T-Cadherin and the Ratio of Its Ligands as Predictors of Carotid Atherosclerosis: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Eligibility Criteria
- Total cholesterol level >8 mmol/L (>310 mg/dL) or blood pressure >180/110 mm Hg
- Diabetes
- Established familial hypercholesterolemia
- The level of triglycerides is more than 5.7 mmol/L
- Established systemic inflammatory diseases
- Malignant neoplasm, or another disease (not related to the heart), limiting life expectancy to <three years
- Current abuse of alcohol and/or psychoactive drugs
- Established liver failure (total bilirubin >3 mg/dl) or a history of cirrhosis with signs of portal hypertension
- Pregnancy, lactation
2.2. Ultrasonography
2.3. Laboratory Tests
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Subjects
3.2. Correlations between Laboratory Data and Carotid IMT
3.3. Association between Laboratory Data and the Presence of Arterial Hypertension (AH)
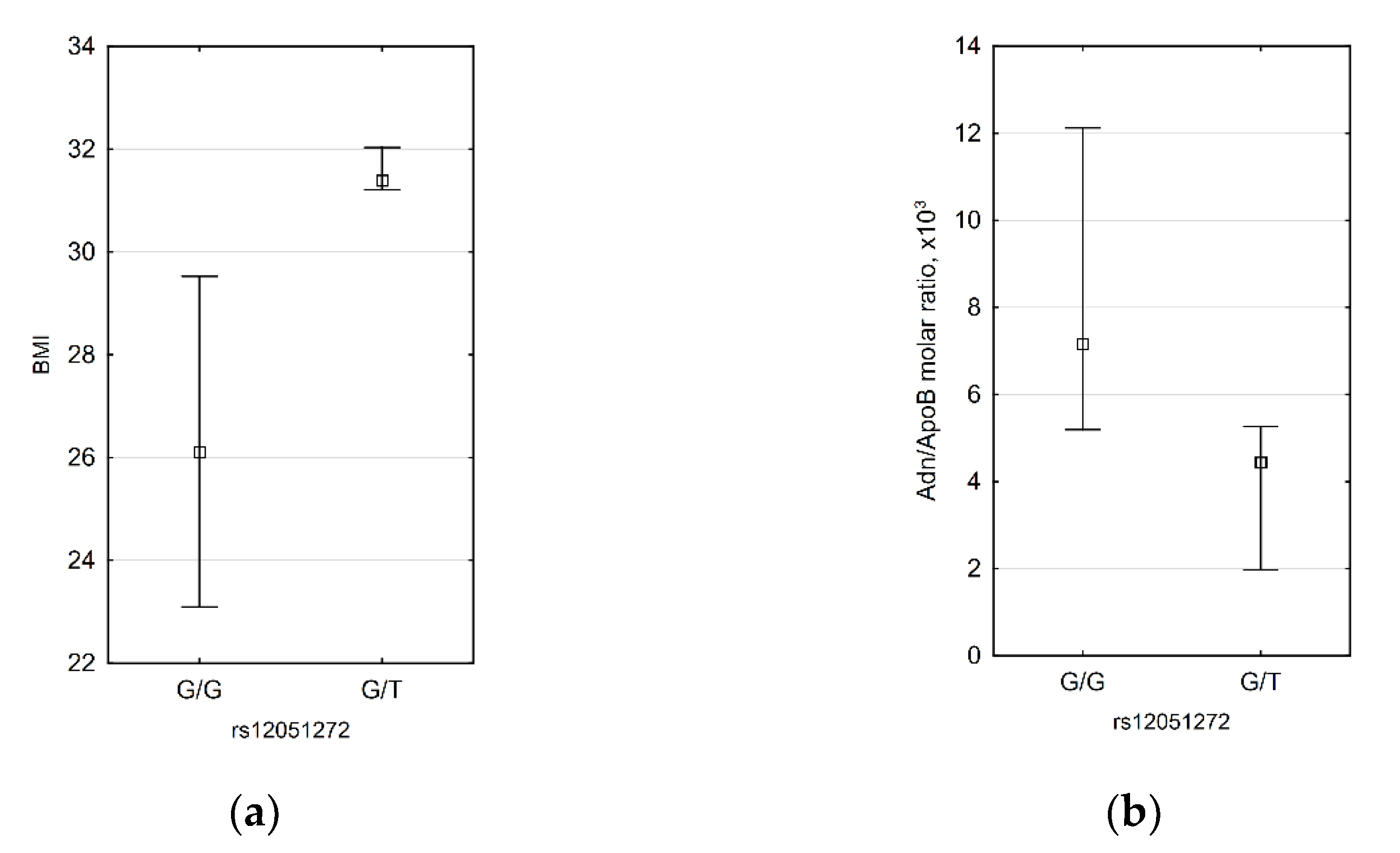
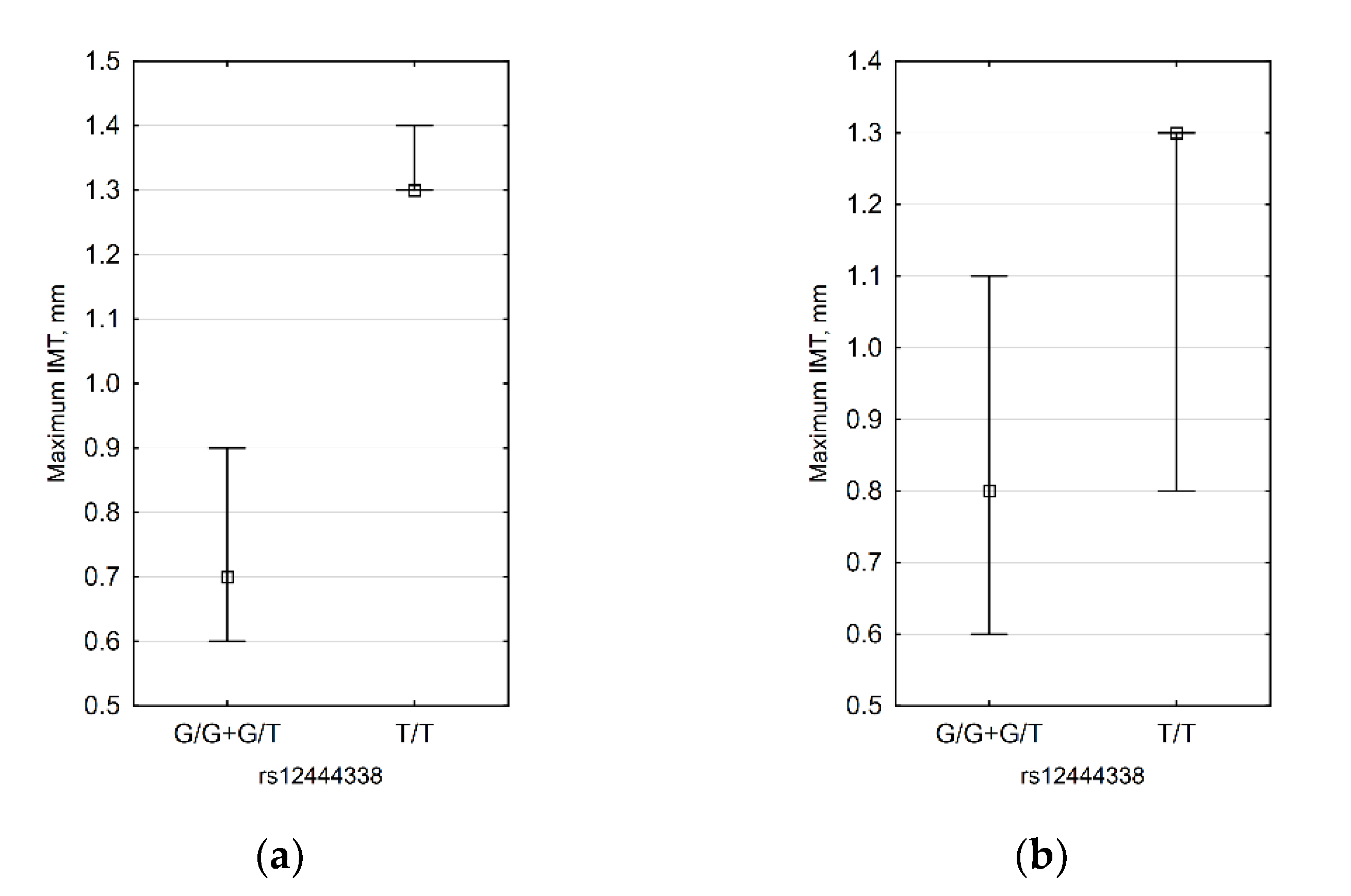
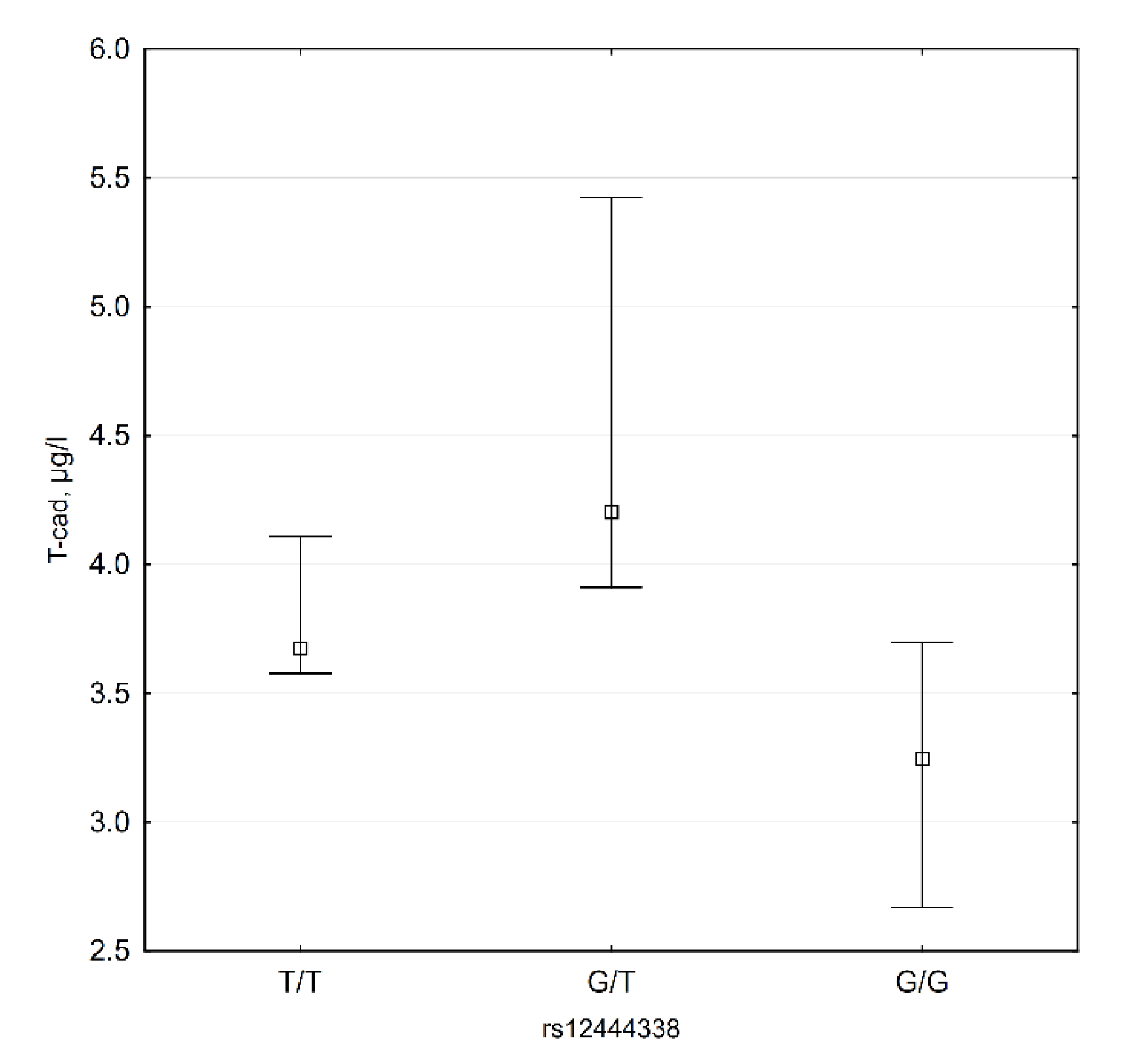
3.4. CDH13 SNP Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| ApoB | HMW Adn 1 | LDL | Adn/ApoB Molar Ratio | Adn/LDL Molar Ratio | T-cad | Carotid IMT, Right | Carotid IMT, Left | Carotid IMT, Max. | |
|---|---|---|---|---|---|---|---|---|---|
| ApoB | −0.277 | 0.805 * | −0.534 * | −0.468 * | −0.098 | −0.058 | −0.025 | −0.140 | |
| HMW Adn | −0.277 | −0.145 | 0.945 * | 0.944 * | 0.303 | −0.075 | −0.145 | −0.104 | |
| LDL | 0.805 * | −0.145 | −0.332 | −0.395 * | 0.187 | 0.146 | 0.114 | 0.022 | |
| Adn/ApoB molar ratio | −0.534 * | 0.945 * | −0.332 | 0.963 * | 0.322 | −0.046 | −0.112 | −0.046 | |
| Adn/LDL molar ratio | −0.468 * | 0.944 * | −0.395 * | 0.963 * | 0.232 | −0.097 | −0.101 | −0.058 | |
| T-cad | −0.098 | 0.303 | 0.187 | 0.322 | 0.232 | 0.075 | 0.068 | −0.006 | |
| Carotid IMT, right | −0.058 | −0.075 | 0.146 | −0.046 | −0.097 | 0.075 | 0.801 * | 0.936 * | |
| Carotid IMT, left | −0.025 | −0.145 | 0.114 | −0.112 | −0.101 | 0.068 | 0.801 * | 0.899 * | |
| Carotid IMT, max. | −0.140 | −0.104 | 0.022 | −0.046 | −0.058 | −0.006 | 0.936 * | 0.899 * |
References
- Goldstein, J.L.; Anderson, R.G.; Brown, M.S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature 1979, 279, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Buhler, F.R.; Tkachuk, V.A.; Hahn, A.W.; Resink, T.J. Low- and high-density lipoproteins as hormonal regulators of platelet, vascular endothelial and smooth muscle cell interactions: Relevance to hypertension. J. Hypertens. Suppl. 1991, 9, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Bjorkerud, S.; Bjorkerud, B. Lipoproteins are major and primary mitogens and growth promoters for human arterial smooth muscle cells and lung fibroblasts in vitro. Arterioscler. Thromb. 1994, 14, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, V.; Tkachuk, V.; Buhler, F.; Resink, T. Phosphoinositide and calcium signalling responses in smooth muscle cells: Comparison between lipoproteins, Ang II, and PDGF. Biochem. Biophys. Res. Commun. 1992, 188, 1295–1304. [Google Scholar] [CrossRef]
- Bochkov, V.N.; Kuz’menko, E.S.; Rezink, T.; Tkachuk, V.A. “Classical” apo B,E-receptor does not mediate the activating effect of low density lipoproteins on the second messenger system in human platelets and vascular smooth muscle cells. Biokhimiia 1994, 59, 1330–1339. [Google Scholar] [PubMed]
- Bochkov, V.N.; Matchin, Y.G.; Fuki, I.V.; Lyakishev, A.A.; Tkachuk, V.A. Platelets in patients with homozygous familial hypercholesterolemia are sensitive to Ca(2+)-mobilizing activity of low density lipoproteins. Atherosclerosis 1992, 96, 119–124. [Google Scholar] [CrossRef]
- Bochkov, V.N.; Tkachuk, V.A.; Hahn, A.W.; Bernhardt, J.; Buhler, F.R.; Resink, T.J. Concerted effects of lipoproteins and angiotensin II on signal transduction processes in vascular smooth muscle cells. Arterioscler. Thromb. 1993, 13, 1261–1269. [Google Scholar] [CrossRef]
- Drobnik, W.; Mollers, C.; Resink, T.; Schmitz, G. Activation of phosphatidylinositol-specific phospholipase C in response to HDL3 and LDL is markedly reduced in cultured fibroblasts from Tangier patients. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1369–1377. [Google Scholar] [CrossRef]
- Kipmen-Korgun, D.; Osibow, K.; Zoratti, C.; Schraml, E.; Greilberger, J.; Kostner, G.; Jürgens, G.; Graier, W. T-Cadherin Mediates Low-Density Lipoprotein–Initiated Cell Proliferation Via the Ca2+-Tyrosine Kinase-Erk 1/2 Phathway. J. Cardiovasc. Pharmacol. 2005, 45, 418–430. [Google Scholar] [CrossRef]
- Orlov, S.; Resink, T.J.; Bernhardt, J.; Ferracin, F.; Buhler, F.R. Vascular smooth muscle cell calcium fluxes. Regulation by angiotensin II and lipoproteins. Hypertension 1993, 21, 195–203. [Google Scholar] [CrossRef]
- Resink, T.J.; Bochkov, V.N.; Tkachuk, V.A.; Buhler, F.R.; Hahn, A.W. Lipoproteins and angiotensin II exert synergistic effects on signalling processes in vascular smooth muscle cells. J. Hypertens. Suppl. 1993, 11, S110–S111. [Google Scholar] [CrossRef] [PubMed]
- Rubina, K.; Talovskaya, E.; Cherenkov, V.; Ivanov, D.; Stambolsky, D.; Storozhevykh, T.; Pinelis, V.; Shevelev, A.; Parfyonova, Y.; Resink, T.; et al. LDL induces intracellular signalling and cell migration via atypical LDL-binding protein T-cadherin. Mol. Cell. Biochem. 2005, 273, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, V.N.; Rozhkova, T.A.; Matchin Yu, G.; Lyakishev, A.A.; Bochkova, N.A.; Borisova Yu, L.; Kukharchuk, V.V.; Tkachuk, V.A. LDL- and agonist-induced Ca(2+)-mobilization in platelets of healthy subjects and in patients with familial hyperlipoproteinemia type II. Thromb. Res. 1991, 61, 403–409. [Google Scholar] [CrossRef]
- Bochkov, V.N.; Voino-Yasenetskaya, T.A.; Tkachuk, V.A. Epinephrine potentiates activation of human platelets by low density lipoproteins. Biochim. Biophys. Acta 1991, 1097, 123–127. [Google Scholar] [CrossRef]
- González-Timón, B.; Gonzalez-Muñoz, M.; Zaragoza, C.; Lamas, S.; Melián, E. Native and oxidized low density lipoproteins oppositely modulate the effects of insulin-like growth factor I on VSMC. Cardiovasc. Res. 2004, 61, 247–255. [Google Scholar] [CrossRef][Green Version]
- Resink, T.J.; Bochkov, V.N.; Hahn, A.W.; Philippova, M.P.; Buhler, F.R.; Tkachuk, V.A. Low- and high-density lipoproteins as mitogenic factors for vascular smooth muscle cells: Individual, additive and synergistic effects. J. Vasc. Res. 1995, 32, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Seewald, S.; Nickenig, G.; Ko, Y.; Vetter, H.; Sachinidis, A. Low density lipoprotein enhances the thrombin-induced growth of vascular smooth muscle cells. Cardiovasc. Res. 1997, 36, 92–100. [Google Scholar] [CrossRef]
- Tkachuk, V.; Bochkov, V.; Philippova, M.; Stambolsky, D. Identification of atypical lipoprotein-binding protein from human aortic smooth muscle as T-cadherin. FEBS Lett. 1998, 421, 208–212. [Google Scholar] [CrossRef]
- Hug, C.; Wang, J.; Ahmad, N.S.; Bogan, J.S.; Tsao, T.S.; Lodish, H.F. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl. Acad. Sci. USA 2004, 101, 10308–10313. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 425, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Fruebis, J.; Tsao, T.S.; Javorschi, S.; Ebbets-Reed, D.; Erickson, M.R.; Yen, F.T.; Bihain, B.E.; Lodish, H.F. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. USA 2001, 98, 2005–2010. [Google Scholar] [CrossRef]
- Hu, E.L.P.; Splegelman, B.M. AdipoQ Is a Novel Adipose-specific Gene Dysregulated in Obesity. J. Biol. Chem. 1996, 271, 10697–10703. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Funahashi, T.; Sakamoto, T.; Miyamoto, S.; Soejima, H.; Hokamaki, J.; Kajiwara, I.; Sugiyama, S.; Yoshimura, M.; Fujimoto, K.; et al. The variation of plasma concentrations of a novel, adipocyte derived protein, adiponectin, in patients with acute myocardial infarction. Heart 2003, 89, 667. [Google Scholar] [CrossRef] [PubMed]
- Kumada, M.; Kihara, S.; Sumitsuji, S. Association of Hypoadiponectinemia With Coronary Artery Disease in Men. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.E.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995, 270, 26746–26749. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Imai, Y.; Shimozawa, N.; Hioki, K.; Uchida, S.; Ito, Y.; Takakuwa, K.; Matsui, J.; et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J. Biol. Chem. 2003, 278, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Zhu, W.; Cheng, K.K.; Vanhoutte, P.M.; Lam, K.S.; Xu, A. Vascular effects of adiponectin: Molecular mechanisms and potential therapeutic intervention. Clin. Sci. 2008, 114, 361–374. [Google Scholar] [CrossRef]
- Ebrahimi-Mamaeghani, M.; Mohammadi, S.; Arefhosseini, S.R.; Fallah, P.; Bazi, Z. Adiponectin as a potential biomarker of vascular disease. Vasc. Health Risk Manag. 2015, 11, 55–70. [Google Scholar] [CrossRef]
- Iglseder, B.; Mackevics, V.; Stadlmayer, A.; Tasch, G.; Ladurner, G.; Paulweber, B. Plasma adiponectin levels and sonographic phenotypes of subclinical carotid artery atherosclerosis: Data from the SAPHIR Study. Stroke 2005, 36, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Gasbarrino, K.; Gorgui, J.; Nauche, B.; Cote, R.; Daskalopoulou, S.S. Circulating adiponectin and carotid intima-media thickness: A systematic review and meta-analysis. Metabolism 2016, 65, 968–986. [Google Scholar] [CrossRef]
- Waki, H.; Yamauchi, T.; Kamon, J.; Ito, Y.; Uchida, S.; Kita, S.; Hara, K.; Hada, Y.; Vasseur, F.; Froguel, P.; et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J. Biol. Chem. 2003, 278, 40352–40363. [Google Scholar] [CrossRef] [PubMed]
- Daniele, A.; De Rosa, A.; De Cristofaro, M.; Monaco, M.L.; Masullo, M.; Porcile, C.; Capasso, M.; Tedeschi, G.; Oriani, G.; Di Costanzo, A. Decreased concentration of adiponectin together with a selective reduction of its high molecular weight oligomers is involved in metabolic complications of myotonic dystrophy type 1. Eur. J. Endocrinol. 2011, 165, 969–975. [Google Scholar] [CrossRef]
- De Rosa, A.; Monaco, M.L.; Nigro, E.; Scudiero, O.; D’Andrea, M.; Pilla, F.; Oriani, G.; Daniele, A. Tissue-specific downregulation of the adiponectin "system": Possible implications for fat accumulation tendency in the pig. Domest. Anim. Endocrinol. 2013, 44, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Horakova, D.; Azeem, K.; Benesova, R.; Pastucha, D.; Horak, V.; Dumbrovska, L.; Martinek, A.; Novotny, D.; Svagera, Z.; Hobzova, M.; et al. Total and High Molecular Weight Adiponectin Levels and Prediction of Cardiovascular Risk in Diabetic Patients. Int. J. Endocrinol. 2015, 545068. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ouchi, N.; Kihara, S.; Walsh, K.; Kumada, M.; Abe, Y.; Funahashi, T.; Matsuzawa, Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ. Res. 2004, 94, e27-31. [Google Scholar] [CrossRef]
- Pajvani, U.B.; Hawkins, M.; Combs, T.P.; Rajala, M.W.; Doebber, T.; Berger, J.P.; Wagner, J.A.; Wu, M.; Knopps, A.; Xiang, A.H.; et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J. Biol. Chem. 2004, 279, 12152–12162. [Google Scholar] [CrossRef] [PubMed]
- Pischon, T.; Hu, F.B.; Girman, C.J.; Rifai, N.; Manson, J.E.; Rexrode, K.M.; Rimm, E.B. Plasma total and high molecular weight adiponectin levels and risk of coronary heart disease in women. Atherosclerosis 2011, 219, 322–329. [Google Scholar] [CrossRef]
- Denzel, M.S.; Scimia, M.C.; Zumstein, P.M.; Walsh, K.; Ruiz-Lozano, P.; Ranscht, B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J. Clin. Investig. 2010, 120, 4342–4352. [Google Scholar] [CrossRef]
- Fujishima, Y.; Maeda, N.; Matsuda, K.; Masuda, S.; Mori, T.; Fukuda, S.; Sekimoto, R.; Yamaoka, M.; Obata, Y.; Kita, S.; et al. Adiponectin association with T-cadherin protects against neointima proliferation and atherosclerosis. FASEB J. 2017, 31, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Parker-Duffen, J.L.; Nakamura, K.; Silver, M.; Kikuchi, R.; Tigges, U.; Yoshida, S.; Denzel, M.S.; Ranscht, B.; Walsh, K. T-cadherin Is Essential for Adiponectin-mediated Revascularization. J. Biol. Chem. 2013, 288, 24886–24897. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.M.; Lin, T.H.; Chen, J.W.; Leu, H.B.; Yang, H.C.; Ho, H.Y.; Ting, C.T.; Sheu, S.H.; Tsai, W.C.; Chen, J.H.; et al. A genome-wide association study reveals a quantitative trait locus of adiponectin on CDH13 that predicts cardiometabolic outcomes. Diabetes 2011, 60, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- Fava, C.; Danese, E.; Montagnana, M.; Sjogren, M.; Almgren, P.; Guidi, G.C.; Hedblad, B.; Engstrom, G.; Lechi, A.; Minuz, P.; et al. A variant upstream of the CDH13 adiponectin receptor gene and metabolic syndrome in Swedes. Am. J. Cardiol. 2011, 108, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Kim, Y.M.; Chen, P.; Igase, M.; Kawamoto, R.; Kim, M.K.; Kohara, K.; Lee, J.; Miki, T.; Ong, R.T.; et al. Genetic variation in CDH13 is associated with lower plasma adiponectin levels but greater adiponectin sensitivity in East Asian populations. Diabetes 2013, 62, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.H.; Sull, J.W.; Lee, J.E.; Shin, C.; Park, J.; Kimm, H.; Cho, E.Y.; Shin, E.S.; Yun, J.E.; Park, J.W.; et al. Adiponectin concentrations: A genome-wide association study. Am. J. Hum. Genet. 2010, 87, 545–552. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.H.; Shin, D.J.; Park, S.; Kang, S.M.; Jang, Y.; Lee, S.H. Association between CDH13 variants and cardiometabolic and vascular phenotypes in a Korean population. Yonsei Med. J. 2013, 54, 1305–1312. [Google Scholar] [CrossRef]
- Morisaki, H.; Yamanaka, I.; Iwai, N.; Miyamoto, Y.; Kokubo, Y.; Okamura, T.; Okayama, A.; Morisaki, T. CDH13 gene coding T-cadherin influences variations in plasma adiponectin levels in the Japanese population. Hum. Mutat. 2012, 33, 402–410. [Google Scholar] [CrossRef]
- Org, E.; Eyheramendy, S.; Juhanson, P.; Gieger, C.; Lichtner, P.; Klopp, N.; Veldre, G.; Doring, A.; Viigimaa, M.; Sober, S.; et al. Genome-wide scan identifies CDH13 as a novel susceptibility locus contributing to blood pressure determination in two European populations. Hum. Mol. Genet. 2009, 18, 2288–2296. [Google Scholar] [CrossRef]
- Teng, M.S.; Hsu, L.A.; Wu, S.; Sun, Y.C.; Juan, S.H.; Ko, Y.L. Association of CDH13 genotypes/haplotypes with circulating adiponectin levels, metabolic syndrome, and related metabolic phenotypes: The role of the suppression effect. PLoS ONE 2015, 10, e0122664. [Google Scholar] [CrossRef]
- Balatsky, A.V.; Konovalov, D.Y.; Samokhodskaya, L.M.; Kochegura, T.N.; Rubina, K.A.; Tkachuk, V.A. Single Nucleotide Polymorphisms in T-Cadherin Gene (CDH13) Have Cumulative Effect on Body Mass in Patients With Ischemic Heart Disease. Kardiologiia 2015, 55, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Consortium, W.T.C.C. Genome-wide association study of 14,000 cases of seven common diseases and 3000 shared controls. Nature 2007, 447, 661–678. [Google Scholar] [CrossRef]
- Levy, D.; Larson, M.G.; Benjamin, E.J.; Newton-Cheh, C.; Wang, T.J.; Hwang, S.J.; Vasan, R.S.; Mitchell, G.F. Framingham Heart Study 100K Project: Genome-wide associations for blood pressure and arterial stiffness. BMC Med. Genet. 2007, 8, 1–11. [Google Scholar] [CrossRef]
- Menzaghi, C.; Trischitta, V. The Adiponectin Paradox for All-Cause and Cardiovascular Mortality. Diabetes 2018, 67, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, V.N.; Tkachuk, V.A.; Kuzmenko, Y.S.; Borisova, Y.L.; Buhler, F.R.; Resink, T.J. Characteristics of low and high density lipoprotein binding and lipoprotein-induced signaling in quiescent human vascular smooth muscle cells. Mol. Pharmacol. 1994, 45, 262–270. [Google Scholar] [PubMed]
- Tkachuk, V.A.; Kuzmenko, Y.S.; Resink, T.J.; Stambolsky, D.V.; Bochkov, V.N. Atypical Low-Density-Lipoprotein Binding-Site That May Mediate Lipoprotein-Induced Signal-Transduction. Mol. Pharmacol. 1994, 46, 1129–1137. [Google Scholar]
- Fukuda, S.; Kita, S.; Obata, Y.; Fujishima, Y.; Nagao, H.; Masuda, S.; Tanaka, Y.; Nishizawa, H.; Funahashi, T.; Takagi, J.; et al. The unique prodomain of T-cadherin plays a key role in adiponectin binding with the essential extracellular cadherin repeats 1 and 2. J. Biol. Chem. 2017. [Google Scholar] [CrossRef]
- Balatskaya, M.N.; Balatskii, A.V.; Sharonov, G.V.; Tkachuk, V.A. T-cadherin as a novel receptor regulating metabolism in the blood vessel and heart cells: From structure to function. J Evol Biochem Phys. 2016, 52, 103–118. [Google Scholar] [CrossRef]
- Rubina, K.A.; Semina, E.A.; Balatskaya, M.N.; Plekhanova, O.S.; Tkachuk, V.A. Mechanisms of regulation of the directed growth of vessels and nerves by the fibrinolytic system components and GPI-anchored navigation receptors. Neurosci. Behav. Physiol. 2018, 1001–1026. [Google Scholar] [CrossRef]
- Matsuda, K.; Fujishima, Y.; Maeda, N.; Mori, T.; Hirata, A.; Sekimoto, R.; Tsushima, Y.; Masuda, S.; Yamaoka, M.; Inoue, K.; et al. Positive feedback regulation between adiponectin and T-cadherin impacts adiponectin levels in tissue and plasma of male mice. Endocrinology 2014, en20141618. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.; Philippova, M.; Antropova, J.; Gubaeva, F.; Iljinskaya, O.; Tararak, E.; Bochkov, V.; Erne, P.; Resink, T.; Tkachuk, V. Expression of cell adhesion molecule T-cadherin in the human vasculature. Histochem. Cell Biol. 2001, 115, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Sole, X.; Guino, E.; Valls, J.; Iniesta, R.; Moreno, V. SNPStats: A web tool for the analysis of association studies. Bioinformatics 2006, 22, 1928–1929. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Putku, M.; Kals, M.; Inno, R.; Kasela, S.; Org, E.; Kozich, V.; Milani, L.; Laan, M. CDH13 promoter SNPs with pleiotropic effect on cardiometabolic parameters represent methylation QTLs. Hum. Genet. 2015, 134, 291–303. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Lange, E.M.; Croteau-Chonka, D.C.; Kuzawa, C.W.; McDade, T.W.; Qin, L.; Curocichin, G.; Borja, J.B.; Lange, L.A.; et al. Genome-wide association study for adiponectin levels in Filipino women identifies CDH13 and a novel uncommon haplotype at KNG1-ADIPOQ. Hum. Mol. Genet. 2010, 19, 4955–4964. [Google Scholar] [CrossRef]
- Uetani, E.; Tabara, Y.; Kawamoto, R.; Onuma, H.; Kohara, K.; Osawa, H.; Miki, T. CDH13 genotype-dependent association of high-molecular weight adiponectin with all-cause mortality: The J-SHIPP study. Diabetes Care 2014, 37, 396–401. [Google Scholar] [CrossRef][Green Version]
- Mansouri, M.; Heshmat, R.; Tabatabaei-Malazy, O.; Sharifi, F.; Badamchizadeh, Z.; Alatab, S.; Omidfar, K.; Fakhrzadeh, H.; Larijani, B. The association of carotid intima media thickness with retinol binding protein-4 and total and high molecular weight adiponectin in type 2 diabetic patients. J. Diabetes Metab. Disord. 2012, 11, 2. [Google Scholar] [CrossRef][Green Version]
- Song, F.; Zou, J.; Song, Z.; Xu, H.; Qian, Y.; Zhu, H.; Liu, S.; Guan, J.; Chen, J.; Yi, H. Association of Adipocytokines With Carotid Intima Media Thickness and Arterial Stiffness in Obstructive Sleep Apnea Patients. Front. Endocrinol. 2020, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.M.; Faienza, M.F.; Altomare, M.; Nacci, C.; Montagnani, M.; Valente, F.; Cortese, F.; Gesualdo, M.; Zito, A.; Mancarella, R.; et al. Endothelial and Metabolic Function Interactions in Overweight/Obese Children. J. Atheroscler. Thromb. 2016, 23, 950–959. [Google Scholar] [CrossRef]
- Tsushima, M.; Terayama, Y.; Momose, A.; Funyu, T.; Ohyama, C. Progression of atherosclerosis in hemodialysis patients: Effect of adiponectin on carotid intima media thickness. J. Atheroscler. Thromb. 2008, 15, 213–218. [Google Scholar] [CrossRef]
- Brovin, D.L.; Belyaeva, O.D.; Pchelina, S.N.; Berezina, A.V.; Karonova, T.L.; Bazhenova, E.A.; Kolodina, D.A.; Bakulina, A.S.; Polyakova, E.A.; Listopad, O.V.; et al. Common Carotid Intima-Media Thickness, Levels of Total and High-Molecular Weight Adiponectin in Women With Abdominal Obesity. Kardiologiia 2018, 58, 29–36. [Google Scholar] [CrossRef] [PubMed]
- von Eynatten, M.; Humpert, P.M.; Bluemm, A.; Lepper, P.M.; Hamann, A.; Allolio, B.; Nawroth, P.P.; Bierhaus, A.; Dugi, K.A. High-molecular weight adiponectin is independently associated with the extent of coronary artery disease in men. Atherosclerosis 2008, 199, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Almer, G.; Haj-Yahya, S.; Pilz, S.; Gasser, R.; Moller, R.; Horejsi, R. Preatherosclerosis and adiponectin subfractions in obese adolescents. Obesity (Silver Spring) 2008, 16, 2578–2584. [Google Scholar] [CrossRef]
- Takamura, N.; Hayashida, N.; Hagane, K.; Kadota, K.; Yamasaki, H.; Abiru, N.; Ozono, Y.; Kamihira, S.; Aoyagi, K.; Ishibashi, K.; et al. Leptin to high-molecular-weight adiponectin ratio is independently correlated with carotid intima-media thickness in men, but not in women. Biomarkers 2010, 15, 340–344. [Google Scholar] [CrossRef]
- Swiger, K.J.; Martin, S.S.; Blaha, M.J.; Toth, P.P.; Nasir, K.; Michos, E.D.; Gerstenblith, G.; Blumenthal, R.S.; Jones, S.R. Narrowing sex differences in lipoprotein cholesterol subclasses following mid-life: The very large database of lipids (VLDL-10B). J. Am. Heart Assoc. 2014, 3, e000851. [Google Scholar] [CrossRef] [PubMed]
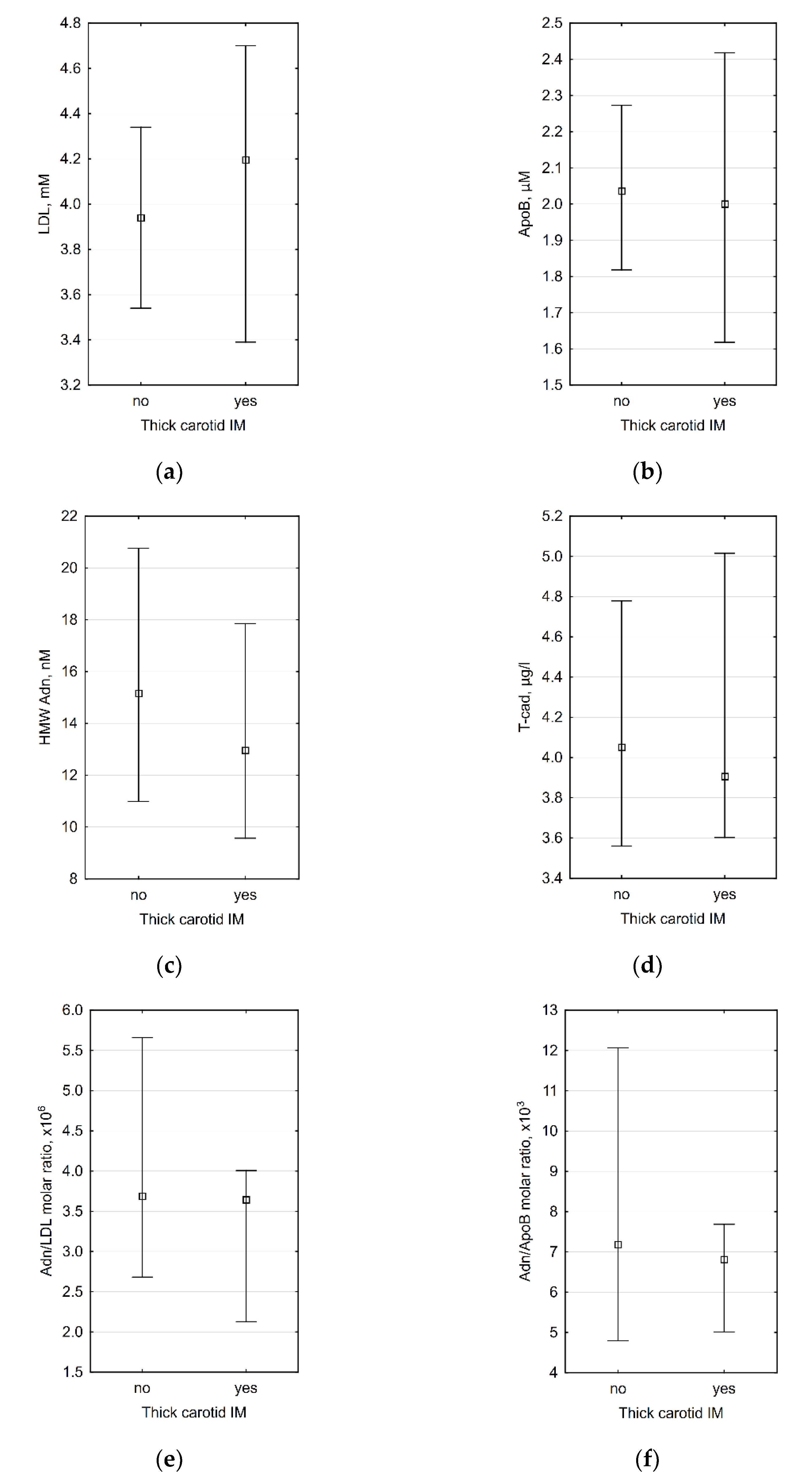
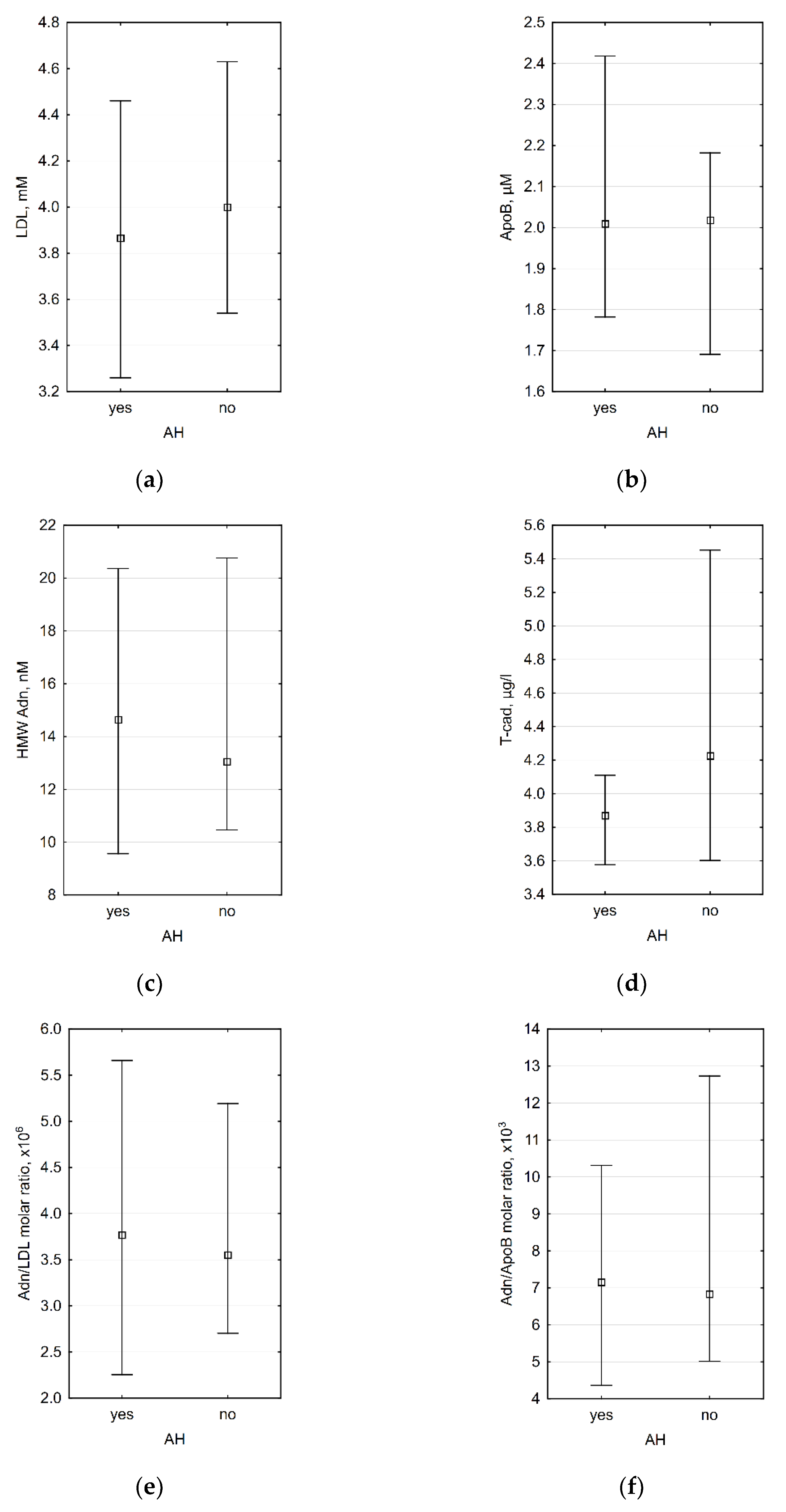
| IMT ≤ 0.9 mm, n = 21 | IMT > 0.9 mm, n = 14 | p IMT ≤ 0.9 vs. IMT > 0.9 mm | AH, n = 18 | No AH, n = 17 | p AH vs. No AH | |
|---|---|---|---|---|---|---|
| Sex Male, n (%) Female, n (%) | ||||||
| 6 (28.6) 15 (71.4) | 4 (28.6) 10 (71.40) | 0.703 | 5 (27. 8) 13 (72.2) | 5 (29.4) 12 (70.6) | 0.789 | |
| Age, years | 54 (50; 59) | 52 (48; 60) | 0.661 | 51.5 (48; 60) | 55 (50; 59) | 0.437 |
| Obesity, n (%) | 6 (28.6) | 4 (28.6) | 0.990 | 7 (38.9) | 3 (17.7) | 0.310 |
| AH, n (%) | 12 (57.1) | 6 (42.9) | 0.629 | 18 (100) | 0 (0) | - |
| IMT >0.9 mm, n (%) | 0 (0) | 14 (100) | - | 6 (33.3) | 8 (47.1) | 0.629 |
| ApoB | HMW Adn | Adn/ApoB Molar Ratio | Adn/LDL Molar Ratio | |
|---|---|---|---|---|
| Carotid IMT, right | 0.308 | −0.828 * | −0.656 * | −0.779 * |
| Carotid IMT, left | 0.080 | −0.722 * | −0.489 | −0.538 |
| Carotid IMT, max. | 0.095 | −0.746 * | −0.502 | −0.575 |
| BMI | 0.693 * | −0.612 | −0.721 * | −0.612 |
| Model | Genotype | IMT ≤ 0.9 mm. n(%) | IMT > 0.9 mm. n(%) | OR (95% CI) | p | AIC |
|---|---|---|---|---|---|---|
| Codominant | T/T | 5 (23.8%) | 8 (57.1%) | 1.00 | 0.032 | 46.2 |
| G/T | 12 (57.1%) | 6 (42.9%) | 0.31 (0.07–1.38) | |||
| G/G | 4 (19.1%) | 0 (0%) | 0.00 (0.00–NA) | |||
| Dominant | T/T | 5 (23.8%) | 8 (57.1%) | 1.00 | 0.045 | 47.1 |
| G/T-G/G | 16 (76.2%) | 6 (42.9%) | 0.23 (0.05–1.01) | |||
| Recessive | T/T-G/T | 17 (81%) | 14 (100%) | 1.00 | 0.035 | 46.7 |
| G/G | 4 (19.1%) | 0 (0%) | 0.00 (0.00–NA) | |||
| Overdominant | T/T-G/G | 9 (42.9%) | 8 (57.1%) | 1.00 | 0.41 | 50.4 |
| G/T | 12 (57.1%) | 6 (42.9%) | 0.56 (0.14–2.21) | |||
| Log-additive | --- | --- | --- | 0.23 (0.06–0.84) | 0.015 | 45.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balatskiy, A.; Teterina, M.; Pisaryuk, A.; Balabanenko, I.; Kadrev, A.; Tishuk, A.; Balatskaya, M.; Samokhodskaya, L.; Boytsov, S.; Kalinina, N.; et al. T-Cadherin and the Ratio of Its Ligands as Predictors of Carotid Atherosclerosis: A Pilot Study. Biomedicines 2021, 9, 1398. https://doi.org/10.3390/biomedicines9101398
Balatskiy A, Teterina M, Pisaryuk A, Balabanenko I, Kadrev A, Tishuk A, Balatskaya M, Samokhodskaya L, Boytsov S, Kalinina N, et al. T-Cadherin and the Ratio of Its Ligands as Predictors of Carotid Atherosclerosis: A Pilot Study. Biomedicines. 2021; 9(10):1398. https://doi.org/10.3390/biomedicines9101398
Chicago/Turabian StyleBalatskiy, Alexander, Marina Teterina, Alexandra Pisaryuk, Irina Balabanenko, Alexey Kadrev, Anastasia Tishuk, Maria Balatskaya, Larisa Samokhodskaya, Sergey Boytsov, Natalia Kalinina, and et al. 2021. "T-Cadherin and the Ratio of Its Ligands as Predictors of Carotid Atherosclerosis: A Pilot Study" Biomedicines 9, no. 10: 1398. https://doi.org/10.3390/biomedicines9101398
APA StyleBalatskiy, A., Teterina, M., Pisaryuk, A., Balabanenko, I., Kadrev, A., Tishuk, A., Balatskaya, M., Samokhodskaya, L., Boytsov, S., Kalinina, N., & Tkachuk, V. (2021). T-Cadherin and the Ratio of Its Ligands as Predictors of Carotid Atherosclerosis: A Pilot Study. Biomedicines, 9(10), 1398. https://doi.org/10.3390/biomedicines9101398







