Abstract
It is a general goal to improve wound healing, especially of chronic wounds. As light therapy has gained increasing attention, the positive influence on healing progression of water-filtered infrared A (wIRA), a special form of thermal radiation, has been investigated and compared to the detrimental effects of UV-B irradiation on wound closure in vitro. Models of keratinocyte and fibroblast scratches help to elucidate effects on epithelial and dermal healing. This study further used the simulation of non-optimal settings such as S. aureus infection, chronic inflammation, and anti-inflammatory conditions to determine how these affect scratch wound progression and whether wIRA treatment can improve healing. Gene expression analysis for cytokines (IL1A, IL6, CXCL8), growth (TGFB1, PDGFC) and transcription factors (NFKB1, TP53), heat shock proteins (HSP90AA1, HSPA1A, HSPD1), keratinocyte desmogleins (DSG1, DSG3), and fibroblast collagen (COL1A1, COL3A1) was performed. Keratinocyte and fibroblast wound healing under non-optimal conditions was found to be distinctly reduced in vitro. wIRA treatment could counteract the inflammatory response in infected keratinocytes as well as under chronic inflammatory conditions by decreasing pro-inflammatory cytokine gene expression and improve wound healing. In contrast, in the anti-inflammatory setting, wIRA radiation could re-initiate the acute inflammatory response necessary after injury to stimulate the regenerative processes and advance scratch closure.
1. Introduction
Wound healing is a complex process in which different epidermal and dermal cell types as well as leukocytes are involved and is coordinated by cytokines and growth factors [1]. The focus here was on fibroblasts, which are involved in the production of extracellular matrix and thus fill the wound with new tissue [2], and keratinocytes, which are involved in the inflammatory reaction and can ultimately close the injured area [3]. Prolonged inflammation delays wound closure by preventing the transition from degrading to proliferative processes [4]. Chronic wounds represent a major challenge in hospitals and health care settings. These wounds may remain non-healing due to a microbial infection [5,6] and continuous inflammatory conditions [7]. Moreover, anti-inflammatory conditions have been reported to affect wound healing processes [8,9,10].
Positive effects on wound healing by photobiomodulation through light therapy have been described [11], so it is of interest to investigate the effects of light treatment on cell viability and cell proliferation during wound healing. Recently, acceleration of healing processes after application of low-level laser therapy (LLLT) has been evaluated [12]. Red light (600–760 nm) was found to induce the migration of human epidermal stem cells [13], stimulate keratinocyte proliferation [14], and increase migration of fibroblasts isolated from diabetic patients [15]. Red or infrared light with less than 200 mW/cm2 irradiance and a wavelength range of 600–1000 nm was further reported to promote the skin repair process in experimental animal and human wounds [16,17,18,19]. This suggests beneficial roles for red light therapy in re-epithelialization and extracellular matrix formation during wound healing, probably by enhancing fibroblast migration and proliferation as well as collagen synthesis [20,21] and by modulating the timing and release of growth factors and cytokines [22].
Water-filtered infrared A (wIRA) is a special form of thermal radiation with high penetration capacity into the tissue with low thermal surface load. It is characterized by a deep-reaching thermal radiation with comparatively low heat-induced pain sensation on the skin surface in its typical irradiance levels between 60 and 120 mW/cm2 [23,24]. Studies have shown that it promotes the healing of acute and chronic wounds through thermal and non-thermal effects [25,26,27]. Indeed, it has been found that wIRA-irradiation evokes an increase in local temperature [26,27], oxygen partial pressure [27], and tissue blood flow in wounds [26]. These factors substantially support the healing process, especially in chronic wounds, where generally ischemic, hypoxic, and hypothermic conditions are found [28]. Additionally, wIRA has nonthermal effects, which are thought to directly stimulate cells and cellular structures by infrared radiation. These range from stimulation of wound repair [29] and cell protective effects [30] to target-oriented growth of neurons [31] and possible effects on pain receptors [27]. So far, Zöller et al. have reported on foreskin and keloid fibroblasts treated with convective heat and/or wIRA to assess their potential as treatment regimens for keloids and hypertrophic scars [32]. They found a temperature dependent induction of a spherical cell shape, a reduction in collagen type I synthesis, and decreased TGF-β1 secretion in the fibroblasts. No influence of wIRA-irradiation on MMP-1 was observed. However, wIRA-treatment restored original cell morphology in foreskin fibroblasts, and beyond that, collagen type I synthesis and TGF-β1 secretion in keloid fibroblasts [32]. Knels et al. evaluated wIRA for the treatment of oxidative stress in cells as it is found in elderly or diabetic patients [33]. Therefore, they exposed fibroblast cultures to glyoxal to induce glycation of proteins and lipids, mimicking the conditions of oxidative stress, and determined their rescue from apoptotic death by wIRA. They found that wIRA-irradiation diminished the effects of glyoxal-induced stress such as ROS production, translocation of phosphatidylserine, and DNA fragmentation, which are considered the main events of apoptosis, while being well tolerated by the cultured fibroblasts [33].
It is therefore of interest to further elucidate the cellular effects of wIRA in order to be able to use this effectively as a treatment option. The knowledge gained provides important information on the cellular influence of wIRA on the processes taking place in keratinocytes and fibroblasts during wound healing. Fibroblasts are involved in the production of extracellular matrix and are therefore essential to fill the wound with new tissue [2]. Keratinocytes are not only involved in the eventual closure of the injured site through epithelialization, but also take part directly in immunological processes and inflammatory reactions as non-professional immune cells [3,34,35]. One of the most studied in vitro models for wound healing is the mechanical damage to confluent cell layers (“scratch wound assay”) [36]. This method enables the direct measurement of cell migration and regeneration of the cell layer. HaCaT cells, primary epidermal keratinocytes, and primary dermal fibroblasts have already been successfully employed in the scratch wound assay [37]. Preliminary studies for wIRA-effects on wound closure in vitro have already shown distinct differences of a 10 min treatment of wounded HaCaT keratinocyte monolayers compared to the untreated control and heat treatment alone [38]. A further advantage of the scratch wound assay is that concentrations of growth factors and cytokines can be easily assessed by flow cytometry or ELISA techniques and gene transcript levels can be determined by real-time qPCR [39]. Furthermore, the milieu of scratch assays can be easily altered to reflect different wound conditions [40]. Here, cells were infected with S. aureus to mimic wound infection, primed with TNF-α and IFN-γ to establish chronic inflammatory conditions [41], and stimulated with IL-4 and IL-13 to yield a TH2-dominant, anti-inflammatory milieu [9,42].
In this study, keratinocyte and fibroblast wound healing in vitro under optimal and non-optimal conditions was investigated using cell scratch wound healing assays. Furthermore, gene expression analysis for pro-inflammatory cytokines (IL1A, IL6, CXCL8), growth factors (TGFB1, PDGFC), transcription factors (NFKB1, TP53), and heat shock proteins (HSP90AA1, HSPA1A, HSPD1) as well as desmogleins (DSG1, DSG3) in keratinocytes and collagen (COL1A1, COL3A1) in fibroblasts was performed to evaluate cellular responses to healing progression (Table 1) under optimal conditions and infection with S. aureus as well as after priming with TNF-α/IFN-γ (chronic inflammatory milieu) or IL-4/IL-13 (anti-inflammatory conditions). Moreover, scratches were treated with wIRA and heating w/o wIRA as the control to discriminate between thermal and non-thermal effects of wIRA [43,44] and scratches under optimal conditions were also irradiated with UV-B for comparison.

Table 1.
Overview of the biological functions of the examined target genes.
2. Materials and Methods
Keratinocyte and fibroblast culture: HaCaT cells (provided by Prof. Fusenig, Heidelberg, Germany) and normal human dermal fibroblasts (NHDF; Promocell, Heidelberg, Germany) were cultured in Dulbecco’s modified Eagle’s Medium (DMEM; BioConcept, Alschwil, Switzerland) supplemented with 1% antibiotic-antimycotic solution (BioConcept, Alschwil, Switzerland) and 10% fetal calf serum (FCS; PAN-Biotech, Aidenbach, Germany). The cells were cultured for seven days in 75-cm2 cell culture flasks (Greiner bio-one, Frickenhausen, Germany) at 37 °C and in a humidified atmosphere containing 5% CO2. For experiments, cells were harvested through trypsin-EDTA (Gibco Thermo Fisher Scientific, Waltham, MA, USA) treatment, seeded into 12-well plates (Greiner bio-one, Frickenhausen, Germany) at a density of 40,000 cells/cm2, and cultured for 48 h to confluence.
Scratch wound assay: Cell monolayers were scratched with a sterile pipette tip and washed with medium to remove any loose cells. Scratches were then treated with the light emitting devices and scratches kept in the incubator at 37 °C served as untreated controls. Cells were incubated for 1, 3, 6, 12, 24, and 48 h before staining with hematoxylin (Merck Millipore, Darmstadt, Germany) for the evaluation of scratch closure progression or gene expression analysis. Microscopic assessment of the scratches was carried out using a VHX 950F digital microscope (KEYENCE DEUTSCHLAND GmbH, Neu-Isenburg, Germany) and images were obtained. The scratch area was determined in mm2 using the VHX 950F software (v01., KEYENCE DEUTSCHLAND GmbH, Neu-Isenburg, Germany). From these results, scratch wound healing was calculated in [%] for each time point relative to the scratch area at 1 h. To test the scratch wound healing under non-optimal conditions, cell culture media were replaced by primed media 24 h before the experiment. Chronic inflammatory conditions were mimicked by the addition of 10 ng/mL TNF-α (7Bioscience, Neuenburg, Germany) and 5 ng/mL IFN-γ (7Bioscience, Neuenburg, Germany) while anti-inflammatory conditions were simulated using 50 ng/mL IL-4 (7Bioscience, Neuenburg, Germany) and 50 ng/mL IL-13 (7Bioscience, Neuenburg, Germany). To investigate wound healing under infection conditions, cells were infected with Staphylococcus aureus 1 h before the start of the experiments using 102 cfu/mL in cell culture media. For preparation of bacteria, S. aureus ATCC 6538 (DSMZ, Braunschweig, Germany) colonies from Columbia agar plates (bioMerieux, Nürtingen, Germany) were suspended in Tryptic Soy broth (TSB, Oxoid, Dardilly, France) and cultivated for 24 h at 37 °C under vigorous shaking. The number of microbes in the solution was determined by serial dilution followed by plating on Columbia agar. After 24 h incubation at 37 °C, colonies were counted and microbial count (in CFU/mL) of the bacterial suspension was calculated.
Treatment with light emitting devices and controls: Treatments were performed in 12-well plates after replacing the culture medium with 800 µL PBS (phosphate buffered saline, Promocell, Heidelberg, Germany) to avoid desiccation of the cells. Samples were treated with wIRA (wIRA-irradiator type 750; Hydrosun®, Müllheim, Germany) at a distance of 33 cm for 20 min. Control experiments were included with a simulated increase in temperature of 8 degrees over 20 min as it may occur during a clinical application of wIRA [26] to distinguish between effects caused by the wIRA-treatment and pure warming of the sample. These samples were designated as heating w/o wIRA. Temperature profiles were recorded locally on the cells using thin thermocouples (type K, TC Direct, Mönchengladbach, Germany). In addition, tests were performed with UV-B irradiation (UV109; Waldmann Medizintechnik, Villingen-Schwenningen, Germany) at a distance of 16 cm for 20 min.
Gene expression analysis: After removal of the cell culture supernatants, human cells were lysed by adding RLT buffer (Qiagen, Hilden, Germany) containing 10 µL/mL β-mercaptoethanol and subsequently incubated for 3 min on ice and 3 min under shaking. Lysates were subsequently loaded to QIA Shredder spin columns (Qiagen, Hilden, Germany), and centrifuged at 4 °C and 10.000× g for 2 min. RNA purification was performed using the RNeasy® Mini Kit and the QIAcube (Qiagen, Hilden, Germany). To avoid DNA contamination, genomic DNA was removed from the extracted RNA using the Ribonuclease assay DNase I, RNase Free (Thermo Fisher Scientific, Waltham, MA, USA). A single master mix prepared on ice contained 41 µL extracted RNA, 5 µL 10× Reaction Buffer (Mg2+), and 4 µL DNase I. The latter was activated at 37 °C for 30 min. Finally, EDTA was added as a chelating agent to stop the Mg2+-dependent nuclease activity of DNase I during 10 min incubation at 65 °C. RNA concentration was determined using the SPECTROstar® Omega with a UV/Vis plate (BMG Labtech, Ortenberg, Germany) by measuring the OD at 260 nm, with the assumption that OD of 1.0 equals 40 ng/mL RNA. The purity of the RNA sample was evaluated using the ratio of the absorbance at 260 nm and 280 nm with a ratio’s threshold between 1.9 and 2.1. All samples fulfilled this requirement. Subsequently, absolute RNA amounts were adjusted to 60–400 ng in 10 µL. For reverse transcription, the High Capacity cDNA Reverse Transcription Kit by Applied Biosystems (Thermo Fisher Scientific, Waltham, MA, USA) was used following the manufacturer’s instructions. The PCR protocol ran with the Mastercycler® gradient thermal cycler (Eppendorf) included primer annealing for 10 min at 25 °C, reverse transcription for 120 min at 37 °C, and termination for 5 min at 85 °C cDNA samples were stored at −80 °C until further use. After reverse transcription, cDNA was diluted respectively to obtain a final test concentration of 0.5 ng/mL. RTqPCR was performed for gene expression analyses using the QuantiNova™ SYBR Green PCR Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. Briefly, master mix prepared on ice contained forward and reversed primers (test concentration each 0.5 µM) and cDNA or Yellow Template Dilution Buffer as no template control. Using the qTOWER3G (Analytik Jena AG, Thuringia, Germany), the real-time amplification protocol was set for polymerase heat activation at 95 °C for 3 min, and 40 cycles with three steps: denaturation at 95 °C for 5 s, annealing at 57 °C for 10 s, and elongation at 72 °C for 10 s. Signals were detected at λex/λem 470 nm/520 nm. Finally, a melting curve from 65 °C to 95 °C served as the amplicon control. All primer sequences or ordering IDs are listed in Table 2. Samples were measured in technical duplicates. Expression levels of target genes were normalized to the housekeeping gene ACTB.

Table 2.
List of primer sequences and ordering IDs used for the SYBR Green-based RTqPCR.
Data analysis: Two independent experiments were executed and measurements were performed in duplicate. Transcription levels are presented as fold changes of the respective untreated control under optimal conditions at 1 h. All values are expressed as means ± SD (standard deviation). One-way analysis of variance was carried out to determine statistical significances (Microsoft® Excel 2010). Differences were considered statistically significant at a level of p < 0.05. Asterisks [*] indicate significant differences in scratch wound healing or transcription level compared to 1 h (* p < 0.05, ** p < 0.01, *** p < 0.001). Hashtags [#] designate significant differences of scratch wound healing or transcription levels at the respective time point compared to the control under optimal conditions (# p < 0.05, ## p < 0.01, ### p < 0.001) while the paragraph character [§] shows significant differences between untreated and treated samples under non-optimal conditions at the respective time point (§ p < 0.05, §§ p < 0.001, §§§ p < 0.001). The plus sign [+] specifies significant differences between wIRA-treated samples and those receiving heating w/o wIRA under non-optimal conditions at the respective time point (+ p < 0.05, ++ p < 0.01, +++ p < 0.001).
3. Results
3.1. Scratch Wound Healing and Gene Expression Analysis over Time
HaCaT keratinocytes (Figure 1) and dermal fibroblasts (Figure 2) were investigated as model cells for epithelial and dermal wound healing. Scratch width declined over time; the first migrating cells could be noted at the time point after 12 h for keratinocytes (Figure 1A) and after 6 h for fibroblasts (Figure 2A). Wound healing progression, displayed as scratch wound healing in [%], reached almost 100% after 48 h for both cell types (p < 0.001; Figure 1B and Figure 2B). Furthermore, gene expression analysis was performed for pro-inflammatory cytokines, growth factors, transcription factors, and stress response genes as well as desmogleins such as cell adhesion molecules and antimicrobial peptides for keratinocytes along with collagen 1 and 3 in the case of fibroblasts to investigate their profiles during wound healing. It was found that with the progression of healing, the expression of pro-inflammatory cytokines gradually decreased in keratinocytes (Figure 1C) over time while a distinct pro-inflammatory reaction was observed in fibroblasts (Figure 2C) accompanied by an increase in IL1A expression most notably after 6 h (p < 0.01) and of CXCL8 expression, reaching a peak as early as 3 h (p < 0.05). TGFB1 expression increased over time in keratinocytes (Figure 1C) and showed a peak as early as 12 h in fibroblasts (p < 0.01; Figure 2C). A slight surge in PDGFC expression was observed for keratinocytes at 3 h with a subsequent decrease (Figure 1C) while fibroblasts demonstrated a steady rise in transcript levels over time under these conditions (p < 0.05; Figure 2C). Correspondingly, gene expression of TP53 and NFKB was elevated during wound healing in keratinocytes (Figure 1C) and fibroblasts (Figure 2C). DSG1 and DSG3 expression by keratinocytes was significantly increased over time (p < 0.05; Figure 1C). Moreover, a significant increase in antimicrobial peptide gene expression by keratinocytes, such as S100A7 (p < 0.01), RNASE7 (p < 0.05), and DEFB1 (p < 0.001) was noted with scratch wound healing (Figure 1B). It was also observed that, here, the fold changes in transcript levels were distinctly higher compared to those in the regulatory mediators. This was also observed for DSG1. Regeneration of the dermal layer was assessed by collagen expression in fibroblasts. COL1A1 and COL3A1 expression increased significantly over time corresponding to the reformation of the fibroblast cell layer (p < 0.01; Figure 2C).
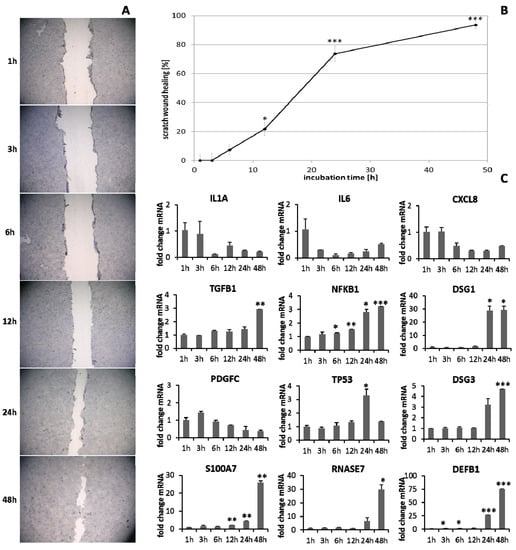
Figure 1.
(A) Scratch wound healing of HaCaT keratinocytes under optimal conditions and (B) evaluation of scratch wound healing in [%]. (C) Gene expression profiles over time during wound healing. Asterisks [*] indicate significant differences in scratch wound healing or transcription level compared to 1 h (* p < 0.05, ** p < 0.01, *** p < 0.001).
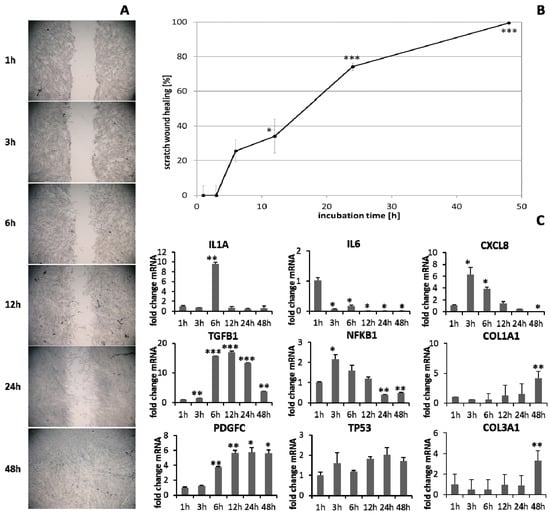
Figure 2.
(A) Scratch wound healing of dermal fibroblasts under optimal conditions and (B) evaluation of scratch wound healing in [%]. (C) Gene expression profiles over time during wound healing. Asterisks [*] indicate significant differences in scratch wound healing or transcription level compared to 1 h (* p < 0.05, ** p < 0.01, *** p < 0.001).
3.2. Scratch Treatment with wIRA and UV-B Radiation
The effect of wIRA radiation, heating w/o wIRA (as thermal control) and UV-B irradiation on scratch wound healing of keratinocytes (Figure 3A,B) and fibroblasts (Figure 3C,D) was investigated under optimal conditions for the cells, referring to a physiological cell environment. Keratinocyte scratches showed an improvement in wound healing after treatment with wIRA (Figure 3A,B) with a significantly increased scratch closure at 24 h (p < 0.01). Moreover, wIRA radiation distinctly improved wound healing of fibroblast scratches in vitro (Figure 3C,D) with a significantly increased scratch closure at 12 h (p < 0.05). Heating w/o wIRA for 20 min slightly improved keratinocyte scratch healing compared to the untreated control (p < 0.05), but delayed healing of fibroblast scratches (p < 0.01). UV-B irradiation resulted in severe damage of the keratinocytes (Figure 3A,B) and exerted a detrimental effect on fibroblasts (Figure 3C,D) resulting in scratch wounds that remained open and showed no tendency for healing (p < 0.001).
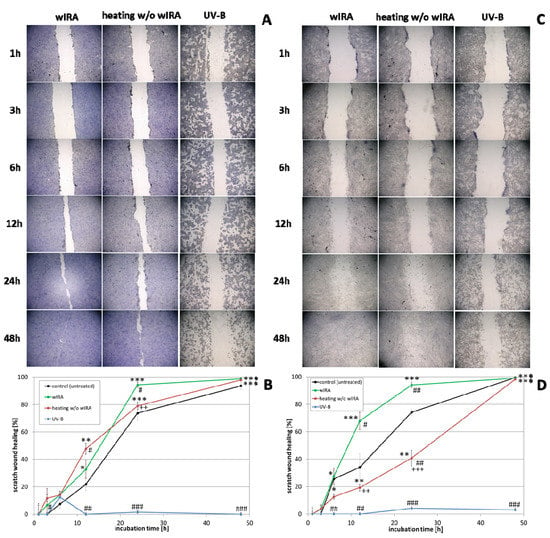
Figure 3.
(A) HaCaT keratinocyte scratch wound closure over time after wIRA radiation, heating w/o wIRA and UV-B irradiation and (B) evaluation of scratch wound healing in [%]. (C) Closure of dermal fibroblast scratches over 48 h after wIRA radiation, heating w/o wIRA and UV-B irradiation, and (D) the evaluation of scratch wound healing progression in [%]. Asterisks [*] indicate significant differences in scratch wound healing or transcription level compared to 1 h (* p < 0.05, ** p < 0.01, *** p < 0.001). Hashtags [#] designate significant differences of scratch wound healing or transcription levels at the respective time point compared to the control under optimal conditions (# p < 0.05, ## p < 0.01, ### p < 0.001) while the plus sign [+] specifies significant differences between wIRA-treated samples and those receiving heating w/o wIRA under non-optimal conditions at the respective time point (++ p < 0.01, +++ p < 0.001).
Transcript levels of IL1A, IL6 (p < 0.01) and CXCL8 (p < 0.05) were distinctly elevated at 6 h after UV-B irradiation in keratinocytes possibly resulting in the detrimental activation of the inflammatory response (Figure 4). Radiation with wIRA did not change pro-inflammatory cytokine expression compared to the untreated control. A significant decrease of IL1A was noted at 6 h after heating w/o wIRA (p < 0.05). Physiological levels of TGFB1 were noted after treatment with wIRA but decreased gene expression was found after heating alone. Surprisingly, UV-B exposure significantly induced TGFB1 expression at 6 h after irradiation (p < 0.05; Figure 4). PDGFC expression showed no distinct changes with wIRA, but was markedly reduced at 6 h after heating w/o wIRA (P < 0.05) and UV-B irradiation (p < 0.05), as were the NFKB1 levels (p < 0.01) and TP53 gene expression (Figure 4). In contrast, TP53 levels were significantly elevated 6 h after wIRA radiation compared to the control (p < 0.05). Heat shock proteins such as HSP90, HSPA1, and HSPD1 possess crucial roles as chaperones in protein folding and as possible signaling regulators inducing cellular stress responses [65,66,67]. In accordance, induction of HSP90AA1, HSPA1A, and HSPD1 gene expression was evaluated after the use of light and heat emitting devices. Stress response genes were differently transcribed in wIRA-treated and UV-B-irradiated keratinocytes after scratching at 12 h (Figure 4). HSPA1A was readily increased by heating w/o wIRA compared to the control (p < 0.05) and significantly decreased by UV-B irradiation (p < 0.05). However, expression of this gene was not altered after wIRA treatment. HSP90AA1 and HSPD1 transcription was slightly increased by UV-B irradiation without reaching statistical significance, while wIRA (p < 0.05), and especially heating alone, markedly decreased HSPD1 levels at 12 h (p < 0.05). Transcript levels of the cell adhesion molecule DSG1 were slightly higher and gene expression of DSG3 significantly elevated (p < 0.01) after wIRA treatment compared to the control (Figure 4), possibly corresponding to the differences observed in the healing progression over time (Figure 3B). Significantly reduced amounts of DSG1 and DSG3 were observed after UV-B irradiation (p < 0.05). The antimicrobial peptide genes were differently transcribed after 48 h dependent on the treatment (Figure 4). DEFB1 levels were elevated by wIRA while heating alone (p < 0.05) and UV-B irradiation (p < 0.01) significantly reduced it. RNASE7 and S100A7 were increased by wIRA (p < 0.05) and UV-B compared to the control while heating alone had a lesser effect.
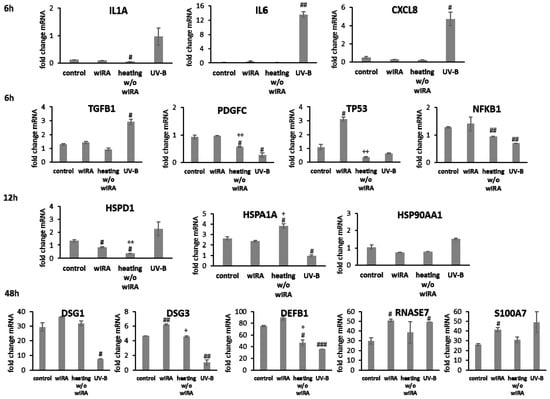
Figure 4.
Gene expression profiles of pro-inflammatory cytokine genes and growth factor genes after 6 h and heat shock protein gene transcript levels after 12 h as well as desmoglein gene and AMP gene expression after 48 h for HaCaT keratinocyte scratches treated with wIRA, heating w/o wIRA and UV-B irradiation. Hashtags [#] designate significant differences of scratch wound healing or transcription levels at the respective time point compared to the control under optimal conditions (# p < 0.05, ## p < 0.01, ### p < 0.001) while the plus sign [+] specifies significant differences between wIRA-treated samples and those receiving heating w/o wIRA under non-optimal conditions at the respective time point (+ p < 0.05, ++ p < 0.01).
In fibroblasts irradiated with UV-B, IL1A expression after 1 h, IL6 levels after 3 h, and CXCL8 amounts after 6 h were distinctly elevated compared to wIRA and heating alone (Figure 5). After wIRA treatment and heating alone, IL1A (p < 0.05) and CXCL8 (p < 0.01) were significantly reduced compared to the control. TGFB1 expression at 6 h after UV-B irradiation was comparable to the control but without eliciting cell migration or proliferation and distinctly higher fold changes in mRNA levels in fibroblasts compared to keratinocytes were noted. PDGFC was distinctly reduced compared to the control (Figure 5). TGFB1 and PDGFC gene expression were also significantly reduced by wIRA treatment and heating w/o wIRA at 6 h (p < 0.001), which would match the absent inflammatory response. However, growth factor gene expression increased at later time points, where it corresponded with the healing progression. TP53 transcription was significantly induced in fibroblasts by wIRA radiation after 6 h (p < 0.01) but not by heating alone (p < 0.01). NFKB gene expression by fibroblasts after treatment with wIRA corresponded to the untreated control while heating w/o wIRA slightly and UV-B irradiation significantly (p < 0.05) reduced NFKB transcript levels in fibroblasts (Figure 5). Stress response gene transcription analysis revealed a distinct induction of HSP90AA1 and HSPA1A expression in fibroblasts by UV-B irradiation after 12 h (p < 0.01) comparable to keratinocytes together with an increase in HSPD1 levels (Figure 5). HSPA1A was also readily induced by heating w/o wIRA (p < 0.05), but affected by wIRA treatment to a much lesser extent (p < 0.05) while HSP90AA1 (p < 0.01) and HSPD1 levels in fibroblasts were reduced by wIRA radiation at this time point (Figure 5). COL3A1 was found to be significantly increased after wIRA treatment (p < 0.01) compared to heating w/o wIRA (Figure 5), corresponding to the healing progression observed (Figure 3D). Interestingly, a most pronounced increase in COL1A1 (p < 0.01) and COL3A1 (p< 0.05) was also observed after UV-B irradiation in the fibroblasts.
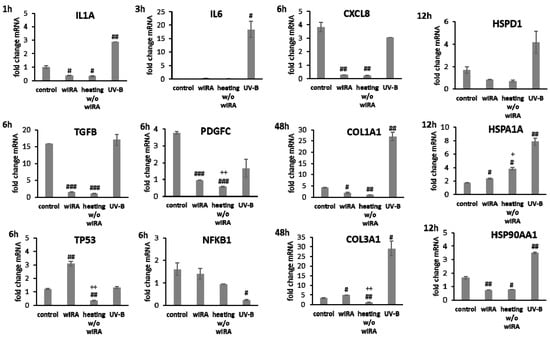
Figure 5.
Gene expression profiles of pro-inflammatory cytokine genes after 1 h (IL1A), 3 h (IL6), and 6 h (CXCL8) for dermal fibroblasts scratches treated with wIRA, heating w/o wIRA and UV-B irradiation. Furthermore, differences for growth factor gene expression were evaluated after 6 h and heat shock protein gene transcript levels after 12 h as well as collagen gene transcription after 48 h. Hashtags [#] designate significant differences of scratch wound healing or transcription levels at the respective time point compared to the control under optimal conditions (# p < 0.05, ## p < 0.01, ### p < 0.001) while the plus sign [+] specifies significant differences between wIRA-treated samples and those receiving heating w/o wIRA under non-optimal conditions at the respective time point (+ p < 0.05, ++ p < 0.01).
3.3. Scratch Wound Healing under Non-Optimal Conditions
The experimental prerequisites in scratch assays mostly account for optimal cell conditions. Hence, the next step of the study included the readjustment of specific milieus more closely mimicking different in vivo situations, explicitly S. aureus infection, chronic inflammation, and anti-inflammatory conditions. Scratch wound healing progression in keratinocytes (Figure 6A,B) and fibroblasts (Figure 6C,D) was distinctly reduced under all non-optimal conditions. The influence was most pronounced for the infection with S. aureus (p < 0.001), which damaged the cells to an extent that scratch wounds could not be closed neither by keratinocytes nor fibroblasts. S. aureus overgrowth was noted after 12 h, most likely inducing cell death. In accordance, no living cells were noted 24 and 48 h after infection and, consequently, mRNA gene expression analysis could not be performed at these time points. Under anti-inflammatory conditions, where cells were primed with IL-4/IL-13 for 24 h prior to scratching, keratinocyte scratch closure was also significantly impeded, reaching only 60% scratch healing after 48 h (p < 0.001; Figure 6B). The scratch wound healing with fibroblasts was also slightly impeded, achieving only 90% closure after 48 h (p < 0.05; Figure 6D). Chronic inflammatory conditions generated by priming with TNF-α/IFN-γ reduced scratch healing compared to the keratinocyte control under optimal conditions (Figure 6B). The effect of the chronic inflammatory setting was even more pronounced on fibroblasts, reducing cell proliferation and migration at early time points (p < 0.05; Figure 6D).
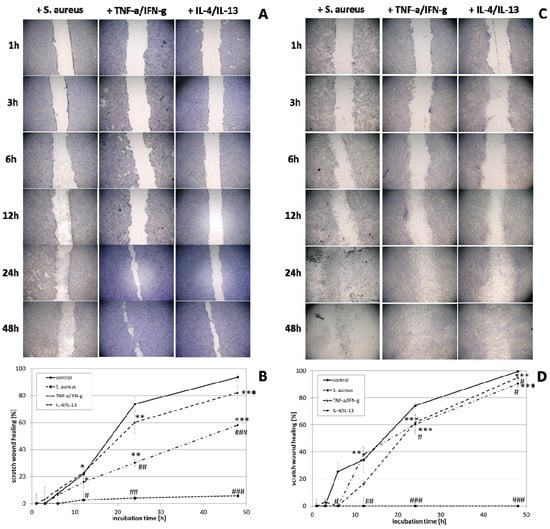
Figure 6.
(A) HaCaT keratinocyte scratch wound closure over time after infection with S. aureus and induction of chronic inflammatory conditions (TNF-α/IFN-γ) as well as anti-inflammatory conditions (IL-4/IL-13) and (B) evaluation of scratch wound healing in [%]. (C) Closure of dermal fibroblast scratches over 48 h after infection with S. aureus and induction of chronic inflammatory conditions (TNF-α/IFN-γ) as well as anti-inflammatory conditions (IL-4/IL-13) and (D) the evaluation of scratch wound healing progression in [%]. Asterisks [*] indicate significant differences in scratch wound healing or transcription level compared to 1 h (* p < 0.05, ** p < 0.01, *** p < 0.001). Hashtags [#] designate significant differences of scratch wound healing or transcription levels at the respective time point compared to the control under optimal conditions (# p < 0.05, ## p < 0.01, ### p < 0.001).
Gene expression analysis revealed a significant induction in pro-inflammatory cytokine transcription in keratinocytes during infection with S. aureus with induction of IL1A (p < 0.05), IL6 (p < 0.01), and CXCL8 (p < 0.05) at early time points (Figure 7). In contrast, infection with S. aureus failed to elicit pro-inflammatory cytokine gene expression in fibroblasts, most likely due to the associated toxic effects of S. aureus (Figure 8). A pro-inflammatory milieu led to slightly increased IL1A mRNA levels in keratinocytes at 6 h compared to the control (Figure 7). IL6 and CXCL8 expression showed no differences to the control (Figure 7). Accordingly, IL1A transcription by fibroblasts was markedly increased (p < 0.01) at one hour under chronic inflammatory conditions (Figure 8). Surprisingly, IL-6 was induced in IL-4/IL-13-stimulated fibroblasts 3 h after scratching (p < 0.05) while CXCL8 was elevated at 6 h under optimal conditions (Figure 8), demonstrating a significant difference to all non-optimal conditions (p < 0.05). PDGFC levels significantly increased under chronic inflammatory conditions (p < 0.01) while TGFB1 was only slightly enhanced (Figure 7), but corresponded to the keratinocyte scratch wound healing progression (Figure 6B). Though, TGFB1 and PDGFC also increased under chronic inflammatory conditions in fibroblasts over time, the response was markedly decreased compared to optimal conditions (p < 0.01; Figure 8), reflecting the slower healing progression (Figure 6D). No noteworthy TGFB1 upregulation was observed under anti-inflammatory conditions in both cell types (Figure 7 and Figure 8), probably accounting for the reduced scratch healing observed (Figure 6B,D). In contrast, PDGFC was significantly increased (p < 0.05) at 12 h in keratinocytes (Figure 7), possibly being the result of a delayed response. Moreover, TP53 was increased in infected keratinocytes (p < 0.05) while NFKB1 was reduced (p < 0.01) at 6 h (Figure 7). In contrast, S. aureus infection did not change transcription factor transcript levels in fibroblast scratches after 6 h (Figure 8). Anti-inflammatory conditions also slightly induced TP53 in scratched keratinocytes at 6 h (Figure 7) and significantly increased NFKB1 levels in fibroblasts (p < 0.05; Figure 8). While the gene expression of transcription factors was not affected by chronic inflammatory conditions in keratinocytes compared to the control under optimal conditions (Figure 7), it was significantly increased in fibroblasts at 6 h (p < 0.01; Figure 8). Heat shock protein gene expression evaluation pointed to condition-specific increases in HSP90AA1, HSPA1A, and HSPD1 transcript levels under the non-optimal conditions compared to the keratinocyte control (Figure 7). Chronic and anti-inflammatory conditions elicited an increase in HSPD1 levels (p < 0.05) while S. aureus reduced its transcription in keratinocytes (p < 0.05). HSPA1A and HSP90AA1 were induced by S. aureus (p < 0.05). HSP90AA1 transcription was also significantly stimulated by pro- and anti-inflammatory conditions compared to the control. Non-optimal conditions in fibroblasts resulted in increased stress response gene transcription with marked effects on HSPD1 for TNF-α/IFN-γ stimulation, which were also found for HSPA1A (p < 0.01) and to a lesser extent HSP90AA1 (Figure 8). Minor effects on HSPD1 were found for infection with S. aureus, which did not reach statistical significance, and scratch wound healing under anti-inflammatory conditions (p < 0.05). DSG1 levels under chronic inflammatory conditions were slightly decreased compared to the keratinocyte control and DSG3 was significantly affected (Figure 7), which possibly reflects the reduced scratch wound healing progression (Figure 6B). A markedly reduced transcription of these cell adhesion molecule genes was found under anti-inflammatory conditions (p < 0.05), also corresponding to the decreased wound healing under these conditions in vitro. Gene expression of the antimicrobial peptides S100A7 and RNASE7 was induced by S. aureus infection at early time points but could not be determined at 48 h due to the loss of viable cells (Figure 7). In contrast, S100A7 was not changed under chronic inflammatory conditions, while RNASE7 transcription was slightly decreased and DEFB1 levels were significantly reduced (p < 0.001) in keratinocytes. Anti-inflammatory conditions generally reduced antimicrobial peptide gene expression, reaching high statistical significance for DEFB1 (p < 0.001). COL1A1 and COL3A1 transcription by fibroblasts increased with the healing progression over time under chronic inflammatory conditions, but while COL3A1 even exceeded the control levels (p < 0.05), COL1A1 was reduced compared to the optimal conditions. After infection with S. aureus, no determination of collagen gene expression was possible after 48 h due to the loss of viable cells. Anti-inflammatory conditioning with IL-4/IL-13 significantly reduced COL1A1 and COL3A1 transcription (p < 0.01; Figure 8).
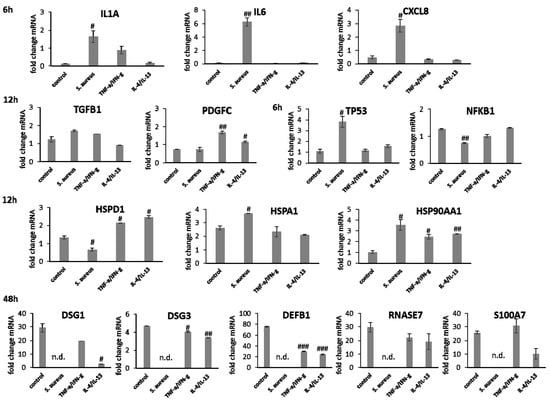
Figure 7.
Gene expression profiles of pro-inflammatory cytokine and growth factor genes after 6 h and heat shock protein gene transcript levels after 12 h as well as desmoglein gene and AMP gene expression after 48 h for HaCaT keratinocyte scratches after infection with S. aureus and induction of chronic inflammatory conditions (TNF-α/IFN-γ) as well as anti-inflammatory conditions (IL-4/IL-13). Hashtags [#] designate significant differences of scratch wound healing or transcription levels at the respective time point compared to the control under optimal conditions (# p < 0.05, ## p < 0.01, ### p < 0.001) and n.d. indicates ‘not determined’.
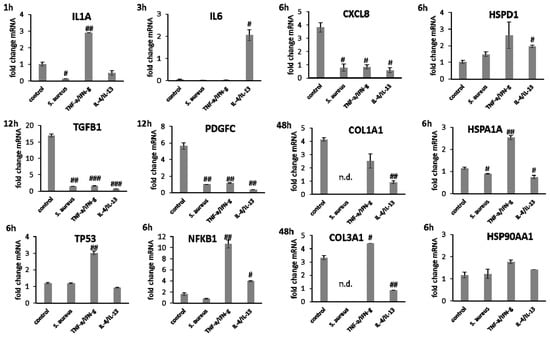
Figure 8.
Gene expression profiles of pro-inflammatory cytokine genes after 1 h (IL1A), 3 h (IL6), and 6 h (CXCL8) for fibroblast scratches after infection with S. aureus and induction of chronic inflammatory conditions (TNF-α/IFN-γ) as well as anti-inflammatory conditions (IL-4/IL-13). Stress protein response was further evaluated after 6 h at the level of TP53 and NFKB1 transcripts as well as heat shock protein and growth factor gene expression were assessed after 12 h. Differences in collagen gene expression were determined after 48 h. Hashtags [#] designate significant differences of scratch wound healing or transcription levels at the respective time point compared to the control under optimal conditions (# p < 0.05, ## p < 0.01, ### p < 0.001) and n.d. indicates ‘not determined’.
3.4. wIRA Treatment of Cell Scratches during S. aureus Infection
Keratinocyte and fibroblast monolayers were infected with S. aureus and scratched before treatment with wIRA or heating w/o wIRA. In all cases, infection with S. aureus damaged the cells so severely that scratch wound closure was inhibited independent of the treatment (Figure 9A,B and Figure 10A,B). Moreover, no living cells were noted after 24 and 48 h under infection conditions in the untreated control and after heating w/o wIRA. Treatment with wIRA slightly stalled the negative outcome in keratinocytes (p < 0.01) and those remained viable up to 24 h but eventually succumbed to the toxic effects after 48 h (Figure 9A). Radiation with wIRA showed no protective effect on fibroblasts (Figure 10A). Consequently, at the time points where cells were killed, no mRNA gene expression analysis was performed.
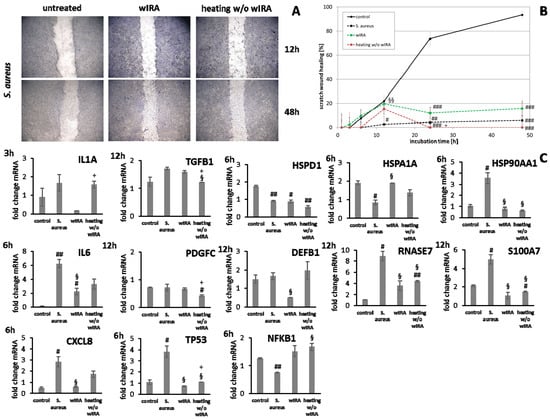
Figure 9.
(A) HaCaT keratinocyte scratch wound closure over time after infection with S. aureus and treatment with wIRA or heating w/o wIRA. (B) Evaluation of scratch wound healing in [%]. (C) Gene expression profiles of these HaCaT keratinocytes for pro-inflammatory cytokine genes after 3 h (IL1A) and 6 h (IL6, CXCL8) together with stress response gene and heat shock protein gene transcript levels as well as growth factor and AMP gene expression after 12 h. Hashtags [#] designate significant differences of scratch wound healing or transcription levels at the respective time point compared to the control under optimal conditions (# p < 0.05, ## p < 0.01, ### p < 0.001) while the paragraph character [§] shows significant differences between untreated and treated samples under non-optimal conditions at the respective time point (§ p < 0.05, §§ p < 0.001). The plus sign [+] specifies significant differences between wIRA-treated samples and those receiving heating w/o wIRA under non-optimal conditions at the respective time point (+ p < 0.05).
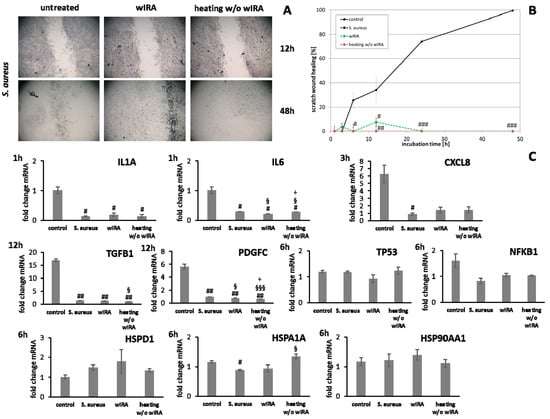
Figure 10.
(A) Dermal fibroblast scratch wound closure over time after infection with S. aureus and treatment with wIRA or heating w/o wIRA. (B) Evaluation of scratch wound healing in [%]. (C) Gene expression profiles of these fibroblast scratches for pro-inflammatory cytokine genes after 1 h (IL1A, IL6) and 3 h (CXCL8) as well as stress response gene and heat shock protein transcript levels. Growth factor gene expression was evaluated after 12 h. Hashtags [#] designate significant differences of scratch wound healing or transcription levels at the respective time point compared to the control under optimal conditions (# p < 0.05, ## p < 0.01, ### p < 0.001) while the paragraph character [§] shows significant differences between untreated and treated samples under non-optimal conditions at the respective time point (§ p < 0.05, §§§ p < 0.001). The plus sign [+] specifies significant differences between wIRA-treated samples and those receiving heating w/o wIRA under non-optimal conditions at the respective time point (+ p < 0.05).
Treatment with wIRA decreased IL1A levels at 3 h as well as IL6 (p < 0.05) and CXCL8 (p < 0.05) transcription after 6 h in infected keratinocytes while heating alone showed no or only slight effects (Figure 9C). In fibroblasts, the inflammatory response after S. aureus infection was decreased and wIRA treatment as well as heating w/o wIRA did not markedly change IL1A and CXCL8 transcription profiles at early time points (Figure 10C), only IL6 levels were significantly decreased (p < 0.05). TGFB1 and PDGFC levels in infected keratinocytes were neither affected by wIRA treatment compared to the infected cells while heating w/o wIRA further decreased their levels (p < 0.05). Similar results were obtained for fibroblasts (Figure 10C). Transcription of TP53 by keratinocytes was increased at 6 h under infection conditions, which was found to be significantly reduced in wIRA-treated keratinocytes and for those treated by heating w/o wIRA (p < 0.05). NFKB1 was augmented with wIRA treatment and especially heating alone (p < 0.05) compared to infected cells (Figure 9C). Moreover, it was found that wIRA radiation, and to some extent, heating w/o wIRA, could restore HSPA1A transcription to control levels while HSPD1 expression was not affected (Figure 9C). In contrast, the HSP90AA1 increase in infected keratinocytes was significantly alleviated at 6 h under wIRA treatment and heating of the cells (p < 0.05; Figure 9C). Changes in heat shock protein transcript levels after infection of fibroblasts with S. aureus and treatment with wIRA were observed for HSPD1 as a slight increase and with heating w/o wIRA for HSPA1A as significant enhancement (p < 0.05) compared to infected cells (Figure 10C). Transcription of the antimicrobial peptides S100A7 and RNASE7, but not DEFB1 was distinctly induced by S. aureus infection in keratinocytes (p < 0.05) compared to the control at 12 h (Figure 9C). Heating w/o wIRA did not affect DEFB1 expression, but significantly reduced S100A7 and RNASE7 expression (p < 0.05). Surprisingly, the wIRA treatment led to overall decreased AMP gene transcript levels in the infected keratinocytes (p < 0.05; Figure 9C).
3.5. Stimulation of Scratch Wound Healing under Chronic Inflammatory Conditions by wIRA
Keratinocytes were primed with TNF-α/INF-γ to induce a state of chronic inflammation. These monolayers were scratched and treated with wIRA or heating w/o wIRA (Figure 11A,B). Heating w/o wIRA exerted a negative effect on the keratinocytes and scratch wound healing was significantly decreased (p < 0.01). In contrast, heating alone was enough to significantly improve fibroblast scratch wound closure as early as 12 h (p < 0.01) compared to the untreated control under chronic inflammatory conditions (Figure 12A,B). Treatment with wIRA significantly enhanced the wound healing capacity of keratinocytes under chronic inflammatory conditions (Figure 11A,B) with an increased scratch closure at 48 h (p < 0.05) and also induced the wound healing progression rate in fibroblast scratches as early as 12 h (p < 0.01) beyond the extent of cells under optimal conditions (Figure 12A,B).
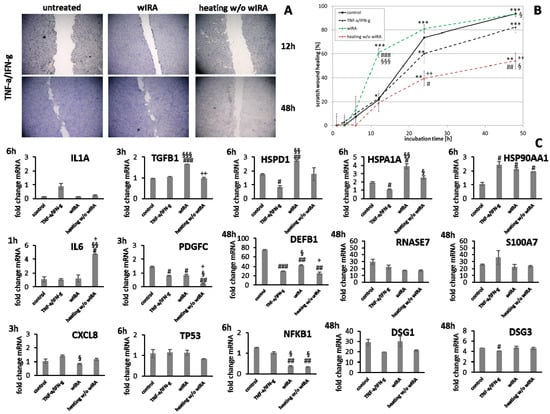
Figure 11.
(A) HaCaT keratinocyte scratch wound closure over time after induction of chronic inflammatory conditions (TNF-α/IFN-γ) and treatment with wIRA or heating w/o wIRA. (B) Evaluation of scratch wound healing in [%]. (C) Gene expression profiles of these HaCaT keratinocytes at specific time points during scratch wound healing after induction of chronic inflammatory conditions (TNF-α/IFN-γ) showed differences for pro-inflammatory cytokine gene expression after 1 h (IL1A, IL6) and 3 h (CXCL8) as well as growth factor gene transcription after 3 h and stress response gene levels after 6 h. In addition, heat shock protein gene expression exhibited alterations after 6 h while AMP gene expression was partly affected at 48 h as well as desmoglein gene expression. Asterisks [*] indicate significant differences in scratch wound healing or transcription level compared to 1 h (* p < 0.05, ** p < 0.01, *** p < 0.001). Hashtags [#] designate significant differences of scratch wound healing or transcription levels at the respective time point compared to the control under optimal conditions (# p < 0.05, ## p < 0.01, ### p < 0.001) while the paragraph character [§] shows significant differences between untreated and treated samples under non-optimal conditions at the respective time point (§ p < 0.05, §§ p < 0.001, §§§ p < 0.001). The plus sign [+] specifies significant differences between wIRA-treated samples and those receiving heating w/o wIRA under non-optimal conditions at the respective time point (+ p < 0.05, ++ p < 0.01).
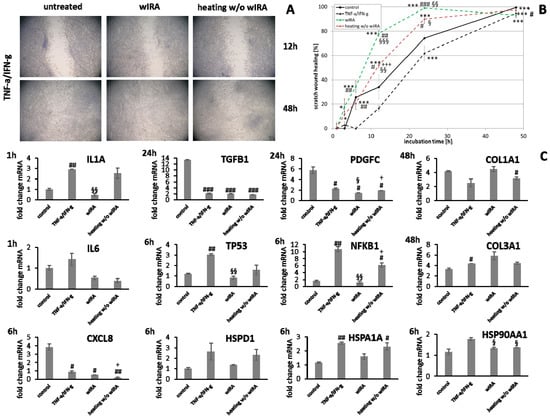
Figure 12.
(A) Dermal fibroblast scratch wound closure over time after induction of chronic inflammatory conditions (TNF-α/IFN-γ) and treatment with wIRA or heating w/o wIRA. (B) Evaluation of scratch wound healing in [%]. (C) Gene expression profiles of these fibroblasts were assessed for pro-inflammatory cytokine gene expression after 1 h (IL1A, IL6) and 6 h (CXCL8) as well as stress response gene together with heat shock protein gene transcript levels. Growth factor gene expression was appraised after 24 h and collagen gene expression was evaluated after 48 h. Asterisks [*] indicate significant differences in scratch wound healing or transcription level compared to 1 h (* p < 0.05, *** p < 0.001). Hashtags [#] designate significant differences of scratch wound healing or transcription levels at the respective time point compared to the control under optimal conditions (# p < 0.05, ## p < 0.01, ### p < 0.001) while the paragraph character [§] shows significant differences between untreated and treated samples under non-optimal conditions at the respective time point (§ p < 0.05, §§ p < 0.001, §§§ p < 0.001). The plus sign [+] specifies significant differences between wIRA-treated samples and those receiving heating w/o wIRA under non-optimal conditions at the respective time point (+ p < 0.05, +++ p < 0.01).
Under chronic inflammatory conditions, an increased expression of IL1A was noted, which could be decreased by wIRA radiation in both keratinocytes (Figure 11C) and also significantly in fibroblasts (p < 0.01; Figure 12C). Heating w/o wIRA also decreased IL1A levels in keratinocytes (Figure 11C), but not in fibroblasts (Figure 12C) while it reduced IL6 levels in the latter (Figure 12C) and significantly stimulated this cytokine’s gene transcription in keratinocytes (Figure 11C). CXCL8 was significantly reduced by wIRA treatment under chronic inflammatory conditions in keratinocytes (p < 0.05) and slightly decreased in fibroblasts. TGFB1 transcription in keratinocytes was significantly enhanced by wIRA (p < 0.001) while it remained unchanged between treatments in fibroblasts and here did not correspond with the overall scratch wound healing progression (Figure 12B). PDGFC transcription in keratinocytes was not altered under chronic inflammatory conditions by wIRA treatment, but it was found to be significantly reduced after heating w/o wIRA (p < 0.05; Figure 11C). No differences in TP53 levels between treated and untreated keratinocyte scratches during wound healing progression were observed but NFKB1 was significantly reduced in treated keratinocytes at 6 h (p < 0.01; Figure 11C). In addition, wIRA radiation significantly decreased TP53 and NFKB1 levels in fibroblasts (p < 0.01; Figure 12C). Keratinocytes transcribed increased levels of the heat shock protein gene HSP90AA1 under chronic inflammatory conditions, which was not affected by treatments. However, HSPD1 and HSPA1A were found to be significantly augmented by wIRA radiation under TNF-α/IFN-γ conditions (p < 0.01) as well as compared to the control (Figure 11C). In contrast, all heat shock proteins HSP90AA1, HSPA1A, and HSPD1 were induced in fibroblasts by chronic inflammatory conditions. Their transcription was markedly decreased in wIRA-treated compared to untreated fibroblast scratches or those treated by heating w/o wIRA with the exception of HSP90AA1, which was also reduced by heating alone (Figure 12C). DSG1 and DSG3 levels under chronic inflammatory conditions after all treatments reflected scratch wound healing progression in keratinocytes. Transcription levels of the antimicrobial peptides S100A7 and RNASE7 were mostly unchanged by treatments compared to the untreated control under chronic inflammatory conditions. In contrast, DEFB1 was significantly increased after wIRA radiation (p < 0.05), but did not reach the control levels of keratinocytes under optimal conditions during wound healing progression (Figure 11C). Treatment with wIRA enhanced COL1A1 and COL3A1 levels in fibroblasts compared to untreated scratches under chronic inflammatory conditions (Figure 12C), but changes did not reach statistical significance, reflecting the improved scratch wound healing progression (Figure 12B).
3.6. wIRA Treatment Accelerates Scratch Wound Healing under Anti-Inflammatory Conditions
Scratch wound healing was found to be distinctly reduced by the addition of IL-4/IL-13, mimicking anti-inflammatory conditions. Heating w/o wIRA increased overall keratinocyte scratch healing (Figure 13A,B, p < 0.01) and induced early scratch healing progression in fibroblasts (Figure 14A,B, p < 0.001) under anti-inflammatory conditions. In contrast, wIRA radiation highly significantly enhanced scratch wound healing in keratinocytes (p < 0.001) and fibroblasts (p < 0.001) beyond the extent of cells under optimal conditions.
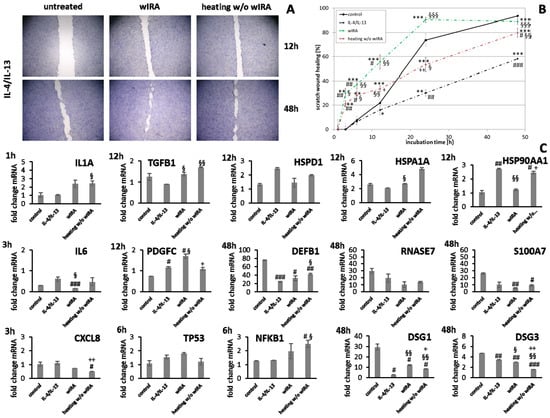
Figure 13.
(A) HaCaT keratinocyte scratch wound closure over time after induction of anti-inflammatory conditions (IL-4/IL-13) and treatment with wIRA or heating w/o wIRA. (B) Evaluation of scratch wound healing in [%]. (C) Gene expression profiles of these HaCaT keratinocytes at specific time points during scratch wound healing after the induction of anti-inflammatory conditions demonstrated differences for pro-inflammatory cytokine gene expression after 1 h (IL1A) and 3 h (IL6, CXCL8) as well as stress response gene transcript levels after 6 h and growth factor gene transcript levels after 12 h. Additionally, heat shock protein gene expression and AMP gene expression exhibited alterations at 48 h as well as desmoglein gene expression. Asterisks [*] indicate significant differences in scratch wound healing or transcription level compared to 1 h (* p < 0.05, ** p < 0.01, *** p < 0.001). Hashtags [#] designate significant differences of scratch wound healing or transcription levels at the respective time point compared to the control under optimal conditions (# p < 0.05, ## p < 0.01, ### p < 0.001) while the paragraph character [§] shows significant differences between untreated and treated samples under non-optimal conditions at the respective time point (§ p < 0.05, §§ p < 0.001, §§§ p < 0.001). The plus sign [+] specifies significant differences between wIRA-treated samples and those receiving heating w/o wIRA under non-optimal conditions at the respective time point (+ p < 0.05, ++ p < 0.01).
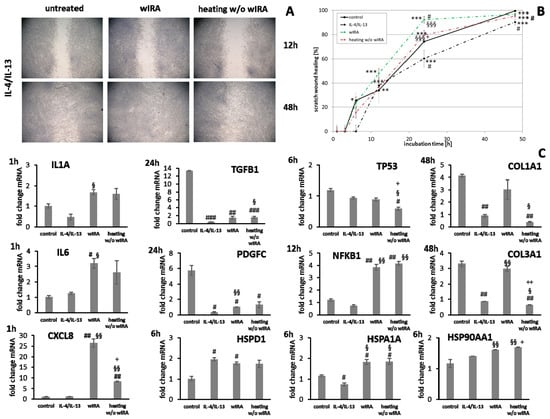
Figure 14.
(A) Dermal fibroblast scratch wound closure over time after induction of anti-inflammatory conditions (IL-4/IL-13) and treatment with wIRA or heating w/o wIRA. (B) Evaluation of scratch wound healing in [%]. (C) Gene expression profiles of these fibroblasts were appraised for pro-inflammatory cytokine gene expression after 1 h and heat shock protein gene transcript levels after 6 h. Furthermore, stress response gene expression exhibited differences after 6 h (TP53) and 12 h (NFKB1). Growth factor gene expression was assessed after 24 h and collagen gene expression was evaluated after 48 h. Asterisks [*] indicate significant differences in scratch wound healing or transcription level compared to 1 h (* p < 0.05, ** p < 0.01, *** p < 0.001). Hashtags [#] designate significant differences of scratch wound healing or transcription levels at the respective time point compared to the control under optimal conditions (# p < 0.05, ## p < 0.01, ### p < 0.001) while the paragraph character [§] shows significant differences between untreated and treated samples under non-optimal conditions at the respective time point (§ p < 0.05, §§ p < 0.001, §§§ p < 0.001). The plus sign [+] specifies significant differences between wIRA-treated samples and those receiving heating w/o wIRA under non-optimal conditions at the respective time point (+ p < 0.05, ++ p < 0.01).
IL1A gene expression by keratinocytes after treatments was distinctly elevated at early time points compared to the untreated control (Figure 13C). IL6 transcription under anti-inflammatory conditions was significantly reduced by wIRA (p < 0.05) and CXCL8 levels were not or only marginally affected by the treatments (Figure 13C). In fibroblasts, IL1A levels were increased by both treatments under anti-inflammatory conditions at one hour while IL6 (p < 0.05) and CXCL8 (p < 0.01) were distinctly induced after wIRA radiation and to a lesser extent by heating w/o wIRA (Figure 14C). Particularly noticeable was the significant increase in mRNA level fold change for CXCL8. A significant TGFB1 upregulation was observed in keratinocytes after treatments compared to the cells under anti-inflammatory conditions alone (p < 0.05) and PDGFC expression was significantly increased by wIRA radiation at 12 h (p < 0.05; Figure 13C). TGFB1 and PDGFC transcription were also found to be increased by treatments in fibroblasts compared to cells under anti-inflammatory conditions, but did not reach the levels of controls under optimal conditions (Figure 14C), despite the observed improved scratch healing (Figure 14B). NFKB1 was increased at 6 h under anti-inflammatory conditions in keratinocytes after treatment with wIRA and heating w/o wIRA (Figure 13C). In fibroblasts, NFKB was found to be significantly increased at 12 h after treatments (p < 0.01; Figure 14C). Increased HSP90AA1 and HSPD1 transcript amounts in keratinocytes under anti-inflammatory settings were reduced by wIRA treatment at 12 h, reaching statistical significance for HSP90AA1. HSPA1A expression was increased by heating w/o wIRA (Figure 13C). Treatments were also found to significantly increase HSPA1A levels (P < 0.05) and HSP90AA1 transcription (p < 0.01) in fibroblasts (Figure 14C), possibly indicating a temperature effect on the cells. HSPD1 was increased in fibroblasts under anti-inflammatory conditions compared to the control and not affected by treatments. Treatments significantly increased keratinocyte DSG1 levels (p < 0.1) in correlation with their effectiveness to enhance scratch closure, but to a lesser extent compared to normal conditions. DSG3 levels were in contrast not enhanced, but rather decreased despite the positive effect on wound healing progression (Figure 13C). Treatments did not significantly affect S100A7 levels under anti-inflammatory conditions and RNASE7 transcription was even reduced. However, DEFB1 gene expression was significantly augmented after heating w/o wIRA as well as to a lesser extent after wIRA radiation (Figure 13C), coinciding with the improved scratch closure (Figure 13B). Furthermore, radiation with wIRA, but not heating w/o wIRA increased COL1A1 and COL3A1 transcript levels in fibroblasts distinctly (Figure 14C).
4. Discussion
Photobiomodulation has been shown to exert beneficial effects on wound healing [26,27], matrix deposition, and cell layers [29,30,90,91]. Significant induction of wound healing processes in acute and chronic wounds by wIRA treatment have been reported [26,27]. Treatment with wIRA radiation improved scratch healing in vitro. While the influence on keratinocytes seems mostly based on a warming effect [92], fibroblasts appear to be directly stimulated by the wIRA radiation. In contrast, UV-B irradiation resulted in severe damage to keratinocytes and fibroblasts and scratch wounds showed no tendency for healing. Positive in vitro effects of wIRA have been previously described [32,33,93]. However, differences between the in vivo and the experimental in vitro conditions exist as chronic wounds, for instance, are characterized by microbial infection [5,6] and continuous conditions of inflammation [7], stalling the healing process. Moreover, TH2-dominant, anti-inflammatory conditions have been reported to affect wound healing processes [8,9,10]. The experimental prerequisites in the scratch assays applied so far accounted for optimal cell conditions. Hence, the study was extended to include the readjustment of specific milieus, more closely mimicking different detrimental in vivo situations, explicitly S. aureus infection, chronic inflammation as well as anti-inflammatory conditions. Scratch wound healing progression under all non-optimal environments was found to be significantly reduced. The influence was most pronounced for the infection with S. aureus, which damaged the cells to an extent that scratch wounds did not heal in vitro. S. aureus is known to induce cell death in keratinocytes in a co-culture model for infection [94]. In accordance, no living cells were noted after 24 and 48 h under infection conditions. Under anti-inflammatory conditions, where keratinocytes and fibroblasts were primed with IL-4 and IL-13 for 24 h prior to scratching, scratch wound healing was also significantly impeded. IL-4 and IL-13 are pleiotropic cytokines involved in cell growth, immune system regulation, induction of anti-inflammatory process, and M2 macrophage promotion [8]. Thus, they are thought to contribute to the wound healing process by stimulation of the repair process [10]. However, they have also been implicated in the pathogenesis of fibroproliferative disorders such as hypertrophic scar formation or systemic sclerosis [8,95]. However, in this study, reduced keratinocyte and fibroblast scratch healing was observed, which is in accordance with results obtained by Serezani et al., demonstrating impaired wound healing in keratinocytes stimulated with IL-4 [9] and consistent with the decreased wound repair found in atopic children [42]. Chronic inflammatory conditions, produced by priming with TNF-α and IFN-γ [41], also reduced scratch healing compared to the controls under optimal conditions mainly by delaying cell layer regeneration. Persistence of inflammation is detrimental and can cause cell damage, thus stalling the healing process [4]. It was found that wIRA treatment improved keratinocyte and fibroblast scratch healing under chronic inflammatory conditions beyond the extent of cells under optimal conditions. The effect was only partly dependent on warming alone and could be an explanation for the positive effects observed in chronic wounds [26]. Moreover, a distinct improvement of the wound healing progression in keratinocytes and fibroblasts under anti-inflammatory conditions was achieved by wIRA radiation, significantly enhancing scratch closure.
Layer repair in the keratinocyte and fibroblast wound healing models was assessed by gene expression analysis of typical proteins in epidermis and dermis. The keratinocytes form a tight cell barrier by connecting neighboring cells to each other through intercellular junctions, which include (corneo) desmosomes, adherens junctions, and tight junctions [88]. Desmosomes are composed of desmosomal cadherins such as desmogleins (Dsg) 1–4 and desmocollins 1–3, which are transmembrane glycoproteins of the cadherin superfamily [89]. DSG1 and DSG3 gene expression by keratinocytes increased over time during scratch wound healing, which is consistent with the regeneration of the epithelial layer and the re-formation of cell–cell-contacts [96]. To allow wound healing, the cell adhesion proteins need to remodel, first detaching to enable cell migration and subsequently reforming to reestablish the paracellular barrier in the skin [97,98]. Transcript levels of the cell adhesion molecule genes DSG1 and DSG3 were found to be increased after wIRA treatment compared to the control corresponding to the differences observed in the healing progression over time. Significantly reduced amounts were observed after UV-B irradiation as well as under non-optimal conditions. Treatment with wIRA restored DSG1 levels under chronic inflammatory to levels under optimal conditions and significantly increased DSG1 levels under anti-inflammatory conditions in correlation with the improvement in scratch wound closure. Regeneration of the dermal cell layer in this in vitro model was assessed by collagen gene transcription in fibroblasts. COL1A1 and COL3A1 levels were found to be significantly increased over time corresponding to the reformation of the fibroblast cell layer. Gene expression of COL3A1 was especially found to be significantly augmented after wIRA treatments corresponding to the healing progression observed. Interestingly, the most pronounced increase in the transcript levels of these genes was found after UV-B irradiation in the fibroblasts. So far, in unscratched fibroblasts, a reduction in the expression of COL1A1 and COL3A1 after UV-B irradiation was observed [99,100]. UV light is known to have an adverse effect on dermal collagen either by direct degradation through increased MMP production [101] or by inhibiting pro-collagen biosynthesis [102] as well as reducing collagen gene expression [99,100]. These processes have been strongly linked to the formation of wrinkles induced by the augmentation of oxidative stress by UV light affecting several downstream events of collagen metabolism, making UV light one of the most powerful physical agents responsible for extrinsic skin aging [103]. However, UV-B exposure has also been reported to exacerbate and prolong the corneal stromal healing response manifesting as augmented sub-epithelial haze and deposition of disorganized collagen [104]. COL1A1 and COL3A1 gene expression was affected by chronic inflammatory conditions, possibly corresponding to the increase in IL1A and NFKB1 levels and was found to be significantly reduced after anti-inflammatory conditioning with IL-4/IL-13. In both cases, radiation with wIRA markedly enhanced COL1A1 and COL3A1 transcription, reestablishing control transcript levels or even exceeding those, reflecting the improved scratch wound healing progression.
From the results obtained in the wound healing studies with keratinocytes and fibroblasts under infection conditions, it was concluded that wIRA does not convey an antimicrobial activity. However, wIRA has been shown to exert antibacterial effects on bacteria that are either heat-sensitive [105] or when used in combination with photosensitizers as photodynamic therapy [106,107]. However, previous studies have also failed to note direct antimicrobial effects of wIRA on wound pathogens [108], which can be explained by the fact that these wound pathogens are not heat-sensitive. Treatment with wIRA slightly stalled the negative effects of S. aureus and keratinocytes remained viable up to 24 h in contrast to untreated keratinocytes, but no protective effect on fibroblasts was observed. It was hence presumed that the better survival of infected keratinocytes after wIRA treatment could depend on the induction of antimicrobial peptides (AMPs) such as psoriasin and defensin by keratinocytes. As innate immune cells, keratinocytes can produce a wide variety of AMPs upon stimulation [34,76], which are important effector molecules with broad spectrum anti-microbial activity against bacteria, fungi, and even viruses [77,78,79,80,81].
AMP gene expression increased during scratch wound healing. Endogenous AMPs have also been found to be upregulated in all stages of wound healing in vivo, indicating a role beyond microbial defense toward regulation of immune response, granulation tissue formation, and re-epithelization [82,83]. It was also observed that antimicrobial peptide transcript levels were significantly elevated after wIRA treatment under optimal conditions, further suggesting the involvement in the improved scratch wound healing outcome. However, the observed increase in S100A7 and RNASE7 transcription after UV-B irradiation was probably due to stimulation by the elicited inflammatory response [109]. Expression of the AMP genes S100A7 and RNASE7 was also significantly induced by S. aureus infection at early time points. S100A7 transcription levels were not affected by chronic inflammatory conditions while RNASE7 levels were slightly decreased and DEFB1 was significantly reduced. Insufficient upregulation of defensins in chronic wounds such as diabetic foot and venous leg ulcers has been suggested to contribute to the chronicity of ulcers by reduced antimicrobial defense [84,85]. Here, it was found that DEFB1 gene expression was significantly increased after wIRA radiation under chronic inflammatory conditions. Although it did not reach expression levels under optimal conditions during scratch wound healing progression, it most likely confers a beneficial influence. Hence, induction of defensins might contribute to the positive effects of wIRA radiation reported in chronic wounds [26]. Moreover, levels of S100A7, RNASE7, and DEFB1 were generally lower under anti-inflammatory conditions compared to optimal conditions. Recent studies indicate that an increased TH2 cytokine expression contributes to the reduction in AMPs observed in atopic dermatitis, resulting in frequent skin infections [86,87]. While wIRA treatment did not increase S100A7 and RNASE7 levels, DEFB1 transcription was augmented in correspondence with the improved scratch closure. In accordance, wIRA could have a potential positive influence on frequent skin infections of patients with atopic dermatitis [86,87] by boosting skin defense mechanisms. However, contrary to expectations after improved survival of infected keratinocytes, wIRA radiation significantly decreased AMP gene transcription under infection conditions. Hence, it is unlikely that an augmented production of AMPs conferred the resistance to S. aureus infection under wIRA treatment.
Keratinocytes and fibroblasts secrete a broad spectrum of cytokines, chemokines, and growth factors including IL-1α, IL-6, IL-8, TGF-β, and PDGF [34,35], all with a possible role in wound healing and therefore potential targets for photobiomodulation by wIRA. Radiation with wIRA did not induce transcription of pro-inflammatory cytokine genes compared to the untreated control under optimal conditions, while UV-B irradiation was found to elicit a detrimental inflammatory response in keratinocytes and fibroblasts. Additionally, bacteria and the formation of biofilms can drive inflammation by interaction with the wound cells [7]. Accordingly, gene expression analysis revealed an early and significant induction of IL1A, IL6, and CXCL8 expression during infection of keratinocytes with S. aureus accounting for the detrimental effects observed. However, infection with S. aureus failed to elicit pro-inflammatory cytokine gene expression in fibroblasts. The fibroblasts rapidly died after the infection with S. aureus, most likely due to the damaging effects of S. aureus (e.g., via the release of toxins). This may also occur due to starving of the cells as bacteria take up the nutrients in the medium. As expected, a chronic inflammatory milieu led to increased IL1A mRNA levels compared to the control while anti-inflammatory conditions reduced IL1A transcription. Acting as the “first alarm system” of the skin, the IL-1 cytokine family plays a crucial role through alerting the body to immediate dangers and initiating the inflammatory cascade in the skin as well as inducing gene expression and synthesis of other inflammatory mediators [45,46]. IL6 gene expression showed no distinct differences to the control under chronic inflammatory conditions, while CXCL8 levels were neither affected in keratinocytes, a significant decrease in fibroblasts was found. Surprisingly, IL-6 gene expression was induced in fibroblasts by the combination of IL-4 and IL-13. Treatment with wIRA had the potential to decrease pro-inflammatory cytokine gene expression in infected keratinocytes as well as under chronic inflammatory conditions. In general, anti-inflammatory therapies have been associated with a positive influence on the wound healing outcomes due to the moderating effects on inciting cytokines and reactive oxygen species [110,111,112]. However, the initial inflammatory response after injury is essential for wound healing and stimulates regenerative processes [41]. The promotion of the acute inflammation can increase wound healing [39,47] and the reduction in IL-6 by anti-inflammatory treatment has been shown to inhibit scratch wound healing [48]. In accordance, scratch wound healing after IL-4/IL-13 stimulation was found to be decreased and could be re-initiated by wIRA treatment, which induced IL1A gene expression under anti-inflammatory conditions at one hour. Similarly, low-level laser therapy and cold atmospheric plasma were able to induce IL-8 secretion and increase wound healing in vitro [90,113].
Photobiomodulation has been demonstrated to promote the production of growth factors such as PDGF, TGF, and bFGF as well as activate the mitogenic signaling pathway in fibroblasts [91]. TGF-β plays an important role in wound healing by stimulating granulation tissue formation [49,50,51,52] and mediating fibroblast proliferation, collagen production, ECM deposition and myofibroblast differentiation [53,54,55] as well as promoting angiogenesis [1]. Hence, increased TGF-β activity can accelerate wound healing [56,57] and positively impact the production of dermal-epidermal junction proteins [114]. Induction of TGFB1 has also been associated with the promotion of keratinocyte proliferation and migration [58]. Here, it was observed that TGFB1 transcript levels significantly increased over time in keratinocytes and showed a peak as early as 12 h in fibroblasts. With the onset of an injury toward healing, expression levels have been shown to be increased [115,116,117]. A slight surge of PDGFC gene expression was observed for keratinocytes at 3 h with a subsequent decrease while fibroblasts demonstrated a steady rise in the gene’s expression over time under these conditions. PDGF-C is a key component of the PDGFR-α signaling pathway and possesses a similar modulation capacity of fibroblast differentiation as TGF-β [59]. TGFB1 and PDGFC gene expression increased under chronic inflammatory conditions over time, corresponding to scratch wound healing. However, the response in fibroblasts was significantly decreased compared to optimal conditions reflecting the slower progression. Overexpression may also contribute to abundant collagen accumulation, tissue fibrosis, and pathological scar formation [8,118,119], making it a double-edged sword. Significantly decreased TGFB1 or PDGFC levels were observed under anti-inflammatory conditions in fibroblasts, probably accounting for the reduced scratch healing found here. TGFB1 levels were also slightly reduced in keratinocytes under anti-inflammatory conditions. Treatment with wIRA significantly increased TGFB1 levels in keratinocytes in the chronic inflammatory milieu and induced TGFB1 as well as PDGFC gene expression under anti-inflammatory conditions in keratinocytes and fibroblasts. In addition, transcription factors play a crucial role in stress response, damage control, and wound healing. The NF-κB transcription factors have been implicated in the regulation of several genes, thus governing many biological effects including apoptosis, immune response, and inflammatory processes [60,61,62]. It has previously been shown that NF-κB is a major regulator of cell proliferation and migration, acting as a transcription factor for both cyclin D and MMP-9 [63], and is essential for wound healing [64]. Similarly, p53 (gene TP53) regulates cell cycle progression after DNA damage, activation of DNA repair mechanisms, and cell apoptosis, if DNA damage proves to be irreparable. Correspondingly, the gene expression of TP53 and NFKB was elevated during wound healing [63,64]. Here, TP53 levels were significantly increased by infection of keratinocytes with S. aureus and in fibroblasts under chronic inflammatory conditions as well as wIRA radiation under optimal conditions. Chronic and anti-inflammatory conditions also significantly induced NFKB1 in fibroblasts. TP53 levels were not affected by UV-B irradiation, although protein levels of p53 in keratinocytes and fibroblasts have been reported to be dramatically increased after UV-B exposure, eventually resulting in cell apoptosis [120]. Under non-optimal conditions, wIRA treatment was found to significantly reduce TP53 levels in keratinocytes during infection and in fibroblasts under chronic inflammation. In contrast, NFKB1 levels were elevated in wIRA-treated, infected keratinocytes and wIRA radiation also induced NFKB1 gene expression in keratinocytes and fibroblasts under anti-inflammatory conditions. The fostered activation of NF-κB has been shown to improve wound healing in vitro [121], which could account for the observed scratch healing induction. However, NFKB1 was significantly reduced in TNF-α/IFN-γ-primed cells through wIRA treatment, despite showing an equal improvement in scratch wound healing.
Heat shock proteins (HSPs) have not been studied extensively so far in scratch wound healing models. They constitute a large group of chaperone proteins found in virtually all organisms where they modulate cellular homeostasis, aid in repair after cellular stress, and promote wound healing [47]. HSPs are classified into several families on the basis of their molecular size and amino acid sequence similarity with highly differentiated expression patterns, intracellular localization, and function [68]. They are induced under conditions of cell stress (e.g., inflammation) [68]. For instance, HSP90 is an abundant cellular protein constituting about 1–2% of total protein in non-stressed cells and about 4–6% in stressed tissues [69,70]. It assists in proper protein folding and prevents aggregation of non-native proteins [71]. The HSPA1 protein (previously designated HSP70) is another cytoprotective agent with very low transcript levels in unstressed normal cells, where it plays a crucial role in guiding conformational status of the proteins during folding and translocation [72] and conferring thermo-resistance [73]. HSPD1 (previously HSP60) is foremost located as a chaperonin in the cytosol and mitochondrial matrix [74]. HSPA1A and HSPD1 genes were more strongly expressed in cell layers after scratch wounding and during scratch healing progression, highlighting their role as chaperones in protein folding and as possible cell regulators [65,66,67]. HSPD1 has also been shown to induce an inflammatory response through several different receptors including TLR2, TLR4, and CD36 [65,67]. Consequently, it is expected that HSPs can further act as potent immune activators outside of cells, where they induce various pro-inflammatory cytokines, interact with antigenic polypeptides, and assist in antigen presentation [66]. HSP90AA1 and HSPD1 levels were slightly increased by UV-B irradiation in keratinocytes. However, transcription of HSPA1A was not altered after UV-B irradiation in keratinocytes, but was significantly induced by heating w/o wIRA under optimal conditions. HSPA1 activation indicates the condition of cellular stress [122], which is not limited to heat shock, but can also range from oxidative stress to ischemia and inflammation or aging [123]. UV-B irradiation also induced HSP90AA1, HSPA1A, and HSPD1 gene expression in fibroblasts and these HSPs might therefore be implicated in the inhibition of scratch healing. Stress response gene transcription level evaluation further pointed to condition-specific increases in the HSPs’ gene expression under infection as well as chronic and anti-inflammatory conditions. TNF-α/IFN-γ stimulation resulted in an increase in HSPD1 and HSP90AA1 gene expression in both cell types as well as a significant elevation in HSPA1A levels in fibroblasts. S. aureus notably stimulated HSPA1A and HSP90AA1 gene expression in keratinocytes, but not in fibroblasts. Anti-inflammatory conditions led to significantly higher HSPD1 transcript levels in the cell scratches compared to the control. In keratinocytes, this was also found for HSP90AA1. It is most likely that non-optimal conditions affect HSP gene expression due to their role as chaperones in protein folding and possible cell regulators [65,66,67]. Under infection, wIRA radiation could restore HSPA1A transcription to control levels and alleviate HSP90AA1 increases in keratinocytes. This might contribute to the prolonged survival of wIRA-treated keratinocytes under infection conditions. For instance, HSF1 is known to initiate host defense against bacterial infection, partly through promoting early TLR2 signaling activation [124]. Treatment with wIRA had no significant effect in infected fibroblasts. Hence, it seems likely that toxic effects of the bacteria are overwhelming the natural cellular defense mechanism and impede potential protective and regulatory events, resulting in the abolished scratch closure. On the other hand, HSPs have been associated with the internalization of S. aureus and their inhibition was implicated in the protection against bacterial infection [125]. HSPD1 and HSPA1A transcription have been found to be significantly augmented by wIRA radiation in keratinocytes under chronic inflammatory conditions. HSDP1 has been implicated in wound healing [75] and might therefore confer the improved wound healing observed under these conditions after wIRA treatment. However, the same gene transcripts were reduced in fibroblasts to control levels, which might be another explanation as to why wIRA radiation exhibits a beneficial effect in fibroblast layer regeneration after scratching. Moreover, under anti-inflammatory settings, the increased gene expression levels of HSP90AA1 and HSPD1 were reduced by wIRA treatment in keratinocytes and in contrast, wIRA radiation induced HSPA1A and HSP90AA1 transcription in fibroblasts. Hence, further research is warranted to illuminate the effect of photobiomodulation on HSPs and their role in wound healing after wIRA treatment.
5. Conclusions
Keratinocyte and fibroblast wound healing under all non-optimal environments was found to be distinctly reduced in vitro. The influence was most pronounced for the infection with S. aureus, which damaged the cells to an extent that scratch wounds did not heal. Under chronic inflammatory and anti-inflammatory conditions, scratch wound healing was also significantly impeded, which was considerably improved by treatment with wIRA. The regenerative effect of wIRA radiation under non-optimal conditions could be an explanation for the positive effects observed previously in chronic wounds [26]. The enhancing effect of wIRA treatment on scratch wound closure was confirmed by a slight increase in DSG1 as well as a significant induction of DSG3 levels in keratinocytes under optimal conditions. Furthermore, wIRA was able to restore DSG1 and DSG3 transcript levels under chronic inflammatory conditions to these control levels and elicited a significant increase in DSG1 gene expression under anti-inflammatory conditions. In addition, wIRA especially induced gene expression of COL3A1 under all conditions and to some extent, promoted COL1A1 transcription under chronic and anti-inflammatory conditions in fibroblasts. It was also found that wIRA treatment had the potential to counteract the inflammatory response in infected keratinocytes as well as under chronic inflammatory conditions by decreasing pro-inflammatory cytokine gene expression. In contrast, in the anti-inflammatory setting, wIRA radiation could re-initiate the acute inflammatory response, which has been found necessary after injury to stimulate regenerative processes [41] by increasing IL1A levels in keratinocytes and early transcription of IL1A, IL6, and CXCL8 in fibroblasts. These results were further confirmed by observed increases of TGFB1 levels in wIRA-treated cells under chronic and anti-inflammatory conditions as well as induction of PDGFC in the latter case. Positive effects of wIRA could also partly be linked to elevation of NFKB1 levels under anti-inflammatory conditions as NF-κB has been shown to improve wound healing in vitro [121]. Most interesting were the results for the expression of heat shock protein genes, which have not been studied extensively so far in scratch wound healing models. HSPA1A and HSPD1 were differently transcribed in cell layers after scratch wounding and during scratch healing progression. Non-optimal conditions promoted increased transcript levels. Under infection, wIRA radiation could restore HSPA1A levels to control levels and alleviate HSP90AA1 increases in keratinocytes, which might have contributed to the slightly improved survival of wIRA-treated, infected keratinocytes. Furthermore, HSPD1, which has been previously implicated in wound healing [1], and HSPA1A gene expression have been found to be significantly augmented by wIRA radiation in keratinocytes under chronic inflammatory conditions. However, contradicting results have been found between keratinocytes and fibroblasts. Hence, further research seems warranted to illuminate the effect of photobiomodulation on HSPs and their role in wound healing after wIRA treatment.
Author Contributions
Conceptualization, C.W. and J.T.; Methodology, C.W. and U.-C.H.; Validation, C.W., P.E., and J.T.; Investigation, C.W.; Resources, C.W., U.-C.H., P.E., and J.T.; Data curation, C.W. and J.T.; Writing—original draft preparation, C.W.; Writing—review and editing, C.W., P.E., and J.T.; Visualization, C.W.; Supervision, P.E.; Project administration, C.W. and U.-C.H.; Funding acquisition, C.W. and J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Dr. med. h.c. Erwin Braun Stiftung, Basel, Switzerland.
Institutional Review Board Statement
Not applicable as studies did not involve humans or animals.
Informed Consent Statement
Not applicable as the study did not involve humans.
Data Availability Statement
The data in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Sylvia Hänßgen for her excellent technical support in the gene expression analysis. We acknowledge support by the German Research Foundation and the Open Access Publication Fund of the Thueringer Universitaets- und Landesbibliothek Jena Projekt-Nr. 433052568.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| AMP | antimicrobial peptide |
| COL | collagen |
| CXCL | C-X-C motif ligand |
| DSG | desmoglein |
| ELISA | enzyme linked immunosorbent assay |
| HSP | heat shock protein |
| IL | interleukin |
| IFN | interferon |
| MMP | matrix metalloproteinase |
| n.d. | not determined |
| NFKB | nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells |
| PDGF | platelet derived growth factor |
| qPCR | quantitative polymerase chain reaction |
| ROS | reactive oxygen species |
| TGF | transforming growth factor |
| TNF | tumor necrosis factor |
| TP | tumor suppressor gene |
| UV-B | ultraviolet light B |
| w/o | without |
| wIRA | water-filtered infrared A |
References
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute; fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Suter, M.M.; Schulze, K.; Bergman, W.; Welle, M.; Roosje, P.; Müller, E.J. The keratinocyte in epidermal renewal and defence. Vet. Dermatol. 2009, 20, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Choudhury, D. Insulin Promotes Wound Healing by Inactivating NFkβP50/P65 and Activating Protein and Lipid Biosynthesis and alternating Pro/Anti-inflammatory Cytokines Dynamics. Biomol. Concepts 2019, 10, 11–24. [Google Scholar] [CrossRef]
- Rahim, K.; Saleha, S.; Zhu, X.; Huo, L.; Basit, A.; Franco, O.L. Bacterial Contribution in Chronicity of Wounds. Microb. Ecol. 2017, 73, 710–721. [Google Scholar] [CrossRef]
- Jockenhöfer, F.; Chapot, V.; Stoffels-Weindorf, M.; Körber, A.; Klode, J.; Buer, J.; Küpper, B.; Roesch, A.; Dissemond, J. Bacterial spectrum colonizing chronic leg ulcers: A 10-year comparison from a German wound care center. J. Dtsch. Dermatol. Ges. 2014, 12, 1121–1127. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Nguyen, J.K.; Austin, E.; Huang, A.; Mamalis, A.; Jagdeo, J. The IL-4/IL-13 axis in skin fibrosis and scarring: Mechanistic concepts and therapeutic targets. Arch. Dermatol. Res. 2020, 312, 81–92. [Google Scholar] [CrossRef]
- Serezani, A.P.M.; Bozdogan, G.; Sehra, S.; Walsh, D.; Krishnamurthy, P.; Sierra Potchanant, E.A.; Nalepa, G.; Goenka, S.; Turner, M.J.; Spandau, D.F.; et al. IL-4 impairs wound healing potential in the skin by repressing fibronectin expression. J. Allergy Clin. Immunol. 2017, 139, 142–151. [Google Scholar] [CrossRef]
- Trautmann, A.; Toksoy, A.; Engelhardt, E.; Bröcker, E.B.; Gillitzer, R. Mast cell involvement in normal human skin wound healing: Expression of monocyte chemoattractant protein-1 is correlated with recruitment of mast cells which synthesize interleukin-4 in vivo. J. Pathol. 2000, 190, 100–106. [Google Scholar] [CrossRef]
- Tada, K.; Ikeda, K.; Tomita, K. Effect of polarized light emitting diode irradiation on wound healing. J. Trauma 2009, 67, 1073–1079. [Google Scholar] [CrossRef]
- Mussttaf, R.A.; Jenkins, D.F.L.; Jha, A.N. Assessing the impact of low level laser therapy (LLLT) on biological systems: A review. Int. J. Radiat. Biol. 2019, 95, 120–143. [Google Scholar] [CrossRef]
- Liao, X.; Xie, G.H.; Liu, H.W.; Cheng, B.; Li, S.H.; Xie, S.; Xiao, L.L.; Fu, X.B. Helium-neon laser irradiation promotes the proliferation and migration of human epidermal stem cells in vitro: Proposed mechanism for enhanced wound re-epithelialization. Photomed. Laser Surg. 2014, 32, 219–225. [Google Scholar] [CrossRef]
- Haas, A.F.; Isseroff, R.R.; Wheeland, R.G.; Rood, P.A.; Graves, P.J. Low-energy helium-neon laser irradiation increases the motility of cultured human keratinocytes. J. Investig. Dermatol. 1990, 94, 822–826. [Google Scholar] [CrossRef]
- Houreld, N.; Abrahamse, H. Low-intensity laser irradiation stimulates wound healing in diabetic wounded fibroblast cells (WS1). Diabetes Technol. 2010, 12, 971–978. [Google Scholar] [CrossRef]
- Leite, S.N.; Andrade, T.A.; Masson-Meyers, D.S.; Leite, M.N.; Enwemeka, C.S.; Frade, M.A. Phototherapy promotes healing of cutaneous wounds in undernourished rats. Bras. Dermatol. 2014, 89, 899–904. [Google Scholar] [CrossRef]
- Caetano, K.S.; Frade, M.A.; Minatel, D.G.; Santana, L.A.; Enwemeka, C.S. Phototherapy improves healing of chronic venous ulcers. Photomed. Laser Surg. 2009, 27, 111–118. [Google Scholar] [CrossRef]
- Minatel, D.G.; Frade, M.A.; França, S.C.; Enwemeka, C.S. Phototherapy promotes healing of chronic diabetic leg ulcers that failed to respond to other therapies. Lasers Surg. Med. 2009, 41, 433–441. [Google Scholar] [CrossRef]
- Powell, M.W.; Carnegie, D.E.; Burke, T.J. Reversal of diabetic peripheral neuropathy and new wound incidence: The role of MIRE. Adv. Ski. Wound Care 2004, 17, 295–300. [Google Scholar] [CrossRef]
- Reddy, G.K.; Stehno-Bittel, L.; Enwemeka, C.S. Laser photostimulation accelerates wound healing in diabetic rats. Wound Repair Regen. 2001, 9, 248–255. [Google Scholar] [CrossRef]
- Reddy, G.K.; Stehno-Bittel, L.; Enwemeka, C.S. Matrix remodeling in healing rabbit Achilles tendon. Wound Repair Regen. 1999, 7, 518–527. [Google Scholar] [CrossRef]
- Hawkins, D.; Abrahamse, H. Effect of multiple exposures of low-level laser therapy on the cellular responses of wounded human skin fibroblasts. Photomed. Laser Surg. 2006, 24, 705–714. [Google Scholar] [CrossRef]
- von Felbert, V.; Schumann, H.; Mercer, J.B.; Strasser, W.; Daeschlein, G.; Hoffmann, G. Therapy of chronic wounds with water-filtered infrared-A (wIRA). GMS Krankenhhyg. Interdiszip. 2008, 2, Doc52. [Google Scholar]
- Hartel, M.; Illing, P.; Mercer, J.B.; Lademann, J.; Daeschlein, G.; Hoffmann, G. Therapy of acute wounds with water-filtered infrared-A (wIRA). GMS Krankenhhyg. Interdiszip. 2007, 2, Doc53. [Google Scholar]
- Winkel, R.; Hoffmann, G.; Hoffmann, R. Wassergefiltertes Infrarot A (wIRA) hilft Wunden heilen [Water-filtered infrared-A (wIRA) promotes wound healing]. Chirurg 2014, 85, 980–992. [Google Scholar] [CrossRef][Green Version]
- Mercer, J.B.; Nielsen, S.P.; Hoffmann, G. Improvement of wound healing by water-filtered infrared-A (wIRA) in patients with chronic venous stasis ulcers of the lower legs including evaluation using infrared thermography. Ger. Med. Sci. 2008, 6, Doc11. [Google Scholar]
- Hartel, M.; Hoffmann, G.; Wente, M.N.; Martignoni, M.E.; Büchler, M.W.; Friess, H. Randomized clinical trial of the influence of local water-filtered infrared A irradiation on wound healing after abdominal surgery. Br. J. Surg. 2006, 93, 952–960. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Danno, K.; Mori, N.; Toda, K.; Kobayashi, T.; Utani, A. Near-infrared irradiation stimulates cutaneous wound repair: Laboratory experiments on possible mechanisms. Photodermatol. Photoimmunol. Photomed. 2001, 17, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Applegate, L.A.; Scaletta, C.; Panizzon, R.; Frenk, E.; Hohlfeld, P.; Schwarzkopf, S. Induction of the putative protective protein ferritin by infrared radiation: Implications in skin repair. Int. J. Mol. Med. 2000, 5, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Ehrlicher, A.; Betz, T.; Stuhrmann, B.; Koch, D.; Milner, V.; Raizen, M.G.; Kas, J. Guiding neuronal growth with light. Proc. Natl. Acad. Sci. USA 2002, 99, 16024–16028. [Google Scholar] [CrossRef]
- Zöller, N.; König, A.; Butting, M.; Kaufmann, R.; Bernd, A.; Valesky, E.; Kippenberger, S. Water-filtered near-infrared influences collagen synthesis of keloid-fibroblasts in contrast to normal foreskin fibroblasts. J. Photochem. Photobiol. B 2016, 163, 194–202. [Google Scholar] [CrossRef]
- Knels, L.; Valtink, M.; Piazena, H.; de la Vega Marin, J.; Gommel, K.; Lupp, A.; Roehlecke, C.; Mehner, M.; Funk, R.H. Effects of narrow-band IR-A and of water-filtered infrared A on fibroblasts. Photochem. Photobiol. 2016, 92, 475–487. [Google Scholar] [CrossRef]
- Mann, E.R.; Smith, K.M.; Bernardo, D.; Al-Hassi, H.O.; Knight, S.C.; Hart, A.L. Review: Skin and the Immune System. J. Clin. Exp. Dermatol. Res. 2012, S2, 3. [Google Scholar] [CrossRef]
- Shiraki, Y.; Ishibashi, Y.; Hiruma, M.; Nishikawa, A.; Ikeda, S. Cytokine secretion profiles of human keratinocytes during Trichophyton tonsurans and Arthroderma benhamiae infections. J. Med. Microbiol. 2006, 55, 1175–1185. [Google Scholar] [CrossRef]
- Büth, H.; Luigi Buttigieg, P.; Ostafe, R.; Rehders, M.; Dannenmann, S.R.; Schaschke, N.; Stark, H.J.; Boukamp, P.; Brix, K. Cathepsin B is essential for regeneration of scratch-wounded normal human epidermal keratinocytes. Eur. J. Cell Biol. 2007, 86, 747–761. [Google Scholar] [CrossRef]
- Wiegand, C.; Abel, M.; Hipler, U.C.; Elsner, P. Effect of non-adhering dressings on promotion of fibroblast proliferation and wound healing in vitro. Sci. Rep. 2019, 9, 4320. [Google Scholar] [CrossRef]
- Wiegand, C.; Tittelbach, J.; Hipler, U.-C.; Elsner, P. Water-filtered infrared A (wIRA): From observations in clinical studies to complex in vitro models. In Water-Filtered Infrared-A Radiation: From Principle to Practice, 1st ed.; Vaupel, P., Ed.; UNI-MED: Bremen, Germany, accepted.
- Wiederholt, T.; Heise, R.; Skazik, C.; Marquardt, Y.; Joussen, S.; Erdmann, K.; Schröder, H.; Merk, H.F.; Baron, J.M. Calcium pantothenate modulates gene expression in proliferating human dermal fibroblasts. Exp. Dermatol. 2009, 18, 969–978. [Google Scholar] [CrossRef]
- Rose, M.T. Effect of growth factors on the migration of equine oral and limb fibroblasts using an in vitro scratch assay. Vet. J. 2012, 193, 539–544. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef]
- Ricci, G.; Patrizi, A.; Baldi, E.; Menna, G.; Tabanelli, M.; Masi, M. Long-term follow-up of atopic dermatitis: Retrospective analysis of related risk factors and association with concomitant allergic diseases. J. Am. Acad. Dermatol. 2006, 55, 765–771. [Google Scholar] [CrossRef]
- Jung, T.; Grune, T. Experimental basis for discriminating between thermal and athermal effects of water-filtered infrared A irradiation. Ann. N. Y. Acad. Sci. 2012, 1259, 33–38. [Google Scholar] [CrossRef]
- Jung, T.; Höhn, A.; Lau, A.M.; Piazena, H.; Grune, T. An experimental setup for the measurement of nonthermal effects during water-filtered infrared A-irradiation of mammalian cell cultures. Photochem. Photobiol. 2012, 88, 371–380. [Google Scholar] [CrossRef]
- Sims, J.; Towne, J.; Blumberg, H. 11 IL-1 family members in inflammatory skin disease. Ernst. Scher. Res. Found Workshop 2006, 56, 187–191. [Google Scholar]
- Sauder, D.N. The role of epidermal cytokines in inflammatory skin diseases. J. Investig. Dermatol. 1990, 95 (Suppl. S5), 27S–28S. [Google Scholar] [CrossRef]
- Choi, J.H.; Jun, J.H.; Kim, J.H.; Sung, H.J.; Lee, J.H. Synergistic effect of interleukin-6 and hyaluronic acid on cell migration and ERK activation in human keratinocytes. J. Korean Med. Sci. 2014, 29 (Suppl. S3), S210–S216. [Google Scholar] [CrossRef]
- Ramezanpour, M.; Smith, J.L.P.; Ooi, M.L.; Gouzos, M.; Psaltis, A.J.; Wormald, P.J.; Vreugde, S. Deferiprone has anti-inflammatory properties and reduces fibroblast migration in vitro. Sci. Rep. 2019, 9, 2378. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Yang, L.; Chan, T.; Demare, J.; Iwashina, T.; Ghahary, A.; Scott, P.G.; Tredget, E.E. Healing of burn wounds in transgenic mice overexpressing transforming growth factor-beta 1 in the epidermis. Am. J. Pathol. 2001, 159, 2147–2157. [Google Scholar] [CrossRef]
- Desmoulière, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef]
- Schmid, P.; Kunz, S.; Cerletti, N.; McMaster, G.; Cox, D. Injury induced expression of TGF-beta 1 mRNA is enhanced by exogenously applied TGF-beta S. Biochem. Biophys. Res. Commun. 1993, 194, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, L.; Li, H.; Shi, H.; Luo, H.; Zhang, Y.; Yu, C.; Jin, Y. Mesenchymal stem cells prevent hypertrophic scar formation via inflammatory regulation when undergoing apoptosis. J. Investig. Dermatol. 2014, 134, 2648–2657. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhao, X.; Ji, S.; He, C.; Wang, G.; Tang, C.; Gu, S.; Yin, C. The use of gene activated matrix to mediate effective SMAD2 gene silencing against hypertrophic scar. Biomaterials 2014, 35, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Ho-Chang, J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp. Eye Res. 2003, 77, 581–592. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, S.; Tang, Q.; Li, S.; Peng, C. KGF-1 accelerates wound contraction through the TGF-β1/Smad signaling pathway in a double-paracrine manner. J. Biol. Chem. 2019, 294, 8361–8370. [Google Scholar] [CrossRef]
- Merlo, S.; Frasca, G.; Canonico, P.L.; Sortino, M.A. Differential involvement of estrogen receptor alpha and estrogen receptor beta in the healing promoting effect of estrogen in human keratinocytes. J. Endocrinol. 2009, 200, 189–197. [Google Scholar] [CrossRef]
- Pinto, D.; Marzani, B.; Minervini, F.; Calasso, M.; Giuliani, G.; Gobbetti, M.; De Angelis, M. Plantaricin A synthesized by Lactobacillus plantarum induces in vitro proliferation and migration of human keratinocytes and increases the expression of TGF-β1; FGF7; VEGF-A and IL-8 genes. Peptides 2011, 32, 1815–1824. [Google Scholar] [CrossRef]
- Glim, J.E.; Niessen, F.B.; Everts, V.; van Egmond, M.; Beelen, R.H. Platelet derived growth factor-CC secreted by M2 macrophages induces alpha-smooth muscle actin expression by dermal and gingival fibroblasts. Immunobiology 2013, 218, 924–929. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Qin, J.Z.; Chaturvedi, V.; Denning, M.F.; Choubey, D.; Diaz, M.O.; Nickoloff, B.J. Role of NF-kappaB in the apoptotic-resistant phenotype of keratinocytes. J. Biol. Chem. 1999, 274, 37957–37964. [Google Scholar] [CrossRef]
- Muresan, X.M.; Sticozzi, C.; Belmonte, G.; Cervellati, F.; Ferrara, F.; Lila, M.A.; Valacchi, G. SR-B1 involvement in keratinocytes in vitro wound closure. Arch. Biochem. Biophys. 2018, 658, 1–6. [Google Scholar] [CrossRef]
- Na, J.; Lee, K.; Na, W.; Shin, J.Y.; Lee, M.J.; Yune, T.Y.; Lee, H.K.; Jung, H.S.; Kim, W.S.; Ju, B.G. Histone H3K27 Demethylase JMJD3 in Cooperation with NF-κB Regulates Keratinocyte Wound Healing. J. Investig. Dermatol. 2016, 136, 847–858. [Google Scholar] [CrossRef]
- Parveen, N.; Varman, R.; Nair, S.; Das, G.; Ghosh, S.; Mukhopadhyay, S. Endocytosis of Mycobacterium tuberculosis heat shock protein 60 is required to induce interleukin-10 production in macrophages. J. Biol. Chem. 2013, 288, 24956–24971. [Google Scholar] [CrossRef]
- Pockley, A.G.; Muthana, M.; Calderwood, S.K. The dual immunoregulatory roles of stress proteins. Trends Biochem. Sci. 2008, 33, 71–79. [Google Scholar] [CrossRef]
- Da Costa, C.U.; Wantia, N.; Kirschning, C.J.; Busch, D.H.; Rodriguez, N.; Wagner, H.; Miethke, T. Heat shock protein 60 from Chlamydia pneumoniae elicits an unusual set of inflammatory responses via Toll-like receptor 2 and 4 in vivo. Eur. J. Immunol. 2004, 34, 2874–2884. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef]
- Welch, W.J.; Feramisco, J.R. Purification of the major mammalian heat shock proteins. J. Biol. Chem. 1982, 257, 14949–14959. [Google Scholar] [CrossRef]
- Welch, W.J. The role of heat-shock proteins as molecular chaperones. Curr. Opin. Cell Biol. 1991, 3, 1033–1038. [Google Scholar] [CrossRef]
- Makhnevych, T.; Houry, W.A. The role of Hsp90 in protein complex assembly. Biochim. Biophys. Acta 2012, 1823, 674–682. [Google Scholar] [CrossRef]
- Arya, R.; Mallik, M.; Lakhotia, S.C. Heat shock genes—integrating cell survival and death. J. Biosci. 2007, 32, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Pei, Q.; Ni, W.; Fu, X.; Zhang, W.; Song, C.; Peng, Y.; Guo, Q.; Dong, J.; Yao, M. HSPA1A Protects Cells from Thermal Stress by Impeding ESCRT-0-Mediated Autophagic Flux in Epidermal Thermoresistance. J. Investig. Dermatol. 2021, 141, 48–58. [Google Scholar] [CrossRef]
- Voos, W.; Röttgers, K. Molecular chaperones as essential mediators of mitochondrial biogenesis. Biochim. Biophys. Acta 2002, 1592, 51–62. [Google Scholar] [CrossRef]
- Pei, W.; Tanaka, K.; Huang, S.C.; Xu, L.; Liu, B.; Sinclair, J.; Idol, J.; Varshney, G.K.; Huang, H.; Lin, S.; et al. Extracellular HSP60 triggers tissue regeneration and wound healing by regulating inflammation and cell proliferation. NPJ Regen. Med. 2016, 1, 16013. [Google Scholar] [CrossRef] [PubMed]
- Niyonsaba, F.; Kiatsurayanon, C.; Chieosilapatham, P.; Ogawa, H. Friends or Foes? Host defense (antimicrobial) peptides and proteins in human skin diseases. Exp. Dermatol. 2017, 26, 989–998. [Google Scholar] [CrossRef]
- Fritz, P.; Beck-Jendroschek, V.; Brasch, J. Inhibition of dermatophytes by the antimicrobial peptides human β-defensin-2; ribonuclease 7 and psoriasin. Med. Mycol. 2012, 50, 579–584. [Google Scholar] [CrossRef]
- Gläser, R.; Harder, J.; Lange, H.; Bartels, J.; Christophers, E.; Schröder, J.M. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat. Immunol. 2005, 6, 57–64. [Google Scholar] [CrossRef]
- Harder, J.; Bartels, J.; Christophers, E.; Schroder, J.M. Isolation and characterization of human beta -defensin-3; a novel human inducible peptide antibiotic. J. Biol. Chem. 2001, 276, 5707–5713. [Google Scholar] [CrossRef]
- Harder, J.; Schroder, J.M. RNase 7; a novel innate immune defense antimicrobial protein of healthy human skin. J. Biol. Chem. 2002, 277, 46779–46784. [Google Scholar] [CrossRef]
- Yasin, B.; Pang, M.; Turner, J.S.; Cho, Y.; Dinh, N.N.; Waring, A.J.; Lehrer, R.I.; Wagar, E.A. Evaluation of the inactivation of infectious Herpes simplex virus by host-defense peptides. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 187–194. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Bárcena-Varela, S.; Heinbockel, L.; Gutsmann, T.; Brandenburg, K.; Martinez-de-Tejada, G.; Weindl, G. Antimicrobial endotoxin-neutralizing peptides promote keratinocyte migration via P2X7 receptor activation and accelerate wound healing in vivo. Br. J. Pharm. 2018, 175, 3581–3593. [Google Scholar] [CrossRef] [PubMed]
- Pfalzgraff, A.; Heinbockel, L.; Su, Q.; Gutsmann, T.; Brandenburg, K.; Weindl, G. Synthetic antimicrobial and LPS-neutralising peptides suppress inflammatory and immune responses in skin cells and promote keratinocyte migration. Sci. Rep. 2016, 6, 31577. [Google Scholar] [CrossRef] [PubMed]
- Galkowska, H.; Olszewski, W.L.; Wojewodzka, U. Expression of natural antimicrobial peptide beta-defensin-2 and Langerhans cell accumulation in epidermis from human non-healing leg ulcers. Folia Histochem. Cytobiol. 2005, 43, 133–136. [Google Scholar]
- Heilborn, J.D.; Nilsson, M.F.; Kratz, G.; Weber, G.; Sørensen, O.; Borregaard, N.; Ståhle-Bäckdahl, M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Investig. Dermatol. 2003, 120, 379–389. [Google Scholar] [CrossRef]
- Howell, M.D.; Fairchild, H.R.; Kim, B.E.; Bin, L.; Boguniewicz, M.; Redzic, J.S.; Hansen, K.C.; Leung, D.Y. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J. Investig. Dermatol. 2008, 128, 2248–2258. [Google Scholar] [CrossRef]
- Rieg, S.; Steffen, H.; Seeber, S.; Humeny, A.; Kalbacher, H.; Dietz, K.; Garbe, C.; Schittek, B. Deficiency of dermcidin-derived antimicrobial peptides in sweat of patients with atopic dermatitis correlates with an impaired innate defense of human skin in vivo. J. Immunol. 2005, 174, 8003–8010. [Google Scholar] [CrossRef]
- Brandner, J.; Haftek, M.; Niessen, C. Adherens Junctions; Desmosomes and Tight Junctions in Epidermal Barrier Function. Open Dermatol. J. 2010, 4, 14–20. [Google Scholar] [CrossRef]
- Niessen, C.M. Tight junctions/adherens junctions: Basic structure and function. J. Investig. Dermatol. 2007, 127, 2525–2532. [Google Scholar] [CrossRef]
- Ebrahiminaseri, A.; Sadeghizadeh, M.; Moshaii, A.; Asgaritarghi, G.; Safari, Z. Combination treatment of dendrosomal nanocurcumin and low-level laser therapy develops proliferation and migration of mouse embryonic fibroblasts and alter TGF-β, VEGF, TNF-α and IL-6 expressions involved in wound healing process. PLoS ONE 2021, 16, e0247098. [Google Scholar] [CrossRef]
- Komine, N.; Ikeda, K.; Tada, K.; Hashimoto, N.; Sugimoto, N.; Tomita, K. Activation of the extracellular signal-regulated kinase signal pathway by light emitting diode irradiation. Lasers Med. Sci. 2010, 25, 531–537. [Google Scholar] [CrossRef]
- Nie, Y.; Xu, X.; Wang, W.; Ma, N.; Lendlein, A. The effects of oscillatory temperature on HaCaT keratinocyte behaviors. Clin. Hemorheol. Microcirc. 2020, 76, 317–327. [Google Scholar] [CrossRef]
- Jung, T.; Höhn, A.; Piazena, H.; Grune, T. Effects of water-filtered infrared A irradiation on human fibroblasts. Free Radic Biol. Med. 2010, 48, 153–160. [Google Scholar] [CrossRef]
- Wiegand, C.; Abel, M.; Ruth, P.; Hipler, U.C. HaCaT keratinocytes in co-culture with Staphylococcus aureus can be protected from bacterial damage by polihexanide. Wound Repair Regen. 2009, 17, 730–738. [Google Scholar] [CrossRef]
- Gaspar, K.; Kukova, G.; Bunemann, E.; Buhren, B.A.; Sonkoly, E.; Szollosi, A.G.; Muller, A.; Savinko, T.; Lauerma, A.I.; Alenius, H.; et al. The chemokine receptor CCR3 participates in tissue remodeling during atopic skin inflammation. J. Dermatol. Sci. 2013, 71, 12–21. [Google Scholar] [CrossRef]
- Ghazi, K.; Deng-Pichon, U.; Warnet, J.M.; Rat, P. Hyaluronan fragments improve wound healing on in vitro cutaneous model through P2X7 purinoreceptor basal activation: Role of molecular weight. PLoS ONE 2012, 7, e48351. [Google Scholar] [CrossRef]
- Volksdorf, T.; Heilmann, J.; Eming, S.A.; Schawjinski, K.; Zorn-Kruppa, M.; Ueck, C.; Vidal-Y-Sy, S.; Windhorst, S.; Jücker, M.; Moll, I.; et al. Tight Junction Proteins Claudin-1 and Occludin Are Important for Cutaneous Wound Healing. Am. J. Pathol. 2017, 187, 1301–1312. [Google Scholar] [CrossRef]
- Rötzer, V.; Hartlieb, E.; Winkler, J.; Walter, E.; Schlipp, A.; Sardy, M.; Spindler, V.; Waschke, J. Desmoglein 3-Dependent Signaling Regulates Keratinocyte Migration and Wound Healing. J. Investig. Dermatol. 2016, 136, 301–310. [Google Scholar] [CrossRef]
- Lee, H.; Park, H.Y.; Jeong, T.S. Pheophorbide a Derivatives Exert Antiwrinkle Effects on UVB-Induced Skin Aging in Human Fibroblasts. Life 2021, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kwon, T.R.; Kim, J.H.; Lee, B.C.; Oh, C.T.; Im, M.; Hwang, Y.K.; Paik, S.H.; Han, S.; Kim, J.Y.; et al. Anti photoaging and anti oxidative activities of natural killer cell conditioned medium following UV B irradiation of human dermal fibroblasts and a reconstructed skin model. Int. J. Mol. Med. 2019, 44, 1641–1652. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef]
- Wlaschek, M.; Thceva-Poor, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schüller, J.; Scharfetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Pthotochem. Photobiol. B 2001, 63, 41–51. [Google Scholar] [CrossRef]
- Cho, Y.H.; Bahuguna, A.; Kim, H.H.; Kim, D.I.; Kim, H.J.; Yu, J.M.; Jung, H.G.; Jang, J.Y.; Kwak, J.H.; Park, G.H.; et al. Potential effect of compounds isolated from Coffea arabica against UV-B induced skin damage by protecting fibroblast cells. J. Photochem. Photobiol. B 2017, 174, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.Z.; Hiscott, P.; Seitz, B.; Shlötzer-Schrehardt, U.; Simon, M., Jr.; Süveges, I.; Naumann, G.O. Ultraviolet-B enhances corneal stromal response to 193-nm excimer laser treatment. Ophthalmology 1997, 104, 375–380. [Google Scholar] [CrossRef]
- Borel, N.; Sauer-Durand, A.M.; Hartel, M.; Kuratli, J.; Vaupel, P.; Scherr, N.; Pluschke, G. wIRA: Hyperthermia as a treatment option for intracellular bacteria; with special focus on Chlamydiae and Mycobacteria. Int. J. Hyperth. 2020, 37, 373–383. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Tennert, C.; Karygianni, L.; Wrbas, K.T.; Hellwig, E.; Altenburger, M.J. Antimicrobial photodynamic therapy using visible light plus water-filtered infrared-A (wIRA). J. Med. Microbiol. 2013, 62, 467–473. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Walankiewicz, A.; Hellwig, E.; Follo, M.; Tennert, C.; Wittmer, A.; Karygianni, L. Photoinactivation Using Visible Light Plus Water-Filtered Infrared-A (vis+wIRA) and Chlorine e6 (Ce6) Eradicates Planktonic Periodontal Pathogens and Subgingival Biofilms. Front. Microbiol. 2016, 7, 1900. [Google Scholar] [CrossRef]
- Daeschlein, G.; Alborova, J.; Patzelt, A.; Kramer, A.; Lademann, J. Kinetics of physiological skin flora in a suction blister wound model on healthy subjects after treatment with water-filtered infrared-A radiation. Ski. Pharm. Physiol. 2012, 25, 73–77. [Google Scholar] [CrossRef]
- Zöller, N.N.; Kippenberger, S.; Thaçi, D.; Mewes, K.; Spiegel, M.; Sättler, A.; Schultz, M.; Bereiter-Hahn, J.; Kaufmann, R.; Bernd, A. Evaluation of beneficial and adverse effects of glucocorticoids on a newly developed full-thickness skin model. Toxicol. Vitr. 2008, 22, 747–759. [Google Scholar] [CrossRef]
- de Christo Scherer, M.M.; Marques, F.M.; Figueira, M.M.; Peisino, M.C.O.; Schmitt, E.F.P.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. Wound healing activity of terpinolene and α-phellandrene by attenuating inflammation and oxidative stress in vitro. J. Tissue Viability 2019, 28, 94–99. [Google Scholar] [CrossRef]
- de Moura Sperotto, N.D.; Steffens, L.; Veríssimo, R.M.; Henn, J.G.; Péres, V.F.; Vianna, P.; Chies, J.A.B.; Roehe, A.; Saffi, J.; Moura, D.J. Wound healing and anti-inflammatory activities induced by a Plantago australis hydroethanolic extract standardized in verbascoside. J. Ethnopharmacol. 2018, 225, 178–188. [Google Scholar] [CrossRef]
- Wedler, J.; Daubitz, T.; Schlotterbeck, G.; Butterweck, V. In vitro anti-inflammatory and wound-healing potential of a Phyllostachys edulis leaf extract—identification of isoorientin as an active compound. Planta Med. 2014, 80, 1678–1684. [Google Scholar] [CrossRef]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.K.; et al. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS ONE 2013, 8, e79325. [Google Scholar] [CrossRef]
- Magne, B.; Dedier, M.; Nivet, M.; Coulomb, B.; Banzet, S.; Lataillade, J.J.; Trouillas, M. IL-1β-Primed Mesenchymal Stromal Cells Improve Epidermal Substitute Engraftment and Wound Healing via Matrix Metalloproteinases and Transforming Growth Factor-β1. J. Investig. Dermatol. 2020, 140, 688–698. [Google Scholar] [CrossRef]
- Dinçer, T.; Boz Er, A.B.; Er, İ.; Toraman, B.; Yildiz, G.; Kalay, E. RIPK4 suppresses the TGF-β1 signaling pathway in HaCaT cells. Cell Biol. Int. 2020, 44, 848–860. [Google Scholar] [CrossRef]
- Premarathna, A.D.; Ranahewa, T.H.; Wijesekera, S.K.; Harishchandra, D.L.; Karunathilake, K.J.K.; Waduge, R.N.; Wijesundara, R.R.M.K.K.; Jayasooriya, A.P.; Wijewardana, V.; Rajapakse, R.P.V.J. Preliminary screening of the aqueous extracts of twenty-three different seaweed species in Sri Lanka with in-vitro and in-vivo assays. Heliyon 2020, 6, e03918. [Google Scholar] [CrossRef]
- Toraldo, G.; Bhasin, S.; Bakhit, M.; Guo, W.; Serra, C.; Safer, J.D.; Bhawan, J.; Jasuja, R. Topical androgen antagonism promotes cutaneous wound healing without systemic androgen deprivation by blocking β-catenin nuclear translocation and cross-talk with TGF-β signaling in keratinocytes. Wound Repair Regen. 2012, 20, 61–73. [Google Scholar] [CrossRef]
- Liu, K.; Gao, Z.; Zhou, G.; Zhang, W.; Wu, X.; Liu, W. Characterization of Smad3 knockout mouse derived skin cells. Vitr. Cell Dev. Biol. Anim. 2017, 53, 458–466. [Google Scholar] [CrossRef]
- Varga, J.; Abraham, D. Systemic sclerosis: A prototypic multisystem fibrotic disorder. J. Clin. Investig. 2007, 117, 557–567. [Google Scholar] [CrossRef]
- D’Errico, M.; Lemma, T.; Calcagnile, A.; Proietti De Santis, L.; Dogliotti, E. Cell type and DNA damage specific response of human skin cells to environmental agents. Mutat. Res. 2007, 614, 37–47. [Google Scholar] [CrossRef]
- Tonello, S.; Rizzi, M.; Migliario, M.; Rocchetti, V.; Renò, F. Low concentrations of neutrophil extracellular traps induce proliferation in human keratinocytes via NF-kB activation. J. Derm. Sci. 2017, 88, 110–116. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Ciocca, D.R. Heat shock proteins: Stress proteins with Janus-like properties in cancer. Int. J. Hyperth. 2008, 24, 31–39. [Google Scholar] [CrossRef]
- Favatier, F.; Bornman, L.; Hightower, L.E.; Günther, E.; Polla, B.S. Variation in hsp gene expression and Hsp polymorphism: Do they contribute to differential disease susceptibility and stress tolerance? Cell Stress Chaperones 1997, 2, 141–155. [Google Scholar] [CrossRef]
- Gally, F.; Minor, M.N.; Smith, S.K.; Case, S.R.; Chu, H.W. Heat shock factor 1 protects against lung mycoplasma pneumoniae infection in mice. J. Innate Immun. 2012, 4, 59–68. [Google Scholar] [CrossRef]
- Donnarumma, G.; Paoletti, I.; Buommino, E.; Tufano, M.A.; Baroni, A. Alpha-MSH reduces the internalization of Staphylococcus aureus and down-regulates HSP 70; integrins and cytokine expression in human keratinocyte cell lines. Exp. Dermatol. 2004, 13, 748–754. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).