A Polyclonal Antibody Raised against the Burkholderia cenocepacia OmpA-like Protein BCAL2645 Impairs the Bacterium Adhesion and Invasion of Human Epithelial Cells In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Culture Conditions
2.2. Cell Line and Cell Culture
2.3. Molecular Biology Techniques
2.4. Construction of a BCAL2645 Deletion Mutant
2.5. Production of a Goat Polyclonal Antibody Anti-BCAL2645
2.6. Western Blot Analyses
2.7. Quantitative Western Blot
2.8. Biofilm Formation Assays
2.9. Mucin’s Adhesion Assays
2.10. Adhesion and Invasion of Epithelial Cells
2.11. Effect of the Polyclonal Antibody Anti-BCAL2645 on Bacterial Adhesion and Invasion of Epithelial Cells
2.12. Bioinformatics Analyses
2.13. Statistical Analysis
3. Results
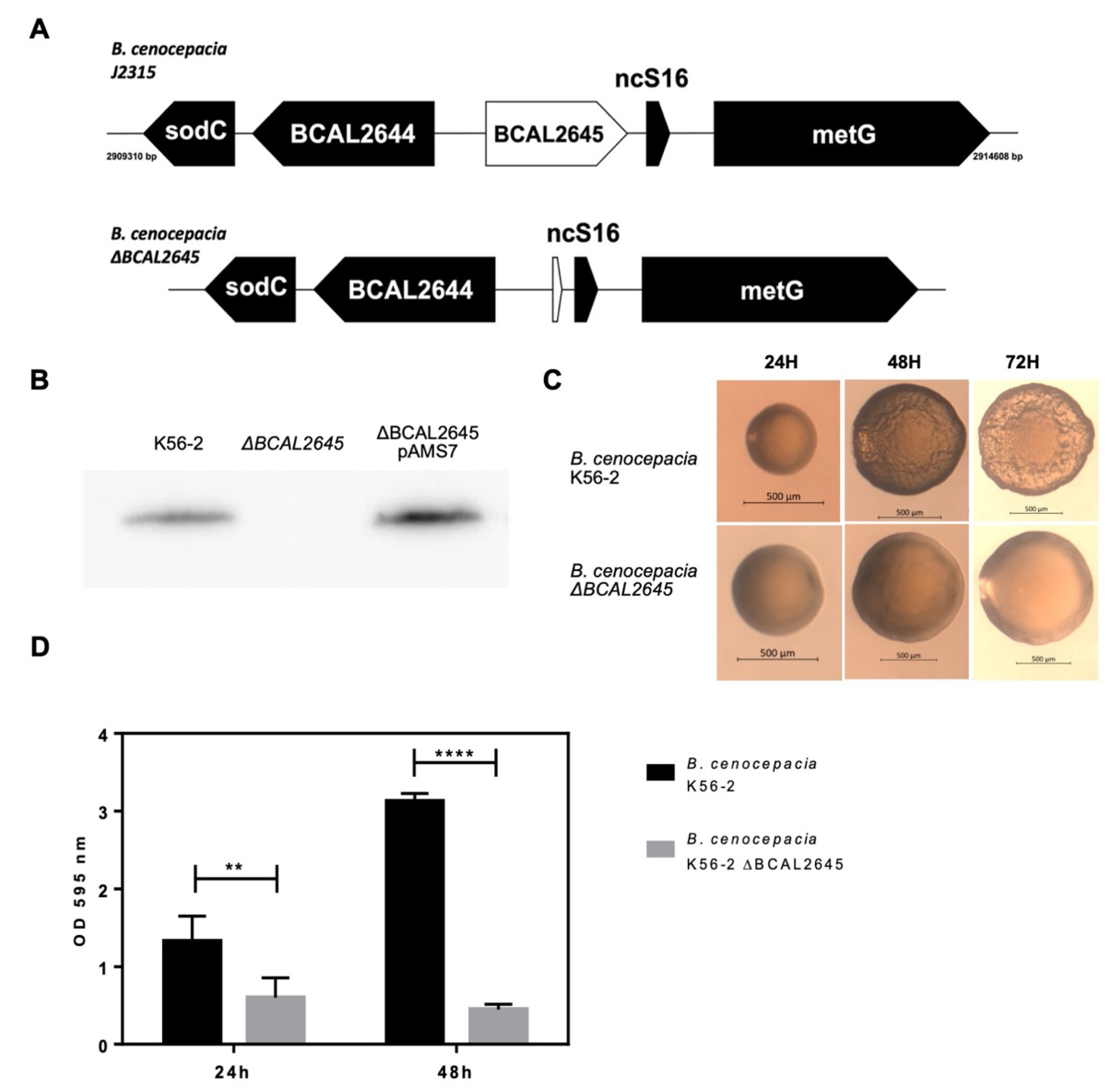
3.1. Construction of a BCAL2645 Gene Deletion Mutant from B. cenocepacia K56-2
3.2. The Deletion of BCAL2645 in B. cenocepacia K56-2 Impairs Biofilm Formation and Alters Colony Morphology
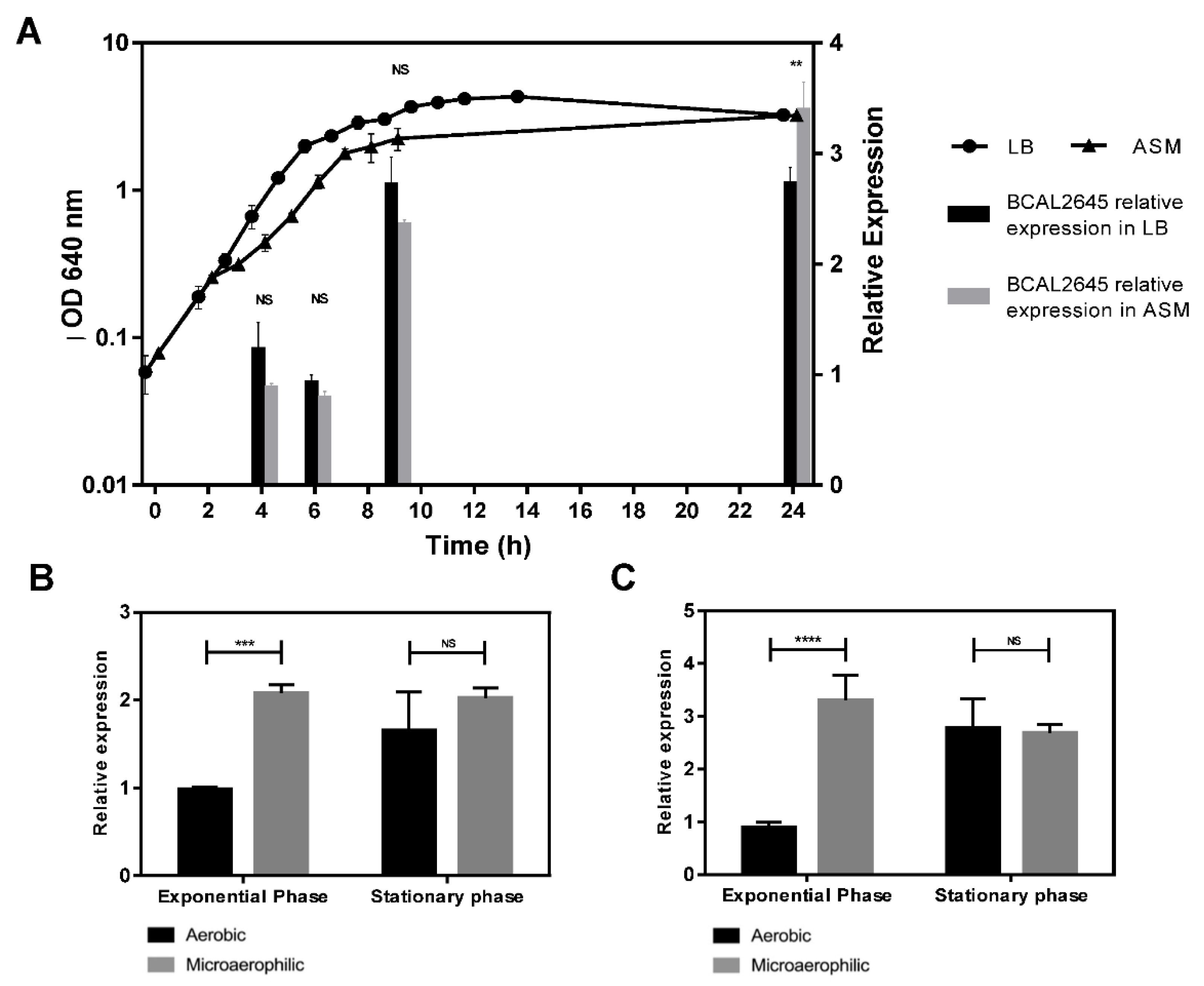
3.3. The BCAL2645 Protein Is More Expressed under Conditions Mimicking the CF Lung
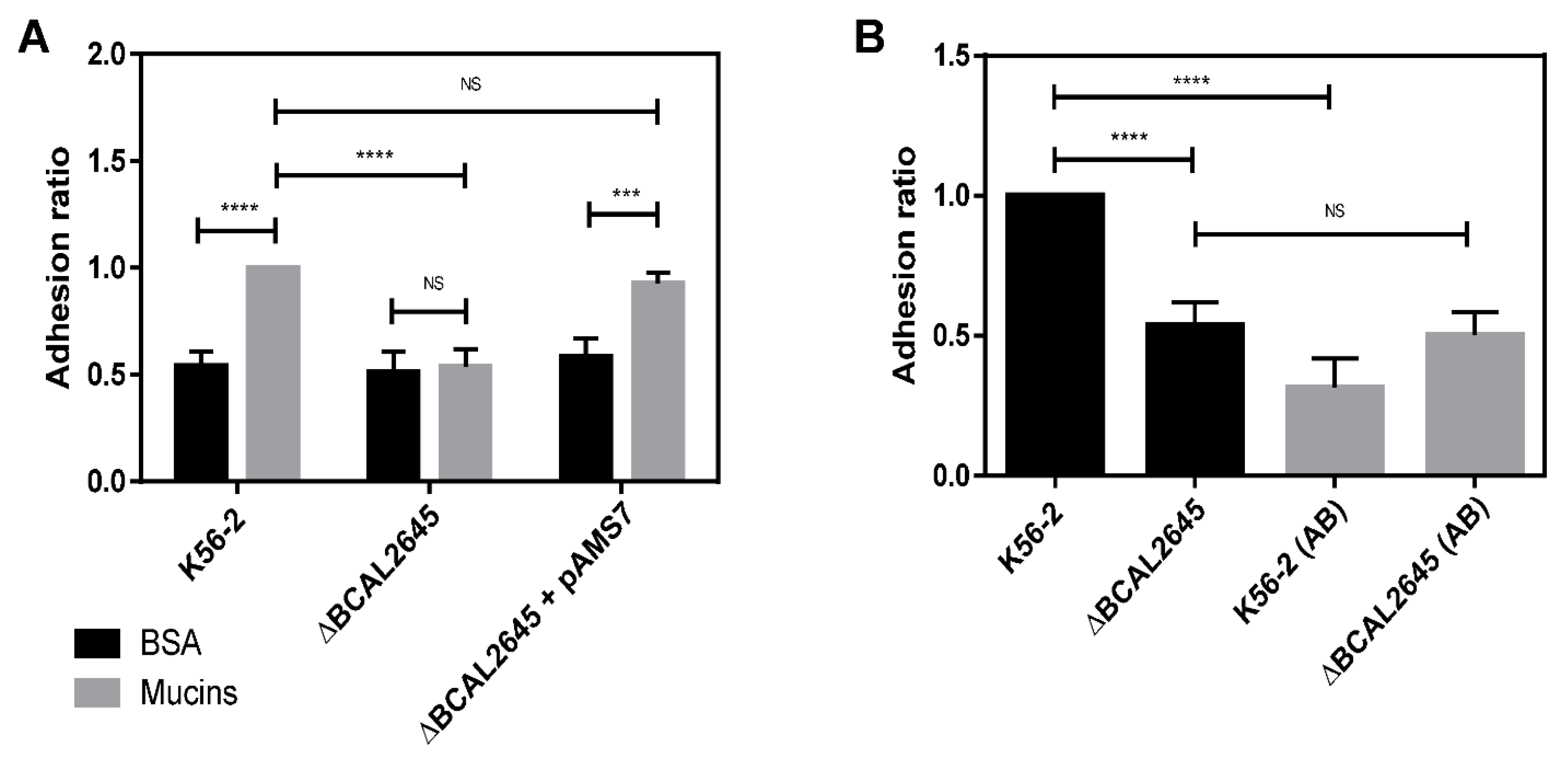
3.4. The BCAL2645 Protein Affects B. cenocepacia K56-2 Adhesion to Mucins
3.5. The BCAL2645 Protein Contributes to Invasion of Human Bronchial Cell Line CFBE41o-
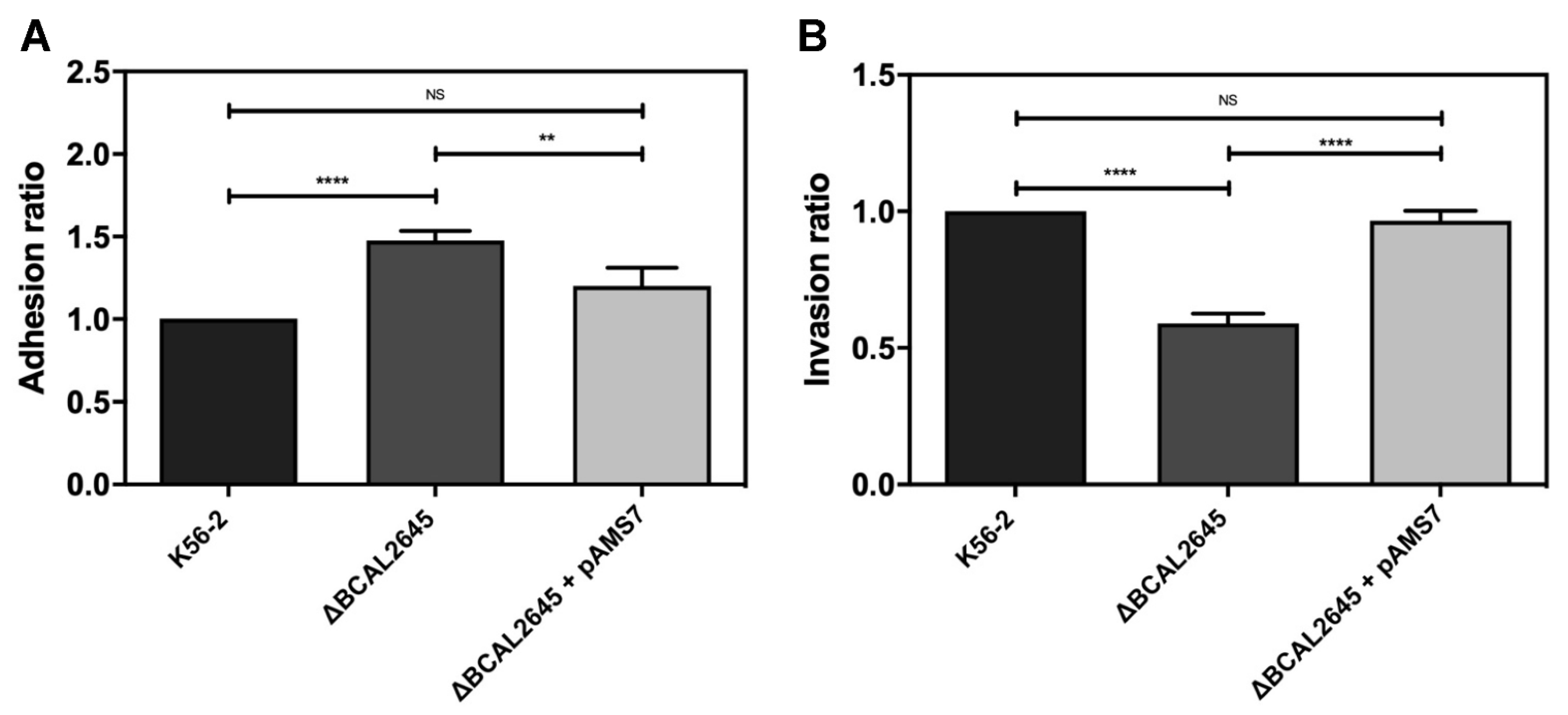
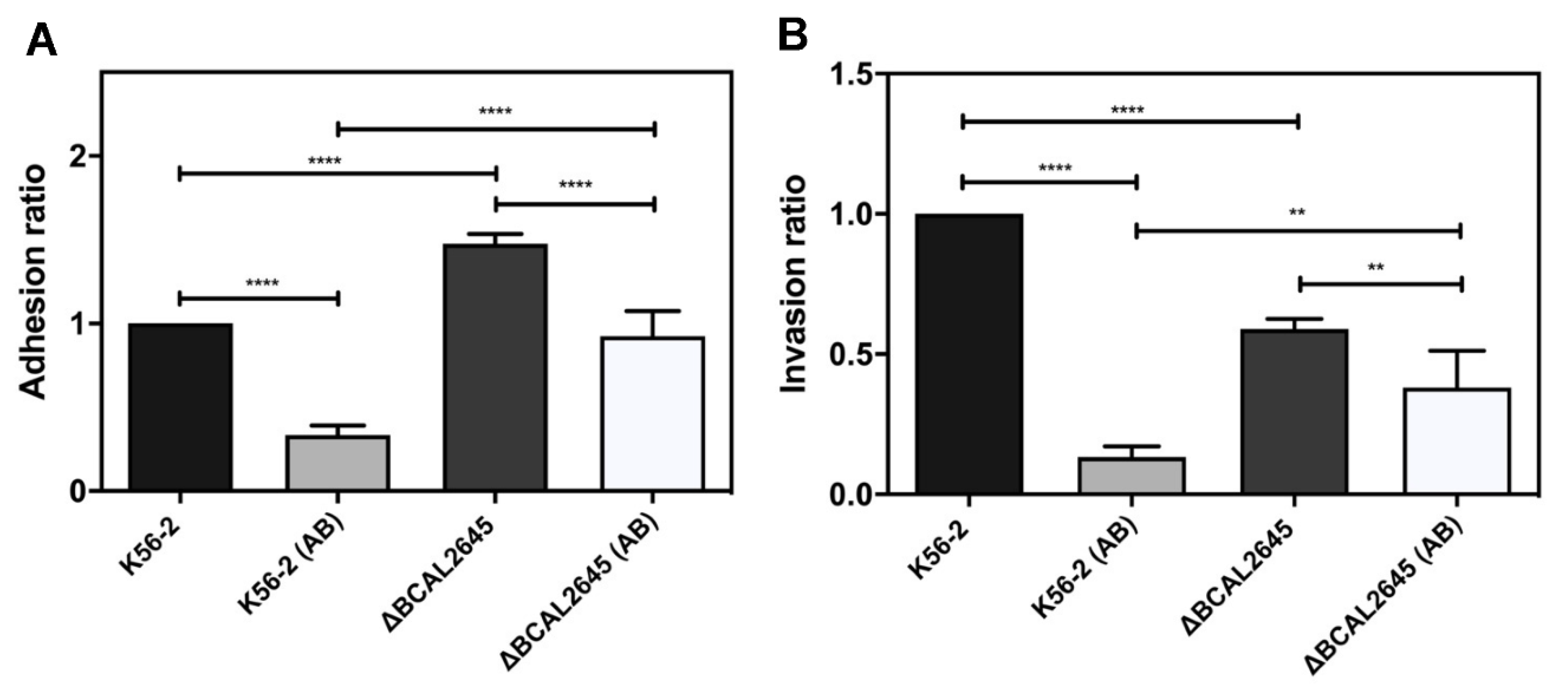
3.6. A Polyclonal Antibody against BCAL2645 Impairs B. cenocepacia K56-2 Adhesion and Invasion of Epithelial Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Govan, J.R.W.; Deretic, V. Microbial pathogenesis in cystic fibrosis: Mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 1996, 60, 539–574. [Google Scholar] [CrossRef]
- De Smet, B.; Mayo, M.; Peeters, C.; Zlosnik, J.; Spilker, T.; Hird, T.J.; Lipuma, J.J.; Kidd, T.; Kaestli, M.; Ginther, J.L.; et al. Burkholderia stagnalis sp. nov. and Burkholderia territorii sp. nov., two novel Burkholderia cepacia complex species from environmental and human sources. Int. J. Syst. Evol. Microbiol. 2015, 65, 2265–2271. [Google Scholar] [CrossRef]
- Weber, C.F.; King, G.M. Volcanic Soils as Sources of Novel CO-Oxidizing ParaBurkholderia and Burkholderia: ParaBurkholderia hiiakae sp. nov., ParaBurkholderia metrosideri sp. nov., ParaBurkholderia paradisi sp. nov., ParaBurkholderia peleae sp. nov., and Burkholderia alpina sp. nov. a Member of the Burkholderia cepacia Complex. Front. Microbiol. 2017, 8, 207. [Google Scholar] [CrossRef]
- Martina, P.; Leguizamon, M.; Prieto, C.I.; Sousa, S.A.; Montanaro, P.; Draghi, W.; Stämmler, M.; Bettiol, M.; de Carvalho, C.; Palau, J.; et al. Burkholderia puraquae sp. nov., a novel species of the Burkholderia cepacia complex isolated from hospital settings and agricultural soils. Int. J. Syst. Evol. Microbiol. 2018, 68, 14–20. [Google Scholar] [CrossRef]
- Drevinek, P.; Mahenthiralingam, E. Burkholderia cenocepacia in cystic fibrosis: Epidemiology and molecular mechanisms of virulence. Clin. Microbiol. Infect. 2010, 16, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.; Kozak, M.; Balola, A.; Sá-Correia, I. Burkholderia cepacia Complex Bacteria: A Feared Contamination Risk in Water-Based Pharmaceutical Products. Clin. Microbiol. Rev. 2020, 33, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Parke, J.L.; Gurian-Sherman, D. Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu. Rev. Phytopathol. 2001, 39, 225–258. [Google Scholar] [CrossRef] [PubMed]
- Leitão, J.H.; Sousa, S.A.; Ferreira, A.S.; Ramos, C.; Silva, I.; Moreira, L. Pathogenicity, virulence factors, and strategies to fight against Burkholderia cepacia complex pathogens and related species. Appl. Microbiol. Biotechnol. 2010, 87, 31–40. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Urban, T.A.; Goldberg, J.B. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Genet. 2005, 3, 144–156. [Google Scholar] [CrossRef]
- Nzula, S.; Vandamme, P.; Govan, J.R.W. Infuence of taxonomic status on the in vitro antimicrobial susceptibility of the Burkholderia cepacia complex. J. Antimicrob. Chemother. 2002, 50, 265–269. [Google Scholar] [CrossRef]
- Peeters, E.; Nelis, H.J.; Coenye, T. In vitro activity of ceftazidime, ciprofloxacin, meropenem, minocycline, tobramycin and trimethoprim/sulfamethoxazole against planktonic and sessile Burkholderia cepacia complex bacteria. J. Antimicrob. Chemother. 2009, 64, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.L.; Burns, J.L.; Ramsey, B.W. Pathophysiology and Management of Pulmonary Infections in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 918–951. [Google Scholar] [CrossRef] [PubMed]
- de Groot, R.; Smith, A.L. Antibiotic Pharmacokinetics in Cystic Fibrosis: Differences and Clinical Significance. Clin. Pharm. 1987, 13, 228–253. [Google Scholar] [CrossRef] [PubMed]
- Prandota, J. Drug disposition in cystic fibrosis: Progress in understanding pathophysiology and pharmacokinetics. Pediatr. Infect. Dis. J. 1987, 6, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Scoffone, V.C.; Chiarelli, L.R.; Trespidi, G.; Mentasti, M.; Riccardi, G.; Buroni, S. Burkholderia cenocepacia Infections in Cystic Fibrosis Patients: Drug Resistance and Therapeutic Approaches. Front. Microbiol. 2017, 8, 1592. [Google Scholar] [CrossRef]
- Leitão, J.H.; Sousa, S.A.; Cunha, M.V.; Salgado, M.J.; Cristino, J.M.; Barreto, M.C.; Sa-Correia, I. Variation of the antimicrobial susceptibility profiles of Burkholderia cepacia complex clonal isolates obtained from chronically infected cystic fibrosis patients: A five-year survey in the major Portuguese treatment center. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Lung Infections Associated with Cystic Fibrosis Lung Infections Associated with Cystic Fibrosis. Clin. Microbiol. Rev. 2002, 15, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Regan, K.H.; Bhatt, J. Eradication therapy for Burkholderia cepacia complex in people with cystic fibrosis. Cochrane Database Syst. Rev. 2019, 4, CD009876. [Google Scholar] [CrossRef]
- Johansen, H.K.; Nørregaard, L.; Gøtzsche, P.C.; Pressler, T.; Koch, C.; Hølby, N. Antibody Response to Pseudomonas aeruginosa in Cystic Fibrosis Patients: A Marker of Therapeutic Success?—A 30-Year Cohort Study of Survival in Danish CF Patients after Onset of Chronic P. aeruginosa Lung Infection. Pediatric Pulmonol. 2004, 37, 427–432. [Google Scholar] [CrossRef]
- Sousa, S.; Seixas, A.; Marques, J.; Leitão, J. Immunization and Immunotherapy Approaches against Pseudomonas aeruginosa and Burkholderia cepacia Complex Infections. Vaccines 2021, 9, 670. [Google Scholar] [CrossRef]
- Sousa, S.A.; Soares-Castro, P.; Seixas, A.M.; Feliciano, J.R.; Balugas, B.; Barreto, C.; Pereira, L.; Santos, P.M.; Leitão, J.H. New insights into the immunoproteome of B. cenocepacia J2315 using serum samples from cystic fibrosis patients. New Biotechnol. 2020, 54, 62–70. [Google Scholar] [CrossRef]
- Johnson, W.M.; Tyler, S.D.; Rozee, K.R. Linkage analysis of geographic and clinical clusters in Pseudomonas cepacia infections by multilocus enzyme electrophoresis and ribotyping. J. Clin. Microbiol. 1994, 32, 924–930. [Google Scholar] [CrossRef]
- Sousa, S.A.; Seixas, A.M.; Mandal, M.; Rodríguez-Ortega, M.J.; Leitão, J.H. Characterization of the Burkholderia cenocepacia J2315 Surface-Exposed Immunoproteome. Vaccines 2020, 8, 509. [Google Scholar] [CrossRef]
- Rollauer, S.E.; Sooreshjani, M.A.; Noinaj, N.; Buchanan, S.K. Outer membrane protein biogenesis in Gram-negative bacteria. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20150023. [Google Scholar] [CrossRef]
- Chaturvedi, D.; Mahalakshmi, R. Transmembrane β-barrels: Evolution, folding and energetics. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2017, 1859, 2467–2482. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.G.; Mahon, V.; Lambert, M.A.; Fagan, R. A molecular Swiss army knife: OmpA structure, function and expression. FEMS Microbiol. Lett. 2007, 273, 1–11. [Google Scholar] [CrossRef]
- Hong, H.; Szabo, G.; Tamm, L.K. Electrostatic couplings in OmpA ion-channel gating suggest a mechanism for pore opening. Nat. Chem. Biol. 2006, 2, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Confer, A.W.; Ayalew, S. The OmpA family of proteins: Roles in bacterial pathogenesis and immunity. Veter Microbiol. 2013, 163, 207–222. [Google Scholar] [CrossRef]
- Torres, A.G.; Li, Y.; Tutt, C.B.; Xin, L.; Eaves-Pyles, T.; Soong, L. Outer Membrane Protein A of Escherichia coli O157:H7 Stimulates Dendritic Cell Activation. Infect. Immun. 2006, 74, 2676–2685. [Google Scholar] [CrossRef] [PubMed]
- Vila-Farrés, X.; Parra-Millán, R.; Encinales, V.S.; Varese, M.; Ayerbe-Algaba, R.; Bayo, N.; Guardiola, S.; Pachón-Ibáñez, M.E.; Kotev, M.; García, J.; et al. Combating virulence of Gram-negative bacilli by OmpA inhibition. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Khalid, S.; Bond, P.J.; Carpenter, T.; Sansom, M.S. OmpA: Gating and dynamics via molecular dynamics simulations. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2008, 1778, 1871–1880. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steere, A.C.; Sikand, V.K.; Meurice, F.; Parenti, D.L.; Fikrig, E.; Schoen, R.T.; Nowakowski, J.; Schmid, C.; Laukamp, S.; Buscarino, C.; et al. Vaccination against Lyme Disease with Recombinant Borrelia burgdorferi Outer-Surface Lipoprotein A with Adjuvant. N. Engl. J. Med. 1998, 339, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Koebnik, R.; Locher, K.P.; Van Gelder, P. Structure and function of bacterial outer membrane proteins: Barrels in a nutshell. Mol. Microbiol. 2000, 37, 239–253. [Google Scholar] [CrossRef]
- Asadi, A.; Razavi, S.; Talebi, M.; Gholami, M. A review on anti-adhesion therapies of bacterial diseases. Infection 2018, 47, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Tipper, J.L.; Ingham, E.; Cove, J.H.; Todd, N.J.; Kerr, K.G. Survival and multiplication of Burkholderia cepacia within respiratory epithelial cells. Clin. Microbiol. Infect. 1998, 4, 450–459. [Google Scholar] [CrossRef][Green Version]
- Darling, P.; Chan, M.; Cox, A.D.; Sokol, P.A. Siderophore Production by Cystic Fibrosis Isolates of Burkholderia cepacia. Infect. Immun. 1998, 66, 874–877. [Google Scholar] [CrossRef]
- Sprynski, N.; Felix, C.; O’Callaghan, D.; Vergunst, A.C. Restoring virulence to mutants lacking subunits of multiprotein machines: Functional complementation of a Brucella virB5 mutant. FEBS Open Bio 2012, 2, 71–75. [Google Scholar] [CrossRef]
- Aubert, D.F.; Hamad, M.A.; Valvano, M.A. A Markerless Deletion Method for Genetic Manipulation of Burkholderia Cenocepacia and Other Multidrug-Resistant Gram-Negative Bacteria; Springer: Singapore, 2014; Volume 1197, pp. 311–327. [Google Scholar]
- Lefebre, M.D.; Valvano, M.A. Construction and Evaluation of Plasmid Vectors Optimized for Constitutive and Regulated Gene Expression in Burkholderia cepacia Complex Isolates. Appl. Environ. Microbiol. 2002, 68, 6283–6291. [Google Scholar] [CrossRef]
- Figurski, D.H.; Helinski, D.R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans (plasmid replication/replication origin/trans-complementation/broad host range/gene cloning). Proc. Natl. Acad. Sci. USA 1979, 76, 1648–1652. [Google Scholar] [CrossRef]
- Bruscia, E.; Sangiuolo, F.; Sinibaldi, P.; Goncz, K.K.; Novelli, G.; Gruenert, D.C. Isolation of CF cell lines corrected at ΔF508-CFTR locus by SFHR-mediated targeting. Gene Ther. 2002, 9, 683–685. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2012. [Google Scholar]
- Burkholderia Cenocepacia—Welcome Sanger Institute. Available online: https://www.sanger.ac.uk/resources/downloads/bacteria/burkholderia-cenocepacia.html (accessed on 30 June 2020).
- Flannagan, R.S.; Linn, T.; Valvano, M.A. A system for the construction of targeted unmarked gene deletions in the genus Burkholderia. Environ. Microbiol. 2008, 10, 1652–1660. [Google Scholar] [CrossRef]
- Aldridge, G.M.; Podrebarac, D.M.; Greenough, W.T.; Weiler, I.J. The use of total protein stains as loading controls: An alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J. Neurosci. Methods 2008, 172, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.; Schindelin, J.E.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.V.; Sousa, S.A.; Leitão, J.H.; Moreira, L.M.; Videira, P.A.; Sá-Correia, I. Studies on the Involvement of the Exopolysaccharide Produced by Cystic Fibrosis-Associated Isolates of the Burkholderia cepacia Complex in Biofilm Formation and in Persistence of Respiratory Infections. J. Clin. Microbiol. 2004, 42, 3052–3058. [Google Scholar] [CrossRef]
- Tomich, M.; Mohr, C.D. Adherence and autoaggregation phenotypes of a Burkholderia cenocepacia cable pilus mutant. FEMS Microbiol. Lett. 2003, 228, 287–297. [Google Scholar] [CrossRef]
- Mil-Homens, D.; Leça, M.I.; Fernandes, F.; Pinto, S.; Fialho, A.M. Characterization of BCAM0224, a Multifunctional Trimeric Autotransporter from the Human Pathogen Burkholderia cenocepacia. J. Bacteriol. 2014, 196, 1968–1979. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Silva, I.; Fernandes, F.; Pilkington, R.; Callaghan, M.; McClean, S.; Moreira, L.M. The Tyrosine Kinase BceF and the Phosphotyrosine Phosphatase BceD of Burkholderia contaminans Are Required for Efficient Invasion and Epithelial Disruption of a Cystic Fibrosis Lung Epithelial Cell Line. Infect. Immun. 2014, 83, 812–821. [Google Scholar] [CrossRef]
- Hulo, N.; Bairoch, A.; Bulliard, V.; Cerutti, L.; De Castro, E.; Langendijk-Genevaux, P.S.; Pagni, M.; Sigrist, C.J.A. The PROSITE database. Nucleic Acids Res. 2006, 34, D227–D230. [Google Scholar] [CrossRef] [PubMed]
- Winsor, G.; Khaira, B.; Van Rossum, T.; Lo, R.; Whiteside, M.D.; Brinkman, F.S.L. The Burkholderia Genome Database: Facilitating flexible queries and comparative analyses. Bioinformatics 2008, 24, 2803–2804. [Google Scholar] [CrossRef]
- Choi, U.; Lee, C.-R. Distinct Roles of Outer Membrane Porins in Antibiotic Resistance and Membrane Integrity in Escherichia coli. Front. Microbiol. 2019, 10, 953. [Google Scholar] [CrossRef]
- Sass, A.; Schmerk, C.; Agnoli, K.; Norville, P.J.; Eberl, L.; Valvano, M.; Mahenthiralingam, E. The unexpected discovery of a novel low-oxygen-activated locus for the anoxic persistence of Burkholderia cenocepacia. ISME J. 2013, 7, 1568–1581. [Google Scholar] [CrossRef] [PubMed]
- Worlitzsch, D.; Tarran, R.; Ulrich, M.; Schwab, U.; Cekici, A.; Meyer, K.C.; Birrer, P.; Bellon, G.; Berger, J.; Weiss, T.; et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patient. J. Clin. Investig. 2002, 109, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, E.P.; Kato, K.; Lu, W.; Kim, K.C. Cellular and Molecular Biology of Airway Mucins. Int. Rev. Cell Mol. Biol. 2013, 303, 139–202. [Google Scholar] [CrossRef]
- Corfield, A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 236–252. [Google Scholar] [CrossRef]
- Sajjan, U.S.; Corey, M.; Karmali, M.A.; Forstner, J.F. Binding of Pseudomonas cepacia to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J. Clin. Investig. 1992, 89, 648–656. [Google Scholar] [CrossRef]
- Jansen, H.-J.; Hart, C.A.; Rhodes, J.M.; Saunders, J.R.; Smalley, J.W. A novel mucin-sulphatase activity found in Burkholderia cepacia and Pseudomonas aeruginosa. J. Med. Microbiol. 1999, 48, 551–557. [Google Scholar] [CrossRef]
- Valvano, M.A. Intracellular survival of Burkholderia cepacia complex in phagocytic cells. Can. J. Microbiol. 2015, 61, 607–615. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, W.C.; Yeo, K.J.; Ryu, K.; Kumarasiri, M.; Hesek, D.; Lee, M.; Mobashery, S.; Song, J.H.; Kim, S.I.; et al. Mechanism of anchoring of OmpA protein to the cell wall peptidoglycan of the gram-negative bacterial outer membrane. FASEB J. 2011, 26, 219–228. [Google Scholar] [CrossRef]
- Ortiz-Suarez, M.L.; Samsudin, F.; Piggot, T.J.; Bond, P.J.; Khalid, S. Full-Length OmpA: Structure, Function, and Membrane Interactions Predicted by Molecular Dynamics Simulations. Biophys. J. 2016, 111, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. The Function of OmpA in Escherichia coli. Biochem. Biophys. Res. Commun. 2002, 292, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Smani, Y.; Fàbrega-Santamaria, A.; Roca, I.; Encinales, V.S.; Vila, J.; Pachón, J. Role of OmpA in the Multidrug Resistance Phenotype of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2014, 58, 1806–1808. [Google Scholar] [CrossRef] [PubMed]
- Fazli, M.; O’Connell, A.; Nilsson, M.; Niehaus, K.; Dow, J.M.; Givskov, M.; Ryan, R.P.; Tolker-Nielsen, T. The CRP/FNR family protein Bcam1349 is a c-di-GMP effector that regulates biofilm formation in the respiratory pathogen Burkholderia cenocepacia. Mol. Microbiol. 2011, 82, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Fazli, M.; Mccarthy, Y.; Givskov, M.; Ryan, R.P.; Tolker-Nielsen, T. The exopolysaccharide gene cluster Bcam1330-Bcam1341 is involved in Burkholderia cenocepacia biofilm formation, and its expression is regulated by c-di-GMP and Bcam1349. Microbiologyopen 2013, 2, 105–122. [Google Scholar] [CrossRef]
- Fazli, M.; Almblad, H.; Rybtke, M.L.; Givskov, M.; Eberl, L.; Tolker-Nielsen, T. Regulation of biofilm formation in Pseudomonas and Burkholderia species. Environ. Microbiol. 2014, 16, 1961–1981. [Google Scholar] [CrossRef]
- Van Acker, H.; Sass, A.; Bazzini, S.; De Roy, K.; Udine, C.; Messiaen, T.; Riccardi, G.; Boon, N.; Nelis, H.J.; Mahenthiralingam, E.; et al. Biofilm-Grown Burkholderia cepacia Complex Cells Survive Antibiotic Treatment by Avoiding Production of Reactive Oxygen Species. PLoS ONE 2013, 8, e58943. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Caraher, E. Residence in biofilms allows Burkholderia cepacia complex (Bcc) bacteria to evade the antimicrobial activities of neutrophil-like dHL60 cells. Pathog. Dis. 2015, 73, 8. [Google Scholar]
- Schwab, U.; Abdullah, L.H.; Perlmutt, O.S.; Albert, D.; Davis, C.W.; Arnold, R.R.; Yankaskas, J.R.; Gilligan, P.; Neubauer, H.; Randell, S.H.; et al. Localization of Burkholderia cepacia Complex Bacteria in Cystic Fibrosis Lungs and Interactions with Pseudomonas aeruginosa in Hypoxic Mucus. Infect. Immun. 2014, 82, 4729–4745. [Google Scholar] [CrossRef] [PubMed]
- Namba, A.; Mano, N.; Takano, H.; Beppu, T.; Ueda, K.; Hirose, H. OmpA is an adhesion factor of Aeromonas veronii, an optimistic pathogen that habituates in carp intestinal tract. J. Appl. Microbiol. 2008, 105, 1441–1451. [Google Scholar] [CrossRef]
- Sajjan, U.S.; Sylvester, F.A.; Forstner, J.F. Cable-Piliated Burkholderia cepacia Binds to Cytokeratin 13 of Epithelial Cells. Infect. Immun. 2000, 68, 1787–1795. [Google Scholar] [CrossRef]
- Martin, D.W.; Mohr, C.D. Invasion and Intracellular Survival of Burkholderia cepacia. Infect. Immun. 2000, 68, 3792. [Google Scholar] [CrossRef]
- Sousa, S.A.; Feliciano, J.R.; Pita, T.; Guerreiro, S.I.; Leitão, J.H. Burkholderia cepacia Complex Regulation of Virulence Gene Expression: A Review. Genes 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, F.; Behrouz, B.; Ranjbar, M.; Gargari, S.L.M. Immunotherapy with IgY Antibodies toward Outer Membrane Protein F Protects Burned Mice against Pseudomonas aeruginosa Infection. J. Immunol. Res. 2020, 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Cozens, D.; Read, R. Anti-adhesion methods as novel therapeutics for bacterial infections. Expert Rev. Anti-Infect. Ther. 2012, 10, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
| Strain or Plasmid | Genotype or Description | References or Source |
|---|---|---|
| Strains | ||
| E. coli DH5α | F− Φ80lacZ∆M15 ∆(lacZYA-argF) U169 recA1 endA1 hsdR17(rk−, mk+) phoA supE44 thi-1gyrA96 relA1 λ− | Invitrogen |
| E. coli BL21 (DE3) | F− ompT hsdSB (rB−mB−) dcm gal λ(DE3) | Stratagene |
| B. cenocepacia K56-2 | Cystic fibrosis clinical isolate (Toronto, Canada); ET12 lineage | [36] |
| B. cenocepacia K56-2 ΔBCAL2645 | B. cenocepacia K56-2 with the BCAL2645 gene deleted | This study |
| Plasmids | ||
| pSAS36 | pET23a+ containing the BCAL2645 gene, cloned downstream of the T7 promoter and upstream of the C-terminal 6× His-Tag, Apr | [23] |
| pIN290 | Suicide plasmid derived from pIN11 [37], sacR/B gene, RK2 oriT, I-SceI and encoding a DsRed fluorescent protein; Cmr | Kindly provided by Dr. Annette Vergunst |
| pDAI-SceI-SacB | pDA17 carrying the I-SceI gene and the counterselectable marker SacB, Tetr | [38] |
| pMLS7 | pBBR1 ori, pS7 promoter, mob+, Tpr | [39] |
| pRK2013 | Mobilizing vector, ColE1 tra (RK2)+, Kmr | [40] |
| pAMS6 | pIN290 with BCAL2645 gene upstream and downstream regions cloned | This study |
| pAMS7 | pMLS7 with BCAL2645 gene cloned | This study |
| Primer Name | Primer Sequence (5′→3′) | Restriction Site | Product Size | Annealing T (°C) |
|---|---|---|---|---|
| UP-Nested BCAL2645 | TAGATGTCGGCGTCGAGAATGC | - | 2200 bp | 60 |
| LW-Nested BCAL2645 | GAACATTTCCTTGACCGGATCGT | - | ||
| UP-Upstream BCAL2645 | ACCTCTAGACGGCAACAGCTTCAC | XbaI | 640 bp | 59.5 |
| LW-Upstream BCAL2645 | ACCGGATCCCTTGGATTCCTCTCTT | BamHI | ||
| UP-Downstream BCAL2645 | AAAGGATCCGCGCGACCACGAGA | BamHI | 710 bp | 59.5 |
| LW-Downstream BCAL2645 | ACCGAGCTCCGGTCGAGTAGAAG | SacI | ||
| UP-BCAL2645Comp | CCCGGATCCATGAACATGAAAATCGC | BamHI | 666 bp | 64 |
| LW-BCAL2645Comp | CCCAAGCTTTTACTGATGCTGTTGC | HindIII |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seixas, A.M.M.; Sousa, S.A.; Feliciano, J.R.; Gomes, S.C.; Ferreira, M.R.; Moreira, L.M.; Leitão, J.H. A Polyclonal Antibody Raised against the Burkholderia cenocepacia OmpA-like Protein BCAL2645 Impairs the Bacterium Adhesion and Invasion of Human Epithelial Cells In Vitro. Biomedicines 2021, 9, 1788. https://doi.org/10.3390/biomedicines9121788
Seixas AMM, Sousa SA, Feliciano JR, Gomes SC, Ferreira MR, Moreira LM, Leitão JH. A Polyclonal Antibody Raised against the Burkholderia cenocepacia OmpA-like Protein BCAL2645 Impairs the Bacterium Adhesion and Invasion of Human Epithelial Cells In Vitro. Biomedicines. 2021; 9(12):1788. https://doi.org/10.3390/biomedicines9121788
Chicago/Turabian StyleSeixas, António M. M., Sílvia A. Sousa, Joana R. Feliciano, Sara C. Gomes, Mirela R. Ferreira, Leonilde M. Moreira, and Jorge H. Leitão. 2021. "A Polyclonal Antibody Raised against the Burkholderia cenocepacia OmpA-like Protein BCAL2645 Impairs the Bacterium Adhesion and Invasion of Human Epithelial Cells In Vitro" Biomedicines 9, no. 12: 1788. https://doi.org/10.3390/biomedicines9121788
APA StyleSeixas, A. M. M., Sousa, S. A., Feliciano, J. R., Gomes, S. C., Ferreira, M. R., Moreira, L. M., & Leitão, J. H. (2021). A Polyclonal Antibody Raised against the Burkholderia cenocepacia OmpA-like Protein BCAL2645 Impairs the Bacterium Adhesion and Invasion of Human Epithelial Cells In Vitro. Biomedicines, 9(12), 1788. https://doi.org/10.3390/biomedicines9121788








