Biological Planning of Radiation Dose Based on In Vivo Dosimetry for Postoperative Vaginal-Cuff HDR Interventional Radiotherapy (Brachytherapy)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Vaginal Brachytherapy
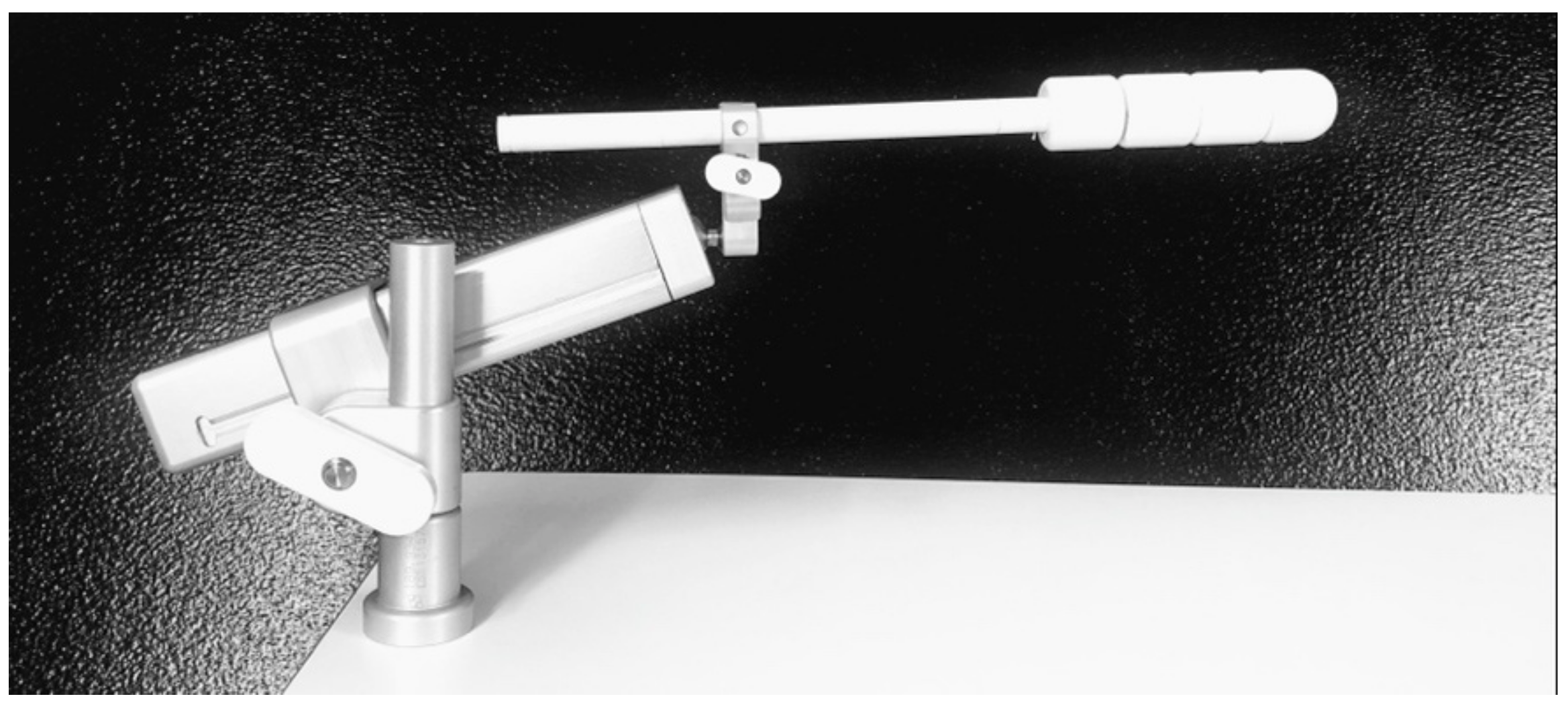
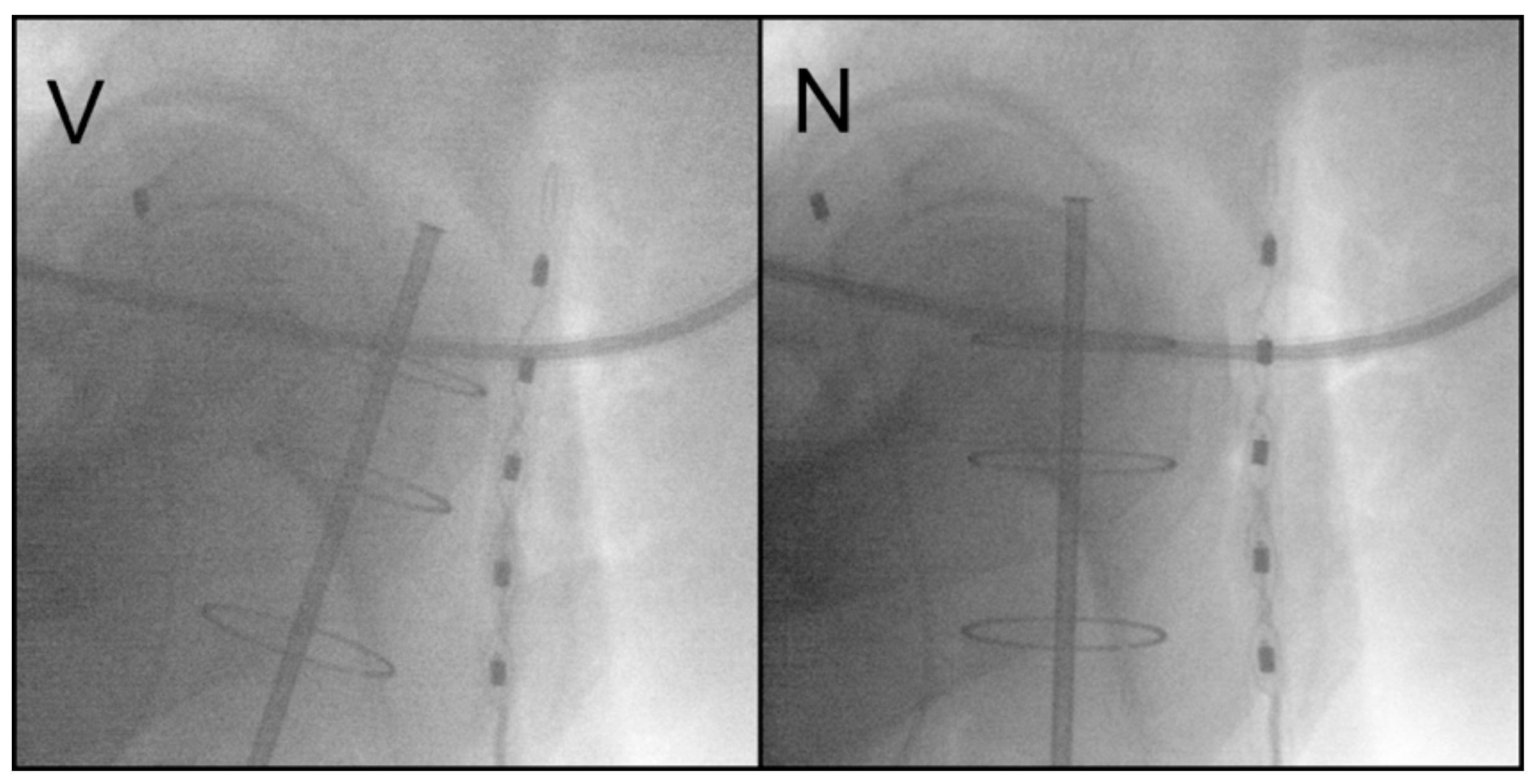
2.3. In Vivo Dosimetry
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Acute and Late Radiation Toxicities
3.2. Treatment Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Nout, R.; Smit, V.; Putter, H.; Jürgenliemk-Schulz, I.; Jobsen, J.; Lutgens, L.; van der Steen-Banasik, E.; Mens, J.; Slot, A.; Kroese, M.S.; et al. Vaginal Brachytherapy versus Pelvic External Beam Radiotherapy for Patients with Endometrial Cancer of High-Intermediate Risk (PORTEC-2): An Open-Label, Non-Inferiority, Randomised Trial. Lancet 2010, 375, 816–823. [Google Scholar] [CrossRef]
- Small, W.; Beriwal, S.; Demanes, D.J.; Dusenbery, K.E.; Eifel, P.; Erickson, B.; Jones, E.; Rownd, J.J.; Santos, J.F.D.L.; Viswanathan, A.N.; et al. American Brachytherapy Society Consensus Guidelines for Adjuvant Vaginal Cuff Brachytherapy after Hysterectomy. Brachytherapy 2012, 11, 58–67. [Google Scholar] [CrossRef]
- Jin, M.; Hou, X.; Sun, X.; Zhang, Y.; Hu, K.; Zhang, F. Impact of Different Adjuvant Radiotherapy Modalities on Women with Early-Stage Intermediate- to High-Risk Endometrial Cancer. Int. J. Gynecol. Cancer 2019, 29, 1264–1270. [Google Scholar] [CrossRef]
- Nout, R.A.; Putter, H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.H.W.; van der Steen-Banasik, E.M.; Mens, J.W.M.; Slot, A.; Kroese, M.C.S.; van Bunningen, B.N.F.M.; et al. Quality of Life After Pelvic Radiotherapy or Vaginal Brachytherapy for Endometrial Cancer: First Results of the Randomized PORTEC-2 Trial. J. Clin. Oncol. 2009, 27, 3547–3556. [Google Scholar] [CrossRef] [PubMed]
- Sorbe, B.G.; Horvath, G.; Andersson, H.; Boman, K.; Lundgren, C.; Pettersson, B. External Pelvic and Vaginal Irradiation Versus Vaginal Irradiation Alone as Postoperative Therapy in Medium-Risk Endometrial Carcinoma: A Prospective, Randomized Study—Quality-of-Life Analysis. Int. J. Gynecol. Cancer 2012, 22, 1281–1288. [Google Scholar] [CrossRef]
- Lancellotta, V.; de Felice, F.; Vicenzi, L.; Antonacci, A.; Cerboneschi, V.; Costantini, S.; di Cristino, D.; Tagliaferri, L.; Cerrotta, A.; Vavassori, A.; et al. The Role of Vaginal Brachytherapy in Stage I Endometrial Serous Cancer: A Systematic Review. J. Contemp. Brachytherapy 2020, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- de Felice, F.; Lancellotta, V.; Vicenzi, L.; Costantini, S.; Antonacci, A.; Cerboneschi, V.; di Cristino, D.; Tagliaferri, L.; Cerrotta, A.; Vavassori, A.; et al. Adjuvant Vaginal Interventional Radiotherapy in Early-Stage Non-Endometrioid Carcinoma of Corpus Uteri: A Systematic Review. J. Contemp. Brachytherapy 2021, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Lachance, J.A.; Stukenborg, G.J.; Schneider, B.F.; Rice, L.W.; Jazaeri, A.A. A Cost-Effective Analysis of Adjuvant Therapies for the Treatment of Stage I Endometrial Adenocarcinoma. Gynecol. Oncol. 2008, 108, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Suidan, R.S.; He, W.; Sun, C.C.; Zhao, H.; Smith, G.L.; Klopp, A.H.; Fleming, N.D.; Lu, K.H.; Giordano, S.H.; Meyer, L.A. National Trends, Outcomes, and Costs of Radiation Therapy in the Management of Low- and High-Intermediate Risk Endometrial Cancer. Gynecol. Oncol. 2019, 152, 439. [Google Scholar] [CrossRef]
- Autorino, R.; Tagliaferri, L.; Campitelli, M.; Smaniotto, D.; Nardangeli, A.; Mattiucci, G.C.; Macchia, G.; Gui, B.; Miccò, M.; Mascilini, F.; et al. EROS Study: Evaluation between High-Dose-Rate and Low-Dose-Rate Vaginal Interventional Radiotherapy (Brachytherapy) in Terms of Overall Survival and Rate of Stenosis. J. Contemp. Brachytherapy 2018, 10, 315. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Endometrial Carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Yashar, C.M.; Bradley, K.; Campos, S.M.; Chino, J.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; Damast, S.; et al. NCCN Guidelines® Insights: Uterine Neoplasms, Version 3.2021: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2021, 19, 888–895. [Google Scholar] [CrossRef]
- Emons, G.; Steiner, E.; Vordermark, D.; Uleer, C.; Bock, N.; Paradies, K.; Ortmann, O.; Aretz, S.; Mallmann, P.; Kurzeder, C.; et al. Interdisciplinary Diagnosis, Therapy and Follow-up of Patients with Endometrial Cancer. Guideline (S3-Level, AWMF Registry Number 032/034-OL, April 2018)—Part 2 with Recommendations on the Therapy and Follow-up of Endometrial Cancer, Palliative Care, Psycho-Oncological/Psychosocial Care/Rehabilitation/Patient Information and Healthcare Facilities. Geburtshilfe Frauenheilkd. 2018, 78, 1089. [Google Scholar] [CrossRef] [Green Version]
- Choo, J.J.; Scudiere, J.; Bitterman, P.; Dickler, A.; Gown, A.M.; Zusag, T.W. Vaginal Lymphatic Channel Location and Its Implication for Intracavitary Brachytherapy Radiation Treatment. Brachytherapy 2005, 4, 236–240. [Google Scholar] [CrossRef]
- Fonseca, G.P.; Johansen, J.G.; Smith, R.L.; Beaulieu, L.; Beddar, S.; Kertzscher, G.; Verhaegen, F.; Tanderup, K. In Vivo Dosimetry in Brachytherapy: Requirements and Future Directions for Research, Development, and Clinical Practice. Phys. Imaging Radiat. Oncol. 2020, 16, 1. [Google Scholar] [CrossRef]
- Chassagne, D.; Dutreix, A.; Almond, P.; Burgers, J.M.V.; Busch, M.; Joslin, C.A. 3. Recommendations for Reporting Absorbed Doses and Volumes in Intracavitary Therapy. Rep. Int. Comm. Radiat. Units Meas. 2019, os-20, 9–14. [Google Scholar] [CrossRef]
- Aalders, J.; Abeler, V.; Kolstad, P.; Onsrud, M. Postoperative External Irradiation and Prognostic Parameters in Stage I Endometrial Carcinoma: Clinical and Histopathologic Study of 540 Patients—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/6999399/ (accessed on 22 September 2021).
- Sorbe, B.G.; Horvath, G.; Andersson, H.; Boman, K.; Lundgren, C.; Pettersson, B. External Pelvic and Vaginal Irradiation Versus Vaginal Irradiation Alone as Postoperative Therapy in Medium-Risk Endometrial Carcinoma—A Prospective Randomized Study. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Sorbe, B.G.; Smeds, A.-C. Postoperative Vaginal Irradiation with High Dose Rate Afterloading Technique in Endometrial Carcinoma Stage I. Int. J. Radiat. Oncol. Biol. Phys. 1990, 18, 305–314. [Google Scholar] [CrossRef]
- Townamchai, K.; Lee, L.; Viswanathan, A.N. A Novel Low Dose Fractionation Regimen for Adjuvant Vaginal Brachytherapy in Early Stage Endometrial Cancer. Gynecol. Oncol. 2012, 127, 351. [Google Scholar] [CrossRef] [Green Version]
- Rovirosa, Á.; Ascaso, C.; Herreros, A.; Sánchez, J.; Holub, K.; Camarasa, A.; Sabater, S.; Oses, G.; García-Miguel, J.; Rivera, Y.; et al. A New Short Daily Brachytherapy Schedule in Postoperative Endometrial Carcinoma. Preliminary Results. Brachytherapy 2017, 16, 147–152. [Google Scholar] [CrossRef]
- Rovirosa, A.; Ascaso, C.; Arenas, M.; Sabater, S.; Herreros, A.; Camarasa, A.; Rios, I.; Holub, K.; Pahisa, J.; Biete, A. Can We Shorten the Overall Treatment Time in Postoperative Brachytherapy of Endometrial Carcinoma? Comparison of Two Brachytherapy Schedules. Radiother. Oncol. 2015, 116, 143–148. [Google Scholar] [CrossRef]
- Ríos, I.; Rovirosa, A.; Ascaso, C.; Valduvieco, I.; Herreros, A.; Castilla, L.; Sabater, S.; Holub, K.; Pahisa, J.; Biete, A.; et al. Vaginal-Cuff Control and Toxicity Results of a Daily HDR Brachytherapy Schedule in Endometrial Cancer Patients. Clin. Transl. Oncol. 2015, 18, 925–930. [Google Scholar] [CrossRef]
- Wortman, B.G.; Astreinidou, E.; Laman, M.S.; van der Steen-Banasik, E.M.; Lutgens, L.C.H.W.; Westerveld, H.; Koppe, F.; Slot, A.; van den Berg, H.A.; Nowee, M.E.; et al. Brachytherapy Quality Assurance in the PORTEC-4a Trial for Molecular-Integrated Risk Profile Guided Adjuvant Treatment of Endometrial Cancer. Radiother. Oncol. 2021, 155, 160–166. [Google Scholar] [CrossRef]
- Russo, J.K.; Armeson, K.E.; Richardson, S. Comparison of 2D and 3D Imaging and Treatment Planning for Postoperative Vaginal Apex High-Dose Rate Brachytherapy for Endometrial Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e75–e80. [Google Scholar] [CrossRef]
- Sabater, S.; Andres, I.; Lopez-Honrubia, V.; Berenguer, R.; Sevillano, M.; Jimenez-Jimenez, E.; Rovirosa, A.; Arenas, M. Vaginal Cuff Brachytherapy in Endometrial Cancer—A Technically Easy Treatment? Cancer Manag. Res. 2017, 9, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Aref, I.; Walker, E.; Movsas, B. Effects of Prescription Depth, Cylinder Size, Treatment Length, Tip Space, and Curved End on Doses in High-Dose-Rate Vaginal Brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Belley, M.D.; Craciunescu, O.; Chang, Z.; Langloss, B.W.; Stanton, I.N.; Yoshizumi, T.T.; Therien, M.J.; Chino, J.P. Real-Time Dose-Rate Monitoring with Gynecologic Brachytherapy: Results of an Initial Clinical Trial. Brachytherapy 2018, 17, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Romanyukha, A.; Carrara, M.; Mazzeo, D.; Tenconi, C.; Al-Salmani, T.; Poder, J.; Cutajar, D.; Fuduli, I.; Petasecca, M.; Bucci, J.; et al. An Innovative Gynecological HDR Brachytherapy Applicator System for Treatment Delivery and Real-Time Verification. Phys. Med. 2019, 59, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Jamalludin, Z.; Jong, W.L.; Abdul Malik, R.; Rosenfeld, A.; Ung, N.M. Characterization of MOSkin Detector for in Vivo Dose Verification during Cobalt-60 High Dose-Rate Intracavitary Brachytherapy. Phys. Med. 2019, 58, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Carrara, M.; Romanyukha, A.; Tenconi, C.; Mazzeo, D.; Cerrotta, A.; Borroni, M.; Cutajar, D.; Petasecca, M.; Lerch, M.; Bucci, J.; et al. Clinical Application of MOSkin Dosimeters to Rectal Wall in Vivo Dosimetry in Gynecological HDR Brachytherapy. Phys. Med. 2017, 41, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Jamalludin, Z.; Malik, R.A.; Ung, N.M. Correlation Analysis of CT-Based Rectal Planning Dosimetric Parameters with in Vivo Dosimetry of MOSkin and PTW 9112 Detectors in Co-60 Source HDR Intracavitary Cervix Brachytherapy. Phys. Eng. Sci. Med. 2021, 44, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Jamalludin, Z.; Jong, W.L.; Malik, R.A.; Rosenfeld, A.B.; Ung, N.M. Evaluation of Rectal Dose Discrepancies between Planned and in Vivo Dosimetry Using MOSkin Detector and PTW 9112 Semiconductor Probe during 60Co HDR CT-Based Intracavitary Cervix Brachytherapy. Phys. Med. 2020, 69, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Poder, J.; Howie, A.; Brown, R.; Bucci, J.; Rosenfeld, A.; Enari, K.; Schreiber, K.; Carrara, M.; Bece, A.; Malouf, D.; et al. Towards Real Time In-Vivo Rectal Dosimetry during Trans-Rectal Ultrasound Based High Dose Rate Prostate Brachytherapy Using MOSkin Dosimeters. Radiother. Oncol. 2020, 151, 273–279. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | ||
|---|---|---|
| Age | Median (range) | 66 (44–87) |
| BMI | Mean (±SD) | 29.9 (±7.8) |
| Menopause | Premenopause | 8 (6.6%) |
| Postmenopause | 113 (93.4%) | |
| Histology | Endometrioid adenocarcinoma | 114 (94.3%) |
| Serous adenocarcinoma | 5 (4.1%) | |
| Clear cell carcinoma | 1 (0.8%) | |
| Undifferentiated carcinoma | 1 (0.8%) | |
| Grading | Grade 1 | 47 |
| Grade 2 | 44 | |
| Grade 3 | 30 | |
| Lymphovascular space invasion | No | 105 (86.8%) |
| Focal | 16 (13.2%) | |
| Substantial | 0 (0%) | |
| Lymphadenectomy | Yes | 100 (82.7%) |
| No | 21 (17.3%) | |
| Myometrial invasion | No | 24 (19.8%) |
| Less than 50% | 45 (37.2%) | |
| More than 50% | 52 (43%) | |
| T-stage | pT1a | 69 (57%) |
| pT1b | 40 (33.1%) | |
| pT2 | 12 (9.9%) | |
| N-stage | Nx | 17 (14%) |
| cN0 | 4 (3.3%) | |
| pN0 | 99 (81.8%) | |
| N1 | 1 (0.8%) | |
| FIGO clinical stage | Stage IA | 69 (57%) |
| Stage IB | 39 (32.2%) | |
| Stage II | 12 (9.9%) | |
| Stage IIIc | 1 (0.8%) | |
| Group A (No. 82) | Group B (No. 39) | All Patients (No.121) | |
|---|---|---|---|
| Dose prescribed to vaginal surface | 54 (65.9%) | 16 (41%) | 70 (57.9%) |
| Dose prescribed to 5 mm depth | 11 (13.4%) | 7 (17.9%) | 18 (14.9%) |
| Dose prescription changed | 17 (20.7%) | 16 (41%) | 33 (27.3%) |
| Group A (No. 82) | Group B (No. 39) | |||
|---|---|---|---|---|
| Mean rectal dose/fraction | Mean rectal total dose | Mean rectal dose/fraction | Mean rectal total dose | |
| Dose prescribed to vaginal surface | 3.9 Gy (±0.5) | 11.5 Gy (±1.6) | 2.7 Gy (±0.5) | 10.6 Gy (±2.1) |
| Dose prescribed to 5 mm depth | 4.1 Gy (±0.4) | 12.4 Gy (±1.1) | 3.2 Gy (±0.5) | 12.8 Gy (±2.1) |
| Dose prescription changed | 4.3 Gy (±0.5) | 12.9 Gy (±1.4) | 2.2 Gy (±0.6) | 9.7 Gy (±2.2) |
| Mean bladder dose/fraction | Mean bladder total dose | Mean bladder dose/fraction | Mean bladder total dose | |
| Dose prescribed to vaginal surface | 2.6 Gy (±0.5) | 7.6 Gy (±1.6) | 1.9 Gy (±0.4) | 7.9 Gy (±2.1) |
| Dose prescribed to 5 mm depth | 2.9 Gy (±0.6) | 8.8 Gy (±1.9) | 2.3 Gy (±0.4) | 9.2 Gy (±1.4) |
| Dose prescription changed | 2.7 Gy (±0.6) | 8.1 Gy (±1.6) | 2.1 Gy (±0.5) | 8.2 Gy (±1.9) |
| Group A (No. 82) | Group B (No. 39) | p | ||
|---|---|---|---|---|
| Acute | GIT | 10 (12.2%) | 4 (10.3%) | 0.6 |
| Urinary | 26 (31.7%) | 5 (12.8%) | 0.02 | |
| Vaginal | 15 (18.3%) | 6 (15.4%) | 0.8 | |
| Late | GIT | 5 (6.1%) | 2 (5.1%) | 0.4 |
| Urinary | 14 (17.1%) | 2 (5.1%) | 0.7 | |
| Vaginal | 15 (18.3%) | 8 (20.5%) | 0.6 |
| Mean Rectal Dose/Fraction | Mean Rectal Total Dose | |||||
|---|---|---|---|---|---|---|
| ≤3 Gy (no. = 28) | ˃3 Gy (no. = 84) | p | ≤12 Gy (no. = 65) | ˃12 Gy (no. = 47) | p | |
| Acute GIT toxicities | 2 (7.1%) | 10 (13.1%) | 0.6 | 8 (12.3%) | 7 (12.8%) | 0.9 |
| Late GIT toxicities | 1 (3.5%) | 6 (7.1) | NA | 1 (4.6%) | 4 (8.5%) | NA |
| Mean bladder dose/fraction | Mean bladder total dose | |||||
| ≤2.5 Gy (no. = 46) | ˃2.5 Gy (no. = 45) | p | ≤7.5 Gy (no. = 45) | ˃7.5 Gy (no. = 46) | p | |
| Acute urinary toxicities | 9 (19.6%) | 15 (33.3%) | 0.04 | 11 (24.4%) | 13 (28.3%) | 0.9 |
| Late urinary toxicities | 3 (6.5%) | 7 (15.6%) | 0.04 | 3 (6.6%) | 7 (15.2%) | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soror, T.; Chafii, R.; Lancellotta, V.; Tagliaferri, L.; Kovács, G. Biological Planning of Radiation Dose Based on In Vivo Dosimetry for Postoperative Vaginal-Cuff HDR Interventional Radiotherapy (Brachytherapy). Biomedicines 2021, 9, 1629. https://doi.org/10.3390/biomedicines9111629
Soror T, Chafii R, Lancellotta V, Tagliaferri L, Kovács G. Biological Planning of Radiation Dose Based on In Vivo Dosimetry for Postoperative Vaginal-Cuff HDR Interventional Radiotherapy (Brachytherapy). Biomedicines. 2021; 9(11):1629. https://doi.org/10.3390/biomedicines9111629
Chicago/Turabian StyleSoror, Tamer, Ramin Chafii, Valentina Lancellotta, Luca Tagliaferri, and György Kovács. 2021. "Biological Planning of Radiation Dose Based on In Vivo Dosimetry for Postoperative Vaginal-Cuff HDR Interventional Radiotherapy (Brachytherapy)" Biomedicines 9, no. 11: 1629. https://doi.org/10.3390/biomedicines9111629
APA StyleSoror, T., Chafii, R., Lancellotta, V., Tagliaferri, L., & Kovács, G. (2021). Biological Planning of Radiation Dose Based on In Vivo Dosimetry for Postoperative Vaginal-Cuff HDR Interventional Radiotherapy (Brachytherapy). Biomedicines, 9(11), 1629. https://doi.org/10.3390/biomedicines9111629






