Novel Biodegradable Composite of Calcium Phosphate Cement and the Collagen I Mimetic P-15 for Pedicle Screw Augmentation in Osteoporotic Bone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cement Formulation
2.2. Cell Culture
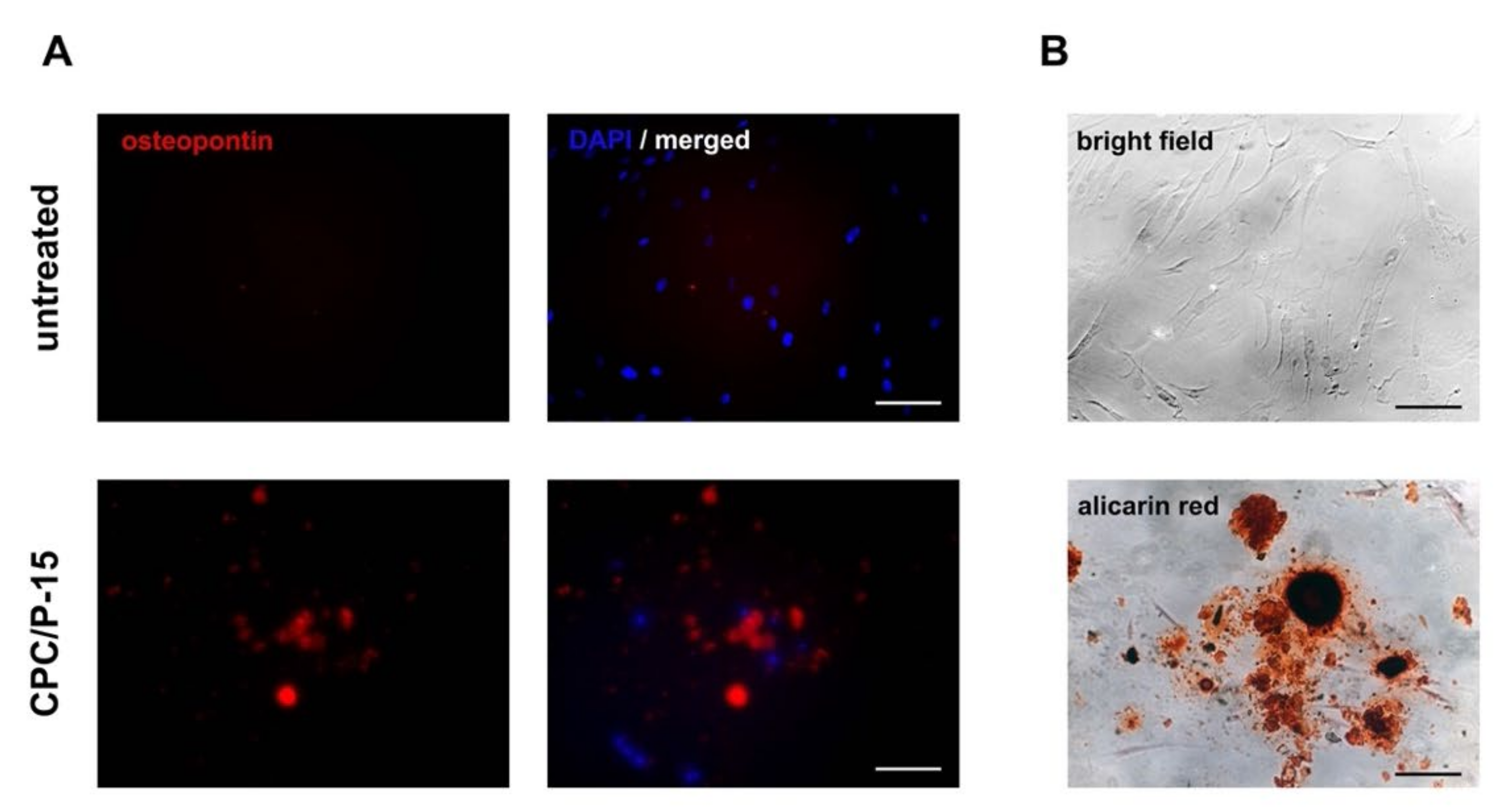
2.3. Immunostaining
2.4. Real-Time PCR
2.5. Specimen Preparation and Screw Insertion
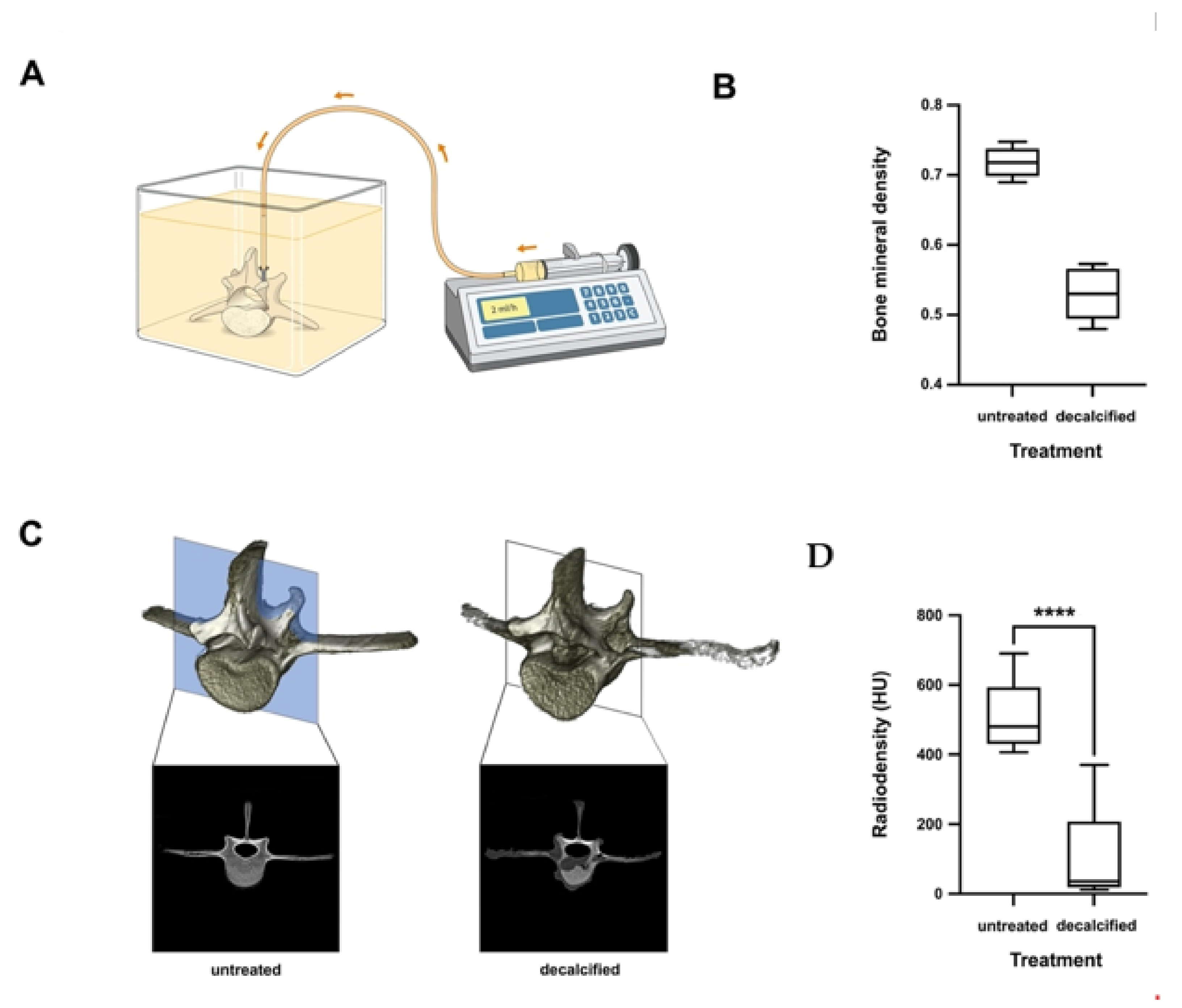
2.6. Decalcification
2.7. Imaging and Bone Mineral Density Measurement
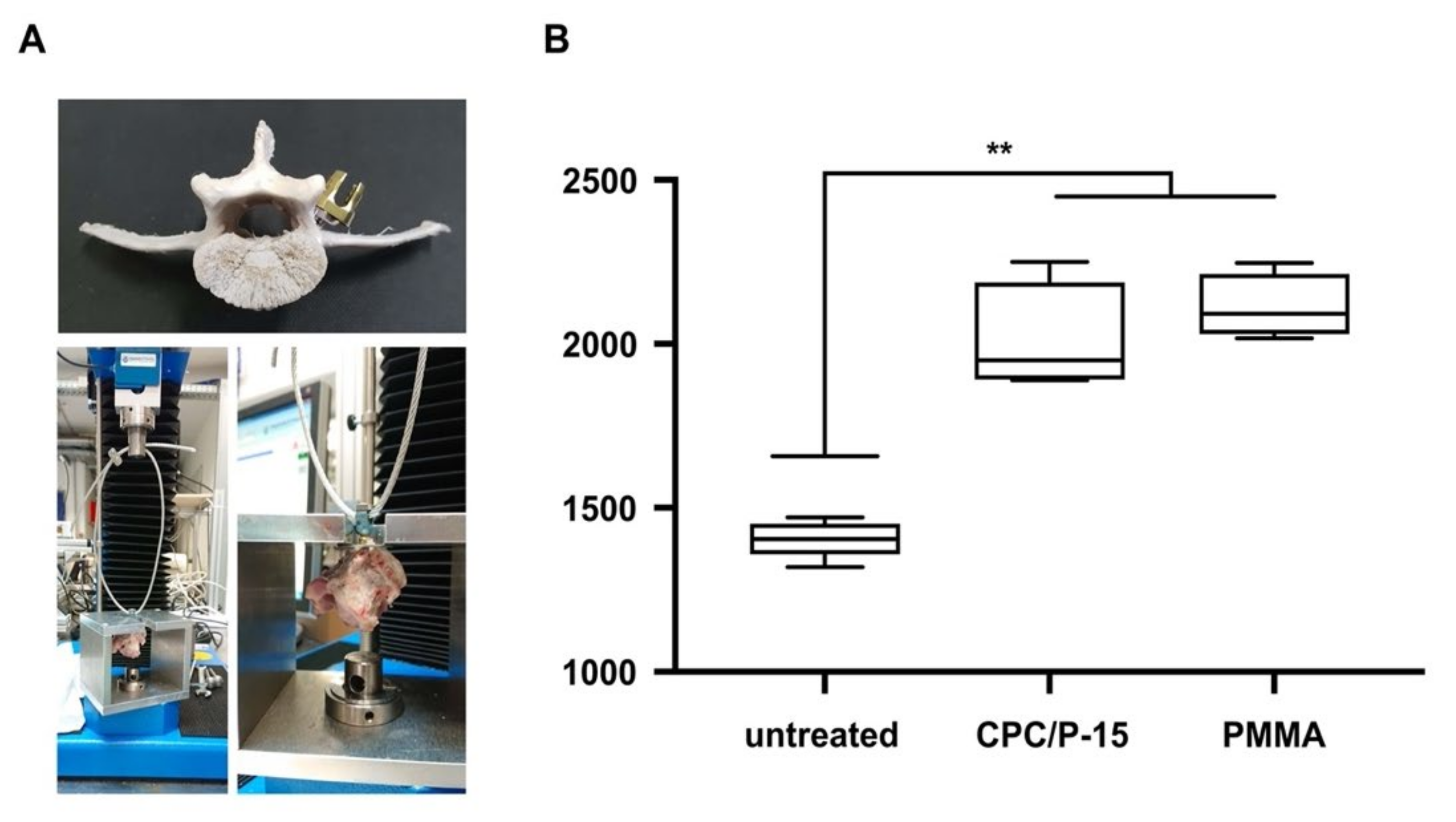
2.8. Pullout Tests
2.9. Statistical Analysis
3. Results
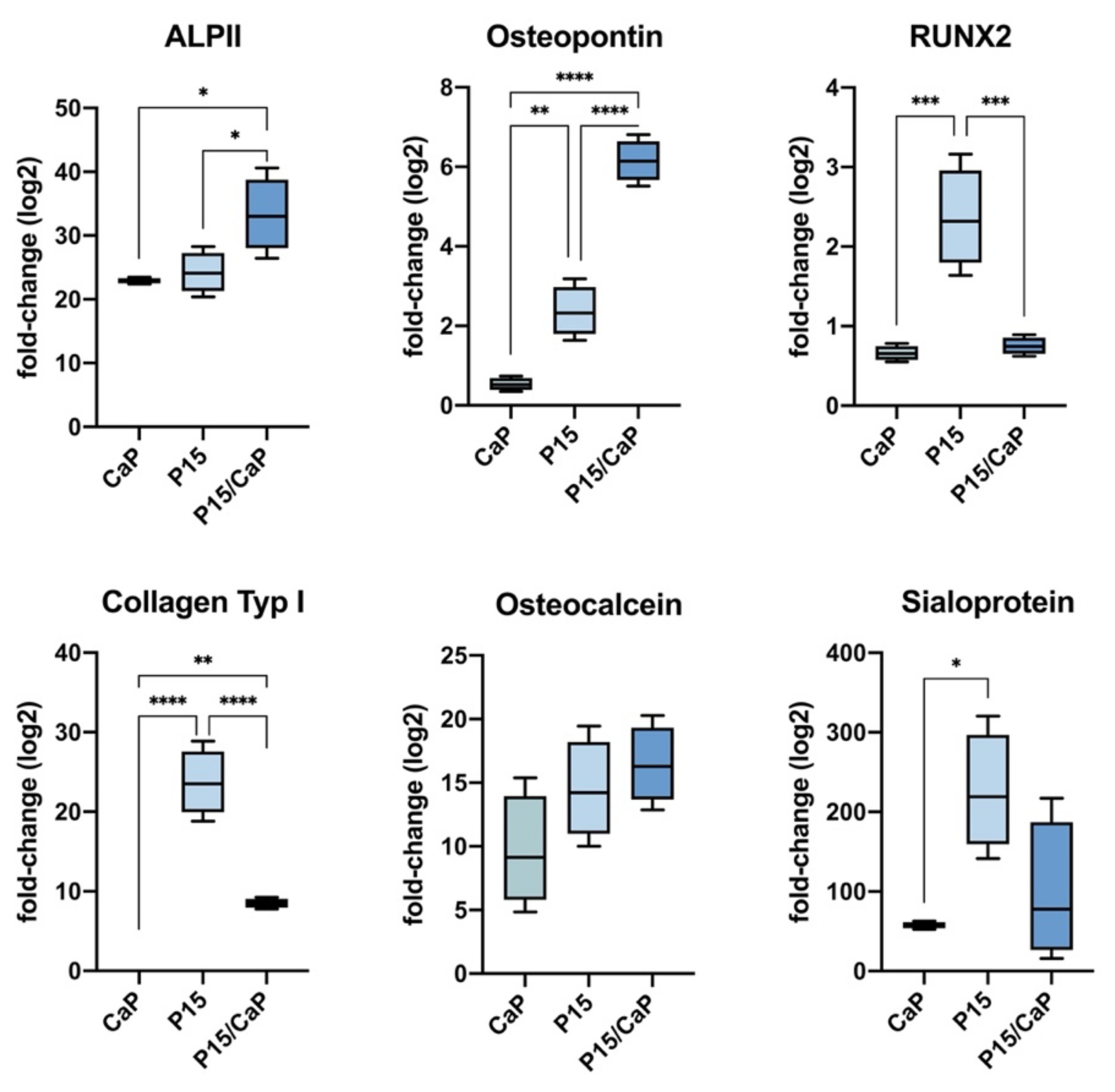
3.1. Osteodifferentiation In Vitro
3.2. Axial Pullout Test and BMD in Non-Osteoporotic Bone
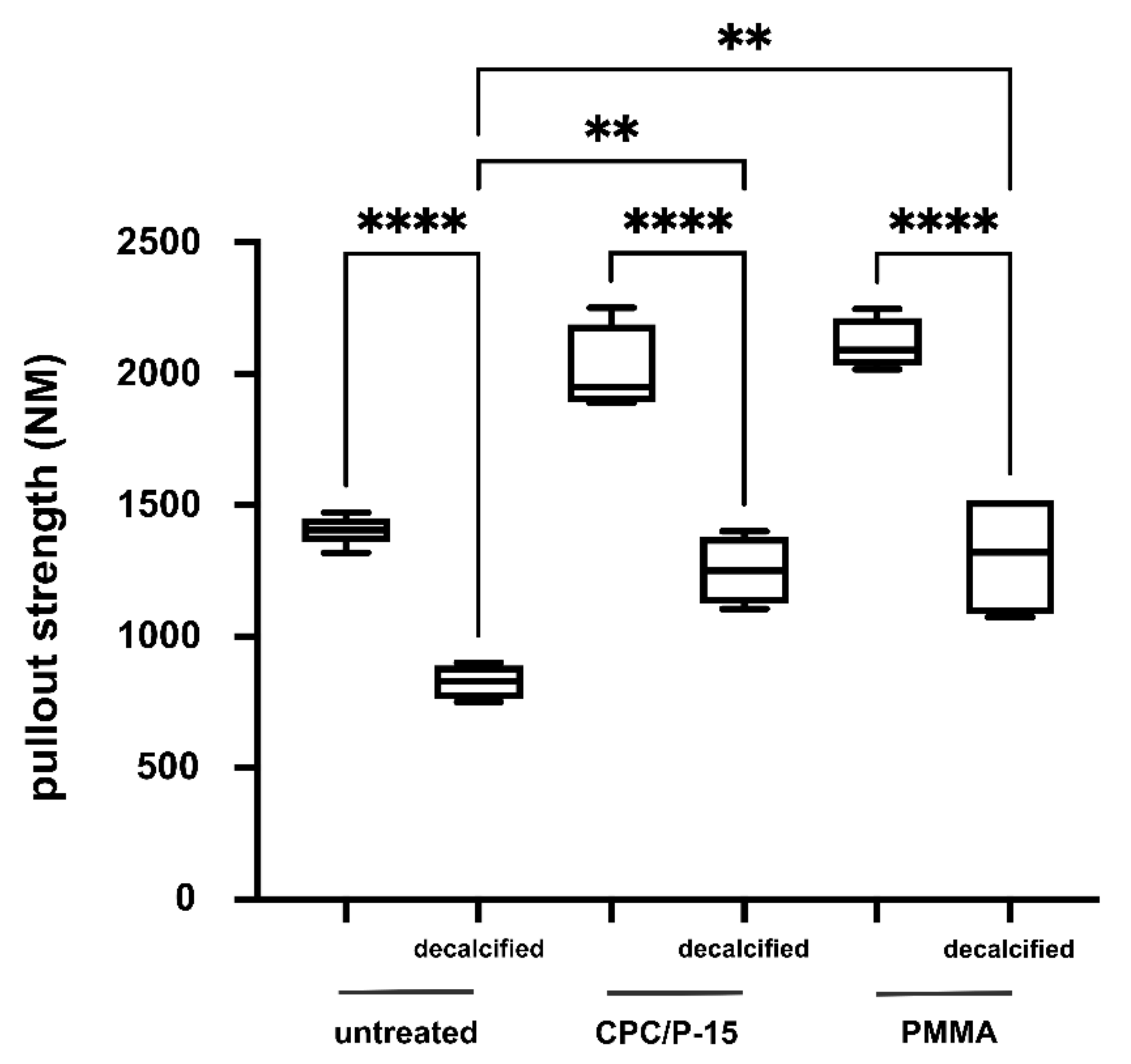
3.3. Axial Pullout Test and BMD in Osteoporotic Bone
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khosla, S. Pathogenesis of Osteoporosis. Transl. Endocrinol. Metab. Metab. Surg. Update 2010, 1, 55–86. [Google Scholar]
- Porter, J.L.; Varacallo, M.; Castono, M. Osteoporosis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK568781/ (accessed on 27 September 2021).
- Rosen, C.J. The Epidemiology and Pathogenesis of Osteoporosis. [Updated 2020 Jun 21]. South Dartmouth 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279134/ (accessed on 27 September 2021).
- Sandhu, S.K.; Hampson, G. The pathogenesis, diagnosis, investigation and management of osteoporosis. J. Clin. Pathol. 2011, 64, 1042–1050. [Google Scholar] [CrossRef] [Green Version]
- Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [CrossRef]
- Styrkarsdottir, U.; Halldórsson, B.; Gretarsdottir, S.; Gudbjartsson, D.; Walters, G.B.; Ingvarsson, T.; Jonsdottir, T.; Saemundsdottir, J.; Snorradóttir, S.; Center, J.; et al. New sequence variants associated with bone mineral density. Nat. Genet. 2008, 41, 15–17. [Google Scholar] [CrossRef]
- Winsloe, C.; Earl, S.; Dennison, E.; Cooper, C.; Harvey, N.C. Early life factors in the pathogenesis of osteoporosis. Curr. Osteoporos. Rep. 2009, 7, 140–144. [Google Scholar] [CrossRef]
- Rowe, P.; Koller, A.; Sharma, S. Physiology, Bone Remodeling. StatPearls Publishing; 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499863/ (accessed on 27 September 2021).
- Braunstein, V.; Sprecher, C.M.; Gisep, A.; Benneker, L.; Yen, K.; Schneider, E.; Heini, P.; Milz, S. Long-term reaction to bone cement in osteoporotic bone: New bone formation in vertebral bodies after vertebroplasty. J. Anat. 2008, 212, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Häussler, B.; Gothe, H.; Göl, D.; Glaeske, G.; Pientka, L.; Felsenberg, D. Epidemiology, treatment and costs of osteoporosis in Germany—the BoneEVA Study. Osteoporos. Int. 2007, 18, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.M.; Aspray, T.; Hide, G.; Sutcliffe, A.M.; Wilkinson, P. Back pain in osteoporotic vertebral fractures. Osteoporos. Int. 2007, 19, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.K.; Lee, S.; Choi, D.J.; Park, S.Y.; Woo, D.G.; Kim, C.H.; Kim, H.S. Mechanical Properties of Blood-Mixed Polymethylmetacrylate in Percutaneous Vertebroplasty. Asian Spine J. 2009, 3, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Bjerke, B.T.; Zarrabian, M.; Aleem, I.S.; Fogelson, J.L.; Currier, B.L.; Freedman, B.A.; Bydon, M.; Nassr, A. Incidence of Osteoporosis-Related Complications Following Posterior Lumbar Fusion. Glob. Spine J. 2018, 8, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, J.J.; Hughes, A.P.; Taher, F.; Girardi, F.P. An Association Can Be Found Between Hounsfield Units and Success of Lumbar Spine Fusion. HSS J. ® 2014, 10, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, S.; Chavanne, A.; Spitaler, R.; Kropik, K.; Aigner, N.; Ogon, M.; Redl, H. Assessment of different screw augmentation techniques and screw designs in osteoporotic spines. Eur. Spine J. 2008, 17, 1462–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, C.R.; Hanson, G.; Eller, M.; Lehman, R.A. A Systematic Review of Treatment Strategies for Degenerative Lumbar Spine Fusion Surgery in Patients with Osteoporosis. Geriatr. Orthop. Surg. Rehabilitation 2016, 7, 188–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, B.J.; Cho, B.Y.; Choi, E.Y.; Zhang, H.Y. Polymethylmethacrylate-Augmented Screw Fixation for Stabilization of the Osteoporotic Spine: A Three-Year Follow-Up of 37 Patients. J. Korean Neurosurg. Soc. 2009, 46, 305–311. [Google Scholar] [CrossRef]
- Yousefi, A.-M. A review of calcium phosphate cements and acrylic bone cements as injectable materials for bone repair and implant fixation. J. Appl. Biomater. Funct. Mater. 2019, 17, 17. [Google Scholar] [CrossRef]
- Frick, C.; Dietz, A.C.; Merritt, K.; Umbreit, T.H.; Tomazic-Jezic, V.J. Effects of prosthetic materials on the host immune response: Evaluation of polymethyl-methacrylate (PMMA), polyethylene (PE), and polystyrene (PS) particles. J. Autom. Inf. Sci. 2006, 16, 423–433. [Google Scholar] [CrossRef]
- Rometsch, E.; Spruit, M.; Zigler, J.E.; Menon, V.K.; Ouellet, J.A.; Mazel, C.; Härtl, R.; Espinoza, K.; Kandziora, F. Screw-Related Complications After Instrumentation of the Osteoporotic Spine: A Systematic Literature Review with Meta-Analysis. Glob. Spine J. 2020, 10, 69–88. [Google Scholar] [CrossRef] [Green Version]
- Esses, S.I.; Sachs, B.L.; Dreyzin, V. Complications Associated with the Technique of Pedicle Screw Fixation a Selected Survey of ABS Members. Spine 1993, 18, 2231–2239. [Google Scholar] [CrossRef]
- Chow, L.; Takagi, S. A natural bone cement—A laboratory novelty led to the development of revolutionary new biomaterials. J. Res. Natl. Inst. Stand. Technol. 2001, 106, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Cicek, G.; Aksoy, E.A.; Durucan, C.; Hasirci, N. Alpha-tricalcium phosphate (α-TCP): Solid state synthesis from different calcium precursors and the hydraulic reactivity. J. Mater. Sci. Mater. Electron. 2011, 22, 809–817. [Google Scholar] [CrossRef]
- Aghyarian, S.; Hu, X.; Haddas, R.; Lieberman, I.H.; Kosmopoulos, V.; Kim, H.K.; Rodrigues, D.C. Biomechanical behavior of novel composite PMMA-CaP bone cements in an anatomically accurate cadaveric vertebroplasty model. J. Orthop. Res. 2017, 35, 2067–2074. [Google Scholar] [CrossRef] [Green Version]
- Gomar, F.; Orozco, R.; Villar, J.L.; Arrizabalaga, F. P-15 small peptide bone graft substitute in the treatment of non-unions and delayed union. A pilot clinical trial. Int. Orthop. 2006, 31, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobbs, R.J.; Maharaj, M.; Rao, P.J. Clinical outcomes and fusion rates following anterior lumbar interbody fusion with bone graft substitute i-FACTOR, an anorganic bone matrix/P-15 composite. J. Neurosurgery: Spine 2014, 21, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Brüstle, I.; Simmet, T.; Nienhaus, G.U.; Landfester, K.; Mailänder, V. Hematopoietic and mesenchymal stem cells: Polymeric nanoparticle uptake and lineage differentiation. Beilstein J. Nanotechnol. 2015, 6, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Chin, D.K.; Park, J.Y.; Yoon, Y.S.; Kuh, S.; Jin, B.H.; Kim, K.S.; Cho, Y.-E. Prevalence of osteoporosis in patients requiring spine surgery: Incidence and significance of osteoporosis in spine disease. Osteoporos. Int. 2007, 18, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Tempel, Z.J.; Gandhoke, G.S.; Okonkwo, D.O.; Kanter, A.S. Impaired bone mineral density as a predictor of graft subsidence following minimally invasive transpsoas lateral lumbar interbody fusion. Eur. Spine J. 2015, 24, 414–419. [Google Scholar] [CrossRef]
- Weiser, L.; Huber, G.; Sellenschloh, K.; Viezens, L.; Püschel, K.; Morlock, M.; Lehmann, W. Insufficient stability of pedicle screws in osteoporotic vertebrae: Biomechanical correlation of bone mineral density and pedicle screw fixation strength. Eur. Spine J. 2017, 26, 2891–2897. [Google Scholar] [CrossRef]
- Ohtori, S.; Inoue, G.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kishida, S.; Kuniyoshi, K.; Aoki, Y.; Nakamura, J.; et al. Comparison of Teriparatide and Bisphosphonate Treatment to Reduce Pedicle Screw Loosening After Lumbar Spinal Fusion Surgery in Postmenopausal Women with Osteoporosis from a Bone Quality Perspective. Spine 2013, 38, E487–E492. [Google Scholar] [CrossRef]
- Ohtori, S.; Inoue, G.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kishida, S.; Kuniyoshi, K.; Aoki, Y.; Nakamura, J.; et al. Teriparatide Accelerates Lumbar Posterolateral Fusion in Women with Postmenopausal Osteoporosis. Spine 2012, 37, E1464–E1468. [Google Scholar] [CrossRef]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, A.M. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Liu, C.; Wang, D.; Liu, H.; Yang, H.; Yang, L. Biomechanical evaluation of calcium phosphate-based nanocomposite versus polymethylmethacrylate cement for percutaneous kyphoplasty. Spine J. 2019, 19, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Blattert, T.R.; Jestaedt, L.; Weckbach, A. Suitability of a Calcium Phosphate Cement in Osteoporotic Vertebral Body Fracture Augmentation. Spine 2009, 34, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Arkin, V.H.; Narendrakumar, U.; Madhyastha, H.; Manjubala, I. Characterization and In Vitro Evaluations of Injectable Calcium Phosphate Cement Doped with Magnesium and Strontium. ACS Omega 2021, 6, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.-P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef]
- Takechi, M.; Miyamoto, Y.; Ishikawa, K.; Nagayama, M.; Kon, M.; Asaoka, K.; Suzuki, K. Effects of added antibiotics on the basic properties of anti-washout-type fast-setting calcium phosphate cement. J. Biomed. Mater. Res. 1998, 39, 308–316. [Google Scholar] [CrossRef]
- Verron, E.; Pissonnier, M.-L.; Lesoeur, J.; Schnitzler, V.; Fellah, B.H.; Pascal-Moussellard, H.; Pilet, P.; Gauthier, O.; Bouler, J.-M. Vertebroplasty using bisphosphonate-loaded calcium phosphate cement in a standardized vertebral body bone defect in an osteoporotic sheep model. Acta Biomater. 2014, 10, 4887–4895. [Google Scholar] [CrossRef]
- Ohe, M.; Moridaira, H.; Inami, S.; Takeuchi, D.; Nohara, Y.; Taneichi, H. Pedicle screws with a thin hydroxyapatite coating for improving fixation at the bone-implant interface in the osteoporotic spine: Experimental study in a porcine model. J. Neurosurgery: Spine 2018, 28, 679–687. [Google Scholar] [CrossRef]
- Sandén, B.; Olerud, C.; Petrén-Mallmin, M.; Larsson, S. Hydroxyapatite coating improves fixation of pedicle screws: A clinical study. J. Bone Jt. Surgery. Br. Vol. 2002, 84, 387–391. [Google Scholar] [CrossRef]
- Moore, D.C.; Maitra, R.S.; Farjo, L.A.; Graziano, G.P.; Goldstein, S.A. Restoration of Pedicle Screw Fixation With an in Situ Setting Calcium Phosphate Cement. Spine 1997, 22, 1696–1705. [Google Scholar] [CrossRef]
- Taniwaki, Y.; Takemasa, R.; Tani, T.; Mizobuchi, H.; Yamamoto, H. Enhancement of pedicle screw stability using calcium phosphatecement in osteoporotic vertebrae: In vivo biomechanical study. J. Orthop. Sci. 2003, 8, 408–414. [Google Scholar] [CrossRef]
- Tomita, S.; Kin, A.; Yazu, M.; Abe, M. Biomechanical evaluation of kyphoplasty and vertebroplasty with calcium phosphate cement in a simulated osteoporotic compression fracture. J. Orthop. Sci. 2003, 8, 192–197. [Google Scholar] [CrossRef]
- Glass, D.; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.; Clevers, H.; Taketo, M.M.; Long, F.; McMahon, A.P.; Lang, R.; et al. Canonical Wnt Signaling in Differentiated Osteoblasts Controls Osteoclast Differentiation. Dev. Cell 2005, 8, 751–764. [Google Scholar] [CrossRef] [Green Version]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, I.A.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplant. 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Hanna, H.; Mir, L.M.; Andre, F.M. In vitro osteoblastic differentiation of mesenchymal stem cells generates cell layers with distinct properties. Stem Cell Res. Ther. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sollazzo, V.; Palmieri, A.; Girardi, A.; Farinella, F.; Carinci, F. Early Effects of P-15 on Human Bone Marrow Stem Cells. J. Oral Maxillofac. Res. 2010, 1, e4. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krenzlin, H.; Foelger, A.; Mailänder, V.; Blase, C.; Brockmann, M.; Düber, C.; Ringel, F.; Keric, N. Novel Biodegradable Composite of Calcium Phosphate Cement and the Collagen I Mimetic P-15 for Pedicle Screw Augmentation in Osteoporotic Bone. Biomedicines 2021, 9, 1392. https://doi.org/10.3390/biomedicines9101392
Krenzlin H, Foelger A, Mailänder V, Blase C, Brockmann M, Düber C, Ringel F, Keric N. Novel Biodegradable Composite of Calcium Phosphate Cement and the Collagen I Mimetic P-15 for Pedicle Screw Augmentation in Osteoporotic Bone. Biomedicines. 2021; 9(10):1392. https://doi.org/10.3390/biomedicines9101392
Chicago/Turabian StyleKrenzlin, Harald, Andrea Foelger, Volker Mailänder, Christopher Blase, Marc Brockmann, Christoph Düber, Florian Ringel, and Naureen Keric. 2021. "Novel Biodegradable Composite of Calcium Phosphate Cement and the Collagen I Mimetic P-15 for Pedicle Screw Augmentation in Osteoporotic Bone" Biomedicines 9, no. 10: 1392. https://doi.org/10.3390/biomedicines9101392
APA StyleKrenzlin, H., Foelger, A., Mailänder, V., Blase, C., Brockmann, M., Düber, C., Ringel, F., & Keric, N. (2021). Novel Biodegradable Composite of Calcium Phosphate Cement and the Collagen I Mimetic P-15 for Pedicle Screw Augmentation in Osteoporotic Bone. Biomedicines, 9(10), 1392. https://doi.org/10.3390/biomedicines9101392




