Transcranial versus Direct Cortical Stimulation for Motor-Evoked Potentials during Resection of Supratentorial Tumors under General Anesthesia (The TRANSEKT-Trial): Study Protocol for a Randomized Controlled Trial
Abstract
:1. Introduction
2. Methods
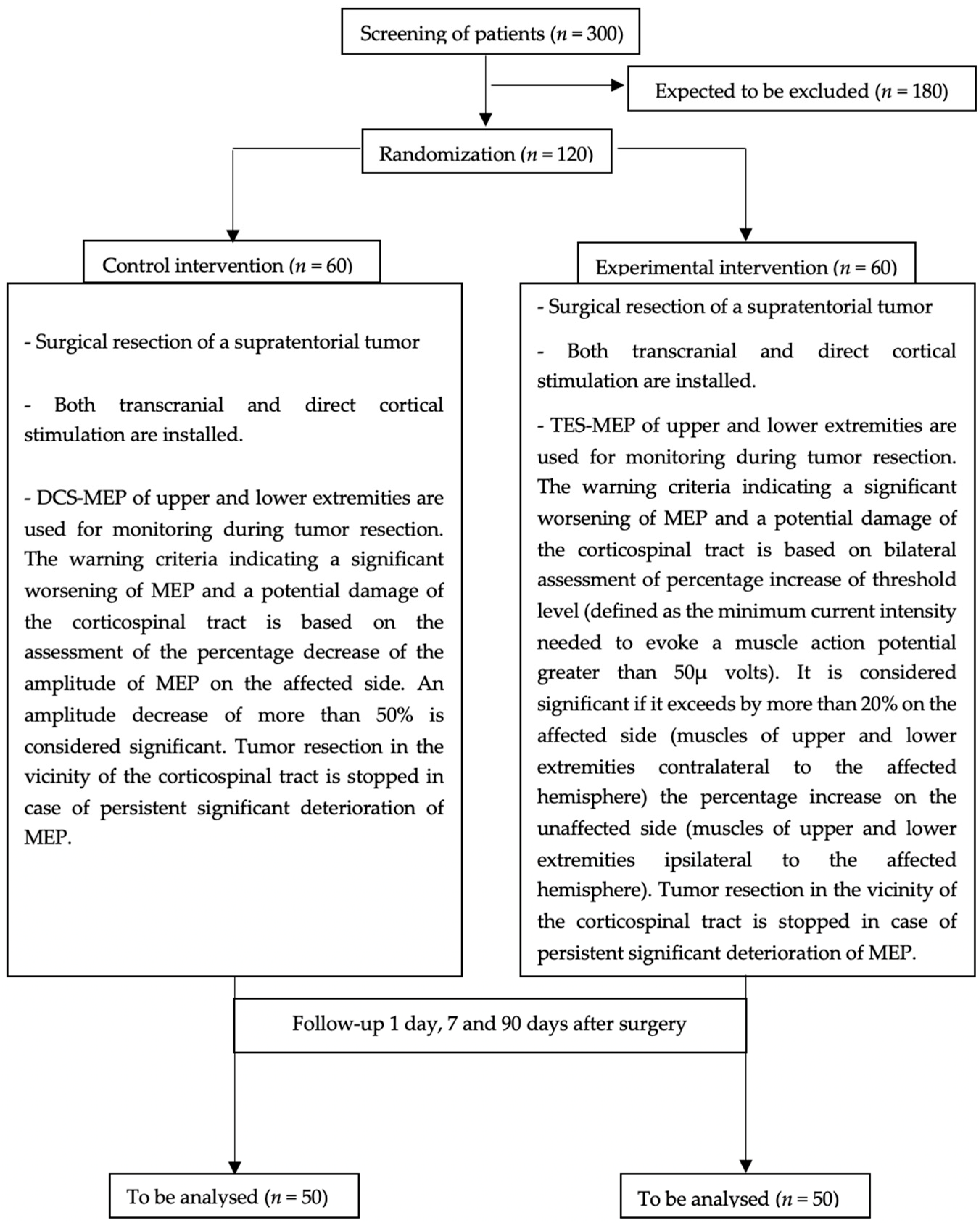
2.1. Trial Design
2.2. Objectives
2.3. Participating Centers and Recruitment
2.4. Inclusion Criteria
- Age ≥ 18 and ≤80 years.
- Ability to give informed consent.
- Indication for surgical resection of a supratentorial tumor.
- Suspected supratentorial glioma or metastasis in close vicinity to the CST confirmed in a preoperative magnetic resonance imaging (MRI).
- Missing or mild preoperative paresis; Medical Research Council scale for muscle strength (MRC) grades 5 or 4.
2.5. Exclusion Criteria
- Tumor infiltration of the precentral gyrus.
- Unavailable preoperative MRI.
- Severe preoperative paralysis (MRC grades 1, 2 or 3).
- One of the stimulation modalities is not appropriate for intraoperative application according to the neurosurgeon.
2.6. Study Timeline
2.7. Interventions
2.7.1. Anesthesia
- All procedures are performed under general total intravenous anesthesia.
- Muscle relaxant (e.g., rocuronium bromide) is applied for intubation only.
- Anesthesia is induced using propofol and continued using perfusion pumps. Analgesia is applied using sufentanil for the intubation and maintained through remifentanil.
- Use of an infusion pump with TCI function (target-controlled infusion) for maintaining anesthesia and analgesia during surgery is recommended.
- Invasive measurement of blood pressure is performed to maintain stable systolic and mean blood pressure. Body temperature is kept at norm values.
- Monitoring of the depth of anesthesia using EEG Monitoring is recommended.
- Performing regional scalp block or local infiltration at the headframe pins is recommended.
2.7.2. Installation of Intraoperative Monitoring
2.7.3. Intraoperative Monitoring
2.7.4. MEP Deterioration/Alarm Criteria
2.8. Outcomes
2.8.1. Primary Outcome Measure
2.8.2. Main Secondary Outcome Measure
2.9. Methods against Bias
2.10. Blinding
2.11. Sample Size
2.12. Definition of Population
2.13. Statistical Analysis
2.13.1. Primary Endpoint
2.13.2. Secondary Endpoints
2.14. Study Initiation
2.15. Study Monitoring
- The rights and well-being of patients are protected.
- The reported trial data are accurate, complete, and verifiable from source documents.
- The conduct of the trial is in compliance with the currently approved protocol/amendment(s), with GCP, and with the applicable regulatory requirement(s).
2.16. Adverse Events
2.17. Drop-Out Criteria and Termination of the Study
2.18. Data Management
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Trial Status
References
- Global, regional, and national burden of brain and other CNS cancer, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 376–393. [CrossRef] [Green Version]
- Kombos, T.; Picht, T.; Derdilopoulos, A.; Suess, O. Impact of intraoperative neurophysiological monitoring on surgery of high-grade gliomas. J. Clin. Neurophysiol. 2009, 26, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Talacchi, A.; Turazzi, S.; Locatelli, F.; Sala, F.; Beltramello, A.; Alessandrini, F.; Manganotti, P.; Lanteri, P.; Gambin, R.; Ganau, M.; et al. Surgical treatment of high-grade gliomas in motor areas. The impact of different supportive technologies: A 171-patient series. J. Neurooncol. 2010, 100, 417–426. [Google Scholar] [CrossRef]
- Spena, G.; Schucht, P.; Seidel, K.; Rutten, G.J.; Freyschlag, C.F.; D’Agata, F.; Costi, E.; Zappa, F.; Fontanella, M.; Fontaine, D.; et al. Brain tumors in eloquent areas: A European multicenter survey of intraoperative mapping techniques, intraoperative seizures occurrence, and antiepileptic drug prophylaxis. Neurosurg. Rev. 2017, 40, 287–298. [Google Scholar] [CrossRef]
- Macdonald, D.B.; Skinner, S.; Shils, J.; Yingling, C. Intraoperative motor evoked potential monitoring—A position statement by the American Society of Neurophysiological Monitoring. Clin. Neurophysiol. 2013, 124, 2291–2316. [Google Scholar] [CrossRef]
- Krieg, S.M.; Shiban, E.; Droese, D.; Gempt, J.; Buchmann, N.; Pape, H.; Ryang, Y.M.; Meyer, B.; Ringel, F. Predictive value and safety of intraoperative neurophysiological monitoring with motor evoked potentials in glioma surgery. Neurosurgery 2012, 70, 1060–1071. [Google Scholar] [CrossRef]
- Krieg, S.M.; Schäffner, M.; Shiban, E.; Droese, D.; Obermüller, T.; Gempt, J.; Meyer, B.; Ringel, F. Reliability of intraoperative neurophysiological monitoring using motor evoked potentials during resection of metastases in motor-eloquent brain regions: Clinical article. J. Neurosurg. 2013, 118, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Seidel, K.; Beck, J.; Stieglitz, L.; Schucht, P.; Raabe, A. The warning-sign hierarchy between quantitative subcortical motor mapping and continuous motor evoked potential monitoring during resection of supratentorial brain tumors. J. Neurosurg. 2013, 118, 287–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abboud, T.; Schaper, M.; Dührsen, L.; Schwarz, C.; Schmidt, N.O.; Westphal, M.; Martens, T. A novel threshold criterion in transcranial motor evoked potentials during surgery for gliomas close to the motor pathway. J. Neurosurg. 2016, 125, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Abboud, T.; Schwarz, C.; Westphal, M.; Martens, T. A comparison between threshold criterion and amplitude criterion in transcranial motor evoked potentials during surgery for supratentorial lesions. J. Neurosurg. 2018, 131, 740–749. [Google Scholar] [CrossRef] [Green Version]
- Asimakidou, E.; Abut, P.A.; Raabe, A.; Seidel, K. Motor Evoked Potential Warning Criteria in Supratentorial Surgery: A Scoping Review. Cancers 2021, 13, 2803. [Google Scholar] [CrossRef]
- McGirt, M.J.; Chaichana, K.L.; Gathinji, M.; Attenello, F.J.; Than, K.; Olivi, A.; Weingart, J.D.; Brem, H.; Quinones-Hinojosa, A.R. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J. Neurosurg. 2009, 110, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Sanai, N.; Berger, M.S. Extent of resection influences outcomes for patients with gliomas. Rev. Neurol. 2011, 167, 648–654. [Google Scholar] [CrossRef]
- Wagner, A.; Ille, S.; Liesenhoff, C.; Aftahy, K.; Meyer, B.; Krieg, S.M. Improved potential quality of intraoperative transcranial motor-evoked potentials by navigated electrode placement compared to the conventional ten-twenty system. Neurosurg. Rev. 2021, 1–9. [Google Scholar] [CrossRef]
- Cordella, R.; Acerbi, F.; Broggi, M.; Vailati, D.; Nazzi, V.; Schiariti, M.; Tringali, G.; Ferroli, P.; Franzini, A.; Broggi, G. Intraoperative neurophysiological monitoring of the cortico-spinal tract in image-guided mini-invasive neurosurgery. Clin. Neurophysiol. 2013, 124, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Neuloh, G.; Pechstein, U.; Cedzich, C.; Schramm, J. Motor evoked potential monitoring with supratentorial surgery. Neurosurgery 2004, 54, 1061–1072. [Google Scholar] [CrossRef]
- Neuloh, G.; Pechstein, U.; Schramm, J. Motor tract monitoring during insular glioma surgery. J. Neurosurg. 2007, 106, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Szelényi, A.; Bello, L.; Duffau, H.; Fava, E.; Feigl, G.C.; Galanda, M.; Neuloh, G.; Signorelli, F.; Sala, F. Intraoperative electrical stimulation in awake craniotomy: Methodological aspects of current practice. Neurosurg. Focus 2010, 28, E7. [Google Scholar] [CrossRef] [PubMed]
- Szelényi, A.; Hattingen, E.; Weidauer, S.; Seifert, V.; Ziemann, U. Intraoperative motor evoked potential alteration in intracranial tumor surgery and its relation to signal alteration in postoperative magnetic resonance imaging. Neurosurgery 2010, 67, 302–313. [Google Scholar] [CrossRef]
- Raabe, A.; Beck, J.; Schucht, P.; Seidel, K. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: Evaluation of a new method. J. Neurosurg. 2014, 120, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.G.; Kim, K.; Shin, H.I.; Bang, M.S.; Kim, H.S.; Choi, J.; Wang, K.C.; Kim, S.K.; Lee, J.Y.; Phi, J.H.; et al. Feasibility of intraoperative monitoring of motor evoked potentials obtained through transcranial electrical stimulation in infants younger than 3 months. J. Neurosurg. Pediatrics 2019, 23, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Conti Nibali, M.; Viganò, L.; Puglisi, G.; Howells, H.; Gay, L.; Sciortino, T.; Leonetti, A.; Riva, M.; Fornia, L.; et al. Resection of tumors within the primary motor cortex using high-frequency stimulation: Oncological and functional efficiency of this versatile approach based on clinical conditions. J. Neurosurg. 2019, 133, 642–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umemura, T.; Nishizawa, S.; Nakano, Y.; Saito, T.; Kitagawa, T.; Miyaoka, R.; Suzuki, K.; Yamamoto, J. Intraoperative monitoring of motor-evoked potential for parenchymal brain tumor removal: An analysis of false-negative cases. J. Clin. Neurosci. 2018, 57, 105–110. [Google Scholar] [CrossRef]
- van der Meche, F.G.; Schmitz, P.I.; Dutch Guillain-Barre Study Group. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barre syndrome. N. Engl. J. Med. 1992, 326, 1123–1129. [Google Scholar] [CrossRef]
- Hughes, R.A.; Donofrio, P.; Bril, V.; Dalakas, M.C.; Deng, C.; Hanna, K.; Hartung, H.P.; Latov, N.; Merkies, I.S.; van Doorn, P.A. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): A randomised placebo-controlled trial. Lancet Neurol. 2008, 7, 136–144. [Google Scholar] [CrossRef]
- Sheehan, J.; Lee, C.C.; Bodach, M.E.; Tumialan, L.M.; Oyesiku, N.M.; Patil, C.G.; Litvack, Z.; Zada, G.; Aghi, M.K. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline for the Management of Patients with Residual or Recurrent Nonfunctioning Pituitary Adenomas. Neurosurgery 2016, 79, E539–E540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caissie, A.; Nguyen, J.; Chen, E.; Zhang, L.; Sahgal, A.; Clemons, M.; Kerba, M.; Arnalot, P.F.; Danjoux, C.; Tsao, M.; et al. Quality of life in patients with brain metastases using the EORTC QLQ-BN20+2 and QLQ-C15-PAL. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1238–1245. [Google Scholar] [CrossRef]
- Liposits, G.; Eshøj, H.R.; Möller, S.; Winther, S.B.; Skuladottir, H.; Ryg, J.; Hofsli, E.; Shah, C.H.; Poulsen, L.; Berglund, Å.; et al. Quality of Life in Vulnerable Older Patients with Metastatic Colorectal Cancer Receiving Palliative Chemotherapy—The Randomized NORDIC9-Study. Cancers 2021, 13, 2604. [Google Scholar] [CrossRef]
- Barca-Hernando, M.; Muñoz-Martin, A.J.; Rios-Herranz, E.; Garcia-Escobar, I.; Beato, C.; Font, C.; Oncala-Sibajas, E.; Revuelta-Rodriguez, A.; Areses, M.C.; Rivas-Jimenez, V.; et al. Case-Control Analysis of the Impact of Anemia on Quality of Life in Patients with Cancer: A Qca Study Analysis. Cancers 2021, 13, 2517. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.; Raschka, J.; Wolff, K.D.; Kolk, A.; Bissinger, O. Hospital Based Quality of Life in Oral Cancer Surgery. Cancers 2020, 12, 2152. [Google Scholar] [CrossRef]
- Warmenhoven, F.; van Rijswijk, E.; Engels, Y.; Kan, C.; Prins, J.; van Weel, C.; Vissers, K. The Beck Depression Inventory (BDI-II) and a single screening question as screening tools for depressive disorder in Dutch advanced cancer patients. Support. Care Cancer 2012, 20, 319–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abboud, T.; Asendorf, T.; Heinrich, J.; Faust, K.; Krieg, S.M.; Seidel, K.; Mielke, D.; Matthies, C.; Ringel, F.; Rohde, V.; et al. Transcranial versus Direct Cortical Stimulation for Motor-Evoked Potentials during Resection of Supratentorial Tumors under General Anesthesia (The TRANSEKT-Trial): Study Protocol for a Randomized Controlled Trial. Biomedicines 2021, 9, 1490. https://doi.org/10.3390/biomedicines9101490
Abboud T, Asendorf T, Heinrich J, Faust K, Krieg SM, Seidel K, Mielke D, Matthies C, Ringel F, Rohde V, et al. Transcranial versus Direct Cortical Stimulation for Motor-Evoked Potentials during Resection of Supratentorial Tumors under General Anesthesia (The TRANSEKT-Trial): Study Protocol for a Randomized Controlled Trial. Biomedicines. 2021; 9(10):1490. https://doi.org/10.3390/biomedicines9101490
Chicago/Turabian StyleAbboud, Tammam, Thomas Asendorf, Jutta Heinrich, Katharina Faust, Sandro M. Krieg, Kathleen Seidel, Dorothee Mielke, Cordola Matthies, Florian Ringel, Veit Rohde, and et al. 2021. "Transcranial versus Direct Cortical Stimulation for Motor-Evoked Potentials during Resection of Supratentorial Tumors under General Anesthesia (The TRANSEKT-Trial): Study Protocol for a Randomized Controlled Trial" Biomedicines 9, no. 10: 1490. https://doi.org/10.3390/biomedicines9101490
APA StyleAbboud, T., Asendorf, T., Heinrich, J., Faust, K., Krieg, S. M., Seidel, K., Mielke, D., Matthies, C., Ringel, F., Rohde, V., & Szelényi, A. (2021). Transcranial versus Direct Cortical Stimulation for Motor-Evoked Potentials during Resection of Supratentorial Tumors under General Anesthesia (The TRANSEKT-Trial): Study Protocol for a Randomized Controlled Trial. Biomedicines, 9(10), 1490. https://doi.org/10.3390/biomedicines9101490







