A New Light on Potential Therapeutic Targets for Colorectal Cancer Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Antibodies
2.2. Cell Culture
2.3. Cell Transfections
2.4. Cell Viability Assay
2.5. Focal Formation Assays
2.6. Cell Cycle Analysis
2.7. Apoptosis Assay
2.8. Autophagy Cytofluorimetric Analysis
2.9. Glucose Uptake Detection
2.10. Glycolysis Stress Test
2.11. BODIPY Staining
2.12. Quantitative Reverse-Transcription Polymerase Chain Reaction (RT-qPCR)
2.13. Luciferase Reporter Assays
2.14. Western Blotting
2.15. Gene Set Enrichment Analysis (GSEA)
2.16. In Situ Hybridization (ISH) and Immunohistochemistry (IHC)
2.17. Statistical Analysis
3. Results
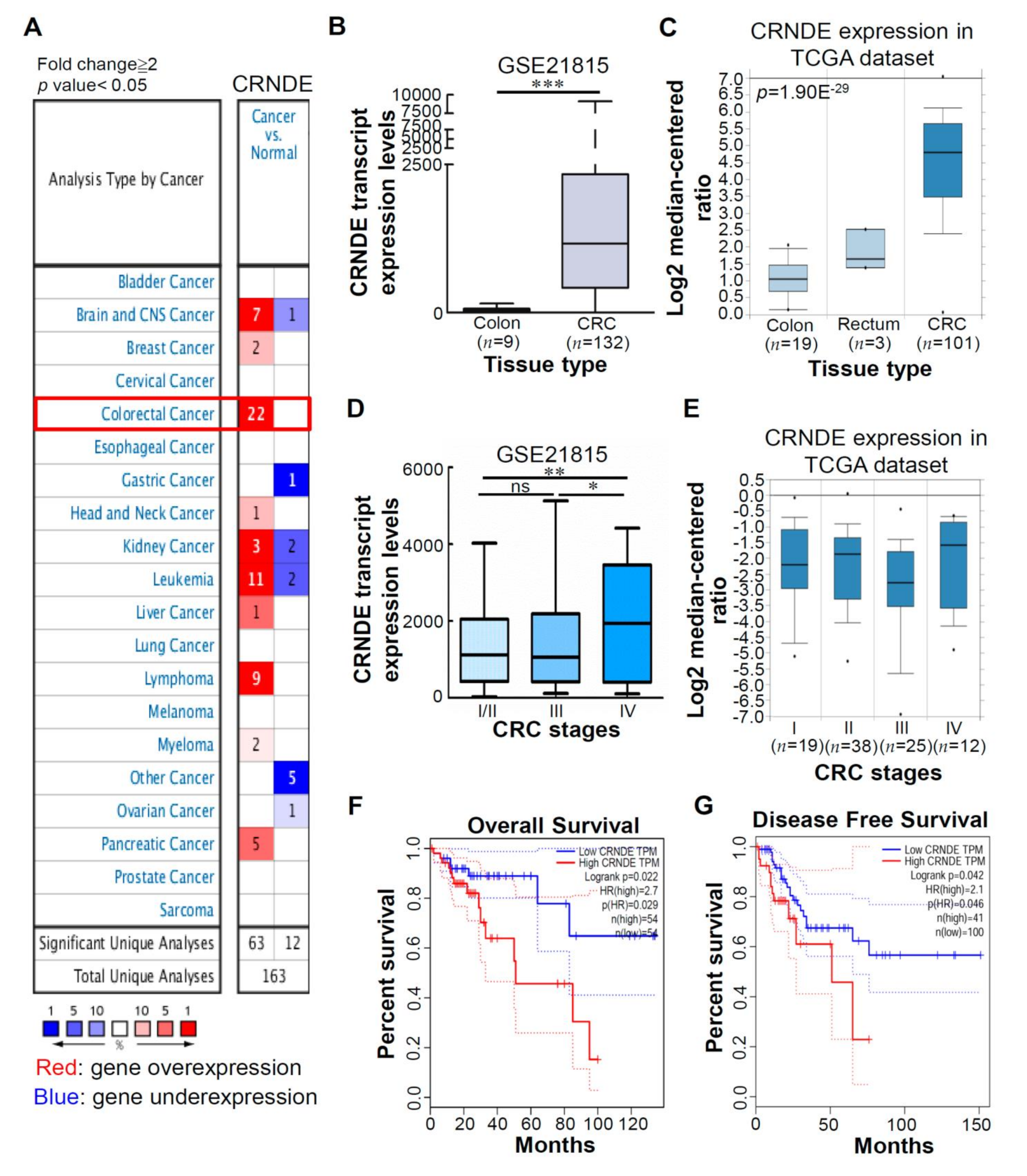
3.1. CRNDE Is Upregulated in CRC Tissues, and High CRNDE Expression Is Correlated with Poor Prognoses of CRC Patients
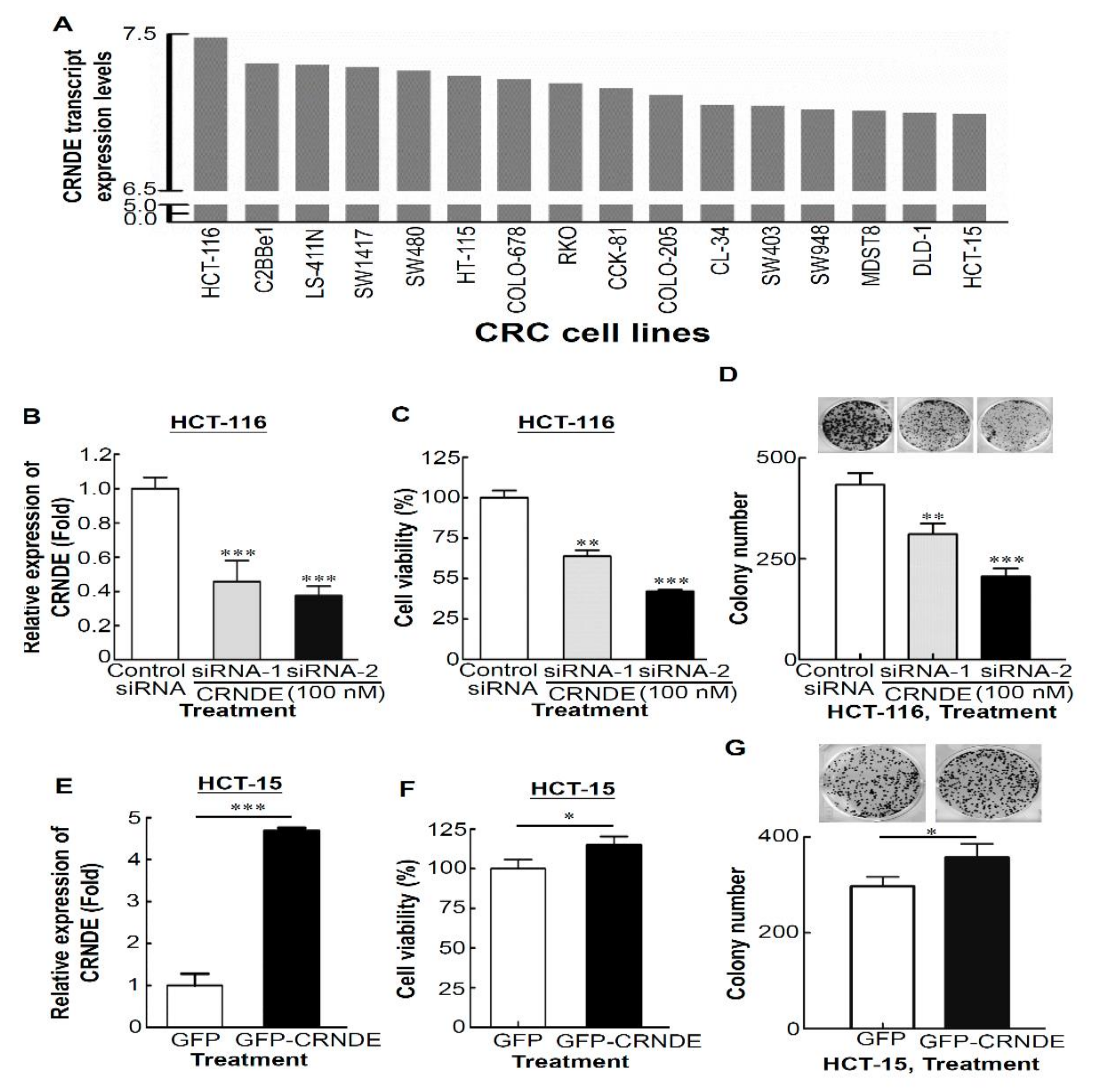
3.2. CRNDE Promotes Proliferation of CRC Cells
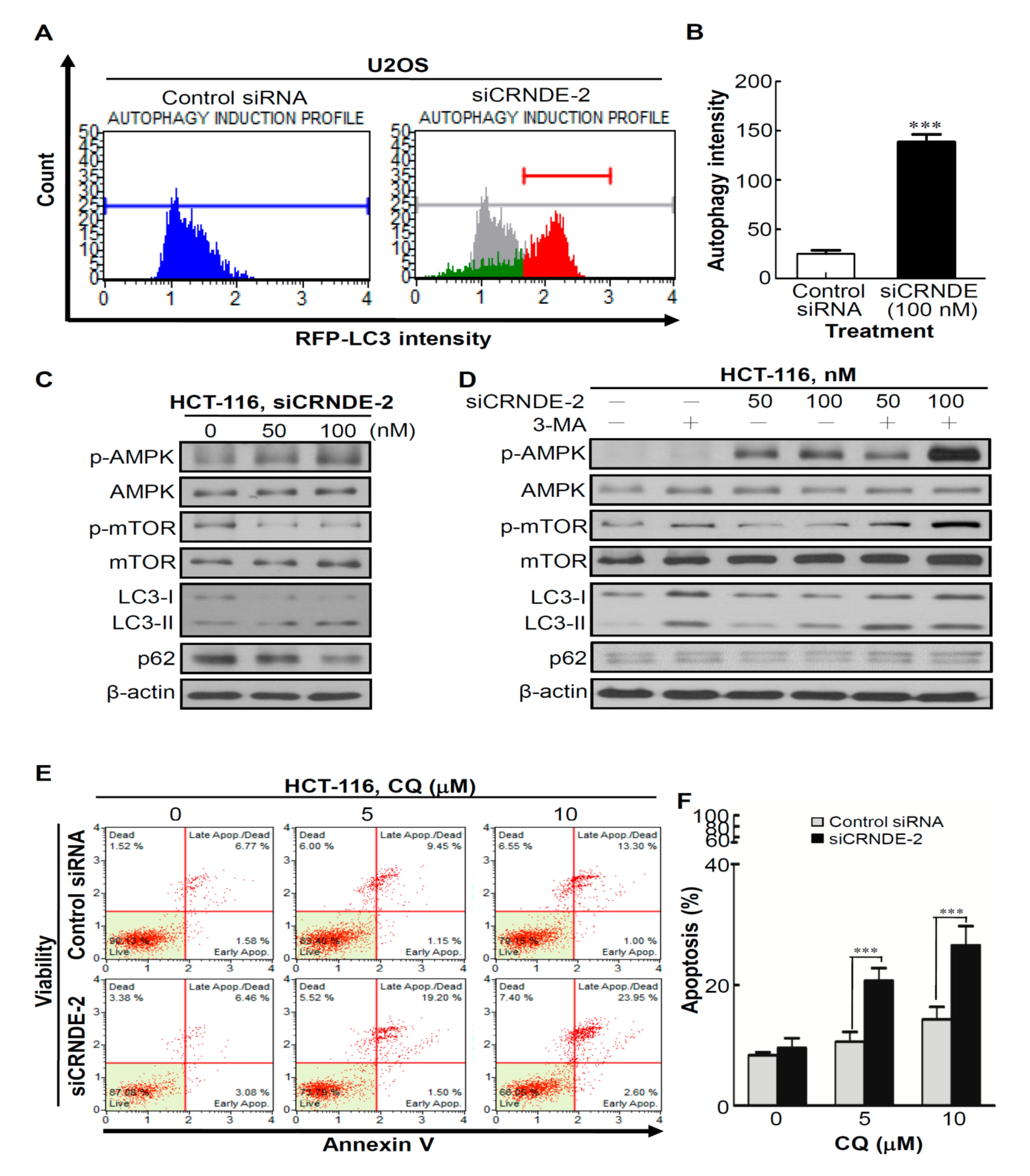
3.3. Knocking Down CRNDE Inhibited Growth of CRC Cells through Cell Cycle Arrest Not Due to Cell Apoptosis
3.4. Knocking Down CRNDE Induced Autophagy in CRC Cells
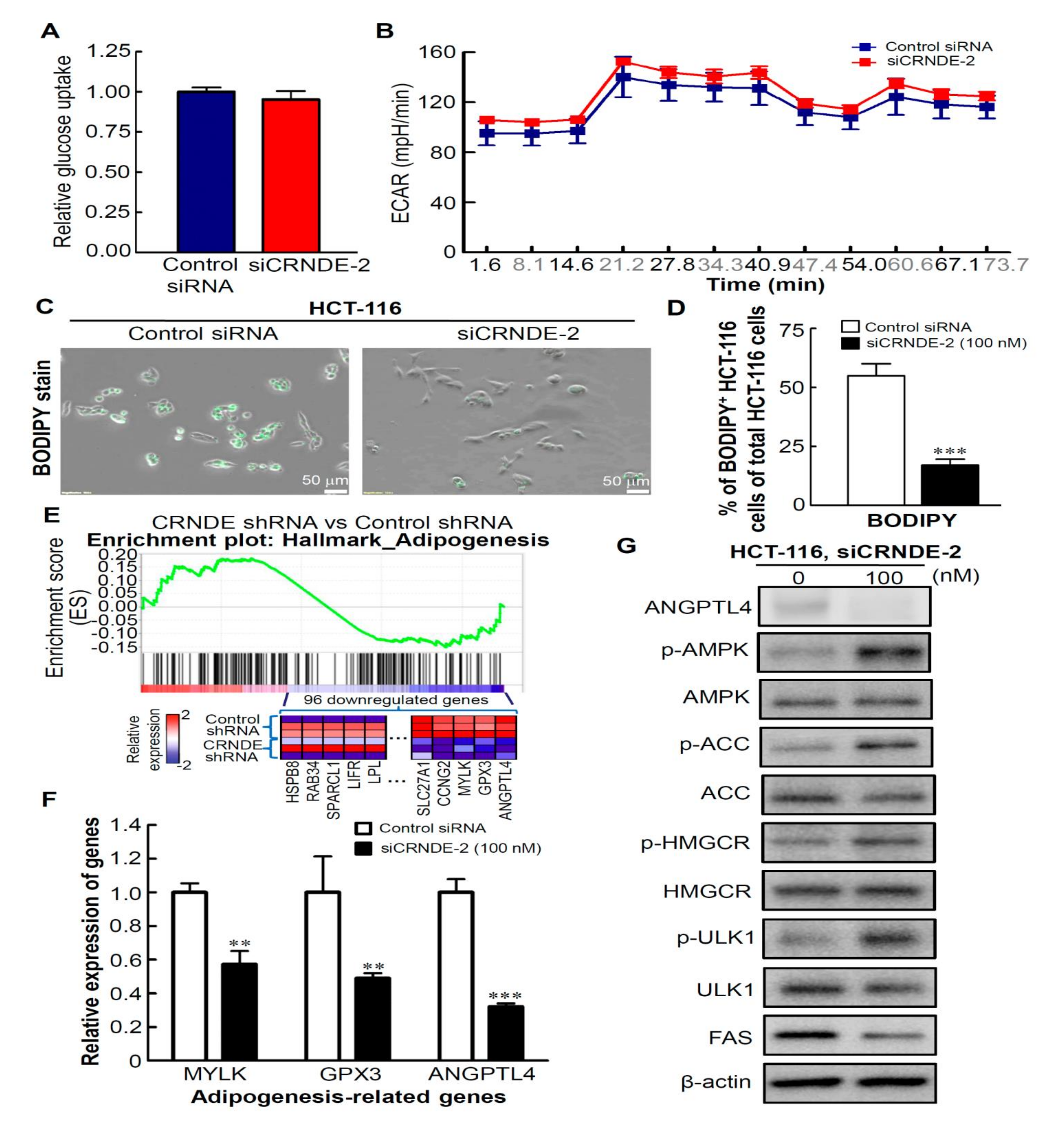
3.5. CRNDE-KD Inhibits Lipid Metabolism by CRC Cells
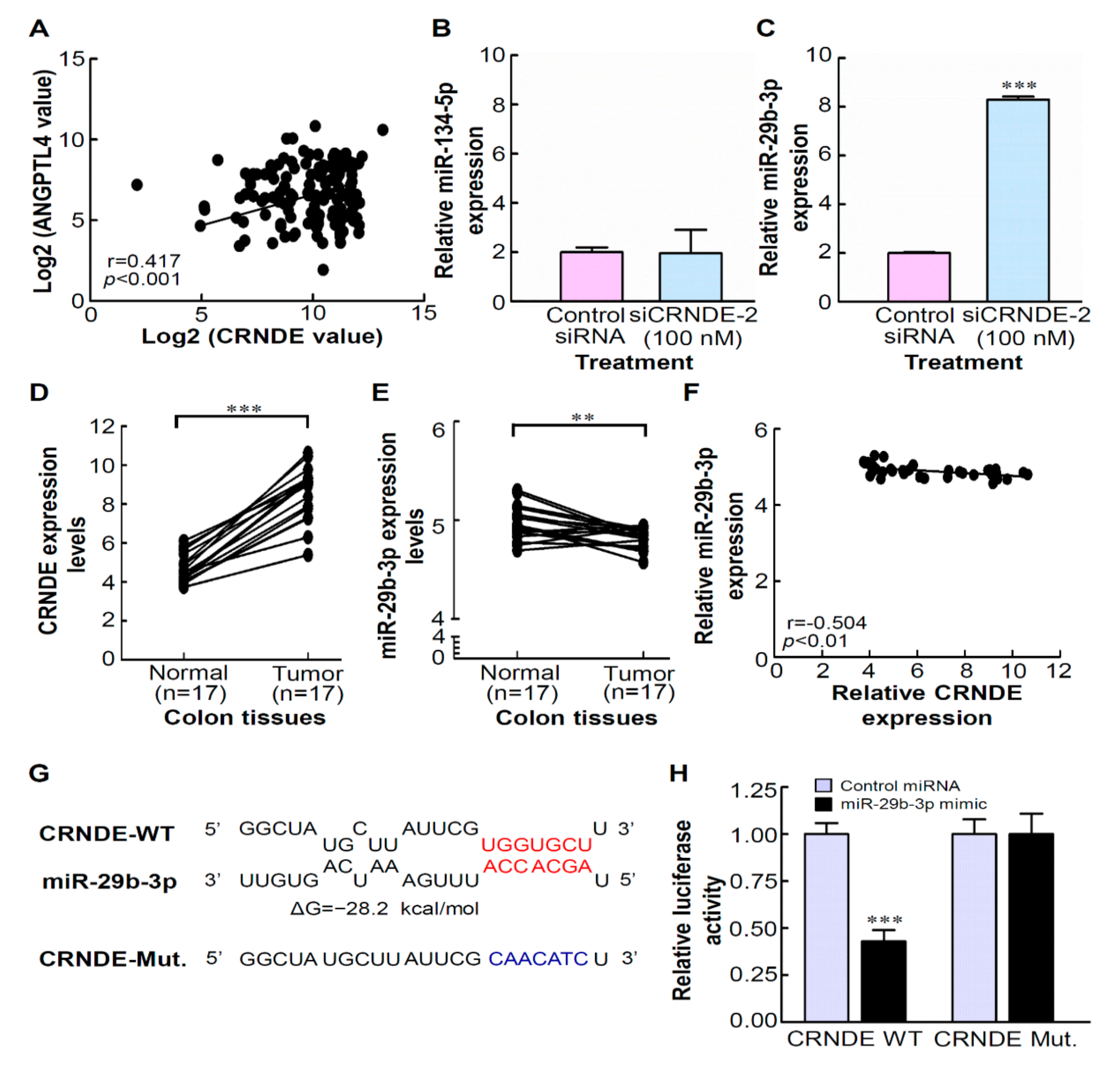
3.6. CRNDE Regulates ANGPTL4 Expression via Competitively Binding with miR-29b-3p
3.7. High Levels of CRNDE and ANGPTL4 and ALow Level of miR-29b-3p in CRC Tissues Are Involved in Regulating Lipid Metabolism by the miR-29b-3p/ANGPTL4 Axis-Mediated Regulation of AMPK/ULK1signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migliore, L.; Migheli, F.; Spisni, R.; Coppedè, F. Genetics, Cytogenetics, and Epigenetics of Colorectal Cancer. J. Biomed. Biotechnol. 2011, 2011, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Kung, J.T.Y.; Colognori, D.; Lee, J.T. Long Noncoding RNAs: Past, Present, and Future. Genetics 2013, 193, 651–669. [Google Scholar] [CrossRef] [Green Version]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and Functions of Long Noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Frith, M.; Pheasant, M.; Mattick, J. Genomics: The amazing complexity of the human transcriptome. Eur. J. Hum. Genet. 2005, 13, 894–897. [Google Scholar] [CrossRef]
- Gutschner, T.; Diederichs, S. The hallmarks of cancer. RNA Biol. 2012, 9, 703–719. [Google Scholar] [CrossRef] [Green Version]
- Brunner, A.L.; Beck, A.H.; Edris, B.; Sweeney, R.T.; Zhu, S.X.; Li, R.; Montgomery, K.; Varma, S.; Gilks, T.; Guo, X.; et al. Transcriptional profiling of long non-coding RNAs and novel transcribed regions across a diverse panel of archived human cancers. Genome Biol. 2012, 13, R75. [Google Scholar] [CrossRef] [Green Version]
- Graham, L.D.; Pedersen, S.K.; Brown, G.S.; Ho, T.; Kassir, Z.; Moynihan, A.T.; Vizgoft, E.K.; Dunne, R.; Pimlott, L.; Young, G.P.; et al. Colorectal Neoplasia Differentially Expressed (CRNDE), a Novel Gene with Elevated Expression in Colorectal Adenomas and Adenocarcinomas. Genes Cancer 2011, 2, 829–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.-H.; Tang, W.-C.; Cheng, Y.-W.; Sia, P.; Huang, C.-C.; Lee, Y.-C.; Jiang, H.-Y.; Wu, M.-H.; Lai, I.-L.; Lee, J.-W.; et al. Targeting of multiple oncogenic signaling pathways by Hsp90 inhibitor alone or in combination with berberine for treatment of colorectal cancer. Biochim. Biophys. Acta (BBA)Bioenerg. 2015, 1853, 2261–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.Z.; Liu, Y.Y.; Liu, X.X.; Dai, M.Y.; Chen, X.L.; Gao, Y.; Chen, J.F.; Dai, S.M. The diagnostic and prognostic significance of long non-coding RNA CRNDE in pan-cancer based on TCGA, GEO and comprehensive meta-analysis. Pathol. Res. Pract. 2019, 215, 256–264. [Google Scholar] [CrossRef]
- Ding, C.; Han, F.; Xiang, H.; Xia, X.; Wang, Y.; Dou, M.; Zheng, J.; Li, Y.; Xue, W.; Ding, X.; et al. LncRNA CRNDE is a biomarker for clinical progression and poor prognosis in clear cell renal cell carcinoma. J. Cell. Biochem. 2018, 119, 10406–10414. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.-Y.; Lu, Y.-Y.; Yang, J.-K.; Deng, W.-Y.; Zhou, Q.; Jiao, B.-H. Expression of long non-coding RNA CRNDE in glioma and its correlation with tumor progression and patient survival. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3992–3996. [Google Scholar]
- Szafron, L.M.; Balcerak, A.; Grzybowska, E.A.; Pienkowska-Grela, B.; Podgorska, A.; Zub, R.; Olbryt, M.; Pamula-Pilat, J.; Lisowska, K.M.; Grzybowska, E.; et al. The putative oncogene, CRNDE, is a negative prognostic factor in ovarian cancer pa-tients. Oncotarget 2015, 6, 43897–43910. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Sha, H.; Sun, X.; Zhang, Y.; Wu, Y.; Zhang, J.; Zhang, H.; Wu, J.; Feng, J. CRNDE: An oncogenic long non-coding RNA in cancers. Cancer Cell Int. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Tsuchihara, K.; Fujii, S.; Esumi, H. Autophagy and cancer: Dynamism of the metabolism of tumor cells and tissues. Cancer Lett. 2009, 278, 130–138. [Google Scholar] [CrossRef]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Wang, S. Targeting disease through novel pathways of apoptosis and autophagy. Expert Opin. Ther. Targets 2012, 16, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Deng, W.; Zhang, S.; Cai, N.; Jiao, S.; Song, J.; Wei, L. Paradoxical roles of autophagy in different stages of tumorigenesis: Protector for normal or cancer cells. Cell Biosci. 2013, 3, 35. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-M.; Talanian, R.V.; Billiar, T.R. Nitric Oxide Inhibits Apoptosis by Preventing Increases in Caspase-3-like Activity via Two Distinct Mechanisms. J. Biol. Chem. 1997, 272, 31138–31148. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-M.; Cheng, C.-H.; Pan, S.-L.; Yang, P.-M.; Lin, D.-Y.; Lee, K.-H. Gene Expression Signature-Based Approach Identifies Antifungal Drug Ciclopirox As a Novel Inhibitor of HMGA2 in Colorectal Cancer. Biomolecules 2019, 9, 688. [Google Scholar] [CrossRef] [Green Version]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Obernosterer, G.; Martinez, J.; Alenius, M. Locked nucleic acid-based in situ detection of microRNAs in mouse tissue sections. Nat. Protoc. 2007, 2, 1508–1514. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Y.; Ai, M.; Wang, H.; Duan, Z.; Wang, H.; Zhao, L.; Yu, J.; Ding, Y.; Wang, S. Long noncoding RNA CRNDE stabilized by hnRNPUL2 accelerates cell proliferation and migration in colorectal carcinoma via activating Ras/MAPK signaling pathways. Cell Death Dis. 2017, 8, e2862. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-F.; Lee, C.-Y.; Lai, L.-C.; Tsai, M.-H.; Lu, T.-P.; Chuang, E.Y. CellExpress: A comprehensive microarray-based cancer cell line and clinical sample gene expression analysis online system. Database 2018, 2018. [Google Scholar] [CrossRef]
- Hovelson, D.H.; Cani, A.K.; McDaniel, A.; Johnson, B.; Rhodes, K.; Williams, P.D.; Bandla, S.; Choppa, P.; Hyland, F.; Liu, G.; et al. The Oncomine Cancer Research Panel, a scalable next-generation sequencing system for relevant somatic variant assessment in solid tumors. J. Clin. Oncol. 2015, 33, e22164. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [Green Version]
- Cirone, M.; Montani, M.S.G.; Granato, M.; Garufi, A.; Faggioni, A.; D’Orazi, G. Autophagy manipulation as a strategy for efficient anticancer therapies: Possible consequences. J. Exp. Clin. Cancer Res. 2019, 38, 1–7. [Google Scholar] [CrossRef]
- Bjørkøy, G.; Lamark, T.; Brech, A.; Outzen, H.; Perander, M.; Øvervatn, A.; Stenmark, H.; Johansen, T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 2005, 171, 603–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altman, B.; Rathmell, J.C. Metabolic Stress in Autophagy and Cell Death Pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a008763. [Google Scholar] [CrossRef]
- Canto, C.; Auwerx, J. Calorie Restriction: Is AMPK a Key Sensor and Effector? Physiology 2011, 26, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Nakayama; Hirakawa, H.; Shibata, K.; Nazneen, A.; Abe, K.; Nagayasu, T.; Taguchi, T. Expression of angiopoietin-like 4 (ANGPTL4) in human colorectal cancer: ANGPTL4 promotes venous invasion and distant metastasis. Oncol. Rep. 2011, 25, 929–935. [Google Scholar] [CrossRef]
- Aryal, B.; Price, N.; Suarez, Y.; Fernández-Hernando, C. ANGPTL4 in Metabolic and Cardiovascular Disease. Trends Mol. Med. 2019, 25, 723–734. [Google Scholar] [CrossRef] [PubMed]
- López-Urrutia, E.; Montes, L.P.B.; Cervantes, D.L.D.G.; Perez-Plasencia, C.; Campos-Parra, A.D. Crosstalk Between Long Non-coding RNAs, Micro-RNAs and mRNAs: Deciphering Molecular Mechanisms of Master Regulators in Cancer. Front. Oncol. 2019, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Lan, G.; Xie, W.; Li, L.; Zhang, M.; Liu, D.; Tan, Y.-L.; Cheng, H.-P.; Gong, D.; Huang, C.; Zheng, X.-L.; et al. MicroRNA-134 actives lipoprotein lipase-mediated lipid accumulation and inflammatory response by targeting angiopoietin-like 4 in THP-1 macrophages. Biochem. Biophys. Res. Commun. 2016, 472, 410–417. [Google Scholar] [CrossRef]
- Chen, S.; Yang, M.; Chang, S. LncRNA CCAL Promotes Angiogenesis Through Regulating the MiR-29b/ANGPTL4 Axis in Osteosarcoma. Cancer Manag. Res. 2020, 12, 10521–10530. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Lin, Y.-C.-D.; Li, J.; Huang, K.-Y.; Shrestha, S.; Hong, H.-C.; Tang, Y.; Chen, Y.-G.; Jin, C.-N.; Yu, Y.; et al. miRTarBase 2020: Updates to the experimentally validated microRNA–target interaction database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef] [Green Version]
- Ding, D.; Li, C.; Zhao, T.; Li, D.; Yang, L.; Zhang, B. LncRNA H19/miR-29b-3p/PGRN Axis Promoted Epithelial-Mesenchymal Transition of Colorectal Cancer Cells by Acting on Wnt Signaling. Mol. Cells 2018, 41, 423–435. [Google Scholar] [CrossRef]
- Beermann, J.; Piccoli, M.-T.; Viereck, J.; Thum, T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol. Rev. 2016, 96, 1297–1325. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Li, Q.; Li, L.; Ma, R. The long non-coding RNA CRNDE promotes cervical cancer cell growth and metastasis. Biol. Chem. 2017, 399, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.E.; Du, P.Z.; Zhang, H.D.; Huang, G.J. Long Noncoding RNA CRNDE Promotes Proliferation of Gastric Cancer Cells by Targeting miR-145. Cell. Physiol. Biochem. 2017, 42, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-X.; Liu, T.; Zhang, X.; Yang, Y.-M.; Du, L.-T. Increased expression of the long noncoding RNA CRNDE-h indicates a poor prognosis in colorectal cancer, and is positively correlated with IRX5 mRNA expression. OncoTargets Ther. 2016, 9, 1437–1448. [Google Scholar] [CrossRef] [Green Version]
- Tariq, K.; Ghias, K. Colorectal cancer carcinogenesis: A review of mechanisms. Cancer Biol. Med. 2016, 13, 120–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F.A. The Role of Autophagy in Cancer: Therapeutic Implications. Mol. Cancer Ther. 2011, 10, 1533–1541. [Google Scholar] [CrossRef] [Green Version]
- Leone, R.D.; Amaravadi, R.K. Autophagy: A targetable linchpin of cancer cell metabolism. Trends Endocrinol. Metab. 2013, 24, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Min, Q.; Ouyang, C.; Lee, J.; He, C.; Zou, M.-H.; Xie, Z. AMPK activation prevents excess nutrient-induced hepatic lipid accumulation by inhibiting mTORC1 signaling and endoplasmic reticulum stress response. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 1844–1854. [Google Scholar] [CrossRef] [Green Version]
- Jia, R.; Wang, C. MiR-29b-3p Reverses Cisplatin Resistance by Targeting COL1A1 in Non-Small-Cell Lung Cancer A549/DDP Cells. Cancer Manag. Res. 2020, 12, 2559–2566. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Shetti, D.; Fan, C.; Wei, K. miR-29b-3p promotes progression of MDA-MB-231 triple-negative breast cancer cells through downregulating TRAF3. Biol. Res. 2019, 52, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Wang, P.; Yang, W.; Shan, Y.; Zhang, Q.; Wu, H. The role of lncRNA MSC-AS1/miR-29b-3p axis-mediated CDK14 modulation in pancreatic cancer proliferation and Gemcitabine-induced apoptosis. Cancer Biol. Ther. 2019, 20, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhong, Z.; Huang, M.; Tian, Q.; Jiang, R.; Chen, J. lncRNA H19 regulates epithelial–mesenchymal transition and metastasis of bladder cancer by miR-29b-3p as competing endogenous RNA. Biochim. Biophys. Acta (BBA) Bioenerg. 2017, 1864, 1887–1899. [Google Scholar] [CrossRef]
- Kwon, J.J.; Factora, T.D.; Dey, S.; Kota, J. A Systematic Review of miR-29 in Cancer. Mol. Ther. Oncolytics 2019, 12, 173–194. [Google Scholar] [CrossRef] [Green Version]
- La Paglia, L.; Listì, A.; Caruso, S.; Amodeo, V.; Passiglia, F.; Bazan, V.; Fanale, D. Potential Role of ANGPTL4 in the Cross Talk between Metabolism and Cancer through PPAR Signaling Pathway. PPAR Res. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Mandard, S.; Zandbergen, F.; van Straten, E.; Wahli, W.; Kuipers, F.; Muller, M.; Kersten, S. The Fasting-induced Adipose Factor/Angiopoietin-like Protein 4 Is Physically Associated with Lipoproteins and Governs Plasma Lipid Levels and Adiposity. J. Biol. Chem. 2006, 281, 934–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Cho, K.B.; Li, Y.; Tao, G.; Xie, Z.; Guo, B. Long Noncoding RNA (lncRNA)-Mediated Competing Endogenous RNA Networks Provide Novel Potential Biomarkers and Therapeutic Targets for Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 5758. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, W.-L.; Wang, C.-Y.; Lee, Y.-C.; Tang, W.-C.; Anuraga, G.; Ta, H.D.K.; Wu, Y.-F.; Lee, K.-H. A New Light on Potential Therapeutic Targets for Colorectal Cancer Treatment. Biomedicines 2021, 9, 1438. https://doi.org/10.3390/biomedicines9101438
Tsai W-L, Wang C-Y, Lee Y-C, Tang W-C, Anuraga G, Ta HDK, Wu Y-F, Lee K-H. A New Light on Potential Therapeutic Targets for Colorectal Cancer Treatment. Biomedicines. 2021; 9(10):1438. https://doi.org/10.3390/biomedicines9101438
Chicago/Turabian StyleTsai, Wei-Lun, Chih-Yang Wang, Yu-Cheng Lee, Wan-Chun Tang, Gangga Anuraga, Hoang Dang Khoa Ta, Yung-Fu Wu, and Kuen-Haur Lee. 2021. "A New Light on Potential Therapeutic Targets for Colorectal Cancer Treatment" Biomedicines 9, no. 10: 1438. https://doi.org/10.3390/biomedicines9101438
APA StyleTsai, W.-L., Wang, C.-Y., Lee, Y.-C., Tang, W.-C., Anuraga, G., Ta, H. D. K., Wu, Y.-F., & Lee, K.-H. (2021). A New Light on Potential Therapeutic Targets for Colorectal Cancer Treatment. Biomedicines, 9(10), 1438. https://doi.org/10.3390/biomedicines9101438









