ATPase Activity of the Subcellular Fractions of Colorectal Cancer Samples under the Action of Nicotinic Acid Adenine Dinucleotide Phosphate
Abstract
:1. Introduction
- To estimate the effect of NAADP on the activity of Na+/K+ ATPase;
- To ascertain the effect of NAADP on the activities of Ca2+ ATPase of endoplasmic reticulum (EPR) and the plasma membrane (PM);
- To research the effect of NAADP on the basal ATPase activity in the patients’ colorectal cancer samples compared to the samples of healthy tissues.
2. Materials and Methods
2.1. Ethical Standards and Characteristics of Patients
2.2. Isolation of Subcellular Post-Mitochondrial Fraction of the Patients’ Colon Mucus
2.3. Assay of ATPase Activity
- The activity of Na+/K+ ATPase was expressed as the difference of Pi in the medium with or without ouabain (Sigma, USA). The samples (without ouabain) instead contained incubation medium in equal measure. Basal Mg2+ ATPase activity was determined in an incubation medium that did not contain CaCl2, but included EGTA, as well as ouabain. Thapsigargin (Sigma, USA) was added to inhibit the activity of the Ca2+ ATP of EPR. The activity of Ca2+ ATP of EPR was also calculated as the difference of Pi in the medium with inhibitors (thapsigargin and ouabain) or without these compounds. The samples (without thapsigargin and ouabain) contained incubation medium in equal measure;
- Thapsigargin and ouabain were previously dissolved in DMSO in a separate aliquot, then dissolved again in another aliquot in internal solution and then was added to the incubation medium in concentration 1 μmol. Other samples (without thapsigargin and ouabain) instead contained incubation medium in equal measure.
2.4. Calculation of Specific ATPase Activity
2.5. Statistical Analysis
3. Results
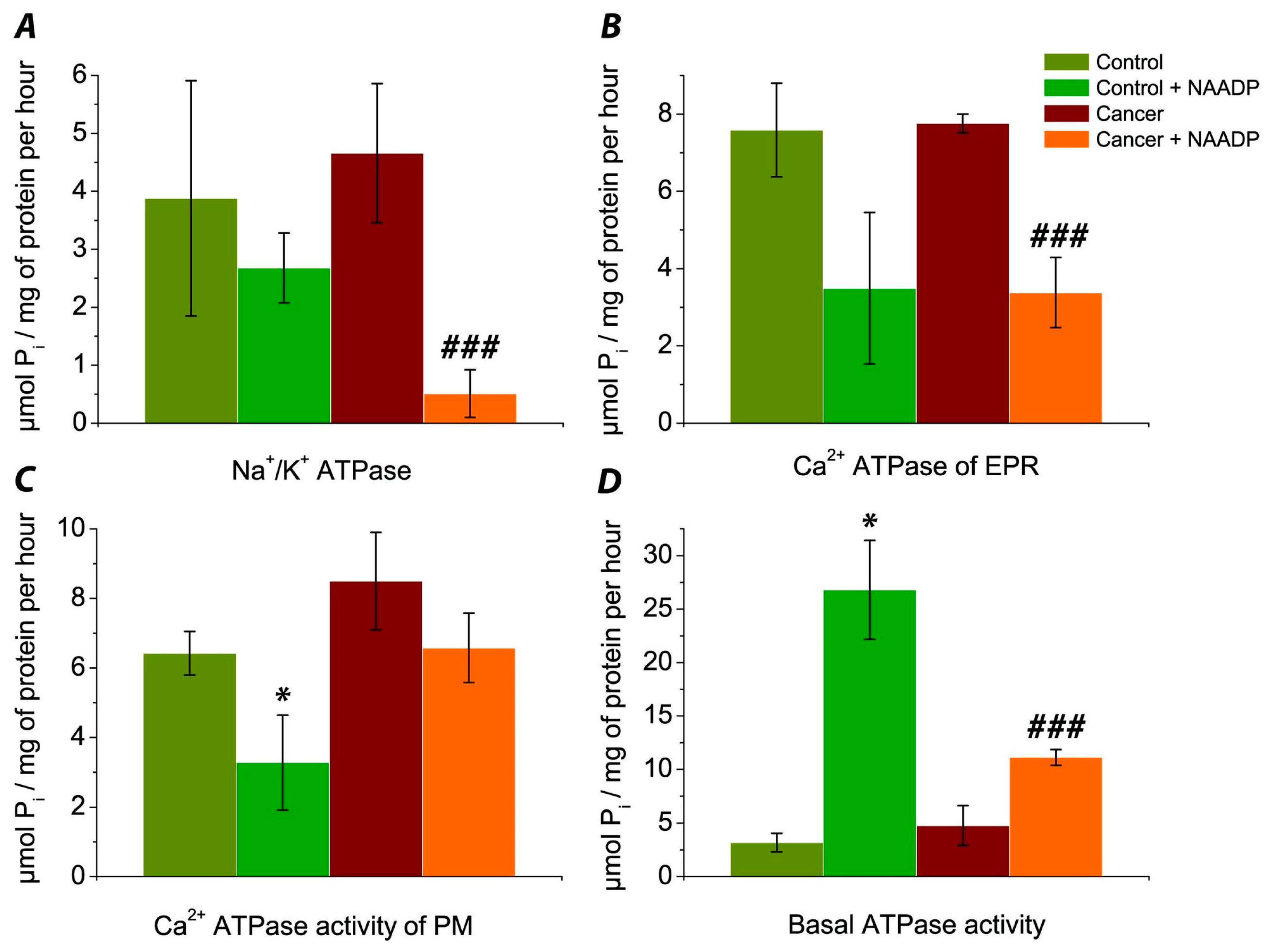
3.1. Evaluation of the Effect of NAADP on the Activity of Na+/K+ ATPase of Post-Mitochondrial Fraction of Human Colorectal Cancer Tissue Samples vs. Normal Tissue
3.2. Determination of the Effect of NAADP on the Activity of Ca2+ ATPase EPR of the Post-Mitochondrial Fraction of Colorectal Cancer Tissue Samples vs. Normal Tissue
3.3. The Effect of NAADP on the Activity of Ca2+ ATPase PM of the Post-Mitochondrial Fraction of Colorectal Cancer Cells vs. Normal Tissue
3.4. Comparison of Basal ATPase Activity without Exposure and under the Action of NAADP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of Colorectal Cancer: Incidence, Mortality, Survival, and Risk Factors. Prz. Gastroenterol 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Macfarlane, G.T.; Macfarlane, S. Intestinal Bacteria and Ulcerative Colitis. Curr. Issues Intest. Microbiol. 2003, 4, 9–20. [Google Scholar] [PubMed]
- Kushkevych, I.; Cejnar, J.; Treml, J.; Dordević, D.; Kollar, P.; Vítězová, M. Recent Advances in Metabolic Pathways of Sulfate Reduction in Intestinal Bacteria. Cells 2020, 9, 698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushkevych, I.; Leščanová, O.; Dordević, D.; Jančíková, S.; Hošek, J.; Vítězová, M.; Buňková, L.; Drago, L. The Sulfate-Reducing Microbial Communities and Meta-Analysis of Their Occurrence during Diseases of Small–Large Intestine Axis. JCM 2019, 8, 1656. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Cummings, J.H.; Macfarlane, G.T. Growth and Activities of Sulphate-Reducing Bacteria in Gut Contents of Healthy Subjects and Patients with Ulcerative Colitis. FEMS Microbiol. Lett. 1991, 86, 103–112. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Kollár, P. Analysis of Physiological Parameters of Desulfovibrio Strains from Individuals with Colitis. Open Life Sci. 2019, 13, 481–488. [Google Scholar] [CrossRef]
- Dordević, D.; Jančíková, S.; Vítězová, M.; Kushkevych, I. Hydrogen Sulfide Toxicity in the Gut Environment: Meta-Analysis of Sulfate-Reducing and Lactic Acid Bacteria in Inflammatory Processes. J. Adv. Res. 2020, 27, 55–69. [Google Scholar] [CrossRef]
- Kushkevych, I.; Kotrsová, V.; Dordević, D.; Buňková, L.; Vítězová, M.; Amedei, A. Hydrogen Sulfide Effects on the Survival of Lactobacilli with Emphasis on the Development of Inflammatory Bowel Diseases. Biomolecules 2019, 9, 752. [Google Scholar] [CrossRef] [Green Version]
- Kushkevych, I.; Dordević, D.; Vítězová, M. Possible Synergy Effect of Hydrogen Sulfide and Acetate Produced by Sulfate-Reducing Bacteria on Inflammatory Bowel Disease Development. J. Adv. Res. 2020, 27, 71–78. [Google Scholar] [CrossRef]
- Yun, C.; Lee, S. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef] [Green Version]
- Yim, W.W.-Y.; Mizushima, N. Lysosome Biology in Autophagy. Cell Discov. 2020, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Alevizopoulos, K.; Calogeropoulou, T.; Lang, F.; Stournaras, C. Na+/K+ ATPase Inhibitors in Cancer. Curr. Drug Targets 2014, 15, 988–1000. [Google Scholar] [CrossRef]
- Lee, H.C. Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP)-Mediated Calcium Signaling. J. Biol. Chem. 2005, 280, 33693–33696. [Google Scholar] [CrossRef] [Green Version]
- Axelrad, J.E.; Lichtiger, S.; Yajnik, V. Inflammatory Bowel Disease and Cancer: The Role of Inflammation, Immunosuppression, and Cancer Treatment. World J. Gastroentero. 2016, 22, 4794. [Google Scholar] [CrossRef] [PubMed]
- Curry, M.C.; Roberts-Thomson, S.J.; Monteith, G.R. Plasma Membrane Calcium ATPases and Cancer. BioFactors 2011, 37, 132–138. [Google Scholar] [CrossRef]
- Prasad, V.; Okunade, G.W.; Miller, M.L.; Shull, G.E. Phenotypes of SERCA and PMCA Knockout Mice. Biochem. Biophys. Res. Commun. 2004, 322, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, T.; Dufrasne, F.; Kiss, R. Na+ /K+ -ATPase and Cancer. Pharm. Pat. Anal. 2012, 1, 91–106. [Google Scholar] [CrossRef]
- Ferents, I.M.; Bychkova, S.V.; Bychkov, M.A. Peculiarities of the Effects of Bile Acids on Atpase Activity of the Colon Mucosa in Patients with Overweight and Irritable Bowel Syndrome. Wiad. Lek. 2020, 73, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Hreniukh, V.; Bychkova, S.; Kulachkovsky, O.; Babsky, A. Effect of Bafilomycin and NAADP on Membrane-Associated ATPases and Respiration of Isolated Mitochondria of the Murine Nemeth-Kellner Lymphoma: Mitochondria and ATPase Activities in Lymphoma. Cell Biochem. Funct. 2016, 34, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Chemaly, E.R.; Troncone, L.; Lebeche, D. SERCA Control of Cell Death and Survival. Cell Calcium 2018, 69, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Khajah, M.A.; Mathew, P.M.; Luqmani, Y.A. Na+/K+ ATPase Activity Promotes Invasion of Endocrine Resistant Breast Cancer Cells. PLoS ONE 2018, 13, e0193779. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Xu, Y.; Yang, S.; Li, B.; Huang, L.; Lou, G. Comprehensive Analysis of the Expression of Sodium/Potassium-ATPase α Subunits and Prognosis of Ovarian Serous Cystadenocarcinoma. Cancer Cell Int. 2020, 20, 309. [Google Scholar] [CrossRef]
- Baker Bechmann, M.; Rotoli, D.; Morales, M.; del Maeso, M.C.; del García, M.P.; Ávila, J.; Mobasheri, A.; Martín-Vasallo, P. Na,K-ATPase Isozymes in Colorectal Cancer and Liver Metastases. Front. Physiol. 2016, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bychkova, S. Influence of NAADP and Bafilomycine A1 on Activity of ATPase in Liver Postmitochondrial Fraction. Biol. Stud. 2015, 9, 31–40. [Google Scholar] [CrossRef]
- Brown, A.M.; Lew, V.L. The Effect of Intracellular Calcium on the Sodium Pump of Human Red Cells. J. Physiol. 1983, 343, 455–493. [Google Scholar] [CrossRef] [Green Version]
- Morgan, A.J.; Davis, L.C.; Wagner, S.K.T.Y.; Lewis, A.M.; Parrington, J.; Churchill, G.C.; Galione, A. Bidirectional Ca2+ Signaling Occurs between the Endoplasmic Reticulum and Acidic Organelles. J. Cell Biol. 2013, 200, 789–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosterin, S.O.; Veklich, T.O.; Pryluts’kyi, I. Kinetic interpretation of the original pH-dependence of enzymatic activity of „basal” Mg2+ ATPase of the smooth muscle sarcolemma. Ukr. Biokhim. Zh. 2005, 2005, 37–45. [Google Scholar]
- Song, Y.; Lee, S.-Y.; Kim, S.; Choi, I.; Kim, S.-H.; Shum, D.; Heo, J.; Kim, A.-R.; Kim, K.M.; Seo, H.R. Inhibitors of Na+/K+ ATPase Exhibit Antitumor Effects on Multicellular Tumor Spheroids of Hepatocellular Carcinoma. Sci. Rep. 2020, 10, 5318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trenti, A.; Grumati, P.; Cusinato, F.; Orso, G.; Bonaldo, P.; Trevisi, L. Cardiac Glycoside Ouabain Induces Autophagic Cell Death in Non-Small Cell Lung Cancer Cells via a JNK-Dependent Decrease of Bcl-2. Biochem. Pharmacol. 2014, 89, 197–209. [Google Scholar] [CrossRef]
- Varga, K.; Hollósi, A.; Pászty, K.; Hegedűs, L.; Szakács, G.; Tímár, J.; Papp, B.; Enyedi, Á.; Padányi, R. Expression of Calcium Pumps Is Differentially Regulated by Histone Deacetylase Inhibitors and Estrogen Receptor Alpha in Breast Cancer Cells. BMC Cancer 2018, 18, 1029. [Google Scholar] [CrossRef] [Green Version]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Targeting Ca2+ Transport in Cancer: Close Reality or Long Perspective? Expert Opin. Ther. Targets 2013, 17, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Ribiczey, P.; Tordai, A.; Andrikovics, H.; Filoteo, A.G.; Penniston, J.T.; Enouf, J.; Enyedi, Á.; Papp, B.; Kovács, T. Isoform-Specific up-Regulation of Plasma Membrane Ca2+ ATPase Expression during Colon and Gastric Cancer Cell Differentiation. Cell Calcium 2007, 42, 590–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aung, C.S.; Ye, W.; Plowman, G.; Peters, A.A.; Monteith, G.R.; Roberts-Thomson, S.J. Plasma Membrane Calcium ATPase 4 and the Remodeling of Calcium Homeostasis in Human Colon Cancer Cells. Carcinogenesis 2009, 30, 1962–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faris, P.; Pellavio, G.; Ferulli, F.; Di Nezza, F.; Shekha, M.; Lim, D.; Maestri, M.; Guerra, G.; Ambrosone, L.; Pedrazzoli, P.; et al. Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) Induces Intracellular Ca2+ Release through the Two-Pore Channel TPC1 in Metastatic Colorectal Cancer Cells. Cancers 2019, 11, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushkevych, I.; Bychkov, M.; Bychkova, S.; Gajdács, M.; Merza, R.; Vítězová, M. ATPase Activity of the Subcellular Fractions of Colorectal Cancer Samples under the Action of Nicotinic Acid Adenine Dinucleotide Phosphate. Biomedicines 2021, 9, 1805. https://doi.org/10.3390/biomedicines9121805
Kushkevych I, Bychkov M, Bychkova S, Gajdács M, Merza R, Vítězová M. ATPase Activity of the Subcellular Fractions of Colorectal Cancer Samples under the Action of Nicotinic Acid Adenine Dinucleotide Phosphate. Biomedicines. 2021; 9(12):1805. https://doi.org/10.3390/biomedicines9121805
Chicago/Turabian StyleKushkevych, Ivan, Mykola Bychkov, Solomiia Bychkova, Márió Gajdács, Romana Merza, and Monika Vítězová. 2021. "ATPase Activity of the Subcellular Fractions of Colorectal Cancer Samples under the Action of Nicotinic Acid Adenine Dinucleotide Phosphate" Biomedicines 9, no. 12: 1805. https://doi.org/10.3390/biomedicines9121805
APA StyleKushkevych, I., Bychkov, M., Bychkova, S., Gajdács, M., Merza, R., & Vítězová, M. (2021). ATPase Activity of the Subcellular Fractions of Colorectal Cancer Samples under the Action of Nicotinic Acid Adenine Dinucleotide Phosphate. Biomedicines, 9(12), 1805. https://doi.org/10.3390/biomedicines9121805







