Selective Anticancer and Antimicrobial Metallodrugs Based on Gold(III) Dithiocarbamate Complexes
Abstract
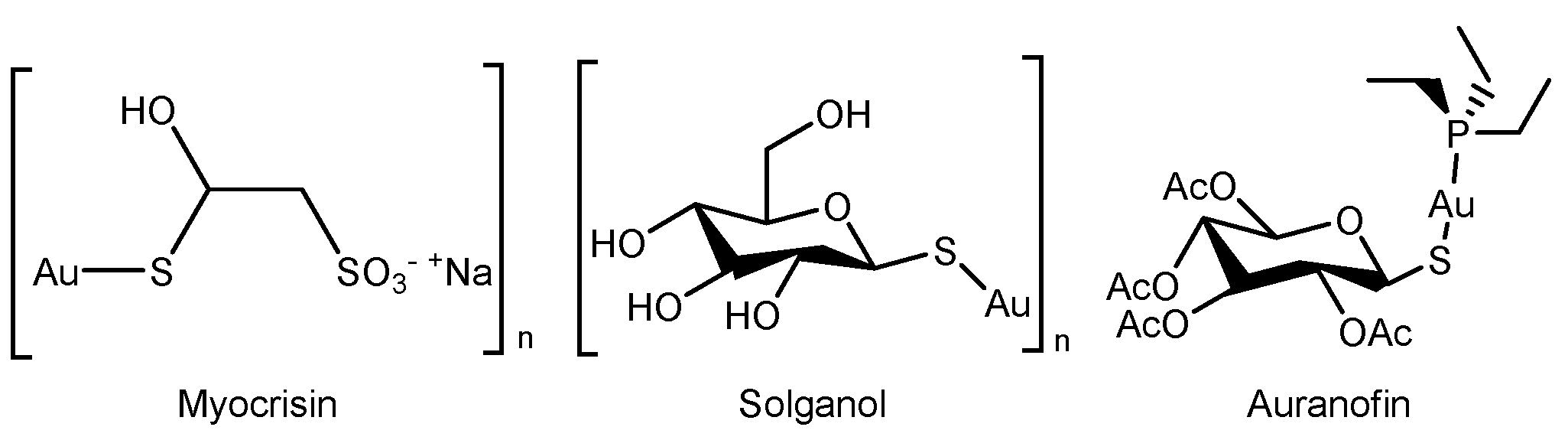
1. Introduction
2. Materials and Methods
2.1. Chemicals
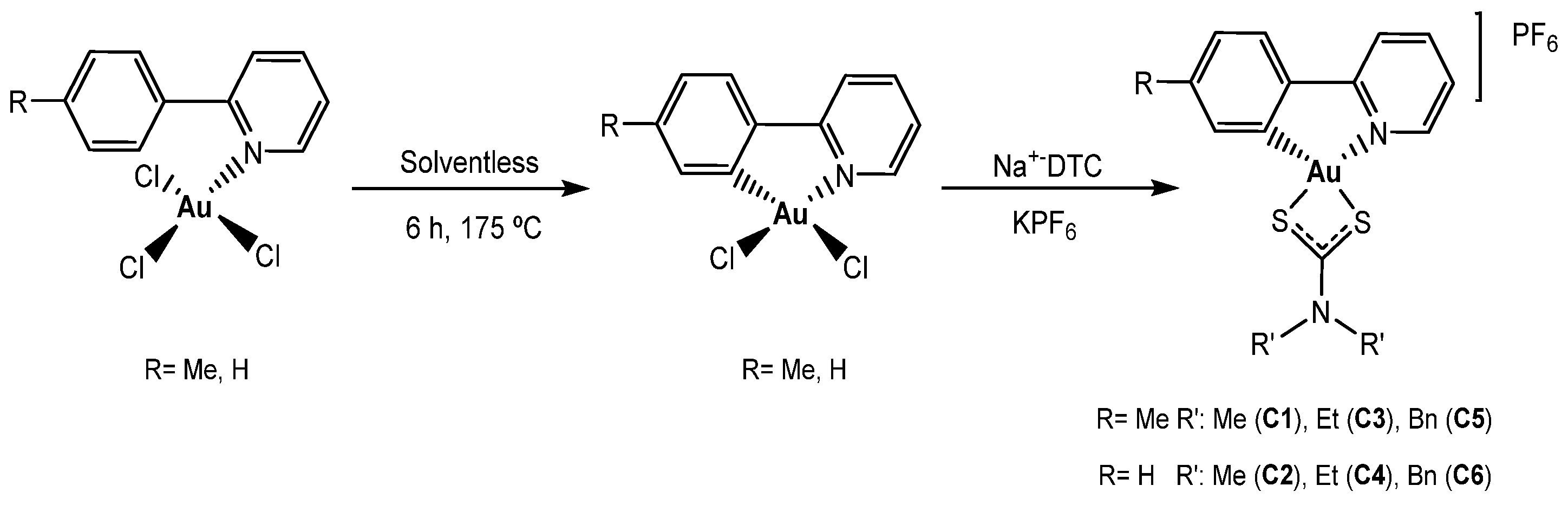
2.2. Synthesis of Dithiocarbamate Gold(III) Cyclometalated Complex
2.3. Distribution Coefficient (Log P7.4)
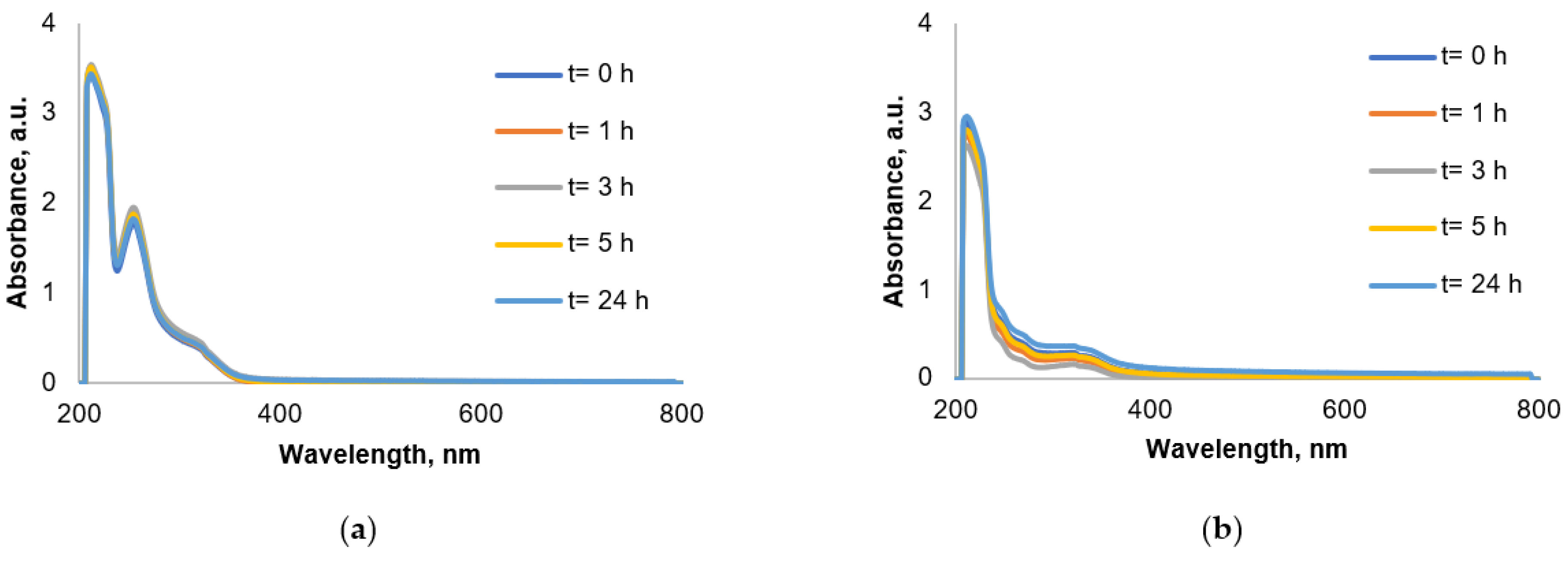
2.4. Solution Chemistry
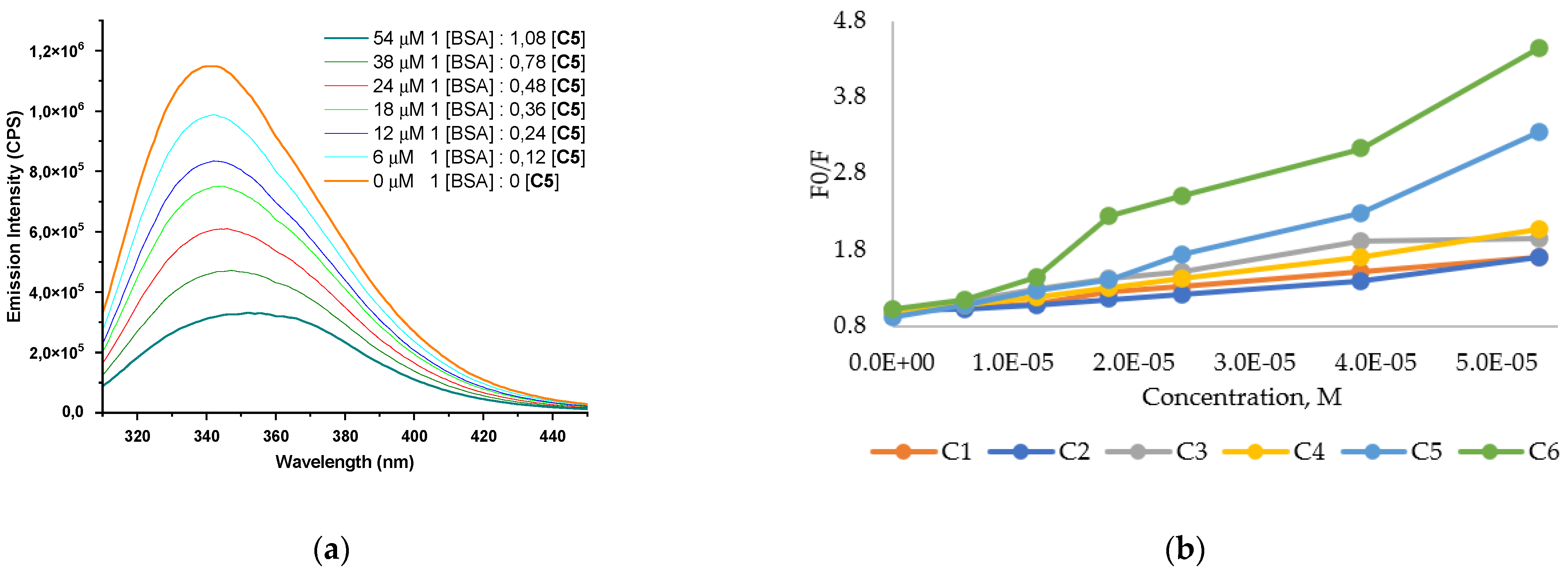
2.5. BSA Interaction Studies
2.6. Cell Culture
2.7. Cell Viability Assay
2.8. Apoptosis Studies
2.9. Cell Cycle Analyses
2.10. Oxidative Stress Damage
2.11. Antimicrobial Assays
2.11.1. Antibacterial Assay
2.11.2. Antifungal Assay
2.12. Statistical Analyses
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Lipophilicity Studies
3.3. Solution Behavior
3.4. Bovine Serum Albumin Interaction
3.5. In Vitro Antiproliferative Activity
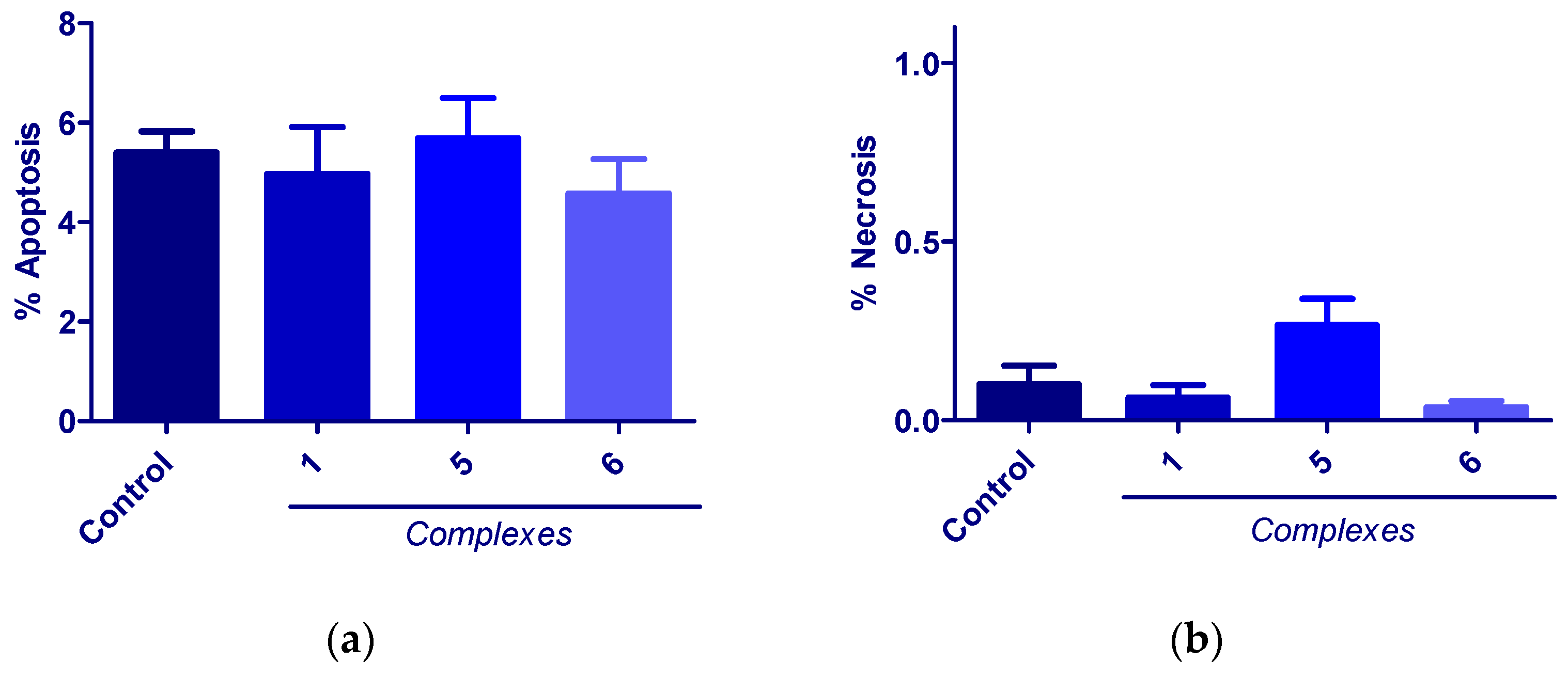
3.6. Apoptosis Studies
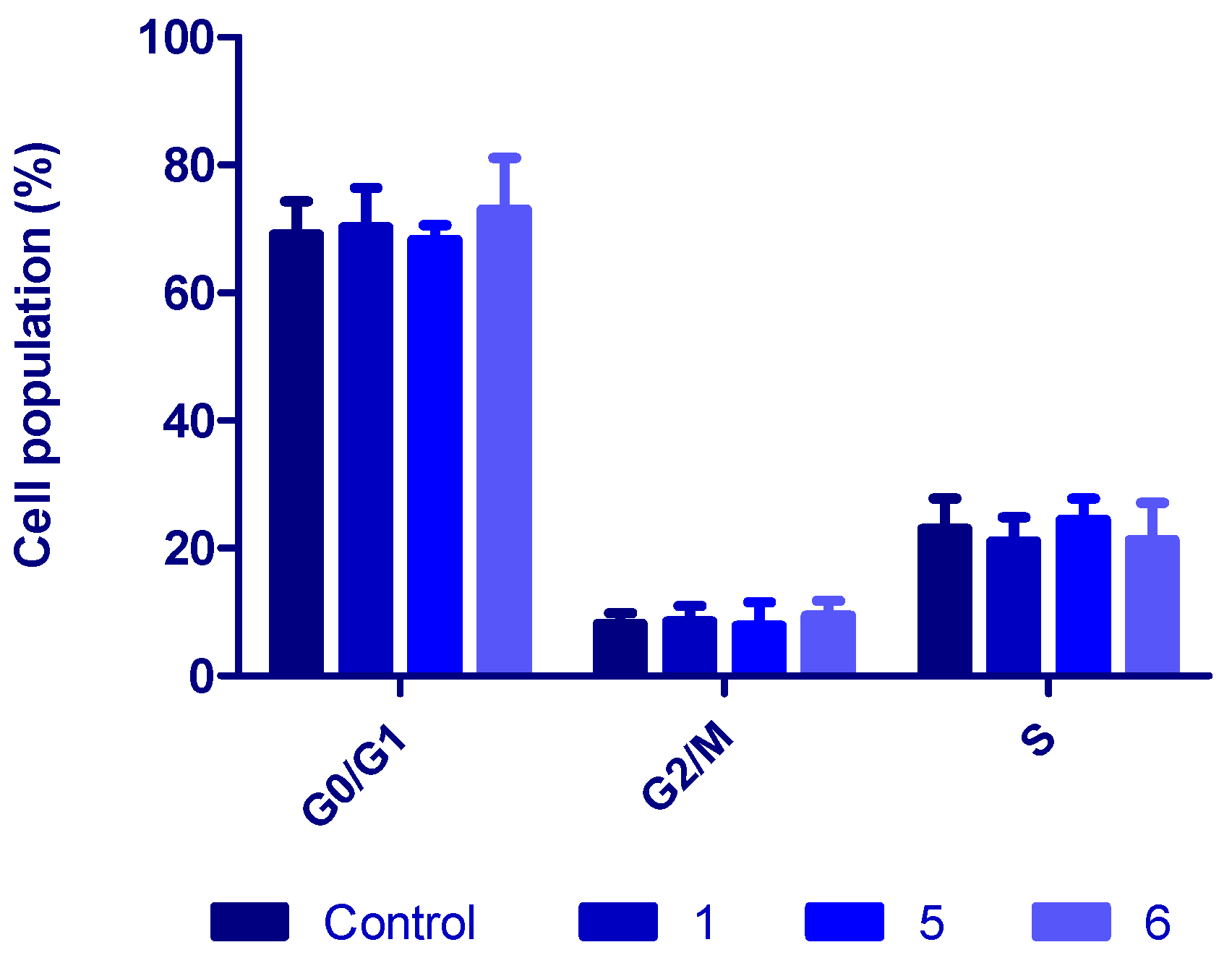
3.7. Cell-Cycle Studies
3.8. Oxidative Stress Damage
3.9. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Ruiz, S.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. On the discovery, biological effects, and use of Cisplatin and metallocenes in anticancer chemotherapy. Bioinorg. Chem. Appl. 2012, 2012, 140284. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorgan. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Sadler, P.J. Metals in Medicine. Angew. Chem. Int. Ed. 1999, 38, 1512–1531. [Google Scholar] [CrossRef]
- Xie, L.; Luo, Z.; Zhao, Z.; Chen, T. Anticancer and Antiangiogenic Iron(II) Complexes That Target Thioredoxin Reductase to Trigger Cancer Cell Apoptosis. J. Med. Chem. 2017, 60, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Kallus, S.; Uhlik, L.; van Schoonhoven, S.; Pelivan, K.; Berger, W.; Enyedy, É.A.; Hofmann, T.; Heffeter, P.; Kowol, C.R.; Keppler, B.K. Synthesis and biological evaluation of biotin-conjugated anticancer thiosemicarbazones and their iron(III) and copper(II) complexes. J. Inorg. Biochem. 2019, 190, 85–97. [Google Scholar] [CrossRef]
- Riccardi, C.; Musumeci, D.; Trifuoggi, M.; Irace, C.; Paduano, L.; Montesarchio, D. Anticancer Ruthenium(III) Complexes and Ru(III)-Containing Nanoformulations: An Update on the Mechanism of Action and Biological Activity. Pharmaceuticals 2019, 12, 146. [Google Scholar] [CrossRef]
- Lazarević, T.; Rilak, A.; Bugarčić, Ž.D. Platinum, palladium, gold and ruthenium complexes as anticancer agents: Current clinical uses, cytotoxicity studies and future perspectives. Eur. J. Med. Chem. 2017, 142, 8–31. [Google Scholar] [CrossRef]
- Mendoza, Z.; Lorenzo-Luis, P.; Scalambra, F.; Padrón, J.M.; Romerosa, A. One Step Up in Antiproliferative Activity: The Ru-Zn Complex [RuCp(PPh3)2-µ-dmoPTA-1ĸP:2ĸ2N,N′-ZnCl2](CF3SO3). Eur. J. Inorg. Chem. 2018, 2018, 4684–4688. [Google Scholar] [CrossRef]
- Milacic, V.; Fregona, D.; Dou, Q.P. Gold complexes as prospective metal-based anticancer drugs. Histol. Histopathol. 2008, 23, 101–108. [Google Scholar] [CrossRef]
- Nobili, S.; Mini, E.; Landini, I.; Gabbiani, C.; Casini, A.; Messori, L. Gold compounds as anticancer agents: Chemistry, cellular pharmacology, and preclinical studies. Med. Res. Rev. 2010, 30, 550–580. [Google Scholar] [CrossRef]
- Omondi, R.O.; Ojwach, S.O.; Jaganyi, D. Review of comparative studies of cytotoxic activities of Pt(II), Pd(II), Ru(II)/(III) and Au(III) complexes, their kinetics of ligand substitution reactions and DNA/BSA interactions. Inorg. Chim. Acta 2020, 512, 119883. [Google Scholar] [CrossRef]
- Murray, B.S.; Dyson, P.J. Recent progress in the development of organometallics for the treatment of cancer. Curr. Opin. Chem. Biol. 2020, 56, 28–34. [Google Scholar] [CrossRef]
- Roder, C.; Thomson, M.J. Auranofin: Repurposing an Old Drug for a Golden New Age. Drugs R D 2015, 15, 13–20. [Google Scholar] [CrossRef]
- Shaw, C.F. Gold-Based Therapeutic Agents. Chem. Rev. 1999, 99, 2589–2600. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Anti-cancer potential of gold complexes. Inflammopharmacology 2008, 16, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Abás, E.; Gómez-Bachiller, M.; Colom, E.; Pardina, E.; Rodríguez-Diéguez, A.; Grasa, L.; Laguna, M. Cyclometallated gold(III) complexes against colon cancer. X-ray structure of [Au(C,NPhenylpyridine)(OAc)2]. J. Organomet. Chem. 2020, 920, 121340. [Google Scholar] [CrossRef]
- Henderson, W.; Nicholson, B.K.; Faville, S.J.; Fan, D.; Ranford, J.D. Gold(III) thiosalicylate complexes containing cycloaurated 2-arylpyridine, 2-anilinopyridine and 2-benzylpyridine ligands. J. Organomet. Chem. 2001, 631, 41–46. [Google Scholar] [CrossRef]
- Ronconi, L.; Marzano, C.; Zanello, P.; Corsini, M.; Miolo, G.; Maccà, C.; Trevisan, A.; Fregona, D. Gold(III) Dithiocarbamate Derivatives for the Treatment of Cancer: Solution Chemistry, DNA Binding, and Hemolytic Properties. J. Med. Chem. 2006, 49, 1648–1657. [Google Scholar] [CrossRef]
- Smith, T.S.; Henderson, W.; Nicholson, B.K. Cycloaurated gold(III) complexes with monoanionic thiourea ligands. Inorg. Chim. Acta 2013, 408, 27–32. [Google Scholar] [CrossRef]
- Zou, T.; Lum, C.T.; Lok, C.-N.; Zhang, J.-J.; Che, C.-M. Chemical biology of anticancer gold(iii) and gold(i) complexes. Chem. Soc. Rev. 2015, 44, 8786–8801. [Google Scholar] [CrossRef] [PubMed]
- Boscutti, G.; Nardon, C.; Marchiò, L.; Crisma, M.; Biondi, B.; Dalzoppo, D.; Dalla Via, L.; Formaggio, F.; Casini, A.; Fregona, D. Anticancer Gold(III) Peptidomimetics: From Synthesis to in vitro and ex vivo Biological Evaluations. ChemMedChem 2018, 13, 1131–1145. [Google Scholar] [CrossRef] [PubMed]
- Saggioro, D.; Rigobello, M.P.; Paloschi, L.; Folda, A.; Moggach, S.A.; Parsons, S.; Ronconi, L.; Fregona, D.; Bindoli, A. Gold(III)-Dithiocarbamato Complexes Induce Cancer Cell Death Triggered by Thioredoxin Redox System Inhibition and Activation of ERK Pathway. Chem. Biol. 2007, 14, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.M.; Bertrand, B.; Hughes, D.L.; Waller, Z.A.E.; Schmidt, C.; Ott, I.; O’Connell, M.; Searcey, M.; Bochmann, M. Cyclometallated Au(iii) dithiocarbamate complexes: Synthesis, anticancer evaluation and mechanistic studies. Metallomics 2018, 10, 1655–1666. [Google Scholar] [CrossRef]
- Nardon, C.; Boscutti, G.; Gabbiani, C.; Massai, L.; Pettenuzzo, N.; Fassina, A.; Messori, L.; Fregona, D. Cell and Cell-Free Mechanistic Studies on Two Gold(III) Complexes with Proven Antitumor Properties. Eur. J. Inorg. Chem. 2017, 2017, 1737–1744. [Google Scholar] [CrossRef]
- Nardon, C.; Fregona, D. Gold(III) Complexes in the Oncological Preclinical Arena: From Aminoderivatives to Peptidomimetics. Curr. Top. Med. Chem. 2016, 16, 360–380. [Google Scholar] [CrossRef]
- Quero, J.; Cabello, S.; Fuertes, T.; Mármol, I.; Laplaza, R.; Polo, V.; Gimeno, M.C.; Rodriguez-Yoldi, M.J.; Cerrada, E. Proteasome versus Thioredoxin Reductase Competition as Possible Biological Targets in Antitumor Mixed Thiolate-Dithiocarbamate Gold(III) Complexes. Inorg. Chem. 2018, 57, 10832–10845. [Google Scholar] [CrossRef]
- Aldin, M.Z.; Zaragoza, G.; Delaude, L. Synthesis of ruthenium–dithiocarbamate chelates bearing diphosphine ligands and their use as latent initiators for atom transfer radical additions. J. Organomet. Chem. 2021, 950, 121993. [Google Scholar] [CrossRef]
- Ekennia, A.C.; Onwudiwe, D.C.; Osowole, A.A. Spectral, thermal stability and antibacterial studies of copper, nickel and cobalt complexes of N-methyl-N-phenyl dithiocarbamate. J. Sulfur Chem. 2015, 36, 96–104. [Google Scholar] [CrossRef]
- Scintilla, S.; Brustolin, L.; Gambalunga, A.; Chiara, F.; Trevisan, A.; Nardon, C.; Fregona, D. Ru(III) anticancer agents with aromatic and non-aromatic dithiocarbamates as ligands: Loading into nanocarriers and preliminary biological studies. J. Inorg. Biochem. 2016, 165, 159–169. [Google Scholar] [CrossRef]
- Tan, Y.S.; Yeo, C.I.; Tiekink, E.R.T.; Heard, P.J. Dithiocarbamate Complexes of Platinum Group Metals: Structural Aspects and Applications. Inorganics 2021, 9, 60. [Google Scholar] [CrossRef]
- Blaskovich, M.A.; Zuegg, J.; Elliott, A.G.; Cooper, M.A. Helping Chemists Discover New Antibiotics. ACS Infect. Dis. 2015, 1, 285–287. [Google Scholar] [CrossRef]
- Chang, E.L.; Simmers, C.; Knight, D.A. Cobalt Complexes as Antiviral and Antibacterial Agents. Pharmaceuticals 2010, 3, 1711–1728. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.J.; Turner, R.J.; Joo, D.A.; Stan, M.A.; Chan, C.S.; Allan, N.D.; Vrionis, H.A.; Olson, M.E.; Ceri, H. Copper and Quaternary Ammonium Cations Exert Synergistic Bactericidal and Antibiofilm Activity against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2008, 52, 2870–2881. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Storr, T.; Thompson, K.H.; Orvig, C. Design of targeting ligands in medicinal inorganic chemistry. Chem. Soc. Rev. 2006, 35, 534–544. [Google Scholar] [CrossRef]
- Constable, E.C.; Leese, T.A. Cycloaurated derivatives of 2-phenylpyridine. J. Organomet. Chem. 1989, 363, 419–424. [Google Scholar] [CrossRef]
- Janzen, D.E.; Doherty, S.R.; VanDerveer, D.G.; Hinkle, L.M.; Benefield, D.A.; Vashi, H.M.; Grant, G.J. Cyclometallated gold(III) complexes with a trithiacrown ligand: Solventless Au(III) cyclometallation, intramolecular gold–sulfur interactions, and fluxional behavior in 1,4,7-trithiacyclononane Au(III) complexes. J. Organomet. Chem. 2014, 755, 47–57. [Google Scholar] [CrossRef]
- Parish, R.V.; Wright, J.P.; Pritchard, R.G. Mercury(II) and gold(III) derivatives of 2-phenyl pyridines and 2-phenyl-4-(methylcarboxylato)quinoline. J. Organomet. Chem. 2000, 596, 165–176. [Google Scholar] [CrossRef]
- Kerns, E.H.; Di, L. Lipophilicity Methods, Drug-like Properties: Concepts, Structure Design and Methods. In Lipophilicity Methods; Elsevier: San Diego, CA, USA, 2008; p. 10. [Google Scholar]
- Wein, A.N.; Stockhausen, A.T.; Hardcastle, K.I.; Saadein, M.R.; Peng, S.; Wang, D.; Shin, D.M.; Chen, Z.; Eichler, J.F. Tumor cytotoxicity of 5,6-dimethyl-1,10-phenanthroline and its corresponding gold(III) complex. J. Inorg. Biochem. 2011, 105, 663–668. [Google Scholar] [CrossRef]
- Mote, U.S.; Bhattar, S.L.; Patil, S.R.; Kolekar, G.B. Interaction between felodipine and bovine serum albumin: Fluorescence quenching study. Lumin. J. Biol. Chem. Lumin. 2010, 25, 1–8. [Google Scholar] [CrossRef]
- Szymańska, M.; Pospieszna-Markiewicz, I.; Mańka, M.; Insińska-Rak, M.; Dutkiewicz, G.; Patroniak, V.; Fik-Jaskółka, M.A. Synthesis and Spectroscopic Investigations of Schiff Base Ligand and Its Bimetallic Ag(I) Complex as DNA and BSA Binders. Biomolecules 2021, 11, 1449. [Google Scholar] [CrossRef]
- Mesonero, J.; Mahraoui, L.; Matosin, M.; Rodolosse, A.; Rousset, M.; Brot-Laroche, E. Expression of the hexose transporters GLUT1-GLUT5 and SGLT1 in clones of Caco-2 cells. Biochem. Soc. Trans. 1994, 22, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Zucco, F.; Batto, A.F.; Bises, G.; Chambaz, J.; Chiusolo, A.; Consalvo, R.; Cross, H.; Dal Negro, G.; de Angelis, I.; Fabre, G.; et al. An inter-laboratory study to evaluate the effects of medium composition on the differentiation and barrier function of Caco-2 cell lines. Altern. Lab. Anim. 2005, 33, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Abas, E.; Espallargas, N.; Burbello, G.; Mesonero, J.E.; Rodriguez-Dieguez, A.; Grasa, L.; Laguna, M. Anticancer Activity of Alkynylgold(I) with P(NMe2)3 Phosphane in Mouse Colon Tumors and Human Colon Carcinoma Caco-2 Cell Line. Inorg. Chem. 2019, 58, 15536–15551. [Google Scholar] [CrossRef] [PubMed]
- Abas, E.; Pena-Martinez, R.; Aguirre-Ramírez, D.; Rodriguez-Dieguez, A.; Laguna, M.; Grasa, L. New selective thiolate gold(i) complexes inhibit the proliferation of different human cancer cells and induce apoptosis in primary cultures of mouse colon tumors. Dalton Trans. 2020, 49, 1915–1927. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Gonzalo, S.; Grasa, L.; Arruebo, M.P.; Plaza, M.Á.; Murillo, M.D. Extracellular signal-regulated kinase (ERK) is involved in LPS-induced disturbances in intestinal motility. Neurogastroenterol. Motil. 2011, 23, e80–e90. [Google Scholar] [CrossRef]
- Brown, S.D.J.; Henderson, W.; Kilpin, K.J.; Nicholson, B.K. Orthomercurated and cycloaurated derivatives of the iminophosphorane Ph3PNPh. Inorg. Chim. Acta 2007, 360, 1310–1315. [Google Scholar] [CrossRef][Green Version]
- Vicente, J.; Chicote, M.T.; Bermúdez, M.D. 2-[(Dimethylamino)methyl]phenylgold(III) complexes. J. Organomet. Chem. 1984, 268, 191–195. [Google Scholar] [CrossRef]
- Cinellu, M.A.; Zucca, A.; Stoccoro, S.; Minghetti, G.; Manassero, M.; Sansoni, M. Synthesis and characterization of gold(III) adducts and cyclometallated derivatives with 2-substituted pyridines. Crystal structure of [Au{NC5H4(CMe2C6H4)-2}Cl2]. J. Chem. Soc. Dalton Trans. 1995, 2865–2872. [Google Scholar] [CrossRef]
- Fuchita, Y.; Ieda, H.; Wada, S.; Kameda, S.; Mikuriya, M. Organogold(III) complexes derived from auration reactions of thienyl-substituted pyridine derivatives. J. Chem. Soc. Dalton Trans. 1999, 4431–4435. [Google Scholar] [CrossRef]
- Khan, E.; Khan, U.A.; Badshah, A.; Tahir, M.N.; Altaf, A.A. Supramolecular dithiocarbamatogold(III) complex a potential DNA binder and antioxidant agent. J. Mol. Struct. 2014, 1060, 150–155. [Google Scholar] [CrossRef]
- Lombardo, F.; Obach, R.S.; Shalaeva, M.Y.; Gao, F. Prediction of volume of distribution values in humans for neutral and basic drugs using physicochemical measurements and plasma protein binding data. J. Med. Chem. 2002, 45, 2867–2876. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, P.; Yu, B.; Chen, Y.; Wang, J.; Ji, L.; Chao, H. Targeting Nucleus DNA with a Cyclometalated Dipyridophenazineruthenium(II) Complex. J. Med. Chem. 2014, 57, 8971–8983. [Google Scholar] [CrossRef]
- Bhal, S.K. LogP: Making Sense of the Value. Available online: http://www.acdlabs.com/resources/knowledgebase/app_notes/-physchem/makingsense.php (accessed on 17 June 2018).
- Forghieri, F.; Preti, C.; Tassi, L.; Tosi, G. Preparation, properties and reactivity of gold complexes with some heterocyclic dithiocarbamates as ligands. Polyhedron 1988, 7, 1231–1237. [Google Scholar] [CrossRef]
- Vogler, A.; Kunkely, H. Photoreactivity of gold complexes. Coord. Chem. Rev. 2001, 219–221, 489–507. [Google Scholar] [CrossRef]
- Calamai, P.; Guerri, A.; Messori, L.; Orioli, P.; Paolo Speroni, G. Structure and DNA binding properties of the gold(III) complex [AuCl2(esal)]. Inorg. Chim. Acta 1999, 285, 309–312. [Google Scholar] [CrossRef]
- Philbert, L.; Xiaoyang, W. Review: Modifications of Human Serum Albumin and their Binding Effect. Curr. Pharm. Des. 2015, 21, 1862–1865. [Google Scholar] [CrossRef]
- Katrahalli, U.; Chanabasappa Yallur, B.; Manjunatha, D.H.; Krishna, P.M. BSA interaction and DNA cleavage studies of anti-bacterial benzothiazol-2-yl-malonaldehyde. J. Mol. Struct. 2019, 1196, 96–104. [Google Scholar] [CrossRef]
- Jiang, S.; Ni, H.; Liu, F.; Gu, S.; Yu, P.; Gou, Y. Binuclear Schiff base copper(II) complexes: Syntheses, crystal structures, HSA interaction and anti-cancer properties. Inorg. Chim. Acta 2020, 499, 119186. [Google Scholar] [CrossRef]
- Sedighipoor, M.; Kianfar, A.H.; Mohammadnezhad, G.; Görls, H.; Plass, W.; Momtazi-Borojeni, A.A.; Abdollahi, E. Synthesis, crystal structure of novel unsymmetrical heterocyclic Schiff base Ni(II)/V(IV) complexes: Investigation of DNA binding, protein binding and in vitro cytotoxic activity. Inorg. Chim. Acta 2019, 488, 182–194. [Google Scholar] [CrossRef]
- Atrián-Blasco, E.; Gascón, S.; Rodríguez-Yoldi, M.J.; Laguna, M.; Cerrada, E. Novel Gold(I) Thiolate Derivatives Synergistic with 5-Fluorouracil as Potential Selective Anticancer Agents in Colon Cancer. Inorg. Chem. 2017, 56, 8562–8579. [Google Scholar] [CrossRef]
- da Silva, E.N.; da Silva, P.A.B.; Graminha, A.E.; de Oliveira, P.F.; Damasceno, J.L.; Tavares, D.C.; Batista, A.A.; Von Poelhsitz, G. Synthesis, Characterization, Cytotoxic Activity, and Interactions with CT-DNA and BSA of Cationic Ruthenium(II) Complexes Containing Dppm and Quinoline Carboxylates. Bioinorg. Chem. Appl. 2017, 2017, 2562780. [Google Scholar] [CrossRef] [PubMed]
- Crouse, H.F.; Potoma, J.; Nejrabi, F.; Snyder, D.L.; Chohan, B.S.; Basu, S. Quenching of tryptophan fluorescence in various proteins by a series of small nickel complexes. Dalton Trans. 2012, 41, 2720–2731. [Google Scholar] [CrossRef] [PubMed]
- Liévin-Le Moal, V.; Servin, A.L. Pathogenesis of human enterovirulent bacteria: Lessons from cultured, fully differentiated human colon cancer cell lines. Microbiol. Mol. Biol. Rev. MMBR 2013, 77, 380–439. [Google Scholar] [CrossRef] [PubMed]
- Meunier, V.; Bourrié, M.; Berger, Y.; Fabre, G. The human intestinal epithelial cell line Caco-2; pharmacological and pharmacokinetic applications. Cell Biol. Toxicol. 1995, 11, 187–194. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Desai, T.J.; Toombs, J.E.; Minna, J.D.; Brekken, R.A.; Udugamasooriya, D.G. Identification of lipid-phosphatidylserine (PS) as the target of unbiasedly selected cancer specific peptide-peptoid hybrid PPS1. Oncotarget 2016, 7, 30678–30690. [Google Scholar] [CrossRef]
- Fadok, V.A.; Voelker, D.R.; Campbell, P.A.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992, 148, 2207–2216. [Google Scholar]
- Sorenson, C.M.; Barry, M.A.; Eastman, A. Analysis of events associated with cell cycle arrest at G2 phase and cell death induced by cisplatin. J. Natl. Cancer Inst. 1990, 82, 749–755. [Google Scholar] [CrossRef] [PubMed]
- William-Faltaos, S.; Rouillard, D.; Lechat, P.; Bastian, G. Cell cycle arrest by oxaliplatin on cancer cells. Fundam. Clin. Pharmacol. 2007, 21, 165–172. [Google Scholar] [CrossRef]
- Coronnello, M.; Mini, E.; Caciagli, B.; Cinellu, M.A.; Bindoli, A.; Gabbiani, C.; Messori, L. Mechanisms of Cytotoxicity of Selected Organogold(III) Compounds. J. Med. Chem. 2005, 48, 6761–6765. [Google Scholar] [CrossRef] [PubMed]
- Dandash, F.; Léger, D.Y.; Fidanzi-Dugas, C.; Nasri, S.; Brégier, F.; Granet, R.; Karam, W.; Diab-Assaf, M.; Sol, V.; Liagre, B. In vitro anticancer activity of new gold(III) porphyrin complexes in colon cancer cells. J. Inorg. Biochem. 2017, 177, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; McMillan-Ward, E.; Kong, J.; Israels, S.J.; Gibson, S.B. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008, 15, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updates 2004, 7, 97–110. [Google Scholar] [CrossRef]
- Grigalius, I.; Petrikaite, V. Relationship between Antioxidant and Anticancer Activity of Trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef]
- Maikoo, S.; Chakraborty, A.; Vukea, N.; Dingle, L.M.K.; Samson, W.J.; de la Mare, J.-A.; Edkins, A.L.; Booysen, I.N. Ruthenium complexes with mono- or bis-heterocyclic chelates: DNA/BSA binding, antioxidant and anticancer studies. J. Biomol. Struct. Dyn. 2020, 39, 4077–4088. [Google Scholar] [CrossRef]
- Altun, Ö.; Şuözer, M. Synthesis, spectral analysis, antimicrobial, cytotoxicity, and antioxidant studies of gold(III) complex of caffeine. J. Coord. Chem. 2019, 72, 2091–2105. [Google Scholar] [CrossRef]
- Sankarganesh, M.; Raja, J.D.; Revathi, N.; Solomon, R.V.; Kumar, R.S. Gold(III) complex from pyrimidine and morpholine analogue Schiff base ligand: Synthesis, characterization, DFT, TDDFT, catalytic, anticancer, molecular modeling with DNA and BSA and DNA binding studies. J. Mol. Liq. 2019, 294, 111655. [Google Scholar] [CrossRef]
- Levine, R.L.; Williams, J.A.; Stadtman, E.R.; Shacter, E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994, 233, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Lauzier, A.; Normandeau-Guimond, J.; Vaillancourt-Lavigueur, V.; Boivin, V.; Charbonneau, M.; Rivard, N.; Scott, M.S.; Dubois, C.M.; Jean, S. Colorectal cancer cells respond differentially to autophagy inhibition in vivo. Sci. Rep. 2019, 9, 11316. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-B.; Wang, F.-Y.; Tang, X.-M.; Feng, H.-W.; Chen, Z.-F.; Liu, Y.-C.; Liu, Y.-N.; Liang, H. Organometallic Gold(III) Complexes Similar to Tetrahydroisoquinoline Induce ER-Stress-Mediated Apoptosis and Pro-Death Autophagy in A549 Cancer Cells. J. Med. Chem. 2018, 61, 3478–3490. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Jiang, M.; Li, S.; Yuan, H.; Sun, H.; Yang, F.; Liang, H. Developing a Novel Gold(III) Agent to Treat Glioma Based on the Unique Properties of Apoferritin Nanoparticles: Inducing Lethal Autophagy and Apoptosis. J. Med. Chem. 2020, 63, 13695–13708. [Google Scholar] [CrossRef] [PubMed]
- Mizdal, C.R.; Stefanello, S.T.; da Costa Flores, V.; Agertt, V.A.; Bonez, P.C.; Rossi, G.G.; da Silva, T.C.; Antunes Soares, F.A.; de Lourenço Marques, L.; de Campos, M.M.A. The antibacterial and anti-biofilm activity of gold-complexed sulfonamides against methicillin-resistant Staphylococcus aureus. Microb. Pathog. 2018, 123, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.A.; Leitão, J.H.; Silva, R.A.L.; Belo, D.; Santos, I.C.; Guerreiro, J.F.; Martins, M.; Fontinha, D.; Prudêncio, M.; Almeida, M.; et al. On the path to gold: Monoanionic Au bisdithiolate complexes with antimicrobial and antitumor activities. J. Inorg. Biochem. 2020, 202, 110904. [Google Scholar] [CrossRef] [PubMed]
- Andrejević, T.P.; Glišić, B.Đ.; Djuran, M.I. Amino Acids and Peptides as Versatile Ligands in the Synthesis of Antiproliferative Gold Complexes. Chemistry 2020, 2, 13. [Google Scholar] [CrossRef]
- Pintus, A.; Aragoni, M.C.; Cinellu, M.A.; Maiore, L.; Isaia, F.; Lippolis, V.; Orrù, G.; Tuveri, E.; Zucca, A.; Arca, M. [Au(pyb-H)(mnt)]: A novel gold(III) 1,2-dithiolene cyclometalated complex with antimicrobial activity (pyb-H=C-deprotonated 2-benzylpyridine; mnt=1,2-dicyanoethene-1,2-dithiolate). J. Inorg. Biochem. 2017, 170, 188–194. [Google Scholar] [CrossRef]
- Noguchi, R.; Hara, A.; Sugie, A.; Nomiya, K. Synthesis of novel gold(I) complexes derived by AgCl-elimination between [AuCl(PPh3)] and silver(I) heterocyclic carboxylates, and their antimicrobial activities. Molecular structure of [Au(R,S-Hpyrrld)(PPh3)] (H2pyrrld=2-pyrrolidone-5-carboxylic acid). Inorg. Chem. Commun. 2006, 9, 355–359. [Google Scholar] [CrossRef]
- Novelli, F.; Recine, M.; Sparatore, F.; Juliano, C. Gold(I) complexes as antimicrobial agents. Il Farm. 1999, 54, 232–236. [Google Scholar] [CrossRef]
- Goss, C.H.A.; Henderson, W.; Wilkins, A.L.; Evans, C. Synthesis, characterisation and biological activity of gold(III) catecholate and related complexes. J. Organomet. Chem. 2003, 679, 194–201. [Google Scholar] [CrossRef]
- Dennis, E.K.; Kim, J.H.; Parkin, S.; Awuah, S.G.; Garneau-Tsodikova, S. Distorted Gold(I)–Phosphine Complexes as Antifungal Agents. J. Med. Chem. 2020, 63, 2455–2469. [Google Scholar] [CrossRef] [PubMed]
- Fontinha, D.; Sousa, S.A.; Morais, T.S.; Prudêncio, M.; Leitão, J.H.; Le Gal, Y.; Lorcy, D.; Silva, R.A.L.; Velho, M.F.G.; Belo, D.; et al. Gold(iii) bis(dithiolene) complexes: From molecular conductors to prospective anticancer, antimicrobial and antiplasmodial agents. Metallomics 2020, 12, 974–987. [Google Scholar] [CrossRef] [PubMed]

| Complex | Pyridine | Phenyl | ||||||
|---|---|---|---|---|---|---|---|---|
| H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 | |
| [Au(tpy)Cl2] | 9.79 | 7.46 | 8.10 | 7.84 | 7.42 | 7.40 | 7.85 | - |
| [Au(ppy)Cl2] | 9.84 | 7.51 | 8.14 | 7.92 | 7.56 | 7.40 | 7.36 | 8.07 |
| [Au(tpy)(dmdtc)]PF6 (C1) | 9.80 | 7.47 | 8.11 | 7.83 | 7.20 | 7.44 | 7.86 | - |
| [Au(ppy)(dmdtc)]PF6 (C2) | 8.69 | 7.21 | 7.71 | 7.80 | 7.59 | 7.21 | 7.21 | 8.02 |
| [Au(tpy)(dedtc)]PF6 (C3) 1 | 8.82 | 7.76 | 8.48 | 8.41 | 7.96 | 7.39 | 7.03 | - |
| [Au(ppy)(dedtc)]PF6 (C4) | 8.69 | 7.21 | 7.72 | 7.86 | 7.58 | 7.21 | 7.21 | 7.97 |
| [Au(tpy)(dbdtc)]PF6 (C5) | 9.66 | 7.43 | 8.16 | 7.96 | 7.16 | 7.48 | 7.73 | - |
| [Au(ppy)(dbdtc)]PF6 (C6) | 9.82 | 7.52 | 8.15 | 7.92 | 7.55 | 7.36 | 7.36 | 8.05 |
| Complex | C1 | C2 | C3 | C4 | C5 | C6 |
|---|---|---|---|---|---|---|
| Log Pow | 0.61 | 1.11 | 1.17 | 1.57 | 0.89 | 1.00 |
| Complex | KSV (M−1) | kq (M−1s−1) | Kb (M−1) | n |
|---|---|---|---|---|
| [Au(tpy)(dmdtc)]PF6 (C1) | 1.30 × 104 | 1.30 × 1012 | 22.23 × 106 | 1.72 (2) |
| [Au(ppy)(dmdtc)]PF6 (C2) | 1.37 × 104 | 1.37 × 1012 | 9.70 × 105 | 1.42 (1) |
| [Au(tpy)(dedtc)]PF6 (C3) | 1.90 × 104 | 1.90 × 1012 | 1.21 × 105 | 0.95 (1) |
| [Au(ppy)(dedtc)]PF6 (C4) | 2.01 × 104 | 2.01 × 1012 | 1.23 × 105 | 1.18 (1) |
| [Au(tpy)(dbdtc)]PF6 (C5) | 4.43 × 104 | 4.43 × 1012 | 12.61 × 106 | 1.57 (1–2) |
| [Au(ppy)(dbdtc)]PF6 (C6) | 6.44 × 104 | 6.44 × 1012 | 5.42 × 106 | 1.43 (1) |
| Complex | Tumor Cells | Normal Cells | Tumor Cells Selectivity over Normal Cells |
|---|---|---|---|
| [Au(tpy)Cl2] 1 | NS | NS | - |
| [Au(ppy)Cl2] 1 | NS | NS | - |
| [Au(tpy)(dmdtc)]PF6 (C1) | 5.11 ± 0.12 | 23.03 ± 0.06 | 4.5-fold |
| [Au(ppy)(dmdtc)]PF6 (C2) | 1.79 ± 0.12 | 15.69 ± 0.06 | 8.8-fold |
| [Au(tpy)(dedtc)]PF6 (C3) | 1.85 ± 0.36 | 12.95 ± 0.11 | 7.0-fold |
| [Au(ppy)(dedtc)]PF6 (C4) | 2.47 ± 0.25 | 16.79 ± 0.13 | 6.8-fold |
| [Au(tpy)(dbdtc)]PF6 (C5) | 2.07 ± 0.11 | >>100 | >>48.3-fold |
| [Au(ppy)(dbdtc)]PF6 (C6) | 4.02 ± 0.30 | 24.74 ± 0.36 | 6.2-fold |
| MIC (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Complex | Sa | Ec | Kp | Pa | Ab | Ca | Cn |
| [Au(tpy)(dmdtc)]PF6 (C1) | ≤0.25 | 32 | >32 | >32 | >32 | >32 | 32 |
| [Au(ppy)(dmdtc)]PF6 (C2) | ≤0.25 | 32 | >32 | >32 | 32 | 32 | 32 |
| [Au(tpy)(dedtc)]PF6 (C3) | ≤0.25 | 32 | >32 | >32 | >32 | >32 | 2 |
| [Au(ppy)(dedtc)]PF6 (C4) | ≤0.25 | 32 | >32 | >32 | >32 | 32 | 32 |
| [Au(tpy)(dbdtc)]PF6 (C5) | 32 | >32 | >32 | >32 | >32 | 32 | 32 |
| [Au(ppy)(dbdtc)]PF6 (C6) | 4 | >32 | >32 | >32 | 32 | 4 | ≤0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abás, E.; Aguirre-Ramírez, D.; Laguna, M.; Grasa, L. Selective Anticancer and Antimicrobial Metallodrugs Based on Gold(III) Dithiocarbamate Complexes. Biomedicines 2021, 9, 1775. https://doi.org/10.3390/biomedicines9121775
Abás E, Aguirre-Ramírez D, Laguna M, Grasa L. Selective Anticancer and Antimicrobial Metallodrugs Based on Gold(III) Dithiocarbamate Complexes. Biomedicines. 2021; 9(12):1775. https://doi.org/10.3390/biomedicines9121775
Chicago/Turabian StyleAbás, Elisa, Diego Aguirre-Ramírez, Mariano Laguna, and Laura Grasa. 2021. "Selective Anticancer and Antimicrobial Metallodrugs Based on Gold(III) Dithiocarbamate Complexes" Biomedicines 9, no. 12: 1775. https://doi.org/10.3390/biomedicines9121775
APA StyleAbás, E., Aguirre-Ramírez, D., Laguna, M., & Grasa, L. (2021). Selective Anticancer and Antimicrobial Metallodrugs Based on Gold(III) Dithiocarbamate Complexes. Biomedicines, 9(12), 1775. https://doi.org/10.3390/biomedicines9121775








