Abstract
The use of mesenchymal stromal cells (MSCs) for tissue engineering of hyaline cartilage is a topical area of regenerative medicine that has already entered clinical practice. The key stage of this procedure is to create conditions for chondrogenic differentiation of MSCs, increase the synthesis of hyaline cartilage extracellular matrix proteins by these cells and activate their proliferation. The first such works consisted in the indirect modification of cells, namely, in changing the conditions in which they are located, including microfracturing of the subchondral bone and the use of 3D biodegradable scaffolds. The most effective methods for modifying the cell culture of MSCs are protein and physical, which have already been partially introduced into clinical practice. Genetic methods for modifying MSCs, despite their effectiveness, have significant limitations. Techniques have not yet been developed that allow studying the effectiveness of their application even in limited groups of patients. The use of MSC modification methods allows precise regulation of cell culture proliferation, and in combination with the use of a 3D biodegradable scaffold, it allows obtaining a hyaline-like regenerate in the damaged area. This review is devoted to the consideration and comparison of various methods used to modify the cell culture of MSCs for their use in regenerative medicine of cartilage tissue.
Keywords:
cartilage; tissue engineering; regeneration; repair; scaffolds; stem cells; growth factors 1. Introduction
Millions of people all over the world face the problems of musculoskeletal diseases and injuries. Damage to the hyaline cartilage of large joints, specifically the knee and the hip, occupies a special place among these problems. There are various causes for articular cartilage such as injuries and excessive physical stress (sports) [1,2], genetic predispositions [3], systemic inflammatory diseases, such as rheumatoid arthritis (RA) [4] and degenerative joint diseases, such as osteoarthritis (OA) and osteochondritis dissecans (OCD) [4,5]. Degenerative lesions of the articular cartilage are observed in 50% of the adult population over 50 years of age [6]. They have a significant negative impact on the quality of life and lead to disability for 10–20% of people over the age of 60 [7,8]. The pronounced deformity caused by osteoarthritis may require arthroplasty. Though this procedure is highly invasive, risky, and very costly [9], the rate of its implementation is growing steadily throughout the world [10,11,12]. Currently, hyaline articular cartilage is considered to have a limited ability for regeneration due to a lack of blood supply combined with significant mechanical stress [13].
Currently, there are many technologies available to surgeons for replacing the damaged layer of the hyaline cartilage [14], the problem of restoring the surface of the articular cartilage has not yet been fully solved [15,16]. One promising approach to replacing defects in the surface layer of large joints is provided by cell and tissue engineering technologies [17,18]. In our opinion, the key factor for obtaining functional regeneration consists in specialized modification of the applied cell culture implanted into the site of a hyaline cartilage lesion [18].
Presently, no standard for preparing an effectively modified cell culture (as well as requirements for its standardization) for its subsequent use in replacing hyaline cartilage defects has been developed. This work is devoted to a review of the currently used methods for modifying cell cultures and conditions for culturing cells for hyaline cartilage repair and a comparative analysis of their advantages and disadvantages.
2. Tissue Engineering Approaches to Cartilage Repair
At the end of the 20th century, Dr. R. Langer and Dr. J.P. Vacanti [19] pioneered the technology of creating vascularized tissue scaffolds on the basis of biocompatible and biodegradable synthetic polymers with the potential to grow de novo tissues and organs and founded a fundamentally new field that combines the principles of engineering and life sciences, specifically tissue engineering (TE).
At present TE makes use of cell cultures, biodegradable scaffolds, methods of bioengineering, and physical and biological factors for creating functional biological constructs to replace (regenerate), support, and repair damaged tissues [20]. In our opinion, the modification of cell cultures for defect replacement is one of the key stages of the TE approach to tissue and organ regeneration. Tissue engineering provides a promising alternative to traditional methods of articular cartilage regeneration because it allows one to create a new autologous tissue in vitro, similar in its biomechanical characteristics to native hyaline cartilage [7].
The key points in the creation of autologous cartilage transplant are the choice of the cell culture and effective method of its modification aimed to enhance the synthesis of target proteins of the extracellular matrix of hyaline cartilage, and to improve the biomechanical characteristics of the regenerate. Early TE works involved the use of “natural” mechanisms of cell modification, for example, replacement of cultured chondrocytes by progenitor cells, viz. mesenchymal multipotent stromal cells from which chondrocytes differentiate. Researchers assumed that stem cells, once in the damage site, would spontaneously (without additional external stimuli) form a hyaline-like regenerate of a suitable structure [21].
2.1. Mesenchymal Stem Cells
The concept of stem cells emerged at the end of the 19th century as a theoretical postulate explaining the ability of certain tissues to undergo self-renewal throughout the entire life of an organism [22]. In 1867, pathologist J. Cohnheim [23] noticed that non-hematopoietic stem cells are present in the bone marrow and may serve a source of fibroblasts involved in wound healing. M. Tavassoli and W.H. Crosby (1968), who were among the first researchers to study stem cells, discovered the proliferative and osteoblastic potentials of bone marrow cells and their autologous transport to extramedullary sites [22]. Later A. Friedenstein (1976) introduced the term mesenchymal stem cells (MSCs), stating that the bone marrow in the postpartum period is a reservoir of stem cells for mesenchymal tissues [21].
Mesenchymal stem cells feature a high proliferative potential and the ability for self-renewal and tissue regeneration, which are important properties for modern tissue engineering and gene therapy [24]. Due to the mesenchymal origin of chondrocytes, they were decided to be replaced by MSCs [20].
At the end of the 20th century, researchers believed that the use of MSCs could solve the problem of de-differentiation (where cells develop from more differentiated to less differentiated state), and also ensure enhanced production of extracellular matrix proteins compared to chondrocytes and thus solve the therapeutic task of restoring the hyaline layer of the articular cartilage [21].
In fact, the use of MSCs for hyaline cartilage regeneration originates from the introduction into clinical practice of microfracture procedure [25], which requires the natural migration of bone marrow mesenchymal stem cells (BM-MSCs) into the defect area after multiple microperforation of the subchondral bone [26]. Along with bone marrow, other sources of MSCs were found, including muscles, dermis, cancellous bone, adipose tissue, periosteum, pericytes, blood, synovium, and other tissue types [24,27]. A number of European studies and clinical trials showed that bone marrow is used as the most common cell source of MSCs due to the renewability of its cellular resource; however, it may not be the most optimal, considering the cell preparation procedure in combination with the differentiation potential and the persistence of the therapeutic effect [28,29,30,31,32]. The second most popular cell source is the adipose derived stromal vascular fraction (ADSVF) because it is abundant in the body and easily available [33].
As far back as the 1980s, evidence was published showing that cells isolated from the postnatal bone marrow of mammals are able, when implanted in vivo, to differentiate into specific cells of the mesenchymal (bone cartilage) tissue [34]. In 1994 S. Wakitani et al. [35] proposed that MSCs to be used to repair large articular cartilage defects. Since MSCs are bone cartilage progenitor cells, the use of various transcription factors and cytokines, for example transforming growth factor β3 (TGF-β3), can direct (enhance) their differentiation in the chondrogenic direction (see Section 2.3.2 for more information) [36,37]. Mesenchymal stem cells can act both directly (due to their ability to differentiate) and indirectly, producing and secreting many factors that enhance the potential of endogenous regeneration of damaged tissue [38].
Among the advantages of MSCs is that their isolation, in contrast with chondrocytes, does not lead to additional traumatization of the intact articular cartilage [37]. Furthermore, the low immunogenicity of autologous MSCs makes cell transplantation well-tolerated by the recipient organism, and a single MSC transplant is safe and does not induce an immune response [38]. Allogeneic MSCs have theoretical advantages over autologous ones in terms of simplifying collection, storage, testing, and implantation procedures. In our opinion, the future of hyaline cartilage cell engineering consists in the use of safe and standardized allogeneic cell products, but today there are concerns about the cell survival and viability rates, as well as immune rejection [38], and at present this issue is debatable [39,40].
C.H. Jo et al. [41] provided evidence for the efficacy of intra-articular MSC injections for cartilage regeneration in OA over a medium-term horizon. F. Davatchi et al. [42] reported a medium-term improvement in the condition of the knee joint in OA patients after the intra-articular injection of immunophenotyped BM-MSCs. An improvement in clinical parameters according to the VAS (Visual Analog Scale) and PGA (Patient Global Assessment) was recorded with a follow-up period of up to 5 years. In the long term, the efficacy of allogeneic MSCs cultured in 3D hyaluronic acid (HA) hydrogel was demonstrated for Kellgren–Lawrence Grade III osteoarthritis patients, as well as patients with ICRS (International Cartilage Repair Society) Grade 4 cartilage defects [43]. The authors found a stable improvement in the VAS and IKDC (International Knee Documentation Committee) scores over 7 years of follow-up. Histological analysis of biopsies taken after 1 year showed that the structure of the regenerate corresponded to hyaline cartilage, and MRIs taken after 3 years indicated the integrity of the regenerate. However, this technique required a long rehabilitation period.
Since the pool of multipotent cells is small, and their number, especially in adult patients, is limited (with a tendency to decrease with age), their isolation is associated with certain difficulties. An alternative to MSCs appeared when Thomson (1998) discovered pluripotency, that is the ability of cells to differentiate into all cell types, except for cells of extraembryonic organs [44].
Embryonic stem cells (ESCs) were the first described pluripotent cells. The chondrogenic potential of ESCs was first discovered in teratomas, where cartilage islets are grouped together with other tissue types in a chaotic manner. Embryonic stem cells originate from embryoblasts at the blastocyst stage and feature a high proliferative activity in an undifferentiated state throughout a long period of culturing [45]. However, the biggest disadvantage of using ESCs lies in their key advantage, that is high but, unfortunately, uncontrollable proliferative potential. At present ESCs are not used in clinical practice because of the high risk of teratoma formation [46].
A more recent development is induced pluripotent stem cells (iPSCs), derived from the epiblast layer of implanted embryos, and induced by four growth factors: Oct4, Sox2, Klf4, and c-Myc (Yamanaka cocktail) [47]. These cells, in contrast with ESCs, have an unlimited ability for self-renewal, and their use is safe and associated with no ethical problems [48,49]. Recently, iPSCs showed encouraging results in in vitro cartilage regeneration experiments [50]. However, the cartilage generated by iPSCs was a heterogeneous combination of hypertrophic, articular, and fibrous cartilage [51]. Thus, it remains unclear whether the de novo formed regenerate possesses the mechanical and functional properties of the native articular cartilage. In our opinion, this is due to the fact the proliferative potential of this subpopulation of cells is quite difficult to MSCs. Moreover, to create autologous iPSCs and transplant them into a patient, a complicated biotechnological problem must be solved for each patient and this is quite expensive [52].
More research is needed to rule out the risks of carcinogenic and tumorigenic effects [53]. Until now the use of MSCs, despite the existing disadvantages (Table 1), remains the prevailing direction in the tissue engineering of hyaline cartilage due to the absence of strict legislative restrictions, relative ease of isolation and culturing, no need for complex and directed differentiation, and enhanced proliferative potential.

Table 1.
Stem cells potency in hyaline cartilage tissue engineering.
Indeed, the tissue differentiated from MSCs can be classified as a cartilage tissue, since it expresses some proteins characteristic and important for hyaline cartilage, such as type II collagen and aggrecan [29]. However, the component ratio of the extracellular matrix (ECM) is generally not the same that of native hyaline articular cartilage, which, in our opinion, has a negative impact on the mechanical characteristics of the de novo regenerate [54,55]. Apparently, this is since the presence of MSCs in the hyaline cartilage is a necessary but insufficient condition for the formation of a full-fledged hyaline-like regenerate, and additional conditions and/or targeted and specialized modification are required to successfully solve this complex problem.
The ability of MSCs to differentiate in a targeted manner and maintain the chondrogenic phenotype is closely related to the local microenvironment, mechanical stress, and need to maintain a certain morphological structure. Obviously, in addition to the local microenvironment, the quality and further proliferation of the resulting MSCs depend on the isolation method and the state of the donor’s (gender, age, genetic characteristics and, concomitant diseases) [56], as well as other unknown factors. It is known that the MSCs from patients suffering from diabetes, obesity, rheumatoid arthritis, and systemic inflammatory diseases partially lose their therapeutic function and the potential for proliferation and differentiation [57].
Thus, the use of MSCs in clinical practice now faces a number of limitations, including attenuation of the therapeutic effect under the influence of endo- and exogenous factors, lack of an optimized protocol for MSC isolation and ex vivo preparation for clinical use, and difficulty in reproducing the spatial organization of the native cartilage and, as a consequence, deterioration of the mechanical properties of regenerate [53,56,57]. Furthermore, as already mentioned, even the most successful results of chondrogenic differentiation of MSCs do not ensure that the biochemical composition allows the ECM to withstand prolonged and significant mechanical stress [54,55].
For correct differentiation and functioning of MSCs, a natural 3D environment should be created, where: (i) correct intercellular interaction is ensured; (ii) signal transmission with ECM components is possible; (iii) the growth factors produced by the cells form a dose-dependent concentration gradient without “forced” polarization/organization; and (iv) ECM fibrils restrict cell proliferation and promote correct spatial distribution of the secreted growth factors [58]. Furthermore, in our opinion, during MSC differentiation, the cultured cells should be subjected to mechanical exposure, which, most likely, is necessary to correctly organize the hyaline cartilage [53].
2.2. Three-Dimensional Environment and Scaffolds
In parallel with the choice of cell culture, researchers focused on the conditions for its proliferation [25,59,60,61]. It was noted that 3D cell culturing repeats the natural conditions of cell proliferation in the body and allows one to obtain a more functional regenerate; as a result, three-dimensional biomimetic structures—scaffolds—were created, which can be considered as one of the main tools for modifying cell culturing conditions. The use of scaffolds in hyaline cartilage regeneration offers a number of advantages: the possibility of reliably fixing the cell-engineered construct (CEC) on the surface of the damaged joint area, protection from significant mechanical stress at the initial stage after implantation, and, most importantly, a natural 3D microenvironment. The 3D structure induces spontaneous cell proliferation in the chondrogenic direction to generate an extracellular matrix characteristic of the hyaline cartilage and drives the synthesis of key proteins, such as type II collagen and aggrecan, thereby preventing cell de-differentiation [40,53,58,61,62].
However, at present there are also scaffold-free technologies, which involve the implantation into the defect area not of individual cellular elements, but of their aggregates, specifically cell spheroids [63]. To form spheroids, MSCs are cultured under conditions that prevent their adhesion to a solid surface. At present the following technologies are used [64]: pellet culture (concentration by precipitation and dispersion of the cell aggregate over U-shaped wells) [65]; liquid overlay (forming a film of a non-adhesive material, such as agarose, on plastic to prevent cell attachment) [64,66]; (hanging drop (culturing in a plate and its further turning upside down) [67]; spinner flask culture (continuous stirring) [68]; rotating way vessel [69]; microfluidics or “spheroids-on-chip” (microwells, microstructures, drop generators, etc.) [64,70]; and magnetic levitation (addition of magnetic particles to the culture to make cells to levitate) [64,71].
The formation of spheroids occurs due to the synthesis and accumulation of the extracellular matrix in the MSC culture: the encapsulation of MSCs takes place in the self-synthesized ECM [72]. The formed spheroids are implanted in the defect site and then special physiotherapeutic procedures are performed to promote cell integration into the cartilage tissue [73,74]. In our opinion, this procedure is technically simple and has understandable and predictable consequences, which attracts practicing specialists.
Scaffolds can be fabricated from synthetic biomaterials or a natural extracellular matrix (Table 2).

Table 2.
Types of scaffold materials used in cartilage tissue engineering and their effect on MSC organization and proliferation.
Biomaterials for tissue engineering should be biodegradable, have a controlled chemical composition and have a porosity to allow optimal cell adhesion, migration, and production of endogenous ECM components [20,95,96,97]. Another important characteristic of the scaffold is stiffness, that is, the ability to resist mechanical deformation [98,99]. Engler et al. were the first to explore the effect of the scaffold stiffness parameter on MSCs and showed that stiff substrates promote the development of a highly organized cytoskeleton with a fusiform morphology, while soft substrates promote the development of a rounded chondrocyte-like morphology and enhanced expression of chondrogenic markers [96].
Later evidence was obtained showing that decreased scaffold stiffness resulted in increased levels of SOX9, type II collagen, aggrecan, as well as oligomeric matrix cartilage protein (COMP) in MSCs [100,101,102]. Thus, in choosing the scaffold, one should bear in mind that the softer the hydrogel substrate, the more likely its deformation during implantation, and the harder it is, the less it meets the conditions for correct chondrogenic differentiation [76,100,101,102].
According to [62,75,76,103,104,105], promising results in articular cartilage regeneration studies were obtained only by those authors who used hybrid (mixed) scaffolds, which we consider quite logical, because hyaline cartilage has a well-defined zoning resulting from the different and strictly defined functions of each layer.
Natural polymers are widespread and biodegradable but inferior in mechanical properties to synthetic polymers. Alginate and chitosan are poor in terms of stiffness/elastic modulus but crosslinking with silk fiber or synthetic polymers (PLA [106], PGA [107], PEG [108]) compensates for this disadvantage [95]. Moreover, alginate lacks adhesion ligands, and its functionalization is achieved with molecules that mimic ECM, the most commonly used of which are RGD [83] and gelatin [92]. Any kind of polymer can also be improved to enhance chondrogenesis by including native ECM molecules (collagen, hyaluronic acid, etc.). Combining synthetic materials to ensure mechanical stiffness and natural components to ensure correct chondrogenesis and to retain a proper phenotype increases the probability of regeneration of the hyaline cartilage in the damaged area. In addition, by varying the structure of the scaffold one can selectively control proliferation, but this is not an easy task [109].
One of the scaffold methods widely used in clinical practice for hyaline cartilage restoration is the implantation of autologous cells (initially chondrocytes, now MSC predominantly) seeded in a scaffold for Autologous Matrix-Induced Chondrogenesis (AMIC) [110]. The essence of the technique is as follows: small areas of the damaged articular surface are pretreated, the defective hyaline cartilage is removed, and then microfracture is performed followed by coating of the defect with a biodegradable membrane/scaffold [111]. As a result, autologous mesenchymal multipotent cells (with an increased proliferative potential) migrate from the subchondral bone through the microfracture holes into the 3D scaffold. A multilayer fixable scaffold (or membrane) allows the cell culture to proliferate under 3D conditions [112,113], and its structure favors the formation of a regenerate resistant to external stress in the mid-term perspective [114,115]. As examples of such scaffolds used in clinical practice, we can mention Chondro-Gide® [116,117], NOVOCART® 3D [118,119,120,121,122], MaioRegen [118,123,124], Agili-C™ [118,125,126], BioSeed®-C [118,127,128,129], etc.
The AMIC technique does not require compliance with strict legal and ethical standards for cell manipulation and is simple, economically accessible, and allows autologous 3D culturing of MSC progenitor cells in the defect area without a preliminary in vitro culturing step [130,131].
Currently, a new 3D bioprinting technology which is used to create CEC based on the MSCs culture and a biodegradable scaffold has become available [132]. This procedure is technologically complicated, and it is necessary to select the optimal conditions for bioprinting, including the composition of the polymer, bio-ink and the use of various stimuli for cell modification [133,134]
3D bioprinting makes it possible to distribute cells accurately and evenly within the scaffold, supplement CEC with the necessary factors for chondrogenic proliferation of hyaline cartilage, for example, recombinant proteins, and also indirectly affects cell proliferation in the chondrogenic direction. [133,135]
In our opinion, 3D bioprinting using MSCs for tissue engineering of hyaline cartilage has been dynamically developing in recent years and, in the nearest future, will become the main method for creating CECs used for tissue engineering of hyaline cartilage. However, a detailed review of all issues related to 3D bioprinting of hyaline cartilage using MSCs is clearly beyond the scope of this work.
To produce a hyaline-like regenerate from an MSC culture and a 3D scaffold is a difficult task; the problem of targeted differentiation and formation of a regenerate with native functions is impossible to solve completely using indirect modification methods, even though a great number of different models of microenvironments to control MSC proliferation have been designed at present. In our opinion, much attention should be paid to direct methods of cell modification, which will be discussed in the next section of our review.
2.3. Direct Modification of Mesenchymal Stem Cells
The above methods for the modification of cell culturing conditions are indirect only and effective within certain limits. These methods have received wide acceptance due to their simplicity, availability, and relative safety. In our opinion, direct cell modification is a more difficult and yet a more promising and interesting task. By creating an artificial regenerate based on cells with a target-modified proliferative potential, we can program them to generate a transplant with biomechanical properties similar to those of the native hyaline cartilage.
Almost all experimental methods of hyaline cartilage regeneration, including those already introduced in clinical practice on a limited scale, make use, to one degree or another, different direct modification techniques. This difficult procedure is necessary for pointwise alteration of cell proliferation with the aim to enhance the synthesis of the required proteins of the extracellular matrix of hyaline cartilage and thus bring the resulting regenerate in its properties closer to the native (intact) hyaline cartilage.
Presently, many physical, chemical and biological factors are known that affect the direction of stem cell differentiation [37,70,136,137,138,139,140,141,142,143,144,145,146]. In principle, these factors can be divided into three groups: physical, chemical, and genetic (Figure 1).
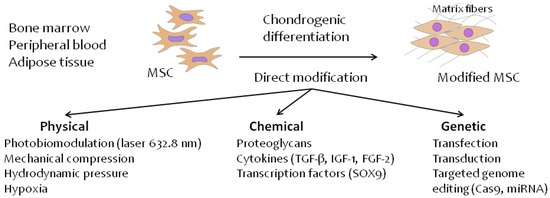
Figure 1.
Direct methods of MSC modification for chondrogenic differentiation in hyaline cartilage tissue.
2.3.1. Physical Modification Methods
The effect of physical factors on MSC proliferation is founded on chain of complex and often poorly understood molecular processes that implicate physical phenomena and lead to changes in gene expression, protein synthesis, and intercellular interactions. Research into such phenomena is still in progress and their detailed description is beyond the scope of this review.
Today, the main physical parameters affecting chondrogenic cell proliferation are periodic physical (mechanical) stress, hypoxia, and electromagnetic radiation (photobiomodulation) [136,137,138,139,140,141], and the consequent transformation of the physical signal into a biochemical signal mediated by integrins and the focal adhesion (FA) protein complex [147].
Dynamic mechanical stress affects the proliferation of the articular cartilage and its degeneration processes, altering the production of extracellular matrix proteins and matrix metalloproteinases [139]. Recent studies provided evidence for a positive effect of long-term dynamic compression (after preliminary initiation of chondrogenic differentiation of cells in vitro) on MSC chondrogenesis with enhanced synthesis of type II collagen, aggrecan [148,149], GAG, SOX9 transcription factor [149], and TGF-β1 [150].
The cartilage ECM is characterized by a high-water content (up to 80%) [151] and low permeability, and consequently, exposure to load creates hydrostatic pressure (HP), which varies depending on the intensity of physical activity from 2 to 18 MPa [141,152]. The physiological levels of HP (5 MPa) significantly enhance the synthesis of the cartilage matrix [152,153], a high hydrostatic pressure (25 MPa) induces proosteoarthritic effects (Col II and aggrecan inhibition, increased expression of metalloproteinases and their synthesis) [154], while a low hydrostatic pressure (100–300 kPa) promotes osteogenic differentiation (increased expression of Runx2, ALP, and osteopontin) [155], resulting in bone tissue from MSCs rather than normal hyaline cartilage being formed (Figure 2).
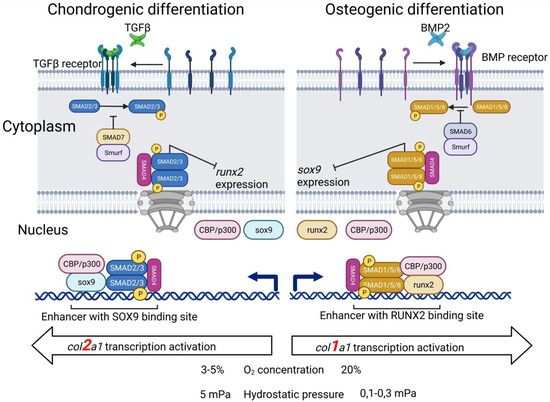
Figure 2.
Chondrogenic and osteogenic differentiation of MSCs depends on various conditions and stimuli.
Hyaline cartilage proliferation occurs under a low (1–3% O2) oxygen environment, whereas the normal oxygen level in the air is 23%; therefore, hypoxic conditions can enhance chondrogenesis [156]. Although the hypoxia-induced factor-1α (HIF-1α) is a key mediator of the beneficial effect of a low oxygen environment on chondrogenesis [157], the underlying mechanisms that mediate hypoxic conditions are still unclear. H. Lee et al. [158] and H. Bae et al. [159] reported evidence for increased mRNA expression of type II collagen, aggrecan, and SOX9 transcription factor under hypoxic conditions (1–5% O2).
While the correlation between chondrogenic differentiation and a low oxygen concentration and a high physical load is, in our opinion, logically predictable (these are natural conditions of hyaline cartilage proliferation in the body), the effect of electromagnetic radiation on chondrogenic differentiation is not directly obvious. For example, as we showed previously [141], the low-intensity coherent electromagnetic radiation with a wavelength of 638.2 nm significantly increases expression of tgfb3, sox9, and col2a1 genes leadings to chondrogenic differentiation of MSCs and initiation aggregation of cellular elements; however, this physical factor had a weaker effect than treatment of the same MSC culture with the recombinant human TGF-β3 protein, a key cytokine responsible for chondrogenesis.
Consequently, the exposure of cell culture and/or CEC to physical factors is important for tissue engineering of MSCs, even though the mechanisms of the changes that occur remain largely unknown, and the biophysical data are recommended to be used when designing CECs to replace hyaline cartilage [160]. An interesting fact is that some physical methods for hyaline cartilage repair had been introduced in clinical practice much earlier than the underlying for molecular mechanisms underlying their effect became understood [141]. In our opinion, the accumulated evidence suggests that the presence of a bioreactor with controlled mechanical load and reduced oxygen concentration is at least desirable, but possibly already imperative to ensure chondrogenic cell proliferation and autologous hyaline cartilage regeneration, which is a challenging technical task.
We consider the choice of physical exposure parameters for directing the proliferation of the cartilage tissue in a desired way to be of no less importance, because the same physical impact with different parameters can act as a “physical switch” between the close but incompatible processes of MSC differentiation in the osteogenic and chondrogenic directions. Nevertheless, some physical methods, for example, low-intensity laser radiation, have already been introduced [161] into clinical practice due to their safety and low invasiveness, lack of legal restrictions, and the ability to locally directly exposure to the hyaline cartilage defective site.
2.3.2. Modification Methods Using Proteins
Further evidence for the fact that MSCs plays an active part in the formation of the dynamic microenvironment of cartilage is provided by the data on their ability to synthesize a wide range of ECM molecules, including fibronectin, collagen(s), glycosaminoglycans, and proteoglycans, as well as various cytokines and growth factors involved in cartilage functioning [162].
In the early 21st century, researchers identified the key regulatory proteins that control cartilage tissue and understood the chain of molecular events that underlie hyaline cartilage proliferation. At the same time, it was proposed to use recombinant proteins for additional modification and to force differentiation of the MSC cell culture in the chondrogenic direction. At present, the modification of MSCs using recombinant growth factors (GF) is one of the simplest, safest, and experimentally and clinically proven approaches to altering cell proliferation [22]. Growth factors are an integral part of the formation of real microenvironment conditions and undoubtedly play a key role in the processes of cell development including chondrogenic differentiation [163].
Currently, a large number of proteins have been described in the scientific literature that in one way or another affect MSC chondrogenesis, but their consideration is definitely beyond the scope of this review. Here we will focus on the most studied, experimentally applied, and fundamentally important families. It is known that the fibroblast growth factors FGF-2, insulin-like growth factor IGF-1, hypoxia factors HIFs, cytokines of the transforming growth factor superfamily TGF-β and associated bone morphogenetic proteins BMP-2,4,6, as well as the SOX9 transcription factor have a direct effect on the chondrogenic differentiation of mesenchymal stem cells [67,141,142,164,165] (Figure 2).
SRY-Related HMG-Box Genes (SOX)
Chondrogenesis begins with the differentiation of condensed chondrogenic cells into chondroblasts, the main function of which is the synthesize of the extracellular matrix cartilage [20,166]. The differentiation of MSCs into a chondrogenic clone requires expression of the sox9 (SRY-box 9) gene, a key chondrogenic transcription factor [166,167,168]. The SOX9 protein cooperatively binds to the SOX5/SOX6 complex on active enhancers and super enhancers associated with hundreds of cartilage-specific genes [169].
Many molecular processes control sox9 expression in the course of chondrogenesis: BMP [170], TGF-β [171], FGF [172], and hypoxia factors HIFs [173] attenuate or increase it, while Notch [174], retinoic acid [175], and inflammation-related signaling pathways (NFκB-mediated) work exclusively to attenuate expression [176,177]. Identification of the transcription factors that control expression of SOX9, as well as establishment of the exact molecular mechanism of the action of proteins on MSC chondrogenesis, remains a difficult and unresolved problem [178,179].
Im et al. [180] developed a plasmid system for the delivery of sox-5,-6,-9 into AD-MSCs seeded in polymeric scaffolds and then implanted the resulting complex into the osteochondral defects in rabbit knees. The plasmid stimulated the synthesis of proteoglycans and type II collagen in the cartilage, while col10a1 expression decreased.
Transforming Growth Factor β (TGF-β) Superfamily
The transforming growth factor-β TGF-β superfamily was discovered and characterized by several independent research groups in the 1980s [181]. Ligands of the TGFβ superfamily are divided into two subfamilies In terms of structural and functional features: TGF-βs and BMPs (bone morphogenetic proteins) [182]. TGF-β, activin, myostatin, and growth differentiation factor GDF11 belong to the TGF-β subfamily, while BMP-2, BMP-4, and BMP-7 belong to the BMP subfamily.
Numerous TGF-β subfamily members modulate the selection of MSC clones and the progression of mesenchymal differentiation into specific cells by controlling the expression and activity of key transcription factors [183]. Members of the TGF-β family transmit an external signal by binding to cellular receptors of Ser/Thr protein kinases and activate intracellular SMAD-dependent signaling pathways [184]. TGF-β signaling has an important anti-inflammatory effect on hyaline cartilage [185]: TGF-β1 counteracts proinflammatory interleukin 1 (IL-1) signaling in vivo [186,187] and in vitro [188], and TGF-β3 helps to replenish the number of proteoglycans after their inflammation-induced depletion [189].
Bone morphogenetic proteins (BMPs) play an important role in chondrogenesis [163] by participating in the TGFβ signaling pathways and cytokine–cytokine receptor interaction. In fact, BMP-2 enhances the production of cartilage matrix and blocks IL-1-induced degeneration of cartilage tissue [190], while TGF-β3 itself increases the expression of the col2a1 gene, the main protein of the ECM of hyaline cartilage [191,192]. It was also reported that BMP-7 inhibits cell proliferation but stimulates ECM synthesis in the BM-MSC culture [193].
M.B. Gugjoo et al. provided in vitro experimental evidence showing that the stimulation of MSCs by TGF-β1 (generally in combination with IGF-1) promotes hyaline cartilage repair in an osteochondral defect in rabbits [194,195].
Insulin-like Growth Factor-I (IGF-I)
IGF-1 was discovered in 1957 by Salmon and Daughaday [196] who defined it as a “sulfation factor” for its ability to stimulate sulfation of the chondroitin chain in the ECM of rat cartilage. Chondroitin sulfates subsequently bind to core proteins (the protein part of the future proteoglycan) and form aggrecan proteoglycans which are important for cartilage [197].
IGF-1 is considered as one of the most essential growth factors involved in maintaining the integrity of the hyaline cartilage [198,199,200]. Recent in vitro experiments showed that IGF-1 can modulate MSC chondrogenesis, irrespective of whether TGFβ signaling is present or absent [201,202]. IGF-1 can accelerate/inhibit the synthesis of ECM components by enhancing collagen II synthesis, and, on the other hand, it can prevent ECM degradation by decreasing MMP synthesis.
According to the in vitro results of Davies et al., the effect of this cytokine is dose-dependent, that is, different doses of IGF-1 induce different biological responses [203]. High doses of IGF-1 increase cell survival in the cartilage defect site and increase the synthesis of type II collagen, as well as improve integrity of the neocartilage tissue. Low doses of IGF-1 induce significant changes in gene expression in the subchondral bone and promote the formation of tissues with better histological characteristics (the cartilage layer and adjacent subchondral bone better correspond in morphology to native tissues) [203,204]. In combination with TGF-β1, IGF-1 stimulates the restoration of hyaline cartilage in bone cartilage defects in rabbits in vivo [194,195].
Fibroblast Growth Factor
Fibroblast growth factor (FGF) was discovered in the bovine brain and pituitary gland in 1973–74s by Armelin and Gospodarowicz [205] and described as a mitogen that initiates DNA synthesis by resting embryonic 3T3 fibroblasts.
There are two key peptides of the FGF superfamily that are involved in hyaline cartilage regeneration: FGF-2 (bFGF/basic FGF) and FGF-18. The former can participate in limited cartilage repair processes and decrease the level of aggrecanase (a matrix metalloproteinase that specifically cleaves aggrecan, one of the main proteins of the ECM of hyaline cartilage [206]). Interestingly, the concentration of FGF-2 in the synovial fluid of osteoarthritis patients is double that of patients with healthy knee joints [207].
L. Xiao et al. [208] studied the effect of blocking the FGF receptor in transgenic mice with overexpressed FGF-2 and found that degenerative changes in the OA cartilage were slowed down due to preventing FGFR binding to FGF-2. T. Yamamoto et al. [209] established that FGF-2 was involved in articular cartilage repair in immature, but not in adult animals. It was also shown that FGF-18 stimulated chondrogenesis and cartilage restoration at individually selected concentrations [210,211,212,213], which like as with IGF-1, points to a dose- dependent effect.
Chuma et al. [214] evaluated the effect of FGF-2 on the proliferation and migration of MSCs in vitro and in vivo. In vitro FGF-2 facilitated the mobilization and migration of replicating BM–MSCs, and a two-week long in vivo treatment led to a restoration of 5 mm articular cartilage defects in rabbits by stimulating MSC recruitment to the damaged site.
Proteoglycans
As noted in the previous sections, chondrogenic differentiation of MSCs depends on, among other things, the interaction with ECM components [215]. Proteoglycans are high molecular weight compounds consisting of a core protein and one or more attached glycosaminoglycan (GAG) chains [216]. Proteoglycans play an important role in the regulation of cell-to-cell signaling. For example, heparin sulfate chains interact with several growth factors, including fibroblast growth factors (FGFs) and TGFβ, ensuring their binding to specific receptors on the cell surface. Although growth factors usually interact with proteoglycan GAG chains, members of the TGF-β family also bind to the decorin proteoglycan core protein. The combination of TGF-β with decorin inhibits TGF-β activity [217].
Thus, to trigger and maintain MSC chondrogenesis and drive the synthesis of key proteins of hyaline cartilage ECM, such as type II collagen and aggrecan, it is sufficient to use some key chondrogenic growth factors and add them to the cell culture during the culturing period (or incorporate them in a biodegradable scaffold in advance) both in vitro and in vivo. In our opinion, this modification procedure offers the advantages of simplicity and safety, and evidence for its efficacy has been provided by numerous studies. When using cytokines and growth factors for MSC modification, one should consider the dose-dependent effect of these proteins, because the direction of cell differentiation depends on their concentration.
The main disadvantage of using recombinant proteins is their high cost, which makes these methods much more expensive in the cases of large CEC. It should also be kept in mind that the growth factor(s) should be added to the culture medium at each change, and this significantly increases the consumption of recombinant proteins. Not all cytokines are commercially available, and if the necessity to modify the protein arises (by adding or removing some domain), one has to contract a commercial company or to isolate and purify the protein in-house, that is a separate biotechnical task, and significantly limits the prospects for widespread use of recombinant proteins in clinical practice.
2.3.3. Genetic Modification Methods
An additional way to affect MSCs is genetic modification of cells to stimulate the synthesis of necessary growth or transcription factors [218,219]. Gene modification of cells allows one to control not only chondrogenic differentiation, but also to obtain cells with enhanced production of ECM proteins, which is an important condition to ensure that the resulting regenerate is close in biomechanical properties to native hyaline cartilage.
This group of methods includes three basic approaches: non-viral gene transfer (transfection), viral vectors (transduction) and direct genome editing. All three approaches have been used for cartilage repair in animals, but have only been introduced in clinical practice on a limited scale; at the same time, a number of publications on this subject are available [218,219,220,221,222,223].
The choice between ex vivo and in vivo methods of genetic modification depends on several factors, including the gene to be delivered and the vector. Vectors based on adenoviruses, retroviruses, herpes simplex virus, adeno-associated viruses, and lentiviruses, as well as non-viral vectors are used [223]. In vivo procedures are simpler, cheaper, and less invasive but involve direct injection of viruses into the body, and, therefore, such procedures are subject to strict safety regulation. Ex vivo gene transfer procedures are generally more invasive, expensive, and labor-consuming, but they allow control of transduced cells and safety testing prior to implantation [218,223].
Transfection and Transduction
Currently, chondrocytes and mesenchymal stem cells are the types of cells most used for genetic modification [224,225]. Some success with genetic modification has been achieved with all cell types via either transfection [226,227,228,229] or transduction with viral vectors [230,231,232,233,234] based on adenoviruses, retroviruses, and at most promisingly recombinant adeno-associated viruses (rAAVs) [235,236,237].
Mesenchymal stem cells, similar to chondrocytes (and any primary cell culture), are almost immune to transfection with plasmid DNA [238,239,240]. Nevertheless, these cells can be transfected by means of electroporation or lipofection [240]. It is known that commercial lipid reagents, such as FuGENE6 and Lipofectamin [241], increase the efficiency of DNA uptake, and pretreatment of cells with hyaluronidase and a mild detergent has a similar effect [242,243]. In our opinion, the efficacy of the transfection procedure is determined not only by the number of genetically modified cells obtained, but also by whether the following conditions are met simultaneously: high cell survival in culture (low cytotoxicity of the procedure), duration of the effect of genetic modification, and reproducibility of modification results. Only if all stages of genetic modification of cells in vitro have been fulfilled with compliance to all the above conditions, success can be achieved in in vivo experiments and, possibly, in further clinical studies. The problem of low efficacy is especially relevant to direct in vivo gene therapy, and, therefore, ex vivo strategies are usually required, with reimplantation of genetically modified cells into the hyaline cartilage defect site [244].
In contrast, transduction is a natural mechanism for introducing foreign genetic material into cells. It is an effective and proven method for promoting (or inhibiting) the synthesis of required proteins in a selected cell culture or subpopulation. However, the efficacy, duration of action, the possibility of protocol standardization, and low cytotoxicity contrast strongly with the main disadvantage of this technique, specifically legislative restrictions on mass implementation even in limited clinical practice.
At present, the scientific literature contains many examples of MSC transduction to enhance chondrogenesis by increasing the expression of genes responsible for the proliferation of hyaline cartilage (tgfb3, sox9, acan, igf1, bmp4,7, etc.), however, all the reported experiments have been carried out either in vitro or in animals [245] (more in the next chapter, Table 3). Nevertheless, there are clinical trials in progress involving use of genetically modified transduced chondrocytes [230,235,246,247], which, in our opinion, is associated with the choice of terminally differentiated cells as a transduced cell culture, as well as the predicted proliferation of such an altered cell culture.
CRISPR-Cas, miRNA, and Other Methods of Targeted MSC Modification
CRISPR/Cas is a new versatile and promising method of genome editing, the discovery of which was awarded the Nobel Prize in Chemistry in 2020. It opens new pathways and opportunities for the effective treatment of OA and other degenerative joint diseases [145,146,147]. The CRISPR/Cas9 prokaryotic system consists of three main components: Cas9 nuclease, crRNA (CRISPR RNA), and tracrRNA (transactivating crRNA). The Cas9 protein in complex with RNA recognizes the complementary sequence of foreign DNA and introduces a double-stranded break into it [248]. This method is used to obtain knockouts for various genes.
In the therapy of inflammatory and degenerative diseases, the IL1-β (inflammation), Has2 (chondrocyte accumulation of aggrecan), miR-140 (chondrocyte homeostasis), Mmp13 (tissue degradation), Foxd1 (transcription factor), and some other genes are the most common targets for knockout [214]. Along with knockout, the CRISPR/Cas technology allows activation of specific gene expression; this approach was successfully applied to produce the ECM proteins in hADSC cells [249] and activate lncRNA GRASLND expression, which in turn activated the synthesis of type II collagen and aggrecan in hMSC cells [250].
Despite the rapid growth in the number of studies over a short period of time and the potential for application in medicine, CRISPR/Cas9, similar to other genome editing tools, has a number of limitations. In addition to ethical problems, there is a possibility of introducing additional mutations into the genome, since Cas9 is not very specific and can introduce double-stranded breaks in non-target DNA sequences [251]. This problem is solved by using a catalytically inactive form of the Cas9 protein fused with a transcription activator to enhance transcription.
Along with gene knockout, another approach to approach to OA gene therapy can be provided using short noncoding RNAs (miRNAs), which affect ECM gene expression. Gurusinghe and Strappe [224] showed that the overexpression of miR-140, miR-21, and miR-675 can stimulate chondrogenesis in cultured MSC cells; however, these studies are still far from complete.
Examples of MSC modification (direct or indirect) for cell engineering of hyaline cartilage have been known since the end of the 20th century. Each subsequent generation of such modification approached features a higher efficacy compared to the previous ones; however, such an improvement was associated with increasing complexity of the therapy. Over the past 10 years, it is the genetic modification of cell culture for use within a cell-engineered scaffolds for the replacement of hyaline defects that has become widely used in in vitro and in vivo experiments due to its effectiveness; however, such cell modification has never been applied in the tissue engineering of hyaline cartilage in humans.
In our opinion, the historical choice of mesenchymal stem cells was inextricably linked with the possibility of their potential clinical use, characteristically increased proliferative potential and ability for natural differentiation into chondrocytes, as well as the ease of their production, isolation, and culturing (or migration in the case of microfracturing). Viral transduction of just MSCs imposes restrictions for clinical use, requires experience in virological experiments with cell cultures, as well as additional studies of the proliferation of genetically modified MSCs, which significantly complicates and increases the cost of the technique. Transfection, while allowing limited clinical application, leads to low efficacy and high cytotoxicity of MSCs [243]. Discussion of all aspects of genetic modification of MSCs (and other cells) for hyaline cartilage repair is undoubtedly beyond the scope of this review; detailed information on this topic can be found in [252,253].

Table 3.
Timeline of MSC modification methods and results of recent studies for each stage (last 5 years publications).
Table 3.
Timeline of MSC modification methods and results of recent studies for each stage (last 5 years publications).
| Method of MSC Modification | Experimental Stage | |||
|---|---|---|---|---|
| In Vitro Studies | Preclinical Studies (Animals) | Clinical Studies | ||
| Microfracture | n/a | + [254,255,256] | + MT [257,258,259] | |
| Spheroid-based | + [260,261,262] | + [73,262,263,264,265] | - | |
| Scaffold-based | + [89,265,266] | + [267,268,269,270] | + ST [271,272] MT [43] | |
| Direct modification | Physical | + [141,273,274,275] | + [276,277,278,279] | + ST [161,280] |
| Chemical (protein) | + [281,282,283,284] | + [168,285,286] | + ST [287] | |
| Genetic | + [288] | + [289] | - | |
n/a—not applicable, ST—short-term, MT—mid-term clinical results.
Modification of MSCs for tissue engineering of hyaline cartilage, however, has some limitations and advantages, which are displayed below.
Advantages:
- (1)
- allows creating more functional hyaline-like regenerate,
- (2)
- allows creating a cell culture with improved properties (increased gene expression and increased synthesis of extracellular matrix proteins), which affects the future properties of the regenerate,
- (3)
- the use of microfracture and scaffolds makes it possible to modify the conditions for the proliferation of MSCs, to restore the damaged area of the hyaline cartilage, and at the same time, to remove the need for a complex stage of cell cultivation in vitro,
- (4)
- some types of MSC modification make it easy to translate it in clinical practice;
Limitations:
- (1)
- in most cases, this is a complicated biological (biophysical) task that prevents the widespread translation of the procedure into clinical practice,
- (2)
- MSC modification can cause the formation that does not match in its biophysical functions to native hyaline cartilage,
- (3)
- the processes occurring during the modification of MSCs have not yet been fully studied,
- (4)
- some types of MSC modification cannot be translated into clinical practice now due to significant law barriers.
3. Conclusions
Despite the fact that tissue engineering of large joints is one of the fastest growing branches of science, to date, most of the methods based on cell engineering technologies are experimental, some of them are under clinical testing, and only a limited list of methods is registered for clinical practice. All the methods of modification of MSCs and the conditions of their culturing analyzed in the review have certain drawbacks and limitations. The analysis of scientific publications shows that the clinical use of various commercial 3D-scaffolds as tools for modifying culturing conditions has demonstrated high medium-term efficacy, which, apparently, will add popularity to this method in real clinical practice. Furthermore, protein and physical methods of MSC modification, which have already been introduced in part into clinical practice, appear to hold the greatest promise. Genetic methods for modifying MSCs, despite their efficacy, have significant limitations and have not yet been developed enough to allow their effectiveness to be tested even in limited groups of patients.
Author Contributions
Conceptualization, M.S.B.; writing—original draft preparation, M.V.S.; writing—review and editing, M.S.B., S.A.B., J.V.S., M.G.K. and M.S.B.; visualization, J.V.S.; supervision, M.G.K. and S.A.B.; project administration M.S.B.; funding acquisition, M.G.K., S.A.B. and J.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of science and Higher Education (State contracts no. 075-15-2021-1063, no. FMFU-2021-0008) and by projects ID 73024105 and ID 73023210 from St. Petersburg State University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The authors are grateful to N.A. Mikhailova, E. Ionina, and N. Khlebnikova for their support of this project. Figures were created with BioRender.com (accessed on 2 November 2021).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| RA | Rheumatoid arthritis |
| OA | Osteoarthritis |
| OCD | Osteochondritis dissecans |
| TE | Tissue engineering |
| MSCs | Mesenchymal stem cells |
| BMSCs | Bone Marrow Mesenchymal Stem Cells |
| ADSVF | adipose derived stromal vascular fraction, |
| TGF-β3 | Transforming growth factor β |
| VAS | Visual analog scale |
| PGA | Patient Global Assessment |
| HA | Hyaluronic acid |
| ICRS | International Cartilage Repair Society |
| IKDC | International Knee Documentation Committee |
| MRI | Magnetic Resonance Imaging |
| ESCs | Embryonic stem cells |
| iPSCs | Induced pluripotent stem cells |
| ECM | Extracellular matrix |
| GAGs | Glycosaminoglycans |
| HBD | Heparin-binding domens |
| PLA | Polylactide |
| PGA | Polyglutamic acid |
| PEG | Poly(ethylene glycol) |
| CEC | Cell-engineered construct |
| COMP | Cartilage oligomeric matrix protein |
| RGD | Arginine-glycine-asparagine protein sequence |
| AMIC | Autologous matrix-induced chondrogenesis |
| FA | Focal adhesion |
| HD | Hydrostatic pressure |
| HIF-1α | Hypoxia-inducible factor-1α |
| GF | Growth factor |
| SOX | SRY-related HMG-box genes |
| FGF-2 | Fibroblast growth factor |
| IGF-I | Insulin-like growth factor-I |
| BMP | Bone morphogenetic proteins |
| GDF | Growth/differentiation factors |
| IL-1 | Interleukin 1 |
| LAP | Latency-associated peptide |
| MMP3 | Metalloproteinase 3 |
| ROS | Reactive oxygen species |
| PAM | Protospacer adjacent motif |
References
- Steinwachs, M.R.; Engebretsen, L.; Brophy, R.H. Scientific Evidence Base for Cartilage Injury and Repair in the Athlete. Cartilage 2012, 3 (Suppl. S1), 11S–17S. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.B. Mechanical loading, cartilage degradation, and arthritis. Ann. N. Y. Acad. Sci. 2010, 1211, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Spector, T.D.; MacGregor, A.J. Risk factors for osteoarthritis: Genetics. Osteoarthr. Cartil. 2004, 12 (Suppl. A), S39–S44. [Google Scholar] [CrossRef] [PubMed]
- Pap, T.; Korb-Pap, A. Cartilage damage in osteoarthritis and rheumatoid arthritis--two unequal siblings. Nat. Rev. Rheumatol. 2015, 11, 606–615. [Google Scholar] [CrossRef]
- Bruns, J.; Werner, M.; Habermann, C. Osteochondritis dissecans: Etiology, pathology, and imaging with a special focus on the knee joint. Cartilage 2018, 9, 346–362. [Google Scholar] [CrossRef]
- Anderson, A.S.; Loeser, R.F. Why is osteoarthritis an age-related disease? Best Pract. Res. Clin. Rheumatol. 2010, 24, 15–26. [Google Scholar] [CrossRef]
- Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular Cartilage Regeneration in Osteoarthritis. Cells 2019, 8, 1305. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Hayes, D.W., Jr.; Brower, R.L.; John, K.J. Articular cartilage. Anatomy, injury, and repair. Clin. Podiatr. Med. Surg. 2001, 18, 35–53. [Google Scholar]
- Yang, X.-G.; Wang, F.; He, X.; Feng, J.-T.; Hu, Y.-C.; Zhang, H.; Yang, L.; Hua, K. Network meta-analysis of knee outcomes following anterior cruciate ligament reconstruction with various types of tendon grafts. Int. Orthop. 2020, 44, 365–380. [Google Scholar] [CrossRef]
- Biscević, M.; Smrke, D. Structural differences between hip endoprostheses, and implications on a hip kinetics. Bosn. J. Basic Med. Sci. 2005, 5, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Hefzy, M.S.; Elahinia, M. State of the art review of knee-ankle-foot orthoses. Ann. Biomed. Eng. 2015, 43, 427–441. [Google Scholar] [CrossRef]
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic science of articular cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Kulyaba, T.A.; Bantser, S.A.; Trachuk, P.A.; Vorontsova, T.N.; Kornilov, N.N. The Effectiveness of Various Surgical Techniques in the Treatment of Local Knee Cartilage Lesions (Review). Traumatol. Orthop. Russia 2020, 26, 170–181. [Google Scholar] [CrossRef]
- Simon, T.M.; Jackson, D.W. Articular Cartilage: Injury Pathways and Treatment Options. Sports Med. Arthrosc. Rev. 2018, 26, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef]
- Hunziker, E.B. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Rocke, J.P.; De Bari, C. Cell-based approaches to joint surface repair: A research perspective. Osteoarthr. Cartil. 2013, 21, 892–900. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Bruder, S.P.; Fox, B.S. Tissue engineering of bone. Cell based strategies. Clin. Orthop. Relat. Res. 1999, 367, S68–S83. [Google Scholar] [CrossRef]
- Afanasyev, B.; Elstner, E.; Zander, A.; Friedenstein, A.J. founder of the mesenchymal stem cell concept. Cell. Ther. Transplant. 2009, 1, 35–38. [Google Scholar] [CrossRef]
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem. Cell. 2008, 2, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Bahsoun, S.; Coopman, K.; Akam, E.C. The impact of cryopreservation on bone marrow-derived mesenchymal stem cells: A systematic review. J. Transl. Med. 2019, 17, 397. [Google Scholar] [CrossRef] [PubMed]
- Mafi, R.; Hindocha, S.; Mafi, P.; Griffin, M.; Khan, W. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications—A systematic review of the literature. Open Orthop. J. 2011, 5 (Suppl. S2), 242–248. [Google Scholar] [CrossRef] [PubMed]
- Orth, P.; Gao, L.; Madry, H. Microfracture for cartilage repair in the knee: A systematic review of the contemporary literature. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 670–706. [Google Scholar] [CrossRef]
- Goldberg, A.; Mitchell, K.; Soans, J.; Kim, L.; Zaidi, R. The use of mesenchymal stem cells for cartilage repair and regeneration: A systematic review. J. Orthop. Surg. Res. 2017, 12, 39. [Google Scholar] [CrossRef]
- Valenti, M.T.; Carbonare, D.L.; Donatelli, L.; Bertoldo, F.; Zanatta, M.; Cascio, V. Gene expression analysis in osteoblastic differentiation from peripheral blood mesenchymal stem cells. Bone 2008, 43, 1084–1092. [Google Scholar] [CrossRef]
- Shariatzadeh, M.; Song, J.; Wilson, S. The efficacy of different sources of mesenchymal stem cells for the treatment of knee osteoarthritis. Cell Tissue Res. 2019, 378, 399–410. [Google Scholar] [CrossRef]
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic differentiation of mesenchymal stem cells: Challenges and unfulfilled expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608. [Google Scholar] [CrossRef]
- Trivanovic, D.; Kocic, J.; Mojsilovic, S.; Krstic, A.; Ilic, V.; Okic-Djordjevic, I.; Santibanez, J.; Jovcic, G.; Terzic, M.; Bugarski, D. Mesenchymal stem cells isolated from peripheral blood and umbilical cord Wharton’s jelly. Srp. Arh. Celok. Lek. 2013, 141, 178–186. [Google Scholar] [CrossRef]
- Ghorbani, A.; Jalali, S.A.; Varedi, M. Isolation of adipose tissue mesenchymal stem cells without tissue destruction: A non-enzymatic method. Tissue Cell. 2014, 46, 54–58. [Google Scholar] [CrossRef]
- Zhang, X.; Hirai, M.; Cantero, S.; Ciubotariu, R.; Dobrila, L.; Hirsh, A.; Igura, K.; Satoh, H.; Yokomi, I.; Nishimura, T.; et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: Reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J. Cell Biochem. 2011, 112, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Yamasaki, S.; Mera, H.; Itokazu, M.; Hashimoto, Y.; Wakitani, S. Cartilage Repair with Autologous Bone Marrow Mesenchymal Stem Cell Transplantation. Rev. Preclin. Clin. Stud. Cartil. 2014, 5, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Wakitani, S.; Goto, T.; Pineda, S.J.; Young, R.G.; Mansour, J.M.; Caplan, A.I.; Goldberg, V.M. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J. Bone Joint Surg Am. 1994, 76, 579–592. [Google Scholar] [CrossRef]
- Wang, W.G.; Lou, S.Q.; Ju, X.D.; Xia, K.; Xia, J.H. In vitro chondrogenesis of human bone marrow-derived mesenchymal progenitor cells in monolayer culture: Activation by transfection with TGF-beta 2. Tissue Cell 2003, 35, 69–77. [Google Scholar] [CrossRef]
- Bozhokin, M.S.; Bozhkova, S.A.; Netylko, G.I. Possibilities of current cellular technologies for articular cartilage repair (analytical review). Traumatol. Orthop. Russia 2016, 22, 122–134. [Google Scholar] [CrossRef][Green Version]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transpl. 2019, 28, 801–812. [Google Scholar] [CrossRef]
- Mautner, K.; Carr, D.; Whitley, J.; Bowers, R. Allogeneic Versus Autologous Injectable Mesenchymal Stem Cells for Knee Osteoarthritis: Review and Current Status. Tech. Orthopaedics. 2019, 34, 244–256. [Google Scholar] [CrossRef]
- Lee, W.Y.; Wang, B. Cartilage repair by mesenchymal stem cells: Clinical trial update and perspectives. J. Orthop. Translat. 2017, 9, 76–88. [Google Scholar] [CrossRef]
- Jo, C.H.; Gil Lee, Y.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266, published correction appears in Stem Cells 2017, 35, 1651–1652. [Google Scholar] [CrossRef]
- Davatchi, F.; Sadeghi Abdollahi, B.; Mohyeddin, M.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int. J. Rheum. Dis. 2016, 19, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Ha, C.W.; Lee, C.H.; Yoon, Y.C.; Park, Y.G. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Reubinoff, B.E.; Pera, M.F.; Fong, C.Y.; Trounson, A.; Bongso, A. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat. Biotechnol. 2000, 18, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Nelakanti, R.V.; Kooreman, N.G.; Wu, J.C. Teratoma formation: A tool for monitoring pluripotency in stem cell research. Curr. Protoc. Stem. Cell Biol. 2015, 32, 4A.8.1–4A.8.17. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Tsumaki, N.; Okada, M.; Yamashita, A. iPS cell technologies and cartilage regeneration. Bone 2015, 70, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem. Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Nejadnik, H.; Diecke, S.; Lenkov, O.D.; Chapelin, F.; Donig, J.; Tong, X.; Derugin, N.; Chan, R.C.F.; Gaur, A.; Yang, F.; et al. Improved approach for chondrogenic differentiation of human induced pluripotent stem cells. Stem. Cell Rev. Rep. 2015, 11, 242–253. [Google Scholar] [CrossRef]
- Diederichs, S.; Klampfleuthner, F.A.M.; Moradi, B.; Richter, W. Chondral Differentiation of Induced Pluripotent Stem Cells Without Progression Into the Endochondral Pathway. Front. Cell Dev. Biol. 2019, 7, 270. [Google Scholar] [CrossRef]
- Medvedev, S.P.; Shevchenko, A.I.; Zakian, S.M. Induced Pluripotent Stem Cells: Problems and Advantages when Applying them in Regenerative Medicine. Acta Nat. 2010, 2, 18–28. [Google Scholar] [CrossRef]
- Monaco, M.L.; Merckx, G.; Ratajczak, J.; Gervois, P.; Hilkens, P.; Clegg, P.; Bronckaers, A.; Vandeweerd, J.-M.; Lambrichts, I. Stem Cells for Cartilage Repair: Preclinical Studies and Insights in Translational Animal Models and Outcome Measures. Stem. Cells Int. 2018, 2018, 9079538. [Google Scholar] [CrossRef]
- Kafienah, W.; Cheung, F.L.; Sims, T.; Martin, I.; Miot, S.; Von Ruhland, C.; Roughley, P.J.; Hollander, A.P. Lumican inhibits collagen deposition in tissue engineered cartilage. Matrix Biol. 2008, 27, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Riesle, J.; Hollander, A.P.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. Collagen in tissue-engineered cartilage: Types, structure, and crosslinks. J. Cell Biochem. 1998, 71, 313–327. [Google Scholar] [CrossRef]
- Dufrane, D. Impact of Age on Human Adipose Stem Cells for Bone Tissue Engineering. Cell Transpl. 2017, 26, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Pachón-Peña, G.; Serena, C.; Ejarque, M.; Petriz, J.; Duran, X.; Oliva-Olivera, W.; Simó, R.; Tinahones, F.J.; Fernández-Veledo, S.; Vendrell, J. Obesity Determines the Immunophenotypic Profile and Functional Characteristics of Human Mesenchymal Stem Cells From Adipose Tissue. Stem. Cells Transl. Med. 2016, 5, 464–475. [Google Scholar] [CrossRef]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Zhou, M.; Yuan, X.; Yin, H.; Gough, J.E. Restoration of chondrocytic phenotype on a two-dimensional micropatterned surface. Biointerphases 2015, 10, 011003. [Google Scholar] [CrossRef]
- Panadero, J.A.; Lanceros-Mendez, S.; Ribelles, J.L. Differentiation of mesenchymal stem cells for cartilage tissue engineering: Individual and synergetic effects of three-dimensional environment and mechanical loading. Acta Biomater. 2016, 33, 1–12. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Role of biological scaffolds, hydro gels and stem cells in tissue regeneration therapy. Adv. Tissue Eng. Regen Med. 2017, 2, 121–135. [Google Scholar] [CrossRef][Green Version]
- Wasyłeczko, M.; Sikorska, W.; Chwojnowski, A. Review of Synthetic and Hybrid Scaffolds in Cartilage Tissue Engineering. Membranes 2020, 10, 348. [Google Scholar] [CrossRef]
- Shimomura, K.; Ando, W.; Fujie, H.; Hart, D.A.; Yoshikawa, H.; Nakamura, N. Scaffold-free tissue engineering for injured joint surface restoration. J. Exp. Orthop. 2018, 5, 2. [Google Scholar] [CrossRef]
- Ryu, N.E.; Lee, S.H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef]
- Zhang, L.; Su, P.; Xu, C.; Yang, J.; Yu, W.; Huang, D. Chondrogenic differentiation of human mesenchymal stem cells: A comparison between micromass and pellet culture systems. Biotechnol. Lett. 2010, 32, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.; de Melo-Diogo, D.; Moreira, A.F.; Carvalho, M.P.; Correia, I.J. Spheroids Formation on Non-Adhesive Surfaces by Liquid Overlay Technique: Considerations and Practical Approaches. Biotechnol. J. 2018, 13, 1700417. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylostalo, J.H. Preparation of anti-inflammatory mesenchymal stem/precursor cells (MSCs) through sphere formation using hanging-drop culture technique. Curr. Protoc. Stem. Cell Biol. 2014, 28, 2B. 6.1–2B. 6.23. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B. Three-dimensional tissue culture models in cancer biology. Semin. Cancer Biol. 2005, 15, 365–377. [Google Scholar] [CrossRef]
- Fu, L.; Li, P.; Li, H.; Gao, C.; Yang, Z.; Zhao, T.; Chen, W.; Liao, Z.; Peng, Y.; Cao, F.; et al. The Application of Bioreactors for Cartilage Tissue Engineering: Advances, Limitations, and Future Perspectives. Stem. Cells Int. 2021, 2021, 6621806. [Google Scholar] [CrossRef]
- Li, F.; Truong, V.X.; Thissen, H.; Frith, J.E.; Forsythe, J.S. Microfluidic Encapsulation of Human Mesenchymal Stem Cells for Articular Cartilage Tissue Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 8589–8601. [Google Scholar] [CrossRef] [PubMed]
- Anil-Inevi, M.; Yaman, S.; Yildiz, A.A.; Mese, G.; Yalcin-Ozuysal, O.; Tekin, H.C.; Ozcivici, E. Biofabrication of in situ Self Assembled 3D Cell Cultures in a Weightlessness Environment Generated using Magnetic Levitation. Sci. Rep. 2018, 8, 7239. [Google Scholar] [CrossRef]
- Cesarz, Z.; Tamama, K. Spheroid Culture of Mesenchymal Stem Cells. Stem. Cells Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef]
- Favreau, H.; Pijnenburg, L.; Seitlinger, J.; Fioretti, F.; Keller, L.; Scipioni, D.; Adriaensen, H.; Kuchler-Bopp, S.; Ehlinger, M.; Mainard, D.; et al. Osteochondral repair combining therapeutics implant with mesenchymal stem cells spheroids. Nanomedicine 2020, 29, 102253. [Google Scholar] [CrossRef] [PubMed]
- Becher, C.; Laute, V.; Fickert, S.; Zinser, W.; Niemeyer, P.; John, T.; Diehl, P.; Kolombe, T.; Siebold, R.; Fay, J. Safety of three different product doses in autologous chondrocyte implantation: Results of a prospective, randomised, controlled trial. J. Orthop. Surg. Res. 2017, 12, 71. [Google Scholar] [CrossRef]
- Souza, P.R.; de Oliveira, A.C.; Vilsinski, B.H.; Kipper, M.J.; Martins, A.F. Polysaccharide-Based Materials Created by Physical Processes: From Preparation to Biomedical Applications. Pharmaceutics 2021, 13, 621. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Shi, B.; Chang, H.; Su, X.; Zhang, L.; Bi, C.; Shuai, Y.; Du, X.; Deng, Z.; Jin, Y. Heparin improves BMSC cell therapy: Anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics. 2017, 7, 106–116. [Google Scholar] [CrossRef]
- Kirn-Safran, C.B.; Gomes, R.R.; Brown, A.J.; Carson, D.D. Heparan sulfate proteoglycans: Coordinators of multiple signaling pathways during chondrogenesis. Birth Defects Res. C Embryo Today 2004, 72, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Tamaddon, M.; Burrows, M.; Ferreira, S.A.R.M.; Dazzi, F.; Apperley, J.F.; Bradshaw, A.; Brand, D.; Czernuszka, J.; Gentleman, E. Monomeric, porous type II collagen scaffolds promote chondrogenic differentiation of human bone marrow mesenchymal stem cells in vitro. Sci. Rep. 2017, 7, 43519. [Google Scholar] [CrossRef]
- Bistolfi, A.; Ferracini, R.; Galletta, C.; Tosto, F.; Sgarminato, V.; Digo, R.; Vernè, E.; Massè, A. Regeneration of articular cartilage: Scaffold used in orthopedic surgery. A short handbook of available products for regenerative joints surgery. Clin. Sci. Res. Rep. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Kitamura, N.; Kurokawa, T.; Fukui, T.; Gong, J.P.; Yasuda, K. Hyaluronic acid enhances the effect of the PAMPS/PDMAAm double-network hydrogel on chondrogenic differentiation of ATDC5 cells. BMC Musculoskelet Disord. 2014, 15, 222. [Google Scholar] [CrossRef]
- Chung, C.; Burdick, J.A. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng. Part A 2009, 15, 243–254. [Google Scholar] [CrossRef]
- Klontzas, M.; Drissi, H.; Mantalatis, A. The Use of Alginate Hydrogels for the Culture of Mesenchymal Stem Cells (MSCs): In vitro and In vivo Paradigms. In Alginates—Recent Uses of This Natural Polymer; Pereira, L., Ed.; IntechOpen: London, UK, 2019; pp. 65–80. [Google Scholar] [CrossRef][Green Version]
- Seo, S.; Na, K. Mesenchymal stem cell-based tissue engineering for chondrogenesis. J. Biomed. Biotechnol. 2011, 2011, 806891. [Google Scholar] [CrossRef]
- Breyner, N.M.; Hell, R.C.; Carvalho, L.R.; Machado, C.B.; Filho, I.N.P.; Valério, P.; Pereira, M.; Goes, A.M. Effect of a three-dimensional chitosan porous scaffold on the differentiation of mesenchymal stem cells into chondrocytes. Cells Tissues Organs. 2010, 191, 119–128. [Google Scholar] [CrossRef]
- Huang, G.S.; Dai, L.G.; Yen, B.L.; Hsu, S.H. Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials 2011, 32, 6929–6945. [Google Scholar] [CrossRef]
- Huang, G.P.; Menezes, R.; Vincent, R.; Hammond, W.; Rizio, L.; Collins, G.; Arinzeh, T.L. Gelatin Scaffolds Containing Partially Sulfated Cellulose Promote Mesenchymal Stem Cell Chondrogenesis. Tissue Eng. Part A 2017, 23, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Cochis, A.; Grad, S.; Stoddart, M.; Farè, S.; Altomare, L.; Azzimonti, B.; Alini, M.; Rimondini, L. Bioreactor mechanically guided 3D mesenchymal stem cell chondrogenesis using a biocompatible novel thermo-reversible methylcellulose-based hydrogel. Sci. Rep. 2017, 7, 45018. [Google Scholar] [CrossRef]
- Calabrese, G.; Gulino, R.; Giuffrida, R.; Forte, S.; Figallo, E.; Fabbi, C.; Salvatorelli, L.; Memeo, L.; Gulisano, M.; Parenti, R. In vivo Evaluation of Biocompatibility and Chondrogenic Potential of a Cell-Free Collagen-Based Scaffold. Front Physiol. 2017, 8, 984. [Google Scholar] [CrossRef]
- Calabrese, G.; Forte, S.; Gulino, R.; Cefalì, F.; Figallo, E.; Salvatorelli, L.; Maniscalchi, E.T.; Angelico, G.; Parenti, R.; Gulisano, M.; et al. Combination of Collagen-Based Scaffold and Bioactive Factors Induces Adipose-Derived Mesenchymal Stem Cells Chondrogenic Differentiation In vitro. Front. Physiol. 2017, 8, 50. [Google Scholar] [CrossRef]
- Campos, D.F.D.; Drescher, W.; Rath, B.; Tingart, M.; Fischer, H. Supporting Biomaterials for Articular Cartilage Repair. Cartilage 2012, 3, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Hernando, A.; Saputri, D.H.A.; Tan, M.I.; Barlian, A. Directing the chondrogenic differentiation of human Wharton’s jelly mesenchymal stem cells using spider silk-based micropattern. AIP Conf. Proc. 2021, 2346, 020001. [Google Scholar] [CrossRef]
- Awad, H.A.; Quinn Wickham, M.; Leddy, H.A.; Gimble, J.M.; Guilak, F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 2004, 25, 3211–3222. [Google Scholar] [CrossRef]
- Neves, M.I.; Araújo, M.; Moroni, L.; da Silva, R.M.P.; Barrias, C.C. Glycosaminoglycan-Inspired Biomaterials for the Development of Bioactive Hydrogel Networks. Molecules 2020, 25, 978. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Luo, J.; Pringle, E.W.; Cantini, M. ChondroGELesis: Hydrogels to harness the chondrogenic potential of stem cells. Mater. Sci. Eng. C 2021, 121, 111822. [Google Scholar] [CrossRef]
- O’Brien, F. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Davis, S.; Roldo, M.; Blunn, G.; Tozzi, G.; Roncada, T. Influence of the Mechanical Environment on the Regeneration of Osteochondral Defects. Front. Bioeng. Biotechnol. 2021, 9, 603408. [Google Scholar] [CrossRef] [PubMed]
- Berthiaume, F.; Yarmush, M.L. Tissue Engineering. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Elsevier Science Ltd.: San Diego, CA, USA, 2003; pp. 817–842. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Little, C.J.; Bawolin, N.K.; Chen, X. Mechanical properties of natural cartilage and tissue-engineered constructs. Tissue Eng. Part B Rev. 2011, 17, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Wang, T.; Lai, J.H.; Yang, F. Effects of Hydrogel Stiffness and Extracellular Compositions on Modulating Cartilage Regeneration by Mixed Populations of Stem Cells and Chondrocytes In Vivo. Tissue Eng. Part A 2016, 22, 1348–1356. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Lee, E.M.; Smith, K.; Hyzy, S.L.; Doroudi, M.; Williams, J.K.; Gall, K.; Boyan, B.D.; Schwartz, Z. Substrate Stiffness Controls Osteoblastic and Chondrocytic Differentiation of Mesenchymal Stem Cells without Exogenous Stimuli. PLoS ONE 2017, 12, e0170312. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Di Matteo, B.; Perdisa, F.; Marcacci, M. Matrix assisted autologous chondrocyte transplantation for cartilage treatment: A systematic review. Bone Joint Res. 2013, 2, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Goh, J.C.; Hutmacher, D.W.; Lee, E.H.; Zigang, G. Repair of large articular osteochondral defects using hybrid scaffolds and bone marrow-derived mesenchymal stem cells in a rabbit model. Tissue Eng. 2006, 12, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Hu, Y.; Zhang, C.; Li, X.; Lv, R.; Qin, L.; Zhu, R. Cartilage regeneration using mesenchymal stem cells and a PLGA-gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials 2006, 27, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Wayne, J.S.; McDowell, C.L.; Shields, K.J.; Tuan, R.S. In vivo response of polylactic acid-alginate scaffolds and bone marrow-derived cells for cartilage tissue engineering. Tissue Eng. 2005, 11, 953–963. [Google Scholar] [CrossRef]
- Shahin, K.; Doran, P.M. Strategies for enhancing the accumulation and retention of extracellular matrix in tissue-engineered cartilage cultured in bioreactors. PLoS ONE 2011, 6, e23119. [Google Scholar] [CrossRef]
- Cao, L.; Cao, B.; Lu, C.; Wang, G.; Yu, L.; Ding, J. An injectable hydrogel formed by in situ cross-linking of glycol chitosan and multi-benzaldehyde functionalized PEG analogues for cartilage tissue engineering. J. Mater. Chem. B 2015, 3, 1268–1280. [Google Scholar] [CrossRef]
- Molina, M.I.E.; Malollari, K.G.; Komvopoulos, K. Design Challenges in Polymeric Scaffolds for Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 617141. [Google Scholar] [CrossRef]
- Lee, Y.H.; Suzer, F.; Thermann, H. Autologous Matrix-Induced Chondrogenesis in the Knee: A Review. Cartilage 2014, 5, 145–153. [Google Scholar] [CrossRef]
- Bark, S.; Piontek, T.; Behrens, P.; Mkalaluh, S.; Varoga, D.; Gille, J. Enhanced microfracture techniques in cartilage knee surgery: Fact or fiction? World J. Orthop. 2014, 5, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lian, M.; Sun, B.; Jia, B.; Wu, Q.; Qiao, Z.; Dai, K. Preparation of high precision multilayer scaffolds based on Melt Electro-Writing to repair cartilage injury. Theranostics 2020, 10, 10214–10230. [Google Scholar] [CrossRef]
- Kang, H.; Zeng, Y.; Varghese, S. Functionally graded multilayer scaffolds for in vivo osteochondral tissue engineering. Acta Biomater. 2018, 78, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Andjelkov, N.; Riyadh, H.; Ivarsson, M.; Kacarevic-Popovic, Z.; Krstic, J.; Wretenberg, P. The enhancement of cartilage regeneration by use of a chitosan-based scaffold in a 3D model of microfracture in vitro: A pilot evaluation. J. Exp. Orthop. 2021, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Di Martino, A.; Busacca, M.; Moio, A.; Perdisa, F.; Marcacci, M. Clinical results and MRI evolution of a nano-composite multilayered biomaterial for osteochondral regeneration at 5 years. Am. J. Sports Med. 2014, 42, 158–165. [Google Scholar] [CrossRef]
- Bryanskiy, A.; Kulyaba, T.; Kornilov, N.; Rumakin, V.; Gornostaev, V. Arthroplasty Using Autologous Multipotent Mesenchymal Cells and Collagen Membrane Chondro-Gide. N.N. Priorov J. Traumatol. Orthop. 2014, 62–66. [Google Scholar] [CrossRef]
- Kohli, N.; Wright, K.T.; Sammons, R.L.; Jeys, L.; Snow, M.; Johnson, W.E. An In vitro Comparison of the Incorporation, Growth, and Chondrogenic Potential of Human Bone Marrow versus Adipose Tissue Mesenchymal Stem Cells in Clinically Relevant Cell Scaffolds Used for Cartilage Repair. Cartilage 2015, 6, 252–263. [Google Scholar] [CrossRef]
- Jiang, S.; Guo, W.; Tian, G.; Luo, X.; Peng, L.; Liu, S.; Sui, X.; Guo, Q.; Li, X. Clinical Application Status of Articular Cartilage Regeneration Techniques: Tissue-Engineered Cartilage Brings New Hope. Stem Cells Int. 2020, 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, C.; Tichy, B.; Nürnberger, S.; Hosiner, S.; Zak, L.; Aldrian, S.; Marlovits, S. Gene expression and cell differentiation in matrix-associated chondrocyte transplantation grafts: A comparative study. Osteoarthr. Cartil. 2011, 19, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Zak, L.; Albrecht, C.; Wondrasch, B.; Widhalm, H.; Vekszler, G.; Trattnig, S.; Marlovits, S.; Aldrian, S. Results 2 Years After Matrix-Associated Autologous Chondrocyte Transplantation Using the Novocart 3D Scaffold. Am. J. Sports Med. 2014, 42, 1618–1627. [Google Scholar] [CrossRef]
- Niethammer, T.R.; Pietschmann, M.F.; Horng, A.; Roßbach, B.P.; Ficklscherer, A.; Jansson, V.; Müller, P.E. Graft hypertrophy of matrix-based autologous chondrocyte implantation: A two-year follow-up study of NOVOCART 3D implantation in the knee. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1329–1336. [Google Scholar] [CrossRef]
- Ochs, B.G.; Müller-Horvat, C.; Albrecht, D.; Schewe, B.; Weise, K.; Aicher, W.K.; Rolauffs, B. Remodeling of Articular Cartilage and Subchondral Bone After Bone Grafting and Matrix-Associated Autologous Chondrocyte Implantation for Osteochondritis Dissecans of the Knee. Am. J. Sports Med. 2010, 39, 764–773. [Google Scholar] [CrossRef]
- Condello, V.; Filardo, G.; Madonna, V.; Andriolo, L.; Screpis, D.; Bonomo, M.; Zappia, M.; Giudici, L.D.; Zorzi, C. Use of a Biomimetic Scaffold for the Treatment of Osteochondral Lesions in Early Osteoarthritis. BioMed Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Delcogliano, M.; de Caro, F.; Scaravella, E.; Ziveri, G.; De Biase, C.F.; Marotta, D.; Marenghi, P.; Delcogliano, A. Use of innovative biomimetic scaffold in the treatment for large osteochondral lesions of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2013, 22, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Di Matteo, B.; Verdonk, P.; Drobnic, M.; Dulic, O.; Gavrilovic, G.; Patrascu, J.M.; Zaslav, K.; Kwiatkowski, G.; Altschuler, N.; et al. Aragonite-Based Scaffold for the Treatment of Joint Surface Lesions in Mild to Moderate Osteoarthritic Knees: Results of a 2-Year Multicenter Prospective Study. Am. J. Sports Med. 2021, 49, 588–598. [Google Scholar] [CrossRef]
- Chubinskaya, S.; Di Matteo, B.; Lovato, L.; Iacono, F.; Robinson, D.; Kon, E. Agili-C implant promotes the regenerative capacity of articular cartilage defects in an ex vivo model. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Kreuz, P.C.; Müller, S.; Ossendorf, C.; Kaps, C.; Erggelet, C. Treatment of focal degenerative cartilage defects with polymer-based autologous chondrocyte grafts: Four-year clinical results. Arthritis Res. Ther. 2009, 11, R33. [Google Scholar] [CrossRef]
- Ossendorf, C.; Kaps, C.; Kreuz, P.C.; Burmester, G.R.; Sittinger, M.; Erggelet, C. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res. Ther. 2007, 9, R41. [Google Scholar] [CrossRef]
- Erggelet, C.; Kreuz, P.C.; Mrosek, E.H.; Schagemann, J.C.; Lahm, A.; Ducommun, P.P.; Ossendorf, C. Autologous chondrocyte implantation versus ACI using 3D-bioresorbable graft for the treatment of large full-thickness cartilage lesions of the knee. Arch. Orthop. Trauma Surg. 2010, 130, 957–964. [Google Scholar] [CrossRef]
- Bartlett, W.; Skinner, J.A.; Gooding, C.R.; Carrington, R.W.J.; Flanagan, A.M.; Briggs, T.W.R.; Bentley, G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: A prospective, randomised study. J. Bone Jt. Surg. Br. Vol. 2005, 87-B, 640–645. [Google Scholar] [CrossRef]
- Gille, J.; Reiss, E.; Freitag, M.; Schagemann, J.; Steinwachs, M.; Piontek, T.; Reiss, E. Autologous Matrix-Induced Chondrogenesis for Treatment of Focal Cartilage Defects in the Knee: A Follow-up Study. Orthop. J. Sports Med. 2021, 9, 2325967120981872. [Google Scholar] [CrossRef]
- Messaoudi, O.; Henrionnet, C.; Bourge, K.; Loeuille, D.; Gillet, P.; Pinzano, A. Stem Cells and Extrusion 3D Printing for Hyaline Cartilage Engineering. Cells 2020, 10, 2. [Google Scholar] [CrossRef]
- Henrionnet, C.; Pourchet, L.; Neybecker, P.; Messaoudi, O.; Gillet, P.; Loeuille, D.; Mainard, D.; Marquette, C.; Pinzano, A. Combining Innovative Bioink and Low Cell Density for the Production of 3D-Bioprinted Cartilage Substitutes: A Pilot Study. Stem Cells Int. 2020, 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Huang, L.; Hao, W.; Zhao, T.; Zhao, H.; Yang, W.; Xie, X.; Qian, L.; Chen, Y.; Dai, J. Long-term stability, high strength, and 3D printable alginate hydrogel for cartilage tissue engineering application. Biomed. Mater. 2021, 16, 064102. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Visser, J.; Planell, J.A.; Engel, E.; Malda, J.; Timoneda, M.A.M. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication 2014, 6, 035020. [Google Scholar] [CrossRef]
- Gómez-Leduc, T.; Desancé, M.; Hervieu, M.; Legendre, F.; Ollitrault, D.; De Vienne, C.; Herlicoviez, M.; Galéra, P.; Demoor, M. Hypoxia Is a Critical Parameter for Chondrogenic Differentiation of Human Umbilical Cord Blood Mesenchymal Stem Cells in Type I/III Collagen Sponges. Int. J. Mol. Sci. 2017, 18, 1933. [Google Scholar] [CrossRef]
- Kim, Y.; Sah, R.; Grodzinsky, A.; Plaas, A.; Sandy, J. Mechanical Regulation of Cartilage Biosynthetic Behavior: Physical Stimuli. Arch. Biochem. Biophys. 1994, 311, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Marks, R. Articular Cartilage Degradation and Photobiomodulation Therapy. CPQ Orthop. 2021, 5, 1–20. [Google Scholar]
- Huang, X.; Das, R.; Patel, A.; Nguyen, T.D. Physical Stimulations for Bone and Cartilage Regeneration. Regen. Eng. Transl. Med. 2018, 4, 216–237. [Google Scholar] [CrossRef]
- Parate, D.; Franco-Obregón, A.; Fröhlich, J.; Beyer, C.; Abbas, A.A.; Kamarul, T.; Hui, J.H.P.; Yang, Z. Enhancement of mesenchymal stem cell chondrogenesis with short-term low intensity pulsed electromagnetic fields. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Bozhokin, M.S.; Vcherashnii, D.B.; Yastrebov, S.G.; Beilinson, L.L.; Zherebtsova, J.V.; Khotin, M.G. Low-intensity photobiomodulation at 632.8 nm increases tgfβ3, col2a1, and sox9 gene expression in rat bone marrow mesenchymal stem cells in vitro Lasers. Med. Sci. 2021, 1–7. [Google Scholar] [CrossRef]
- Kozhemyakina, E.; Lassar, A.B.; Zelzer, E. A pathway to bone: Signaling molecules and transcription factors involved in chondrocyte development and maturation. Development 2015, 142, 817–831. [Google Scholar] [CrossRef]
- Samsa, W.E.; Zhou, X.; Zhou, G. Signaling pathways regulating cartilage growth plate formation and activity. Semin. Cell Dev. Biol. 2017, 62, 3–15. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, J.; Fan, Y.; Li, J.; You, T.; He, S.; Xiao, G.; Chen, D. Exploration of CRISPR/Cas9-based gene editing as therapy for osteoarthritis. Ann. Rheum. Dis. 2019, 78, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Adkar, S.S.; Brunger, J.M.; Willard, V.P.; Wu, C.-L.; Gersbach, C.A.; Guilak, F. Genome Engineering for Personalized Arthritis Therapeutics. Trends Mol. Med. 2017, 23, 917–931. [Google Scholar] [CrossRef]
- Mali, P.; Esvelt, K.M.; Church, G. Cas9 as a versatile tool for engineering biology. Nat. Methods 2013, 10, 957–963. [Google Scholar] [CrossRef]
- Potter, C.M.; Lao, K.H.; Zeng, L.; Xu, Q. Role of Biomechanical Forces in Stem Cell Vascular Lineage Differentiation. Arter. Thromb. Vasc. Biol. 2014, 34, 2184–2190. [Google Scholar] [CrossRef]
- Sawatjui, N.; Limpaiboon, T.; Schrobback, K.; Klein, T. Biomimetic scaffolds and dynamic compression enhance the properties of chondrocyte- and MSC -based tissue-engineered cartilage. J. Tissue Eng. Regen. Med. 2018, 12, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Lin, W.; Cai, H.; Chen, Y.; Man, Y.; Liang, J.; Wang, Q.; Sun, Y.; Fan, Y.; Zhang, X. Dynamic mechanical loading facilitated chondrogenic differentiation of rabbit BMSCs in collagen scaffolds. Regen. Biomater. 2019, 6, 99–106. [Google Scholar] [CrossRef]
- Gardner, O.F.W.; Fahy, N.; Alini, M.; Stoddart, M.J. Joint mimicking mechanical load activates TGFβ1 in fibrin-poly(ester-urethane) scaffolds seeded with mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2017, 11, 2663–2666. [Google Scholar] [CrossRef]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Heal. A Multidiscip. Approach 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Correia, C.; Pereira, A.L.; Duarte, A.; Frias, A.M.; Pedro, A.; Oliveira, J.T.; Sousa, R.; Reis, R.L. Dynamic Culturing of Cartilage Tissue: The Significance of Hydrostatic Pressure. Tissue Eng. Part A 2012, 18, 1979–1991. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Yang, X.; Jiang, Y.; Gui, J. Intermittent hydrostatic pressure maintains and enhances the chondrogenic differentiation of cartilage progenitor cells cultivated in alginate beads. Dev. Growth Differ. 2016, 58, 180–193. [Google Scholar] [CrossRef]
- Montagne, K.; Onuma, Y.; Ito, Y.; Aiki, Y.; Furukawa, K.S.; Ushida, T. High hydrostatic pressure induces pro-osteoarthritic changes in cartilage precursor cells: A transcriptome analysis. PLoS ONE 2017, 12, e0183226. [Google Scholar] [CrossRef]
- Stavenschi, E.; Corrigan, M.A.; Johnson, G.P.; Riffault, M.; Hoey, D.A. Physiological cyclic hydrostatic pressure induces osteogenic lineage commitment of human bone marrow stem cells: A systematic study. Stem Cell Res. Ther. 2018, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Robins, J.C.; Akeno, N.; Mukherjee, A.; Dalal, R.R.; Aronow, B.J.; Koopman, P.; Clemens, T.L. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone 2005, 37, 313–322. [Google Scholar] [CrossRef]
- Kanichai, M.; Ferguson, D.; Prendergast, P.J.; Campbell, V.A. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: A role for AKT and hypoxia-inducible factor (HIF)-1α. J. Cell. Physiol. 2008, 216, 708–715. [Google Scholar] [CrossRef]
- Lee, H.-H.; Chang, C.-C.; Shieh, M.-J.; Wang, J.-P.; Chen, Y.-T.; Young, T.-H.; Hung, S.-C. Hypoxia Enhances Chondrogenesis and Prevents Terminal Differentiation through PI3K/Akt/FoxO Dependent Anti-Apoptotic Effect. Sci. Rep. 2013, 3, 2683. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.C.; Park, H.J.; Wang, S.Y.; Yang, H.R.; Lee, M.C.; Han, H.-S. Hypoxic condition enhances chondrogenesis in synovium-derived mesenchymal stem cells. Biomater. Res. 2018, 22, 271–278. [Google Scholar] [CrossRef]
- Vinatier, C.; Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann. Phys. Rehabilitation Med. 2016, 59, 139–144. [Google Scholar] [CrossRef]
- Langella, L.G.; Casalechi, H.L.; Tomazoni, S.S.; Johnson, D.S.; Albertini, R.; Pallotta, R.C.; Marcos, R.L.; de Tarso Camillo de Carvalho, P.; Leal-Junior, E.C.P. Photobiomodulation therapy (PBMT) on acute pain and inflammation in patients who underwent total hip arthroplasty—a randomized, triple-blind, placebo-controlled clinical trial. Lasers Med. Sci. 2018, 33, 1933–1940. [Google Scholar] [CrossRef]
- Fuentes-Mera, L.; Camacho, A.; Moncada-Saucedo, N.K.; Peña-Martínez, V. Current Applications of Mesenchymal Stem Cells for Cartilage Tissue Engineering. In Mesenchymal Stem Cells-Isolation, Characterization and Applications; Books on Demand: Norderstedt, Germany, 2017. [Google Scholar] [CrossRef][Green Version]
- Yu, D.-A.; Han, J.; Kim, B.-S. Stimulation of Chondrogenic Differentiation of Mesenchymal Stem Cells. Int. J. Stem Cells 2012, 5, 16–22. [Google Scholar] [CrossRef]
- Gentili/snm, C.; Cancedda, R. Cartilage and Bone Extracellular Matrix. Curr. Pharm. Des. 2009, 15, 1334–1348. [Google Scholar] [CrossRef]
- Khan, W.S.; Tew, S.; Adesida, A.B.; Hardingham, T.E. Human infrapatellar fat pad-derived stem cells express the pericyte marker 3G5 and show enhanced chondrogenesis after expansion in fibroblast growth factor-2. Arthritis Res. Ther. 2008, 10, R74. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Angelozzi, M.; Haseeb, A. SOX9 in cartilage development and disease. Curr. Opin. Cell Biol. 2019, 61, 39–47. [Google Scholar] [CrossRef]
- Quintana, L.; Nieden, N.I.Z.; Semino, C.E. Morphogenetic and Regulatory Mechanisms During Developmental Chondrogenesis: New Paradigms for Cartilage Tissue Engineering. Tissue Eng. Part B Rev. 2009, 15, 29–41. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, S.; Naccarato, T.; Prakash-Damani, M.; Chou, Y.; Chu, C.-Q.; Zhu, Y. Regeneration of hyaline-like cartilage in situ with SOX9 stimulation of bone marrow-derived mesenchymal stem cells. PLoS ONE 2017, 12, e0180138. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Park, K.-H. Regulation and function of SOX9 during cartilage development and regeneration. Semin. Cancer Biol. 2020, 67, 12–23. [Google Scholar] [CrossRef]
- Majumdar, M.K.; Wang, E.; Morris, E.A. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J. Cell. Physiol. 2001, 189, 275–284. [Google Scholar] [CrossRef]
- Zuscik, M.; Ma, L.; Buckley, T.; Puzas, J.E.; Drissi, H.; Schwarz, E.M.; O’Keefe, R.J. Lead Induces Chondrogenesis and Alters Transforming Growth Factor-β and Bone Morphogenetic Protein Signaling in Mesenchymal Cell Populations. Environ. Heal. Perspect. 2007, 115, 1276–1282. [Google Scholar] [CrossRef]
- Kabiri, A.; Esfandiari, E.; Hashemibeni, B.; Kazemi, M.; Mardani, M.; Esmaeili, A. Effects of FGF-2 on human adipose tissue derived adult stem cells morphology and chondrogenesis enhancement in Transwell culture. Biochem. Biophys. Res. Commun. 2012, 424, 234–238. [Google Scholar] [CrossRef]
- Zhou, N.; Hu, N.; Liao, J.-Y.; Lin, L.-B.; Zhao, C.; Si, W.-K.; Yang, Z.; Yi, S.-X.; Fan, T.-X.; Bao, W.; et al. HIF-1α as a Regulator of BMP2-Induced Chondrogenic Differentiation, Osteogenic Differentiation, and Endochondral Ossification in Stem Cells. Cell. Physiol. Biochem. 2015, 36, 44–60. [Google Scholar] [CrossRef]
- Hardingham, T.E.; Oldershaw, R.A.; Tew, S. Cartilage, SOX9 and Notch signals in chondrogenesis. J. Anat. 2006, 209, 469–480. [Google Scholar] [CrossRef]
- Riedl, M.; Witzmann, C.; Koch, M.; Lang, S.; Kerschbaum, M.; Baumann, F.; Krutsch, W.; Docheva, D.; Alt, V.; Pfeifer, C. Attenuation of Hypertrophy in Human MSCs via Treatment with a Retinoic Acid Receptor Inverse Agonist. Int. J. Mol. Sci. 2020, 21, 1444. [Google Scholar] [CrossRef]
- Sitcheran, R.; Cogswell, P.C.; Baldwin, J.A.S. NF- B mediates inhibition of mesenchymal cell differentiation through a posttranscriptional gene silencing mechanism. Genes Dev. 2003, 17, 2368–2373. [Google Scholar] [CrossRef]
- Murakami, S.; Lefebvre, V.; de Crombrugghe, B. Potent Inhibition of the Master Chondrogenic FactorSox9 Gene by Interleukin-1 and Tumor Necrosis Factor-α. J. Biol. Chem. 2000, 275, 3687–3692. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, X.; Jiang, T.; Zheng, L.; Zhao, J.; Zhang, X. Correction: The role of Sox9 in collagen hydrogel-mediated chondrogenic differentiation of adult mesenchymal stem cells (MSCs). Biomater. Sci. 2019, 7, 3926. [Google Scholar] [CrossRef]
- Pan, Q.; Yu, Y.; Chen, Q.; Li, C.; Wu, H.; Wan, Y.; Ma, J.; Sun, F. Sox9, a key transcription factor of bone morphogenetic protein-2-induced chondrogenesis, is activated through BMP pathway and a CCAAT box in the proximal promoter. J. Cell. Physiol. 2008, 217, 228–241. [Google Scholar] [CrossRef]
- Im, G.-I.; Kim, H.-J.; Lee, J.H. Chondrogenesis of adipose stem cells in a porous PLGA scaffold impregnated with plasmid DNA containing SOX trio (SOX-5,-6 and -9) genes. Biomaterials 2011, 32, 4385–4392. [Google Scholar] [CrossRef]
- Moses, H.L.; Roberts, A.B.; Derynck, R. The Discovery and Early Days of TGF-beta: A Historical Perspective. Cold Spring Harb. Perspect. Biol. 2016, 8, a021865. [Google Scholar] [CrossRef]
- David, C.J.; Massagué, J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol. 2018, 19, 419–435. [Google Scholar] [CrossRef]
- Grafe, I.; Alexander, S.; Peterson, J.R.; Snider, T.N.; Levi, B.; Lee, B.; Mishina, Y. TGF-β Family Signaling in Mesenchymal Differentiation. Cold Spring Harb. Perspect. Biol. 2018, 10, a022202. [Google Scholar] [CrossRef]
- Hata, A.; Chen, Y.-G. TGF-β Signaling from Receptors to Smads. Cold Spring Harb. Perspect. Biol. 2016, 8, a022061. [Google Scholar] [CrossRef]
- Thielen, N.G.M.; van der Kraan, P.M.; van Caam, A.P.M. TGFβ/BMP Signaling Pathway in Cartilage Homeostasis. Cells 2019, 9, 969. [Google Scholar] [CrossRef]
- Van Beuningen, H.M.; Van Der Kraan, P.M.; Arntz, O.J.; van den Berg, W.B. In vivo protection against interleukin-1-induced articular cartilage damage by transforming growth factor-beta 1: Age-related differences. Ann. Rheum. Dis. 1994, 53, 593–600. [Google Scholar] [CrossRef]
- Takahashi, N.; Rieneck, K.; van der Kraan, P.; van Beuningen, H.; Vitters, E.; Bendtzen, K.; Berg, W.V.D. Elucidation of IL-1/TGF-β interactions in mouse chondrocyte cell line by genome-wide gene expression. Osteoarthr. Cartil. 2005, 13, 426–438. [Google Scholar] [CrossRef]
- Miyamoto, C.; Matsumoto, T.; Sakimura, K.; Shindo, H. Osteogenic protein-1 with transforming growth factor-β1: Potent inducer of chondrogenesis of synovial mesenchymal stem cells in vitro. J. Orthop. Sci. 2007, 12, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.N.B.; Scharstuhl, A.; Vitters, E.L.; Van Der Kraan, P.M.; Berg, W.B.V.D. Reduced transforming growth factor-beta signaling in cartilage of old mice: Role in impaired repair capacity. Arthritis Res. 2005, 7, R1338–R1347. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.N.B.; Vitters, E.L.; van Lent, P.L.; van de Loo, F.A.; van den Berg, W.B.; van der Kraan, P.M. Elevated extracellular matrix production and degradation upon bone morphogenetic protein-2 (BMP-2) stimulation point toward a role for BMP-2 in cartilage repair and remodeling. Arthritis Res. Ther. 2007, 9, R102–R111. [Google Scholar] [CrossRef]
- Timur, U.T.; Caron, M.; Akker, G.V.D.; Van Der Windt, A.; Visser, J.; Van Rhijn, L.; Weinans, H.; Welting, T.; Emans, P.; Jahr, H. Increased TGF-β and BMP Levels and Improved Chondrocyte-Specific Marker Expression In vitro under Cartilage-Specific Physiological Osmolarity. Int. J. Mol. Sci. 2019, 20, 795. [Google Scholar] [CrossRef] [PubMed]
- Bozhokin, M.S.; Sopova, Y.V.; Kachkin, D.V.; Rubel, A.A.; Khotin, M.G. Mechanisms of TGFβ3 Action as a Therapeutic Agent for Promoting the Synthesis of Extracellular Matrix Proteins in Hyaline Cartilage. Biochemistry (Moscow) 2020, 85, 436–447. [Google Scholar] [CrossRef]
- Shen, B.; Wei, A.; Whittaker, S.; Williams, L.A.; Tao, H.; Ma, D.D.; Diwan, A. The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J. Cell. Biochem. 2009, 109, 406–416. [Google Scholar] [CrossRef]
- Gugjoo, M.B.; Amarpal; Abdelbaset-Ismail, A.; Aithal, H.P.; Kinjavdekar, P.; Pawde, A.M.; Kumar, G.S.; Sharma, G.T. Mesenchymal stem cells with IGF-1 and TGF- β1 in laminin gel for osteochondral defects in rabbits. Biomed. Pharmacother. 2017, 93, 1165–1174. [Google Scholar] [CrossRef]
- Gugjoo, M.B.; Amarpal; Abdelbaset-Ismail, A.; Aithal, H.P.; Kinjavdekar, P.; Kumar, G.S.; Sharma, G.T. Allogeneic mesenchymal stem cells and growth factors in gel scaffold repair osteochondral defect in rabbit. Regen. Med. 2020, 15, 1261–1275. [Google Scholar] [CrossRef]
- Salmon, W.D., Jr.; Daughaday, W.H. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J. Lab. Clin. Med. 1957, 49, 825–836. [Google Scholar] [PubMed]
- Djerbal, L.; Lortat-Jacob, H.; Kwok, J. Chondroitin sulfates and their binding molecules in the central nervous system. Glycoconj. J. 2017, 34, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Bonnevie, E.D.; Puetzer, J.; Bonassar, L.J. Enhanced boundary lubrication properties of engineered menisci by lubricin localization with insulin-like growth factor I treatment. J. Biomech. 2014, 47, 2183–2188. [Google Scholar] [CrossRef]
- Madry, H.; Kaul, G.; Zurakowski, D.; Vunjak-Novakovic, G.; Cucchiarini, M. Cartilage constructs engineered from chondrocytes overexpressing IGF-I improve the repair of osteochondral defects in a rabbit model. Eur. Cells Mater. 2013, 25, 229–247. [Google Scholar] [CrossRef]
- Widowati, W.; Afifah, E.; Mozef, T.; Sandra, F.; Rizal, R.; Amalia, A.; Arinta, Y.; Bachtiar, I.; Murti, H. Effects of insulin-like growth factor-induced Wharton jelly mesenchymal stem cells toward chondrogenesis in an osteoarthritis model. Iran J. Basic Med. Sci. 2018, 21, 745–752. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Q.; Wang, E.; Zhang, C.; Wang, G.; Yuan, Q. Dynamic compression of rabbit adipose-derived stem cells transfected with insulin-like growth factor 1 in chitosan/gelatin scaffolds induces chondrogenesis and matrix biosynthesis. J. Cell. Physiol. 2012, 227, 2003–2012. [Google Scholar] [CrossRef]
- Longobardi, L.; O’Rear, L.; Aakula, S.; Johnstone, B.; Shimer, K.; Chytil, A.; Horton, W.A.; Moses, H.L.; Spagnoli, A. Effect of IGF-I in the Chondrogenesis of Bone Marrow Mesenchymal Stem Cells in the Presence or Absence of TGF-β Signaling. J. Bone Miner. Res. 2005, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Blain, E.J.; Gilbert, S.J.; Caterson, B.; Duance, V.C. The Potential of IGF-1 and TGFβ1 for Promoting “Adult” Articular Cartilage Repair: An In vitro Study. Tissue Eng. Part A 2008, 14, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, L.; Yang, W.; Cao, Y.; Shi, Y.; Li, X.; Zhang, Q. The effects of different doses of IGF-1 on cartilage and subchondral bone during the repair of full-thickness articular cartilage defects in rabbits. Osteoarthr. Cartil. 2017, 25, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Gospodarowicz, D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nat. Cell Biol. 1974, 249, 123–127. [Google Scholar] [CrossRef]
- Tortorella, M.D.; Liu, R.-Q.; Burn, T.; Newton, R.C.; Arner, E. Characterization of human aggrecanase 2 (ADAM-TS5): Substrate specificity studies and comparison with aggrecanase 1 (ADAM-TS4). Matrix Biol. 2002, 21, 499–511. [Google Scholar] [CrossRef]
- Im, H.-J.; Muddasani, P.; Natarajan, V.; Schmid, T.M.; Block, J.; Davis, F.; van Wijnen, A.J.; Loeser, R.F. Basic Fibroblast Growth Factor Stimulates Matrix Metalloproteinase-13 via the Molecular Cross-talk between the Mitogen-activated Protein Kinases and Protein Kinase Cδ Pathways in Human Adult Articular Chondrocytes. J. Biol. Chem. 2007, 282, 11110–11121. [Google Scholar] [CrossRef]
- Xiao, L.; Williams, D.; Hurley, M.M. Inhibition of FGFR Signaling Partially Rescues Osteoarthritis in Mice Overexpressing High Molecular Weight FGF2 Isoforms. Endocrinology 2020, 161, 016. [Google Scholar] [CrossRef]
- Yamamoto, T.; Wakitani, S.; Imoto, K.; Hattori, T.; Nakaya, H.; Saito, M.; Yonenobu, K. Fibroblast growth factor-2 promotes the repair of partial thickness defects of articular cartilage in immature rabbits but not in mature rabbits. Osteoarthr. Cartil. 2004, 12, 636–641. [Google Scholar] [CrossRef]
- Moore, E.; Bendele, A.; Thompson, D.; Littau, A.; Waggie, K.; Reardon, B.; Ellsworth, J. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthr. Cartil. 2005, 13, 623–631. [Google Scholar] [CrossRef]
- Mori, Y.; Saito, T.; Chang, S.H.; Kobayashi, H.; Ladel, C.H.; Guehring, H.; Chung, U.-I.; Kawaguchi, H. Identification of Fibroblast Growth Factor-18 as a Molecule to Protect Adult Articular Cartilage by Gene Expression Profiling. J. Biol. Chem. 2014, 289, 10192–10200. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yi, L.; Zhang, C.; He, Y.; Zhou, L.; Liu, Y.; Qian, L.; Hou, S.; Weng, T. Synergistic Effects of FGF-18 and TGF-β3 on the Chondrogenesis of Human Adipose-Derived Mesenchymal Stem Cells in the Pellet Culture. Stem Cells Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barr, L.; Getgood, A.; Guehring, H.; Rushton, N.; Henson, F.M.D. The effect of recombinant human fibroblast growth factor-18 on articular cartilage following single impact load. J. Orthop. Res. 2014, 32, 923–927. [Google Scholar] [CrossRef]
- Chuma, H.; Mizuta, H.; Kudo, S.; Takagi, K.; Hiraki, Y. One day exposure to FGF-2 was sufficient for the regenerative repair of full-thickness defects of articular cartilage in rabbits. Osteoarthr. Cartil. 2004, 12, 834–842. [Google Scholar] [CrossRef]
- Barry, F.; Boynton, R.E.; Liu, B.; Murphy, M. Chondrogenic Differentiation of Mesenchymal Stem Cells from Bone Marrow: Differentiation-Dependent Gene Expression of Matrix Components. Exp. Cell Res. 2001, 268, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.; Akslen-Hoel, L.K.; Grøndahl, F.; Kjos, I.; Prydz, K. Proteoglycan Synthesis and Golgi Organization in Polarized Epithelial Cells. J. Histochem. Cytochem. 2012, 60, 926–935. [Google Scholar] [CrossRef]
- Burton-Wurster, N.; Liu, W.; Matthews, G.; Lust, G.; Roughley, P.; Glant, T.; Cs-Szabó, G. TGF beta 1 and biglycan, decorin, and fibromodulin metabolism in canine cartilage. Osteoarthr. Cartil. 2003, 11, 167–176. [Google Scholar] [CrossRef]
- Steinert, A.F.; Nöth, U.; Tuan, R.S. Concepts in gene therapy for cartilage repair. Injury 2008, 39 (Suppl. S1), 97–113. [Google Scholar] [CrossRef] [PubMed]
- Trippel, S.B.; Ghivizzani, S.C.; Nixon, A.J. Gene-based approaches for the repair of articular cartilage. Gene Ther. 2004, 11, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Orth, P.; Cucchiarini, M. Gene Therapy for Cartilage Repair. Cartilage 2011, 2, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.P.T.; Brunger, J.M.; Gloss, C.C.; Moutos, F.T.; Gersbach, C.A.; Guilak, F. Genetic Engineering of Mesenchymal Stem Cells for Differential Matrix Deposition on 3D Woven Scaffolds. Tissue Eng. Part A 2018, 24, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Feng, G.; Yan, W. Mesenchymal stem cell-based treatment for cartilage defects in osteoarthritis. Mol. Biol. Rep. 2011, 39, 5683–5689. [Google Scholar] [CrossRef]
- Chen, F.H.; Rousche, K.T.; Tuan, R.S. Technology Insight: Adult stem cells in cartilage regeneration and tissue engineering. Nat. Clin. Pr. Rheumatol. 2006, 2, 373–382. [Google Scholar] [CrossRef]
- Gurusinghe, S.; Strappe, P. Gene Modification of Mesenchymal Stem Cells and Articular Chondrocytes to Enhance Chondrogenesis. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Longo, U.G.; Petrillo, S.; Franceschetti, E.; Berton, A.; Maffulli, N.; Denaro, V. Stem Cells and Gene Therapy for Cartilage Repair. Stem Cells Int. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nita, I.; Ghivizzani, S.C.; Galea-Lauri, J.; Bandara, G.; Georgescu, H.I.; Robbins, P.D.; Evans, C.H. Direct gene delivery to synovium: An evaluation of potential vectors in vitro and in vivo. Arthritis Rheum. 1996, 39, 820–828. [Google Scholar] [CrossRef]
- Madry, H.; Kaul, G.; Cucchiarini, M.; Stein, U.; Zurakowski, D.; Remberger, K.; Menger, M.D.; Kohn, D.; Trippel, S.B. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I). Gene Ther. 2005, 12, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Emkey, G.; Zurakowski, D.; Trippel, S.B. Overexpression of human fibroblast growth factor 2 stimulates cell proliferation in an ex vivo model of articular chondrocyte transplantation. J. Gene Med. 2004, 6, 238–245. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Kitoh, H.; Sugiura, F.; Ishiguro, N. Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2003, 301, 338–343. [Google Scholar] [CrossRef]
- Li, Y.; Tew, S.; Russell, A.M.; Gonzalez, K.R.; Hardingham, T.; Hawkins, R.E. Transduction of Passaged Human Articular Chondrocytes with Adenoviral, Retroviral, and Lentiviral Vectors and the Effects of Enhanced Expression of SOX. Tissue Eng. 2004, 10, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Attur, M.G.; Dave, M.N.; Leung, M.Y.; Cipolletta, C.; Meseck, M.; Woo, S.L.C.; Amin, A.R. Functional Genomic Analysis of Type II IL-1β Decoy Receptor: Potential for Gene Therapy in Human Arthritis and Inflammation. J. Immunol. 2002, 168, 2001–2010. [Google Scholar] [CrossRef]
- Smith, P.; Shuler, F.D.; Georgescu, H.I.; Ghivizzani, S.C.; Johnstone, B.; Niyibizi, C.; Robbins, P.D.; Evans, C.H. Genetic enhancement of matrix synthesis by articular chondrocytes: Comparison of different growth factor genes in the presence and absence of interleukin-1. Arthritis Rheum. 2000, 43, 1156–1164. [Google Scholar] [CrossRef]
- Cucchiarini, M.; Madry, H.; Ma, C.; Thurn, T.; Zurakowski, D.; Menger, M.D.; Kohn, D.; Trippel, S.B.; Terwilliger, E.F. Improved Tissue Repair in Articular Cartilage Defects in vivo by rAAV-Mediated Overexpression of Human Fibroblast Growth Factor 2. Mol. Ther. 2005, 12, 229–238. [Google Scholar] [CrossRef]
- Pagnotto, M.R.; Wang, Z.; Karpie, J.C.; Ferretti, M.; Xiao, X.; Chu, C.R. Adeno-associated viral gene transfer of transforming growth factor-β1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007, 14, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Cucchiarini, M.; Terwilliger, E.F.; Trippel, S.B. Recombinant Adeno-Associated Virus Vectors Efficiently and Persistently Transduce Chondrocytes in Normal and Osteoarthritic Human Articular Cartilage. Hum. Gene Ther. 2003, 14, 393–402. [Google Scholar] [CrossRef]
- Jennings, K.; Miyamae, T.; Traister, R.; Marinov, A.; Katakura, S.; Sowders, D.; Trapnell, B.; Wilson, J.M.; Gao, G.; Hirsch, R. Proteasome Inhibition Enhances AAV-Mediated Transgene Expression in Human Synoviocytes in vitro and in vivo. Mol. Ther. 2005, 11, 600–607. [Google Scholar] [CrossRef]
- Ito, H.; Goater, J.J.; Tiyapatanaputi, P.; Rubery, P.T.; O’Keefe, R.J.; Schwarz, E.M. Light-activated gene transduction of recombinant adeno-associated virus in human mesenchymal stem cells. Gene Ther. 2003, 11, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Makki, M.S.; Akhtar, N.; Haqqi, T.M. An effective and efficient method of transfecting primary human chondrocytes in suspension. Anal. Biochem. 2017, 526, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Bozhokin, M.S.; Bozhkova, S.A.; Naschekina, Y.A.; Sopova, Y.V.; Rubel, A.A.; Khotin, M.G. Transfection of mesenchymal stem cells (msc) for modifying cell culture for recovery hyaline cartilage defects. Mod. Probl. Sci. Educ. 2021, 4, 90. [Google Scholar] [CrossRef]
- Hamann, A.; Nguyen, A.; Pannier, A.K. Nucleic acid delivery to mesenchymal stem cells: A review of nonviral methods and applications. J. Biol. Eng. 2019, 13, 1–16. [Google Scholar] [CrossRef]
- Madry, H.; Trippel, S.B. Efficient lipid-mediated gene transfer to articular chondrocytes. Gene Ther. 2000, 7, 286–291. [Google Scholar] [CrossRef]
- Stöve, J.; Fiedler, J.; Huch, K.; Günther, K.-P.; Puhl, W.; Brenner, R. Lipofection of rabbit chondrocytes and long lasting expression of a lacZ reporter system in alginate beads. Osteoarthr. Cartil. 2002, 10, 212–217. [Google Scholar] [CrossRef][Green Version]
- Goomer, R.; Deftos, L.; Terkeltaub, R.; Maris, T.; Lee, M.; Harwood, F.; Amiel, D. High-efficiency non-viral transfection of primary chondrocytes and perichondrial cells for ex-vivo gene therapy to repair articular cartilage defects. Osteoarthr. Cartil. 2001, 9, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Madry, H. Genetic modification of mesenchymal stem cells for cartilage repair. Bio-Medical Mater. Eng. 2010, 20, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Merchan, E.C.; Valentino, L.A. The role of gene therapy for cartilage repair. Arch. Bone Jt. Surg. 2018, 7, 79–90. [Google Scholar]
- Lee, B.; Parvizi, J.; Bramlet, D.; Romness, D.W.; Guermazi, A.; Noh, M.; Sodhi, N.; Khlopas, A.; Mont, M.A. Results of a Phase II Study to Determine the Efficacy and Safety of Genetically Engineered Allogeneic Human Chondrocytes Expressing TGF-β1. J. Knee Surg. 2019, 33, 167–172. [Google Scholar] [CrossRef]
- Ha, C.-W.; Noh, M.J.; Choi, K.B.; Lee, K.H. Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy 2012, 14, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Tanikella, A.; Hardy, M.J.; Frahs, S.M.; Cormier, A.G.; Gibbons, K.D.; Fitzpatrick, C.K.; Oxford, J.T. Emerging Gene-Editing Modalities for Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 6046. [Google Scholar] [CrossRef]
- Farhang, M.N.; Davis, B.; Weston, J.; Ginley-Hidinger, M.; Gertz, J.; Bowles, R.D. Synergistic CRISPRa-Regulated Chondrogenic Extracellular Matrix Deposition Without Exogenous Growth Factors. Tissue Eng. Part A 2020, 26, 1169–1179. [Google Scholar] [CrossRef]
- Huynh, N.P.; Gloss, C.C.; Lorentz, J.; Tang, R.; Brunger, J.M.; McAlinden, A.; Zhang, B.; Guilak, F. Long non-coding RNA GRASLND enhances chondrogenesis via suppression of the interferon type II signaling pathway. eLife 2020, 9, e49558. [Google Scholar] [CrossRef]
- Nishimasu, H.; Shi, X.; Ishiguro, S.; Gao, L.Y.; Hirano, S.; Okazaki, S.; Noda, T.; Abudayyeh, O.O.; Gootenberg, J.S.; Mori, H.; et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science 2018, 361, 1259–1262. [Google Scholar] [CrossRef]
- Ahmed, T.; Hincke, M. Mesenchymal stem cell—Based tissue engineering strategies for repair of articular cartilage. Histol. Histopathol. 2014, 29, 669–689. [Google Scholar] [CrossRef] [PubMed]
- Le, H.; Xu, W.; Zhuang, X.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal stem cells for cartilage regeneration. J. Tissue Eng. 2020, 11, 2041731420943839. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; He, Z.; Zhang, X.; Hu, X.; Yuan, L.; Qiang, M.; Zhu, J.; Shao, Z.; Zhou, C.; Ao, Y. One-Step Repair for Cartilage Defects in a Rabbit Model. Am. J. Sports Med. 2014, 42, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Hu, X.; Huang, H.; Liu, Z.; Yuan, L.; Shao, Z.; Jiang, Y.; Zhang, J.; Fu, X.; Duan, X.; et al. Microfracture combined with functional pig peritoneum-derived acellular matrix for cartilage repair in rabbit models. Acta Biomater. 2017, 53, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Shen, X.; Sun, X.; Yin, H.; Yang, S.; Lu, C.; Wang, Y.; Liu, Y.; Huang, Y.; Yang, Z.; et al. Increased recruitment of endogenous stem cells and chondrogenic differentiation by a composite scaffold containing bone marrow homing peptide for cartilage regeneration. Theranostics 2018, 8, 5039–5058. [Google Scholar] [CrossRef]
- Qiao‡, Z.; Tang‡, J.; Yue, B.; Wang, J.; Zhang, J.; Xuan, L.; Dai, C.; Li, S.; Li, M.; Xu, C.; et al. Human adipose-derived mesenchymal progenitor cells plus microfracture and hyaluronic acid for cartilage repair: A Phase IIa trial. Regen. Med. 2020, 15, 1193–1214. [Google Scholar] [CrossRef]
- Toan, D.D.; Binh, N.T.; Dung, T.T.; Thuy, L.Q.; Hoa, N.D.; Long, N.H.; Tung, P.S. The effectiveness of knee osteoarthritis treatment by arthroscopic microfracture technique in combination with autologous bone marrow stem cells transplantation. J. Back Musculoskelet. Rehabil. 2020, 33, 397–403. [Google Scholar] [CrossRef]
- Volz, M.; Schaumburger, J.; Frick, H.; Grifka, J.; Anders, S. A randomized controlled trial demonstrating sustained benefit of Autologous Matrix-Induced Chondrogenesis over microfracture at five years. Int. Orthop. 2017, 41, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, B.; Laflin, A.D.; Detamore, M.S. Generating Chondromimetic Mesenchymal Stem Cell Spheroids by Regulating Media Composition and Surface Coating. Cell. Mol. Bioeng. 2017, 11, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkova, A.V.; Vakhrushev, I.V.; Basok, Y.B.; Grigor’Ev, A.M.; Kirsanova, L.A.; Lupatov, A.Y.; Sevastianov, V.I.; Yarygin, K.N. Chondrogeneic Potential of MSC from Different Sources in Spheroid Culture. Bull. Exp. Biol. Med. 2021, 170, 528–536. [Google Scholar] [CrossRef]
- Le, H.T.N.; Nguyen, P.; Dao, T.; To, X.; Pham, P.V. Production of engineered cartilage from mesenchymal stem cell spheroids. Front. Biosci. Landmark 2021, 26, 266–285. [Google Scholar] [CrossRef]
- Tu, V.T.-K.; Le, H.T.-N.; To, X.H.-V.; Nguyen, P.D.-N.; Huynh, P.D.; Le, T.M.; Vu, N.B. Method for in vitro production of cartilage microtissues from scaffold-free spheroids composed of human adipose-derived stem cells. Biomed. Res. Ther. 2020, 7, 3697–3708. [Google Scholar] [CrossRef]
- Kondo, S.; Nakagawa, Y.; Mizuno, M.; Katagiri, K.; Tsuji, K.; Kiuchi, S.; Ono, H.; Muneta, T.; Koga, H.; Sekiya, I. Transplantation of Aggregates of Autologous Synovial Mesenchymal Stem Cells for Treatment of Cartilage Defects in the Femoral Condyle and the Femoral Groove in Microminipigs. Am. J. Sports Med. 2019, 47, 2338–2347. [Google Scholar] [CrossRef]
- Dao, T.T.-T.; Vu, N.B.; Pham, L.H.; Van Gia, L.; Le, H.T.-N.; Phi, L.T.; Bui, K.H.-T.; Le, P.T.-B.; Van Pham, P. In vitro Production of Cartilage Tissue from Rabbit Bone Marrow-Derived Mesenchymal Stem Cells and Polycaprolactone Scaffold. Evol. Syst. Biol. 2017, 1084, 45–60. [Google Scholar] [CrossRef]
- Agrawal, P.; Pramanik, K.; Biswas, A. Chondrogenic differentiation of mesenchymal stem cells on silk fibroin:chitosan–glucosamine scaffold in dynamic culture. Regen. Med. 2018, 13, 545–558. [Google Scholar] [CrossRef]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H.; et al. Structurally and Functionally Optimized Silk-Fibroin-Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In vitro and In vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, H.; Shen, H.; Wang, B.; Lei, G.; Tuan, R.S. Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype of and cartilage formation by encapsulated chondrocytes in vitro and in vivo. Acta Biomater. 2018, 69, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, K.; Ramesh, S.; Walter, N.M.; Arora, A.; Katti, D.S.; Madhuri, V. In vivo cartilage regeneration in a multi-layered articular cartilage architecture mimicking scaffold. Bone Jt. Res. 2020, 9, 601–612. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Sun, X.; Liu, D.; Huang, C.; Wu, J.; Yang, C.; Zhang, Q. Biomimetic cartilage scaffold with orientated porous structure of two factors for cartilage repair of knee osteoarthritis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Brittberg, M.; Busacca, M.; Condello, V.; Engebretsen, L.; Marlovits, S.; Niemeyer, P.; Platzer, P.; Posthumus, M.; et al. A multilayer biomaterial for osteochondral regeneration shows superiority vs microfractures for the treatment of osteochondral lesions in a multicentre randomized trial at 2 years. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2704–2715. [Google Scholar] [CrossRef]
- Wolf, M.T.; Zhang, H.; Sharma, B.; Marcus, N.A.; Pietzner, U.; Fickert, S.; Lueth, A.; Albers, G.H.R.; Elisseeff, J.H. Two-Year Follow-Up and Remodeling Kinetics of ChonDux Hydrogel for Full-Thickness Cartilage Defect Repair in the Knee. Cartilage 2018, 11, 447–457. [Google Scholar] [CrossRef]
- Schneider, C.; Dungel, P.; Priglinger, E.; Danzer, M.; Schädl, B.; Nürnberger, S. The impact of photobiomodulation on the chondrogenic potential of adipose-derived stromal/stem cells. J. Photochem. Photobiol. B Biol. 2021, 221, 112243. [Google Scholar] [CrossRef] [PubMed]
- Dusfour, G.; Maumus, M.; Cañadas, P.; Ambard, D.; Jorgensen, C.; Noël, D.; Le Floc’H, S. Mesenchymal stem cells-derived cartilage micropellets: A relevant in vitro model for biomechanical and mechanobiological studies of cartilage growth. Mater. Sci. Eng. C 2020, 112, 110808. [Google Scholar] [CrossRef] [PubMed]
- Shahmoradi, S.R.; Salmani, M.K.; Soleimanpour, H.R.; Tavakoli, A.H.; Hosaini, K.; Haghighipour, N.; Bonakdar, S. Induction of Chondrogenic Differentiation in Human Mesenchymal Stem Cells Cultured on Human Demineralized Bone Matrix Scaffold under Hydrostatic Pressure. Tissue Eng. Regen. Med. 2019, 16, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Eslaminejad, M.B.; Shayan, A.M.; Kalhori, K.A.; Abbas, F.M.; Taghiyar, L.; Pedram, M.S.; Ghuchani, M.S. Effects of Photobiomodulation and Mesenchymal Stem Cells on Articular Cartilage Defects in a Rabbit Model. Photomed. Laser Surg. 2016, 34, 543–549. [Google Scholar] [CrossRef]
- Cheng, B.; Tu, T.; Shi, X.; Liu, Y.; Zhao, Y.; Zhao, Y.; Li, Y.; Chen, H.; Chen, Y.; Zhang, M. A novel construct with biomechanical flexibility for articular cartilage regeneration. Stem Cell Res. Ther. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Pattappa, G.; Krueckel, J.; Schewior, R.; Franke, D.; Mench, A.; Koch, M.; Weber, J.; Lang, S.; Pfeifer, C.G.; Johnstone, B.; et al. Physioxia Expanded Bone Marrow Derived Mesenchymal Stem Cells Have Improved Cartilage Repair in an Early Osteoarthritic Focal Defect Model. Biology 2020, 9, 230. [Google Scholar] [CrossRef]
- Branly, T.; Bertoni, L.; Contentin, R.; Rakic, R.; Gomez-Leduc, T.; Desancé, M.; Hervieu, M.; Legendre, F.; Jacquet, S.; Audigié, F.; et al. Characterization and use of Equine Bone Marrow Mesenchymal Stem Cells in Equine Cartilage Engineering. Study of their Hyaline Cartilage Forming Potential when Cultured under Hypoxia within a Biomaterial in the Presence of BMP-2 and TGF-ß1. Stem Cell Rev. Rep. 2017, 13, 611–630. [Google Scholar] [CrossRef]
- Ferraresi, C.; Freire, F.; Hamblin, M.R. Photobiomodulation in Cartilage: In vitro, in vivo, and Clinical Trials. In Low-Level Light Therapy: Photobiomodulation; SPIE eBooks: Bellingham, WA, USA, 2018. [Google Scholar] [CrossRef]
- Monaco, G.; El Haj, A.J.; Alini, M.; Stoddart, M.J. Sodium Hyaluronate Supplemented Culture Media as a New hMSC Chondrogenic Differentiation Media-Model for in vitro/ex vivo Screening of Potential Cartilage Repair Therapies. Front. Bioeng. Biotechnol. 2020, 8, 243. [Google Scholar] [CrossRef]
- Armakolas, N.; Dimakakos, A.; Armakolas, A.; Antonopoulos, A.; Koutsilieris, M. Possible role of the Ec peptide of IGF-1Ec in cartilage repair. Mol. Med. Rep. 2016, 14, 3066–3072. [Google Scholar] [CrossRef][Green Version]
- Gibson, J.D.; O’Sullivan, M.B.; Alaee, F.; Paglia, D.N.; Yoshida, R.; Guzzo, R.M.; Drissi, H. Regeneration of Articular Cartilage by Human ESC-Derived Mesenchymal Progenitors Treated Sequentially with BMP-2 and Wnt5a. Stem Cells Transl. Med. 2016, 6, 40–50. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, S.; Liang, Y.; Liu, Q. Combination of kartogenin and transforming growth factor-β3 supports synovial flu-id-derived mesenchymal stem cell-based cartilage regeneration. Am. J. Transl. Res. 2019, 11, 2056–2069. [Google Scholar]
- Fu, H.; Wang, H.; Li, D. BMP-7 accelerates the differentiation of rabbit mesenchymal stem cells into cartilage through the Wnt/β-catenin pathway. Exp. Ther. Med. 2017, 14, 5424–5428. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, H.; Hu, X.; Zhang, X.; Duan, X.; Zhang, J.; Fu, X.; Dai, L.; Yuan, L.; Zhou, C.; Ao, Y. Codelivery of Synovium-Derived Mesenchymal Stem Cells and TGF-β by a Hybrid Scaffold for Cartilage Regeneration. ACS Biomater. Sci. Eng. 2019, 5, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Pike, M.C.; Jonas, B.L.; Kissin, E.; Krop, J.; McAlindon, T. Phase 1 safety and tolerability study of BMP-7 in symptomatic knee osteoarthritis. BMC Musculoskelet. Disord. 2010, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Frisch, J.; Rey-Rico, A.; Venkatesan, J.K.; Schmitt, G.; Madry, H.; Lin, J.; Cucchiarini, M. Co-overexpression of TGF-β and SOX9 via rAAV gene transfer modulates the metabolic and chondrogenic activities of human bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Han, R.; He, C.; Wang, G.; Wang, J.; Zheng, J.; Pei, M.; Wei, L. Demineralized Bone Matrix Combined Bone Marrow Mesenchymal Stem Cells, Bone Morphogenetic Protein-2 and Transforming Growth Factor-β3 Gene Promoted Pig Cartilage Defect Repair. PLoS ONE 2014, 9, e116061. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).