mg/dL) ± SD | 1.3 ± 1.8 | 0.9 ± 1.3 | 0.5570 |
| AST(U/L) ± SD | 142.3 ± 44.5 | 144.5 ± 55.7 | 0.9591 |
| ALT(U/L) ± SD | 141.4 ± 63.6 | 89.5 ± 41.6 | 0.3968 |
| Albumin (g/dL) ± SD | 3.6 ± 0.4 | 3.8 ± 0.7 | 0.2487 |
| Platelet count (×103/mL) ± SD | 179.2 ± 84.3 | 157.2 ± 70.0 | 0.2459 |
| AFP (ng/mL), median (range) | 7.9 (2.7–488.1) | 10.7 (2.1–20844.1) | 0.6931 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Y.-D.; Kee, K.-M.; Lin, W.-R.; Lai, M.-W.; Lu, S.-N.; Chung, W.-H.; Pang, S.-T.; Yeh, C.-T. SYNE1 Exonic Variant rs9479297 Contributes to Concurrent Hepatocellular and Transitional Cell Carcinoma Double Primary Cancer. Biomedicines 2021, 9, 1819. https://doi.org/10.3390/biomedicines9121819
Chu Y-D, Kee K-M, Lin W-R, Lai M-W, Lu S-N, Chung W-H, Pang S-T, Yeh C-T. SYNE1 Exonic Variant rs9479297 Contributes to Concurrent Hepatocellular and Transitional Cell Carcinoma Double Primary Cancer. Biomedicines. 2021; 9(12):1819. https://doi.org/10.3390/biomedicines9121819
Chicago/Turabian StyleChu, Yu-De, Kwong-Ming Kee, Wey-Ran Lin, Ming-Wei Lai, Sheng-Nan Lu, Wen-Hung Chung, See-Tong Pang, and Chau-Ting Yeh. 2021. "SYNE1 Exonic Variant rs9479297 Contributes to Concurrent Hepatocellular and Transitional Cell Carcinoma Double Primary Cancer" Biomedicines 9, no. 12: 1819. https://doi.org/10.3390/biomedicines9121819
APA StyleChu, Y.-D., Kee, K.-M., Lin, W.-R., Lai, M.-W., Lu, S.-N., Chung, W.-H., Pang, S.-T., & Yeh, C.-T. (2021). SYNE1 Exonic Variant rs9479297 Contributes to Concurrent Hepatocellular and Transitional Cell Carcinoma Double Primary Cancer. Biomedicines, 9(12), 1819. https://doi.org/10.3390/biomedicines9121819







