Wnt/β-Catenin Pathway in Experimental Model of Fibromyalgia: Role of Hidrox®
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Fibromyalgia
2.3. Experimental Groups
- -
- Vehicle: an FM-like model was induced in rats as described in the previous paragraph and they were treated with saline (orally, o.s.) for 7 days, starting from the day after the last reserpine injection.
- -
- Vehicle + HD: an FM-like model was induced in the rats as previously described and they were treated with HD (10 mg/kg, o.s.) for 7 days, starting after the last reserpine injection.
- -
- Sham: The rats received no reserpine administrations as described in previous paragraph and were treated orally with saline or HD for 7 days, starting from the day after the last vehicle injection. Since no significant changes were found between the groups, we present data from the sham + saline group.
2.4. Western Blot Analysis
2.5. Immunohistochemical Analysis
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Behavioural Testing
2.7.1. Von Frey Hair Test
2.7.2. Hot Plate Test
2.7.3. Tail-Flick Warm Water Test
2.8. Statistical Evaluation
3. Results
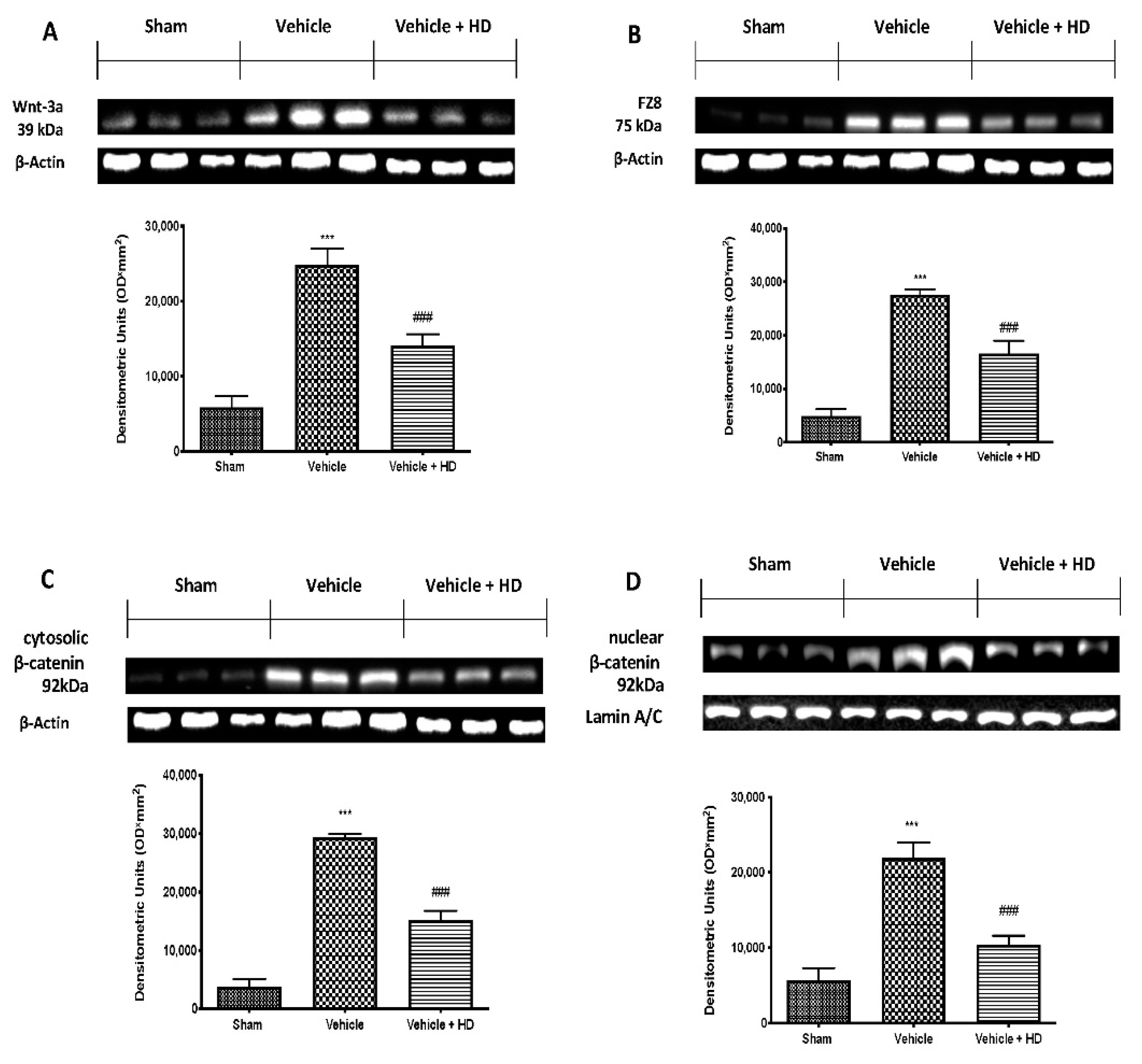
3.1. Effect of HD on WNT/FZ/β-Catenin Signaling Pathway
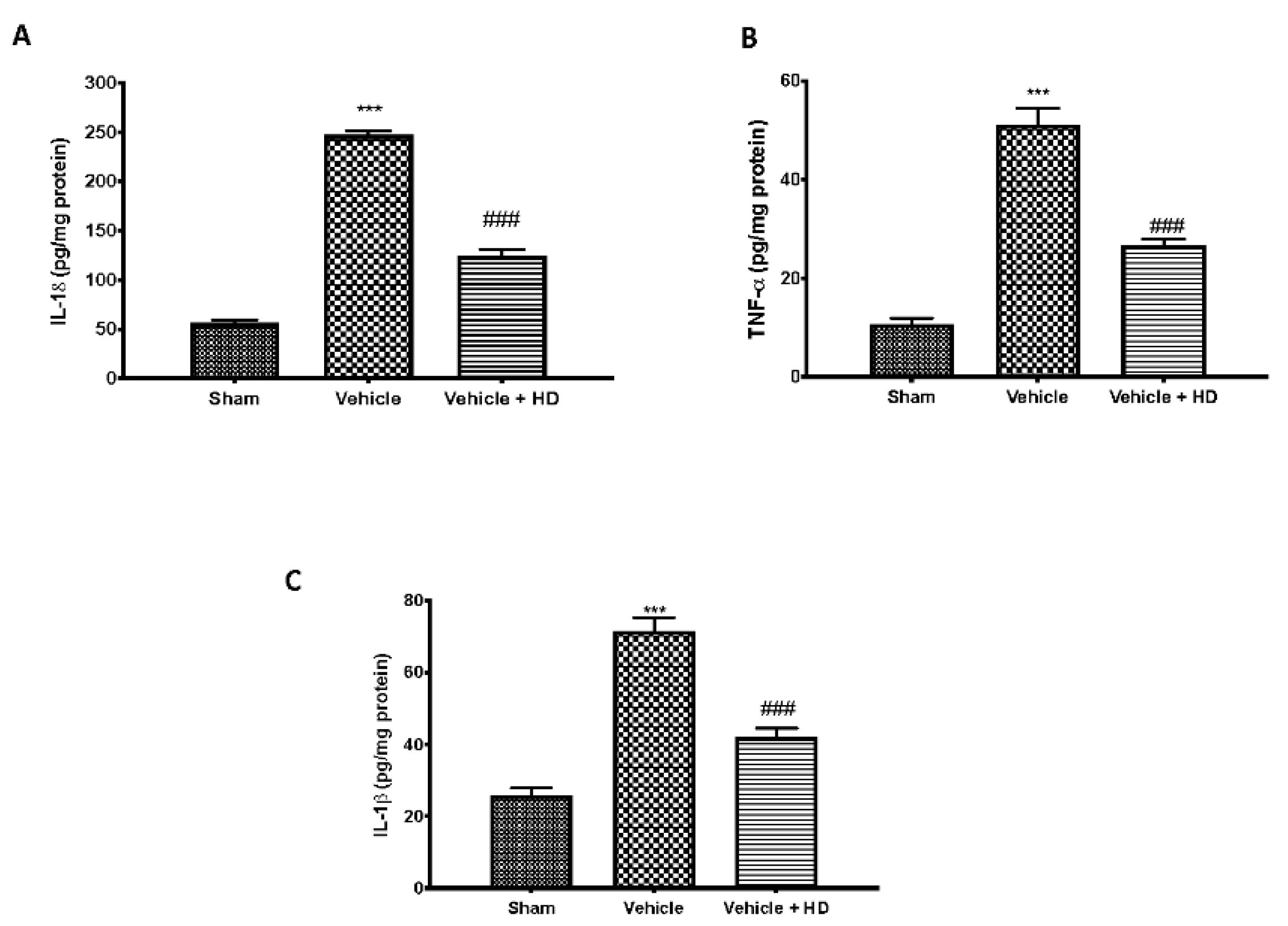
3.2. Effect of HD on Pro-Inflammatory Cytokines Levels
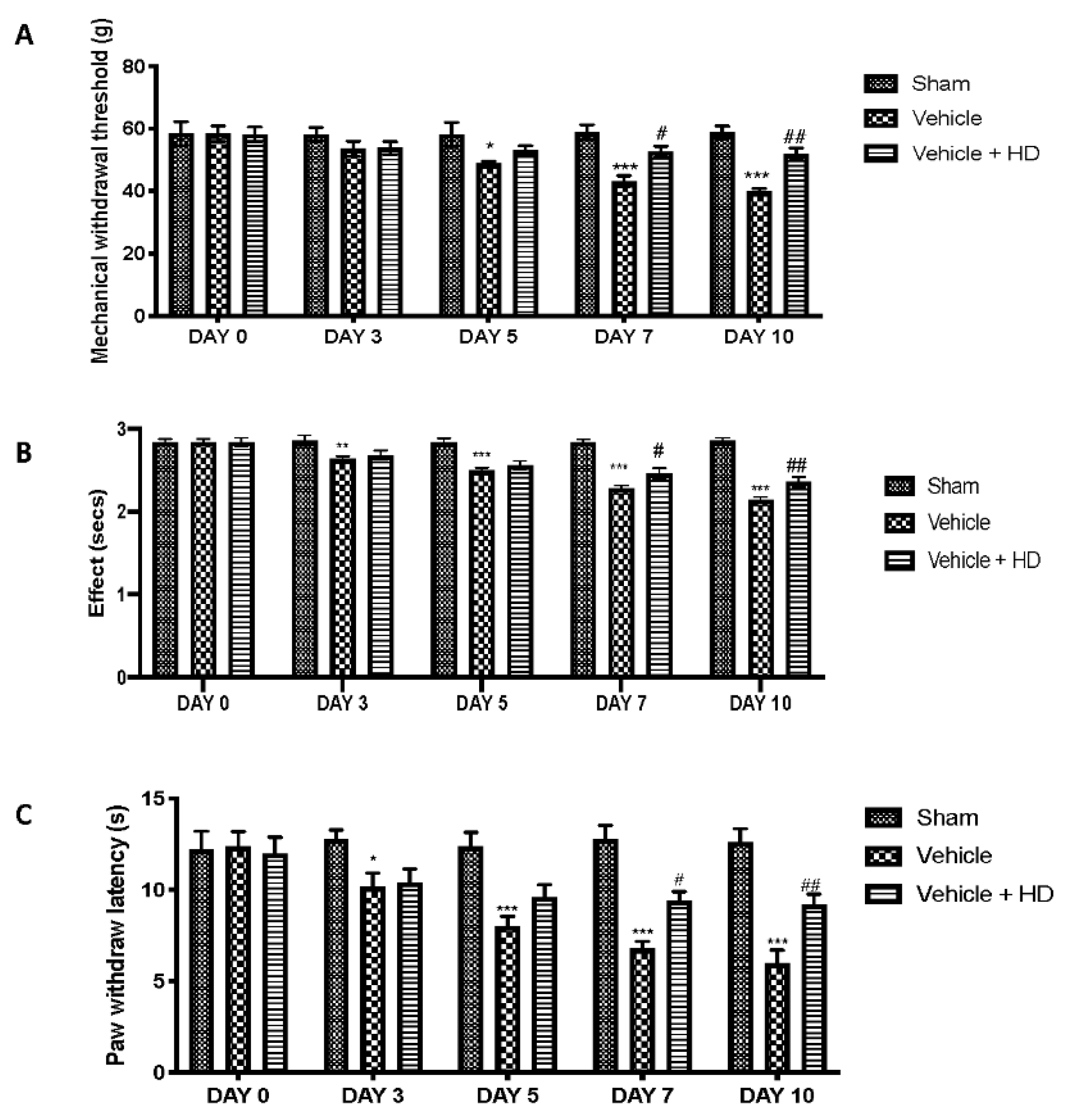
3.3. Effect of HD on Mechanical and Thermal Hyperalgesia
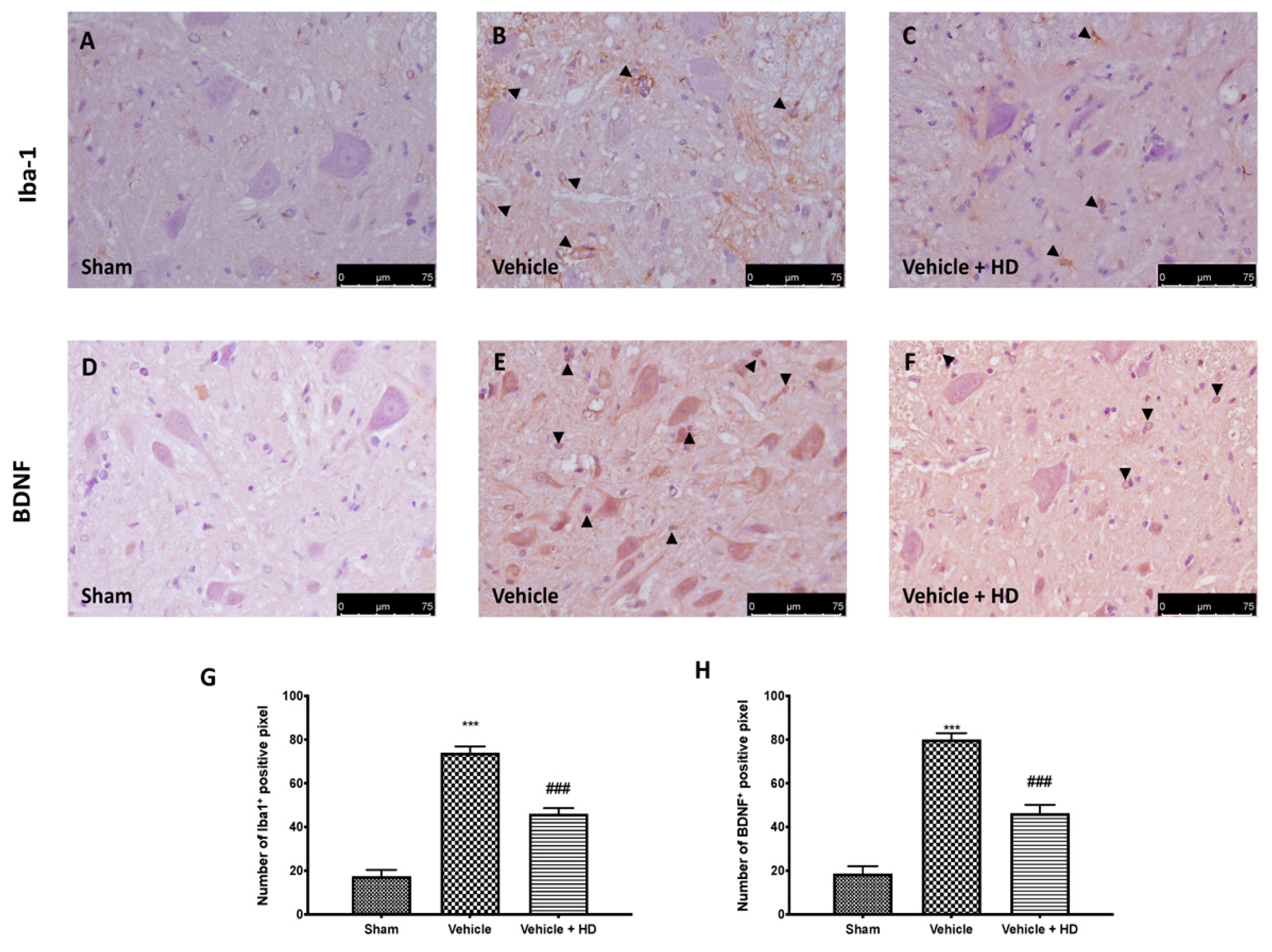
3.4. Effect of HD on Microglia Activation and BDNF Expression
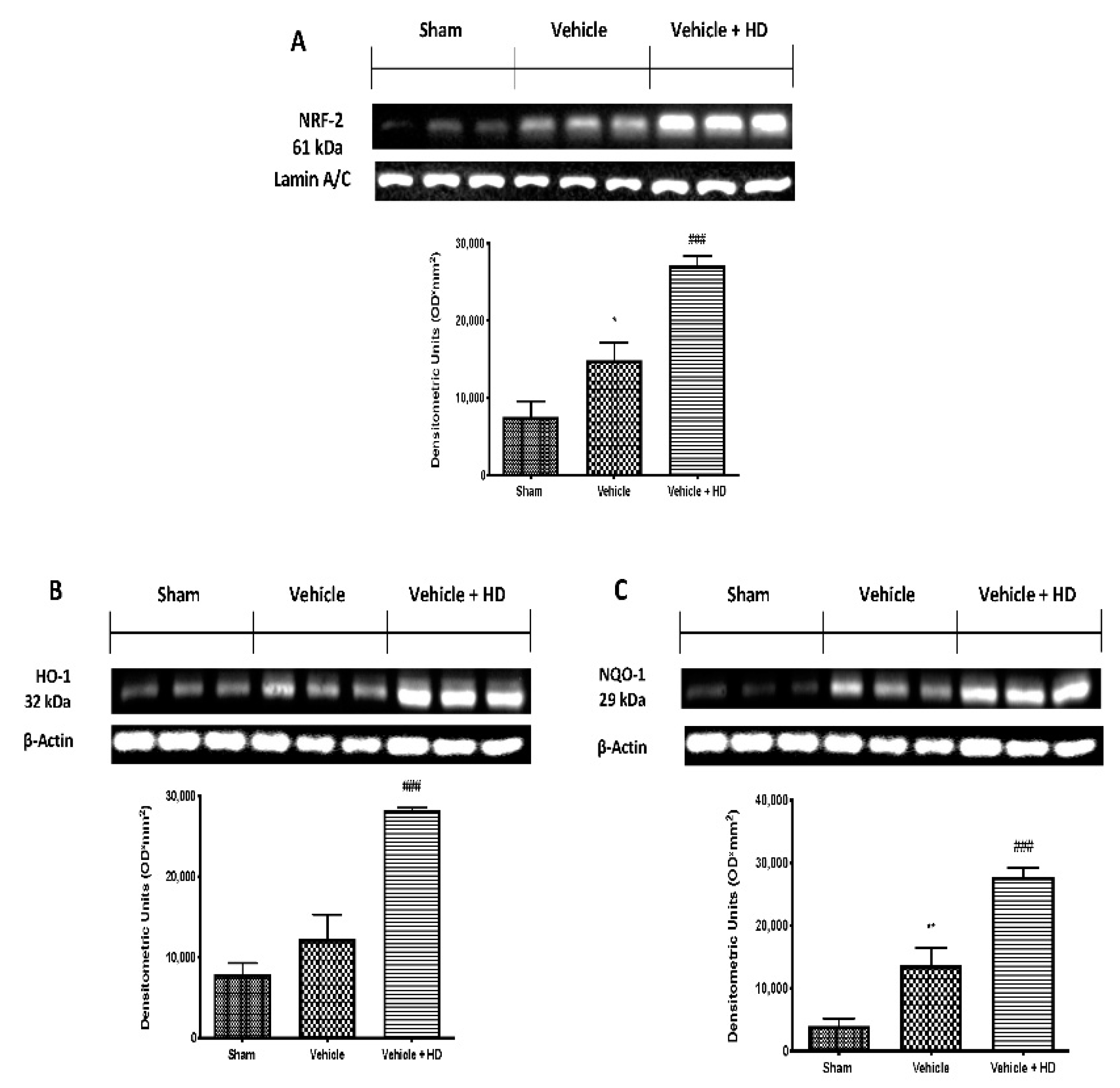
3.5. Effect of HD on Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- De la Luz-Cuellar, Y.E.; Rodriguez-Palma, E.J.; Franco-Enzastiga, U.; Salinas-Abarca, A.B.; Delgado-Lezama, R.; Granados-Soto, V. Blockade of spinal alpha5-GABAA receptors differentially reduces reserpine-induced fibromyalgia-type pain in female rats. Eur. J. Pharmacol. 2019, 858, 172443. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. ALIAmides Update: Palmitoylethanolamide and Its Formulations on Management of Peripheral Neuropathic Pain. Int. J. Mol. Sci. 2020, 21, 5330. [Google Scholar] [CrossRef]
- Jensen, K.B.; Loitoile, R.; Kosek, E.; Petzke, F.; Carville, S.; Fransson, P.; Marcus, H.; Williams, S.C.; Choy, E.; Mainguy, Y.; et al. Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol. Pain 2012, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Fusco, R.; Siracusa, R.; D’Amico, R.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants 2019, 8, 628. [Google Scholar] [CrossRef] [Green Version]
- Simonetti, M.; Kuner, R. Spinal Wnt5a Plays a Key Role in Spinal Dendritic Spine Remodeling in Neuropathic and Inflammatory Pain Models and in the Proalgesic Effects of Peripheral Wnt3a. J. Neurosci. 2020, 40, 6664–6677. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Z. Effect of Wnt signaling pathway on pathogenesis and intervention of neuropathic pain. Exp. Ther. Med. 2018, 16, 3082–3088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siracusa, R.; Monaco, F.; D’Amico, R.; Genovese, T.; Cordaro, M.; Interdonato, L.; Gugliandolo, E.; Peritore, A.F.; Crupi, R.; Cuzzocrea, S.; et al. Epigallocatechin-3-Gallate Modulates Postoperative Pain by Regulating Biochemical and Molecular Pathways. Int. J. Mol. Sci. 2021, 22, 6879. [Google Scholar] [CrossRef]
- Itokazu, T.; Hayano, Y.; Takahashi, R.; Yamashita, T. Involvement of Wnt/beta-catenin signaling in the development of neuropathic pain. Neurosci. Res. 2014, 79, 34–40. [Google Scholar] [CrossRef]
- Arnes, M.; Tinto, S.C. Aberrant Wnt signaling: A special focus in CNS diseases. J. Neurogenet. 2017, 31, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Stenman, J.M.; Rajagopal, J.; Carroll, T.J.; Ishibashi, M.; McMahon, J.; McMahon, A.P. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 2008, 322, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Huang, Z.J.; Liu, S.; Liu, Y.P.; Song, A.A.; Song, X.J. WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J. Clin. Investig. 2013, 123, 2268–2286. [Google Scholar] [CrossRef] [PubMed]
- Karimaian, A.; Majidinia, M.; Bannazadeh Baghi, H.; Yousefi, B. The crosstalk between Wnt/beta-catenin signaling pathway with DNA damage response and oxidative stress: Implications in cancer therapy. DNA Repair 2017, 51, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yuan, S.; Li, B.; Wang, J.; Carlton, S.M.; Chung, K.; Chung, J.M.; Tang, S.J. Regulation of Wnt signaling by nociceptive input in animal models. Mol. Pain 2012, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Gong, G.; Hu, L.; Qin, F.; Yin, L.; Yi, X.; Yuan, L.; Wu, W. Spinal WNT pathway contributes to remifentanil induced hyperalgesia through regulating fractalkine and CX3CR1 in rats. Neurosci. Lett. 2016, 633, 21–27. [Google Scholar] [CrossRef]
- Siniscalco, D.; Giordano, C.; Rossi, F.; Maione, S.; de Novellis, V. Role of neurotrophins in neuropathic pain. Curr. Neuropharmacol. 2011, 9, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Teodoro, A.J. Bioactive Compounds of Food: Their Role in the Prevention and Treatment of Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 3765986. [Google Scholar] [CrossRef] [Green Version]
- Aruoma, O.I.; Bahorun, T.; Jen, L.S. Neuroprotection by bioactive components in medicinal and food plant extracts. Mutat. Res. 2003, 544, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Cordaro, M.; Siracusa, R.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Biochemical Evaluation of the Antioxidant Effects of Hydroxytyrosol on Pancreatitis-Associated Gut Injury. Antioxidants 2020, 9, 781. [Google Scholar] [CrossRef]
- Cordaro, M.; Trovato Salinaro, A.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Crea, R.; Cuzzocrea, S.; Di Paola, R.; et al. Hidrox® Roles in Neuroprotection: Biochemical Links between Traumatic Brain Injury and Alzheimer’s Disease. Antioxidants 2021, 10, 818. [Google Scholar] [CrossRef]
- Siracusa, R.; Scuto, M.; Fusco, R.; Trovato, A.; Ontario, M.L.; Crea, R.; Di Paola, R.; Cuzzocrea, S.; Calabrese, V. Anti-inflammatory and Anti-oxidant Activity of Hidrox® in Rotenone-Induced Parkinson’s Disease in Mice. Antioxidants 2020, 9, 824. [Google Scholar] [CrossRef]
- Fusco, R.; Salinaro, A.T.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Crea, R.; Cordaro, M.; Cuzzocrea, S.; et al. Hidrox® Counteracts Cyclophosphamide-Induced Male Infertility through NRF2 Pathways in a Mouse Model. Antioxidants 2021, 10, 778. [Google Scholar] [CrossRef]
- Ricelli, A.; Gionfra, F.; Percario, Z.; De Angelis, M.; Primitivo, L.; Bonfantini, V.; Antonioletti, R.; Bullitta, S.M.; Saso, L.; Incerpi, S.; et al. Antioxidant and Biological Activities of Hydroxytyrosol and Homovanillic Alcohol Obtained from Olive Mill Wastewaters of Extra-Virgin Olive Oil Production. J. Agric. Food Chem. 2020, 68, 15428–15439. [Google Scholar] [CrossRef] [PubMed]
- Alblihed, M.A. Hydroxytyrosol ameliorates oxidative challenge and inflammatory response associated with lipopolysaccharide-mediated sepsis in mice. Hum. Exp. Toxicol. 2021, 40, 342–354. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- D’Amico, R.; Fusco, R.; Siracusa, R.; Impellizzeri, D.; Peritore, A.F.; Gugliandolo, E.; Interdonato, L.; Sforza, A.M.; Crupi, R.; Cuzzocrea, S.; et al. Inhibition of P2X7 Purinergic Receptor Ameliorates Fibromyalgia Syndrome by Suppressing NLRP3 Pathway. Int. J. Mol. Sci. 2021, 22, 6471. [Google Scholar] [CrossRef]
- Cordaro, M.; Trovato Salinaro, A.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Interdonato, L.; Crea, R.; Fusco, R.; et al. Hidrox® and Endometriosis: Biochemical Evaluation of Oxidative Stress and Pain. Antioxidants 2021, 10, 720. [Google Scholar] [CrossRef]
- D’Amico, R.; Trovato Salinaro, A.; Cordaro, M.; Fusco, R.; Impellizzeri, D.; Interdonato, L.; Scuto, M.; Ontario, M.L.; Crea, R.; Siracusa, R.; et al. Hidrox® and Chronic Cystitis: Biochemical Evaluation of Inflammation, Oxidative Stress, and Pain. Antioxidants 2021, 10, 1046. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Scuto, M.; Cuzzocrea, S.; Di Paola, R.; et al. Modulation of NLRP3 Inflammasome through Formyl Peptide Receptor 1 (Fpr-1) Pathway as a New Therapeutic Target in Bronchiolitis Obliterans Syndrome. Int. J. Mol. Sci. 2020, 21, 2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peritore, A.F.; Crupi, R.; Scuto, M.; Gugliandolo, E.; Siracusa, R.; Impellizzeri, D.; Cordaro, M.; D’Amico, R.; Fusco, R.; Di Paola, R.; et al. The Role of Annexin A1 and Formyl Peptide Receptor 2/3 Signaling in Chronic Corticosterone-Induced Depression-Like behaviors and Impairment in Hippocampal-Dependent Memory. CNS Neurol. Disord. Drug Targets 2020, 19, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Peritore, A.F.; D’Amico, R.; Gugliandolo, E.; Di Paola, R.; Cuzzocrea, S. 2-Pentadecyl-2-Oxazoline Reduces Neuroinflammatory Environment in the MPTP Model of Parkinson Disease. Mol. Neurobiol. 2018, 55, 9251–9266. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Crupi, R.; Peritore, A.F.; Gugliandolo, E.; D’Amico, R.; Petrosino, S.; Evangelista, M.; Di Paola, R.; et al. N-Palmitoylethanolamine-oxazoline (PEA-OXA): A new therapeutic strategy to reduce neuroinflammation, oxidative stress associated to vascular dementia in an experimental model of repeated bilateral common carotid arteries occlusion. Neurobiol. Dis. 2019, 125, 77–91. [Google Scholar] [CrossRef]
- Fusco, R.; Gugliandolo, E.; Siracusa, R.; Scuto, M.; Cordaro, M.; D’Amico, R.; Evangelista, M.; Peli, A.; Peritore, A.F.; Impellizzeri, D.; et al. Formyl Peptide Receptor 1 Signaling in Acute Inflammation and Neural Differentiation Induced by Traumatic Brain Injury. Biology 2020, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- Peritore, A.F.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Crupi, R.; Genovese, T.; Impellizzeri, D.; Cuzzocrea, S.; et al. Ultramicronized Palmitoylethanolamide and Paracetamol, a New Association to Relieve Hyperalgesia and Pain in a Sciatic Nerve Injury Model in Rat. Int. J. Mol. Sci. 2020, 21, 3509. [Google Scholar] [CrossRef]
- Favero, G.; Trapletti, V.; Bonomini, F.; Stacchiotti, A.; Lavazza, A.; Rodella, L.F.; Rezzani, R. Oral Supplementation of Melatonin Protects against Fibromyalgia-Related Skeletal Muscle Alterations in Reserpine-Induced Myalgia Rats. Int. J. Mol. Sci. 2017, 18, 1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruce, B.K.; Allman, M.E.; Rivera, F.A.; Wang, B.; Berianu, F.; Butendieck, R.R.; Calamia, K.T.; Hines, S.L.; Rummans, T.A.; Niazi, S.K.; et al. Intensive Multicomponent Fibromyalgia Treatment: A Translational Study to Evaluate Effectiveness in Routine Care Delivery. J. Clin. Rheumatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, L.; Bertuccelli, M.; Formaggio, E.; Rubega, M.; Bosco, G.; Tenconi, E.; Cattelan, M.; Masiero, S.; Del Felice, A. Beyond physiotherapy and pharmacological treatment for fibromyalgia syndrome: Tailored tACS as a new therapeutic tool. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 199–210. [Google Scholar] [CrossRef]
- Schulze, N.B.; Salemi, M.M.; de Alencar, G.G.; Moreira, M.C.; de Siqueira, G.R. Efficacy of Manual Therapy on Pain, Impact of Disease, and Quality of Life in the Treatment of Fibromyalgia: A Systematic Review. Pain Physician 2020, 23, 461–476. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef]
- Rus, A.; Robles-Fernandez, I.; Martinez-Gonzalez, L.J.; Carmona, R.; Alvarez-Cubero, M.J. Influence of Oxidative Stress-Related Genes on Susceptibility to Fibromyalgia. Nurs. Res. 2021, 70, 44–50. [Google Scholar] [CrossRef]
- Patapoutian, A.; Reichardt, L.F. Roles of Wnt proteins in neural development and maintenance. Curr. Opin. Neurobiol. 2000, 10, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Teng, R.; Zhao, Y.; Gao, J.; Chu, H. Epigenetic modulation of Wnt signaling contributes to neuropathic pain in rats. Mol. Med. Rep. 2015, 12, 4727–4733. [Google Scholar] [CrossRef]
- Cruz-Lozano, M.; Gonzalez-Gonzalez, A.; Marchal, J.A.; Munoz-Muela, E.; Molina, M.P.; Cara, F.E.; Brown, A.M.; Garcia-Rivas, G.; Hernandez-Brenes, C.; Lorente, J.A.; et al. Hydroxytyrosol inhibits cancer stem cells and the metastatic capacity of triple-negative breast cancer cell lines by the simultaneous targeting of epithelial-to-mesenchymal transition, Wnt/beta-catenin and TGFbeta signaling pathways. Eur. J. Nutr. 2019, 58, 3207–3219. [Google Scholar] [CrossRef]
- Peng, Z.; Zha, L.; Yang, M.; Li, Y.; Guo, X.; Feng, Z. Effects of ghrelin on pGSK-3beta and beta-catenin expression when protects against neuropathic pain behavior in rats challenged with chronic constriction injury. Sci. Rep. 2019, 9, 14664. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xiang, Y.; Li, F.; Yin, C.; Li, B.; Ke, X. WNT/beta-Catenin Signaling Pathway Regulating T Cell-Inflammation in the Tumor Microenvironment. Front. Immunol. 2019, 10, 2293. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.F.; Xu, H.J.; He, Z.L.; Yin, Q.; Cheng, W. Crocin Alleviates Pain Hyperalgesia in AIA Rats by Inhibiting the Spinal Wnt5a/beta-Catenin Signaling Pathway and Glial Activation. Neural Plast. 2020, 2020, 4297483. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H. Serum brain-derived neurotrophic factor levels in patients with diabetic neuropathic pain. Neurosci. Lett. 2021, 752, 135655. [Google Scholar] [CrossRef]
- Rojo, A.I.; McBean, G.; Cindric, M.; Egea, J.; Lopez, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox control of microglial function: Molecular mechanisms and functional significance. Antioxid Redox Signal. 2014, 21, 1766–1801. [Google Scholar] [CrossRef] [Green Version]
- Peres Klein, C.; Rodrigues Cintra, M.; Binda, N.; Montijo Diniz, D.; Gomez, M.V.; Souto, A.A.; de Souza, A.H. Coadministration of Resveratrol and Rice Oil Mitigates Nociception and Oxidative State in a Mouse Fibromyalgia-Like Model. Pain Res. Treat. 2016, 2016, 3191638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordaro, M.; D’Amico, R.; Morabito, R.; Fusco, R.; Siracusa, R.; Peritore, A.F.; Impellizzeri, D.; Genovese, T.; Crupi, R.; Gugliandolo, E.; et al. Physiological and Biochemical Changes in NRF2 Pathway in Aged Animals Subjected to Brain Injury. Cell Physiol. Biochem. 2021, 55, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Fusco, R.; D’Amico, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Mandalari, G.; Cuzzocrea, S.; et al. Cashew (Anacardium occidentale L.) Nuts Modulate the Nrf2 and NLRP3 Pathways in Pancreas and Lung after Induction of Acute Pancreatitis by Cerulein. Antioxidants 2020, 9, 992. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amico, R.; Cordaro, M.; Siracusa, R.; Impellizzeri, D.; Trovato Salinaro, A.; Scuto, M.; Ontario, M.L.; Crea, R.; Cuzzocrea, S.; Di Paola, R.; et al. Wnt/β-Catenin Pathway in Experimental Model of Fibromyalgia: Role of Hidrox®. Biomedicines 2021, 9, 1683. https://doi.org/10.3390/biomedicines9111683
D’Amico R, Cordaro M, Siracusa R, Impellizzeri D, Trovato Salinaro A, Scuto M, Ontario ML, Crea R, Cuzzocrea S, Di Paola R, et al. Wnt/β-Catenin Pathway in Experimental Model of Fibromyalgia: Role of Hidrox®. Biomedicines. 2021; 9(11):1683. https://doi.org/10.3390/biomedicines9111683
Chicago/Turabian StyleD’Amico, Ramona, Marika Cordaro, Rosalba Siracusa, Daniela Impellizzeri, Angela Trovato Salinaro, Maria Scuto, Maria Laura Ontario, Roberto Crea, Salvatore Cuzzocrea, Rosanna Di Paola, and et al. 2021. "Wnt/β-Catenin Pathway in Experimental Model of Fibromyalgia: Role of Hidrox®" Biomedicines 9, no. 11: 1683. https://doi.org/10.3390/biomedicines9111683
APA StyleD’Amico, R., Cordaro, M., Siracusa, R., Impellizzeri, D., Trovato Salinaro, A., Scuto, M., Ontario, M. L., Crea, R., Cuzzocrea, S., Di Paola, R., Fusco, R., & Calabrese, V. (2021). Wnt/β-Catenin Pathway in Experimental Model of Fibromyalgia: Role of Hidrox®. Biomedicines, 9(11), 1683. https://doi.org/10.3390/biomedicines9111683












