Short-Term Epileptiform Activity Potentiates Excitatory Synapses but Does Not Affect Intrinsic Membrane Properties of Pyramidal Neurons in the Rat Hippocampus In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Brain Slice Preparation
2.2. Induction of Short-Term Epileptiform Activity In Vitro
2.3. Field Excitatory Postsynaptic Potential (fEPSP) Recordings
2.4. Patch-Clamp Experiments
2.5. Data Analysis and Statistics
3. Results
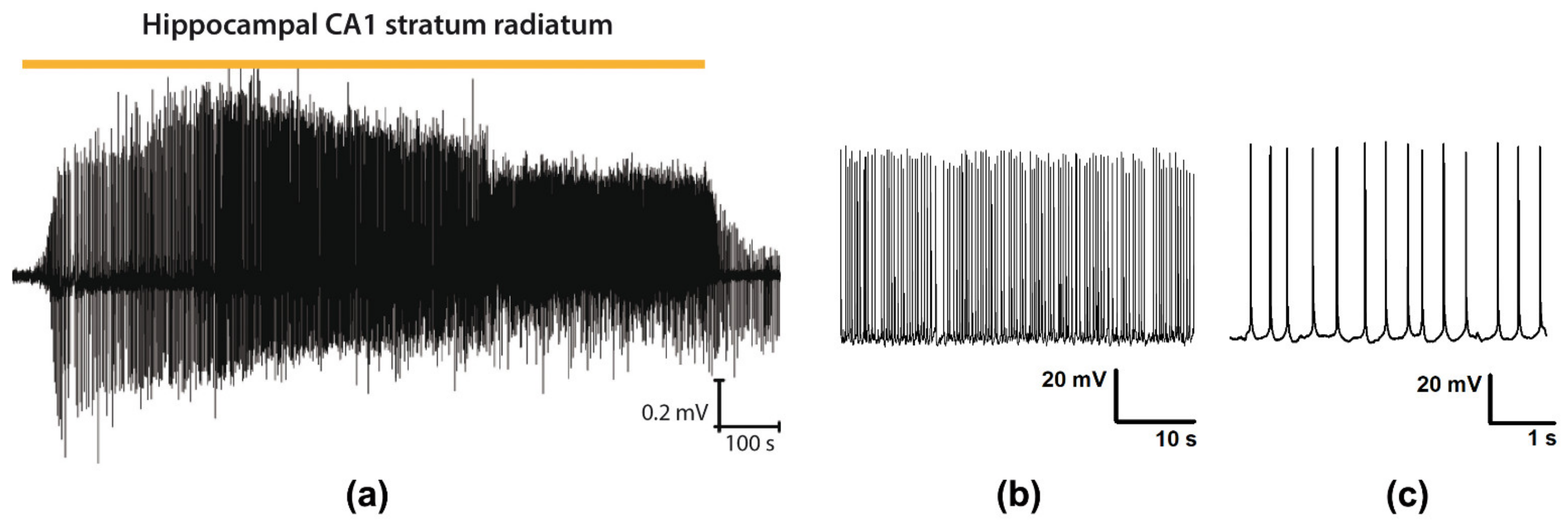
3.1. Epileptiform Activity in Entorhinal-Hippocampal Slices
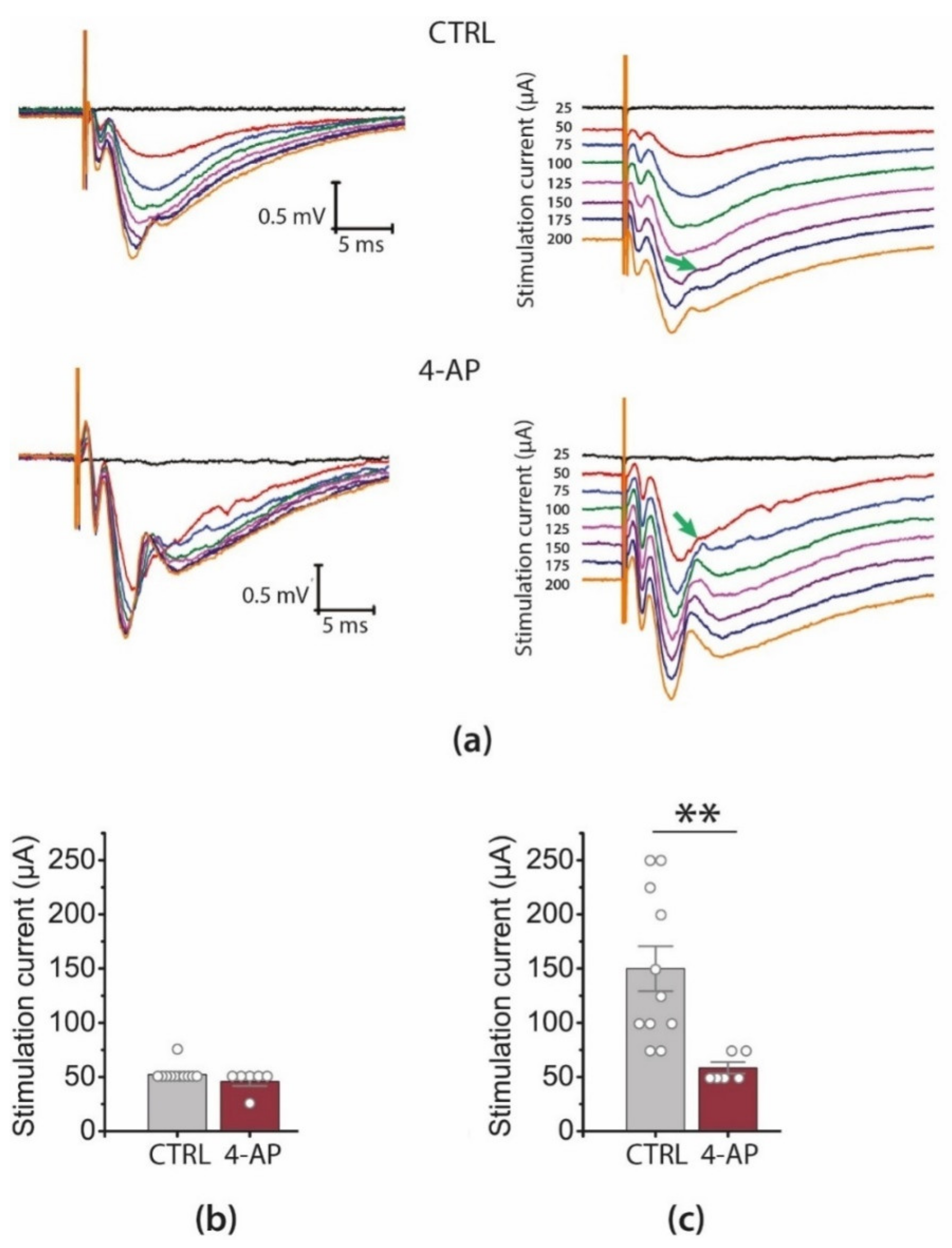
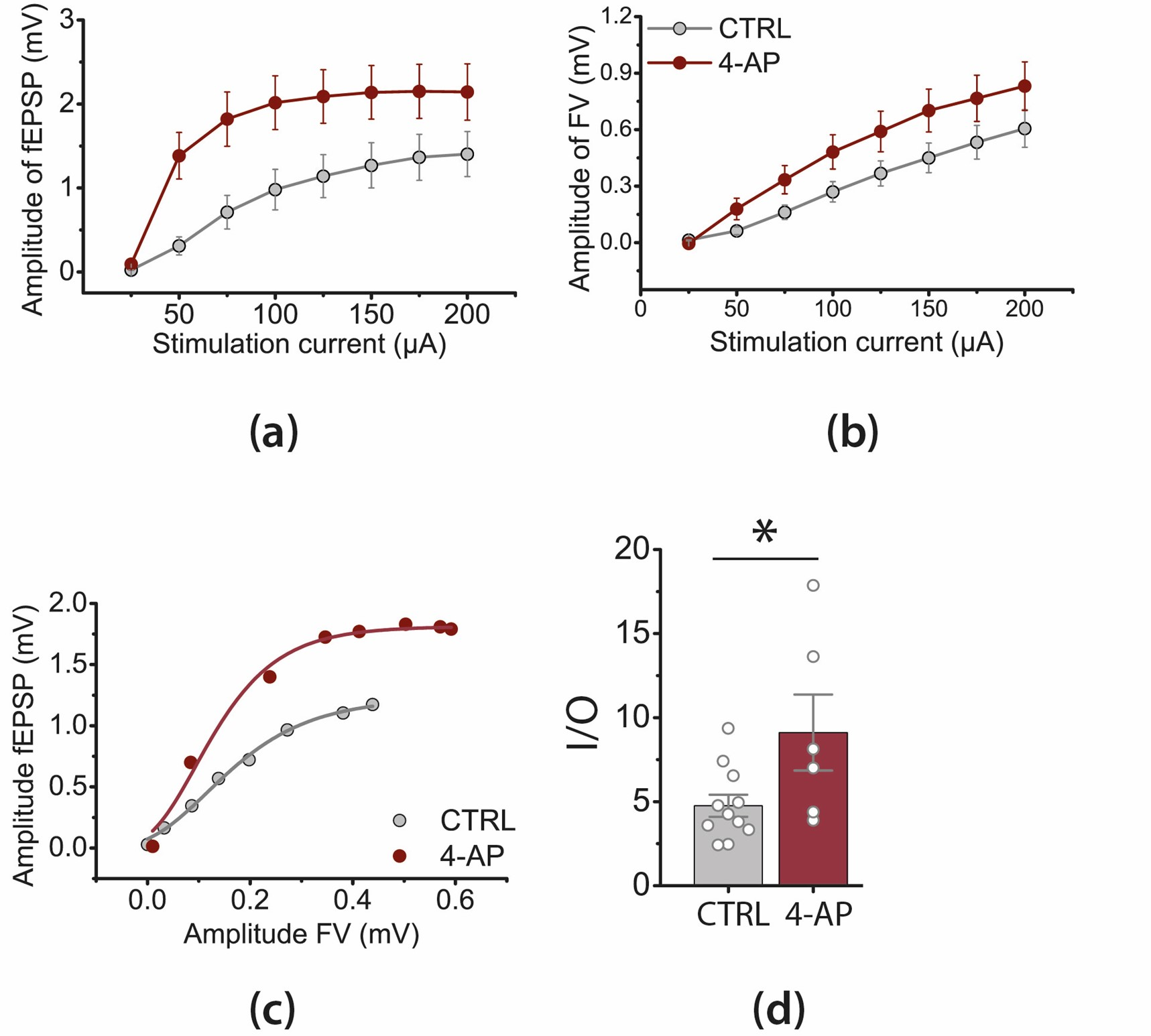
3.2. Epileptiform Activity Increases the Gain of Input–Output Relationship in CA3-CA1 Synapses
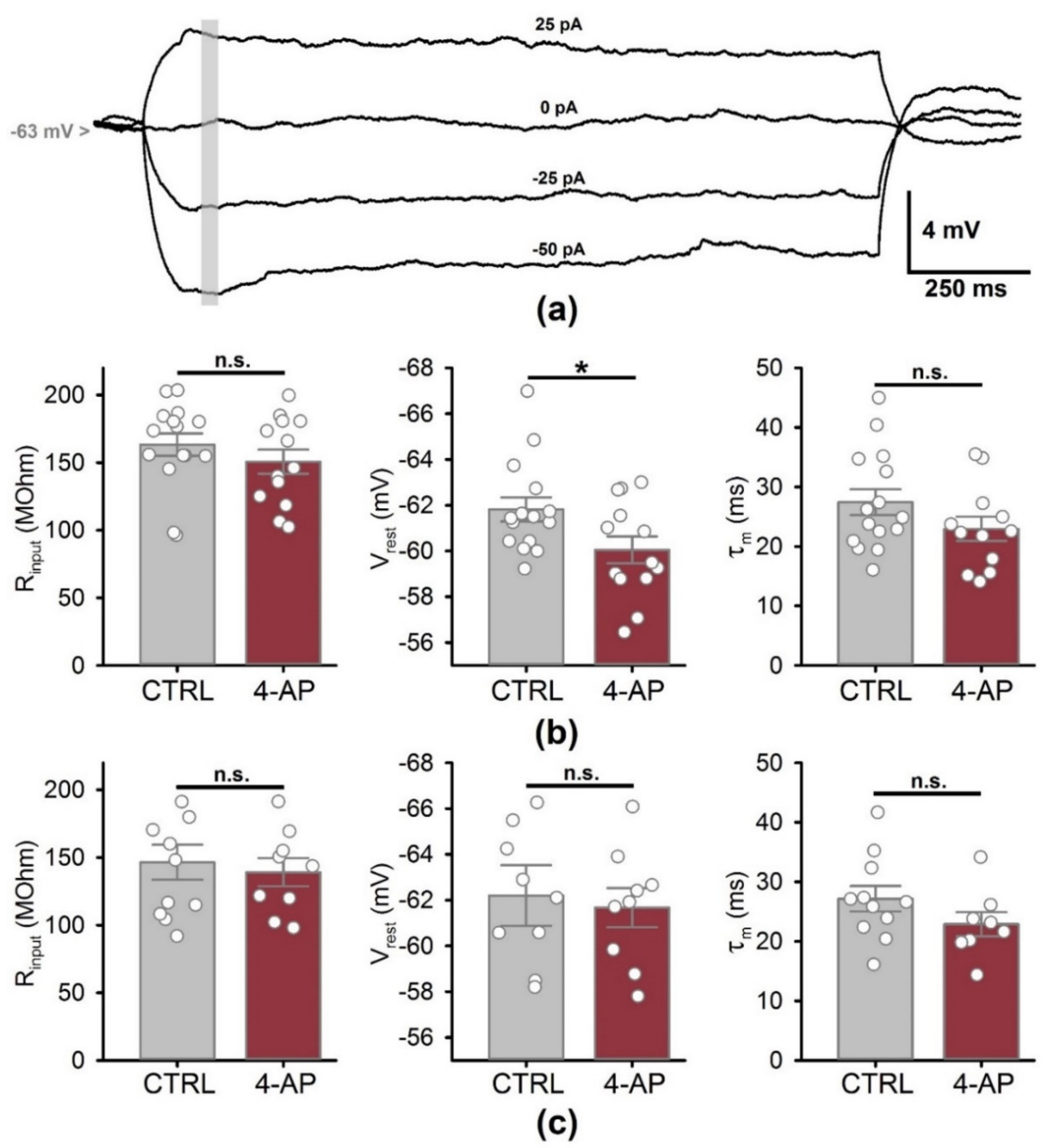
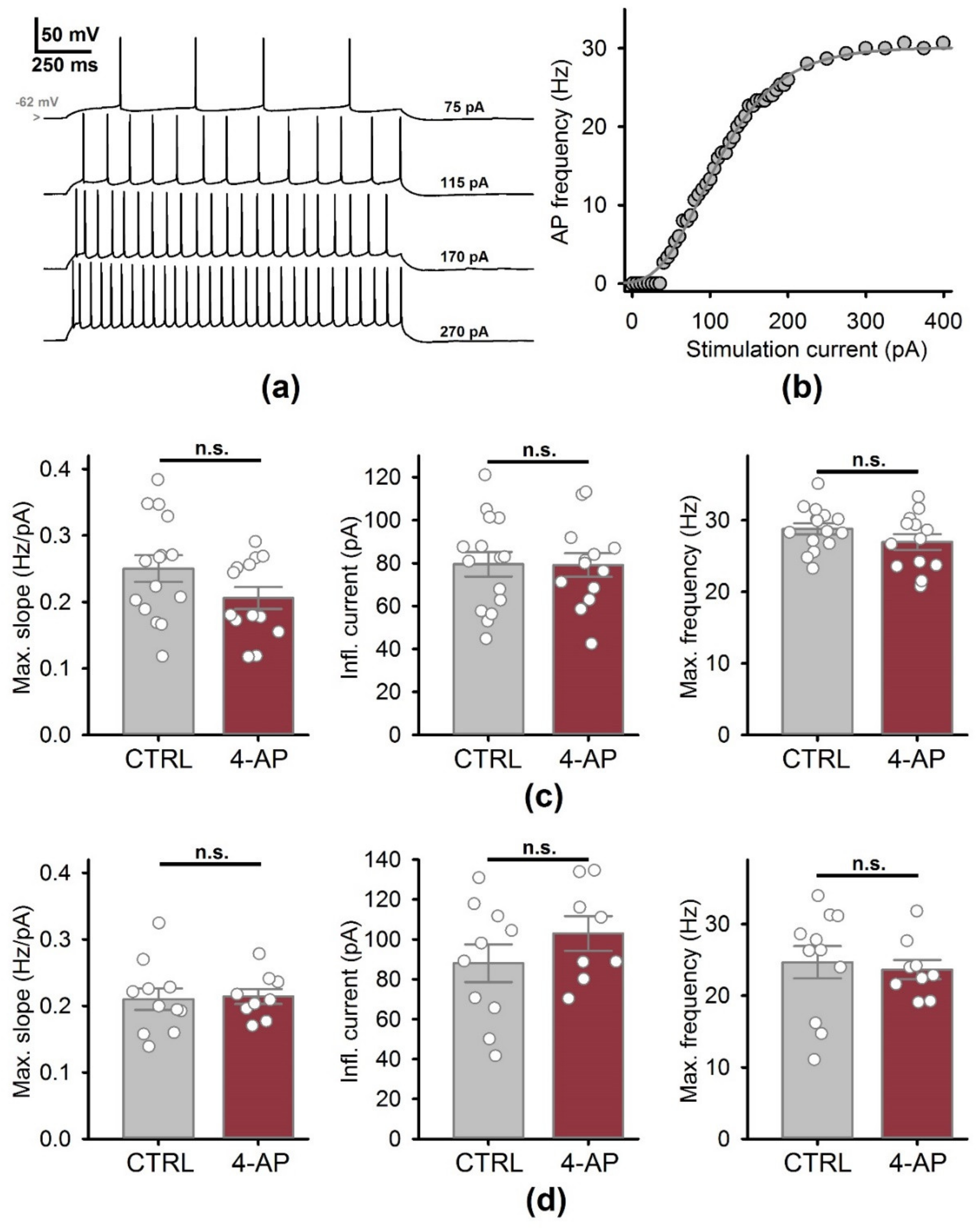
3.3. Biophysical Properties of CA1 Pyramidal Neurons
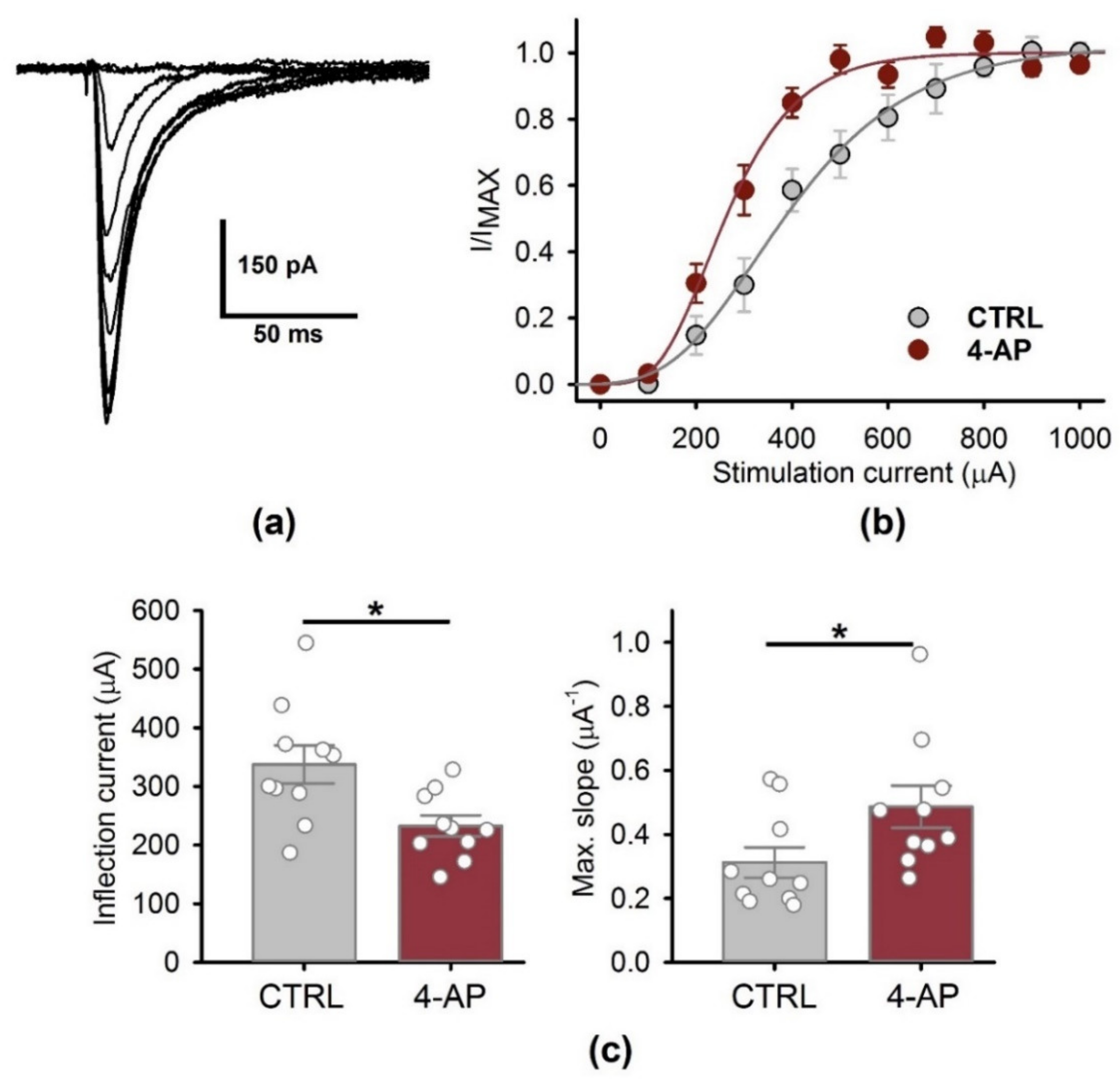
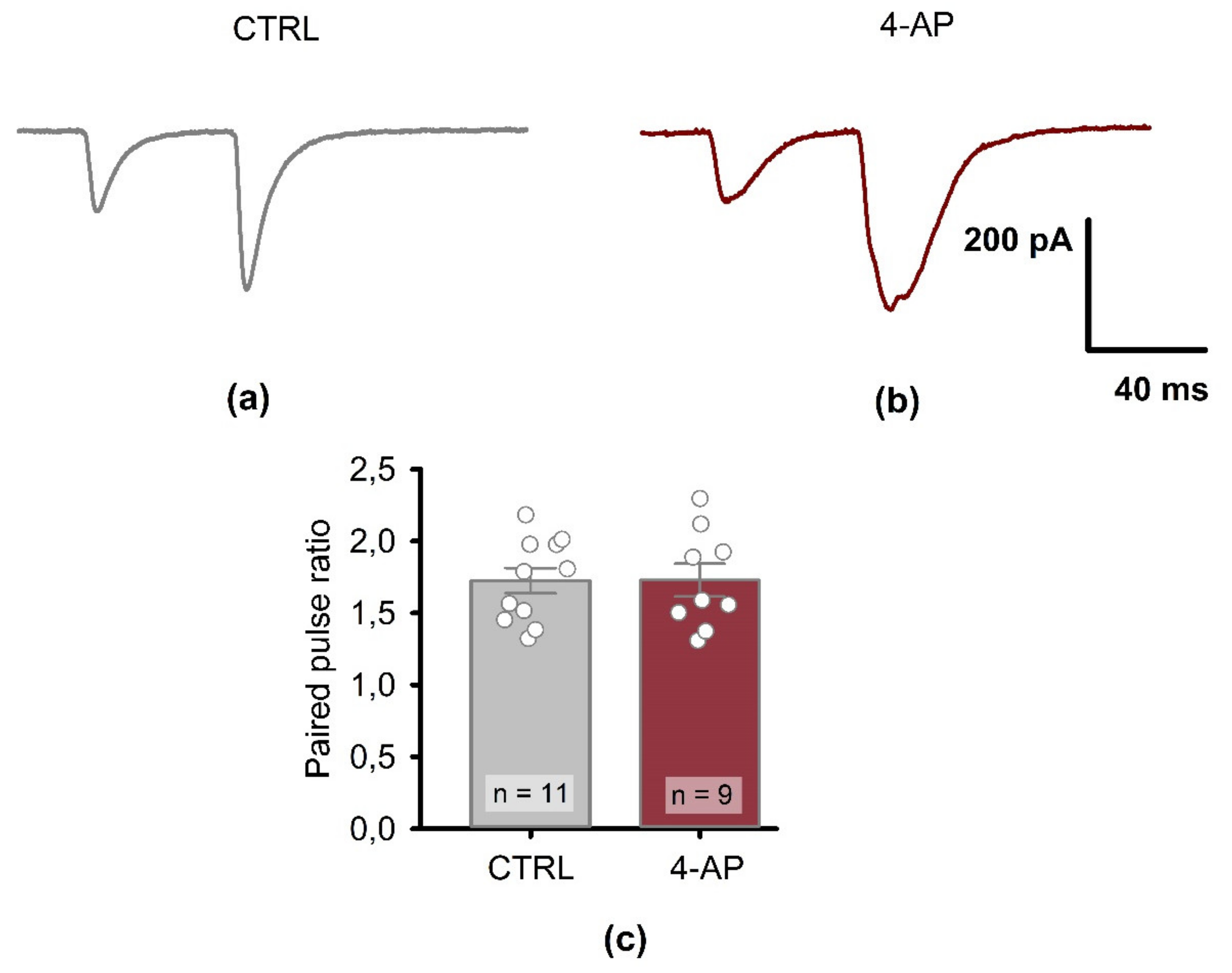
3.4. Presynaptic Properties of CA1 Pyramidal Neurons 1 H after the Epileptiform Activity
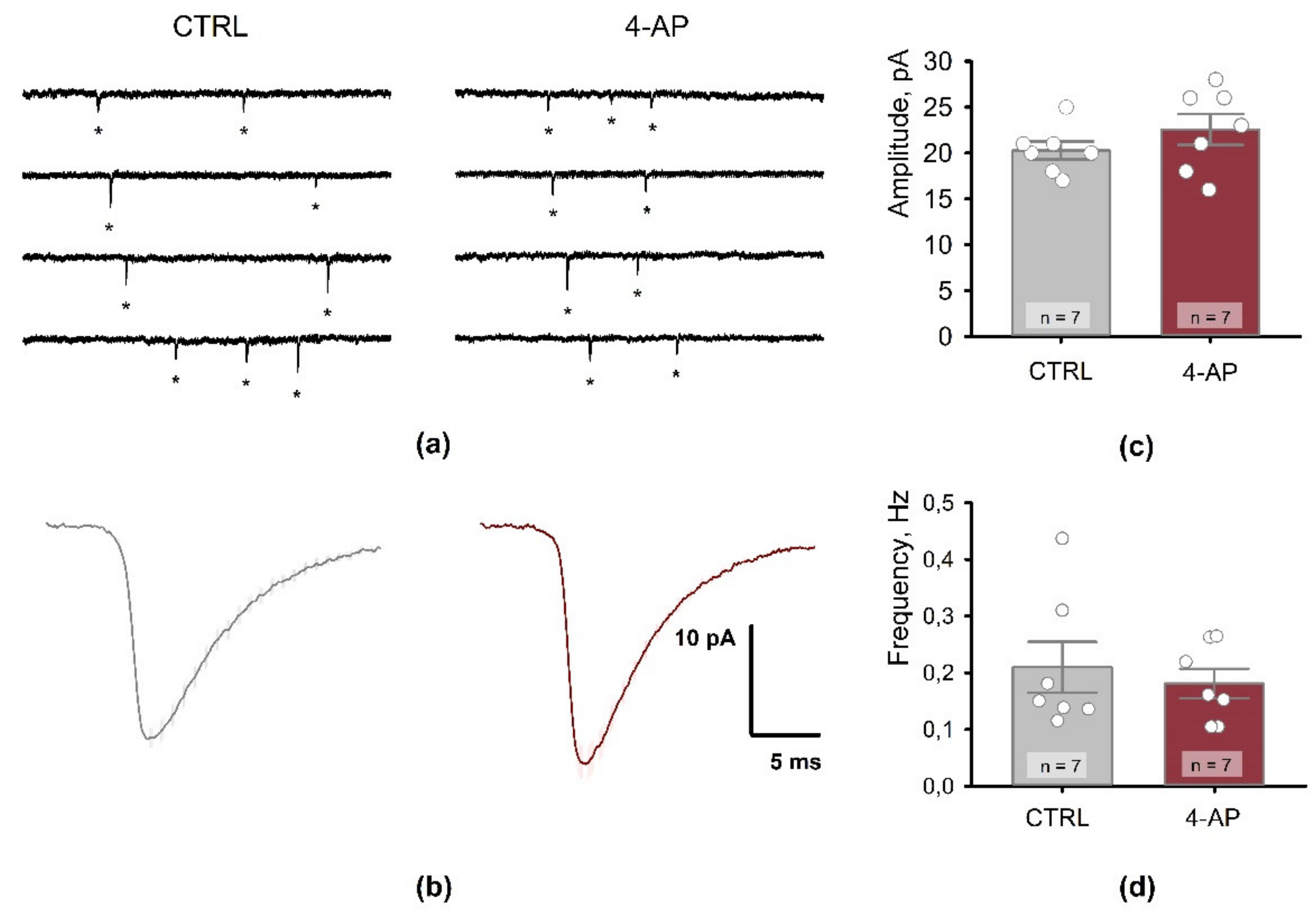
3.5. Postsynaptic Properties of CA1 Pyramidal Neurons
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitkänen, A.; Sutula, T.P. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002, 1, 173–181. [Google Scholar] [CrossRef]
- Herman, S.T. Epilepsy after brain insult: Targeting epileptogenesis. Neurology 2002, 59, S21–S26. [Google Scholar] [CrossRef]
- Arzimanoglou, A.; Hirsch, E.; Nehlig, A.; Castelnau, P.; Gressens, P.; De Vasconcelos, A.P. Epilepsy and neuroprotection: An illustrated review. Epileptic Disord. 2002, 4, 173–182. [Google Scholar]
- Dulla, C.G.; Janigro, D.; Jiruska, P.; Raimondo, J.V.; Ikeda, A.; Lin, C.K.; Goodkin, H.P.; Galanopoulou, A.S.; Bernard, C.; de Curtis, M. How do we use in vitro models to understand epileptiform and ictal activity? A report of the TASK 1- WG 4 group of the ILAE / AES Joint Translational Task Force. Epilepsia Open 2018, 3, 460–473. [Google Scholar] [CrossRef]
- Martin, E.D.; Pozo, M.A. Valproate Reduced Synaptic Activity Increase Induced by 4-Aminopyridine at the Hippocampal CA3-CA1 Synapse. Epilepsia 2004, 45, 436–440. [Google Scholar] [CrossRef]
- Heuzeroth, H.; Wawra, M.; Fidzinski, P.; Dag, R.; Holtkamp, M. The 4-Aminopyridine Model of Acute Seizures in vitro Elucidates Efficacy of New Antiepileptic Drugs. Front. Neurosci. 2019, 13, 677. [Google Scholar] [CrossRef] [PubMed]
- Avoli, M.; Jefferys, J.G. Models of drug-induced epileptiform synchronization in vitro. J. Neurosci. Methods 2016, 260, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Perreault, P.; Avoli, M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J. Neurophysiol. 1991, 65, 771–785. [Google Scholar] [CrossRef]
- Gu, Y.; Ge, S.-Y.; Ruan, D.-Y. Effect of 4-aminopyridine on synaptic transmission in rat hippocampal slices. Brain Res. 2004, 1006, 225–232. [Google Scholar] [CrossRef]
- Ziburkus, J.; Cressman, J.R.; Barreto, E.; Schiff, S.J. Interneuron and Pyramidal Cell Interplay During In Vitro Seizure-Like Events. J. Neurophysiol. 2006, 95, 3948–3954. [Google Scholar] [CrossRef]
- Amakhin, D.V.; Smolensky, I.V.; Soboleva, E.B.; Zaitsev, A.V. Paradoxical Anticonvulsant Effect of Cefepime in the Pentylenetetrazole Model of Seizures in Rats. Pharmaceuticals 2020, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Chizhov, A.V.; Amakhin, D.; Zaitsev, A.V. Computational model of interictal discharges triggered by interneurons. PLoS ONE 2017, 12, e0185752. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.A.; Contreras, D. On The Cellular and Network Bases of Epileptic Seizures. Annu. Rev. Physiol. 2001, 63, 815–846. [Google Scholar] [CrossRef] [PubMed]
- Thom, M. Review: Hippocampal sclerosis in epilepsy: A neuropathology review. Neuropathol. Appl. Neurobiol. 2014, 40, 520–543. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.; Anderson, A.; Becker, A.; Poolos, N.P.; Beck, H.; Johnston, D. Acquired Dendritic Channelopathy in Temporal Lobe Epilepsy. Science 2004, 305, 532–535. [Google Scholar] [CrossRef]
- Smirnova, E.Y.; Amakhin, D.; Malkin, S.L.; Chizhov, A.V.; Zaitsev, A.V. Acute Changes in Electrophysiological Properties of Cortical Regular-Spiking Cells Following Seizures in a Rat Lithium–Pilocarpine Model. Neuroscience 2018, 379, 202–215. [Google Scholar] [CrossRef]
- Postnikova, T.Y.; Amakhin, D.V.; Trofimova, A.M.; Smolensky, I.V.; Zaitsev, A.V. Changes in Functional Properties of Rat Hippocampal Neurons Following Pentylenetetrazole-induced Status Epilepticus. Neuroscience 2019, 399, 103–116. [Google Scholar] [CrossRef]
- Fleck, M.W.; Palmer, A.M.; Barrionuevo, G. Potassium-induced long-term potentiation in rat hippocampal slices. Brain Res. 1992, 580, 100–105. [Google Scholar] [CrossRef]
- Debanne, D.; Thompson, S.M.; Gähwiler, B.H. A Brief Period of Epileptiform Activity Strengthens Excitatory Synapses in the Rat Hippocampus in Vitro. Epilepsia 2006, 47, 247–256. [Google Scholar] [CrossRef]
- Postnikova, T.Y.; Amakhin, D.V.; Trofimova, A.M.; Zaitsev, A.V. Calcium-permeable AMPA receptors are essential to the synaptic plasticity induced by epileptiform activity in rat hippocampal slices. Biochem. Biophys. Res. Commun. 2020, 529, 1145–1150. [Google Scholar] [CrossRef]
- Amakhin, D.; Soboleva, E.; Ergina, J.L.; Malkin, S.; Chizhov, A.; Zaitsev, A.V. Seizure-Induced Potentiation of AMPA Receptor-Mediated Synaptic Transmission in the Entorhinal Cortex. Front. Cell. Neurosci. 2018, 12, 486. [Google Scholar] [CrossRef]
- Gibbs, I.J.W.; Sombati, S.; DeLorenzo, R.J.; Coulter, D.A. Physiological and Pharmacological Alterations in Postsynaptic GABAA Receptor Function in a Hippocampal Culture Model of Chronic Spontaneous Seizures. J. Neurophysiol. 1997, 77, 2139–2152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joshi, S.; Rajasekaran, K.; Hawk, K.M.; Brar, J.; Ross, B.M.; Tran, C.A.; Chester, S.J.; Goodkin, H. Phosphatase inhibition prevents the activity-dependent trafficking of GABAAreceptors during status epilepticus in the young animal. Epilepsia 2015, 56, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Burman, R.J.; Selfe, J.S.; Lee, J.H.; Berg, M.V.D.; Calin, A.; Codadu, N.K.; Wright, R.; E Newey, S.; Parrish, R.R.; A Katz, A.; et al. Excitatory GABAergic signalling is associated with benzodiazepine resistance in status epilepticus. Brain 2019, 142, 3482–3501. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, A.V. The Role of GABAergic Interneurons in the Cortex and Hippocampus in the Development of Epilepsy. Neurosci. Behav. Physiol. 2017, 47, 913–922. [Google Scholar] [CrossRef]
- Feng, Y.; Duan, C.; Luo, Z.; Xiao, W.; Tian, F. Silencing miR-20a-5p inhibits axonal growth and neuronal branching and prevents epileptogenesis through RGMa-RhoA-mediated synaptic plasticity. J. Cell. Mol. Med. 2020, 24, 10573–10588. [Google Scholar] [CrossRef] [PubMed]
- Curia, G.; Lucchi, C.; Vinet, J.; Gualtieri, F.; Marinelli, C.; Torsello, A.; Costantino, L.; Biagini, G. Pathophysiogenesis of Mesial Temporal Lobe Epilepsy: Is Prevention of Damage Antiepileptogenic? Curr. Med. Chem. 2014, 21, 663–688. [Google Scholar] [CrossRef] [PubMed]
- Dingledine, R.; Varvel, N.H.; Dudek, F.E. When and How Do Seizures Kill Neurons, and Is Cell Death Relevant to Epileptogenesis? In Issues in Clinical Epileptology: A View from the Bench; Springer: Dordrecht, The Netherlands, 2014; Volume 813, pp. 109–122. [Google Scholar]
- Amakhin, D.V.; Malkin, S.L.; Ergina, J.L.; Kryukov, K.A.; Veniaminova, E.A.; Zubareva, O.E.; Zaitsev, A.V. Alterations in Properties of Glutamatergic Transmission in the Temporal Cortex and Hippocampus Following Pilocarpine-Induced Acute Seizures in Wistar Rats. Front. Cell. Neurosci. 2017, 11, 264. [Google Scholar] [CrossRef]
- Amakhin, D.; Ergina, J.L.; Chizhov, A.; Zaitsev, A.V. Synaptic Conductances during Interictal Discharges in Pyramidal Neurons of Rat Entorhinal Cortex. Front. Cell. Neurosci. 2016, 10, 233. [Google Scholar] [CrossRef]
- Citri, A.; Malenka, R.C. Synaptic Plasticity: Multiple Forms, Functions, and Mechanisms. Neuropsychopharmacology 2007, 33, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Zucker, R.S.; Regehr, W.G. Short-Term Synaptic Plasticity. Annu. Rev. Physiol. 2002, 64, 355–405. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.L.; Teyler, T.J. Epileptic-like activity induces multiple forms of plasticity in hippocampal area CA1. Brain Res. 2001, 917, 90–96. [Google Scholar] [CrossRef]
- Iyengar, S.S.; Mott, D.D. Neuregulin blocks synaptic strengthening after epileptiform activity in the rat hippocampus. Brain Res. 2008, 1208, 67–73. [Google Scholar] [CrossRef]
- Lopantsev, V.; Both, M.; Draguhn, A. Rapid plasticity at inhibitory and excitatory synapses in the hippocampus induced by ictal epileptiform discharges. Eur. J. Neurosci. 2009, 29, 1153–1164. [Google Scholar] [CrossRef]
- Schneiderman, J. The role of long-term potentiation in persistent epileptiform burst-induced hyperexcitability following GABAA receptor blockade. Neuroscience 1997, 81, 1111–1122. [Google Scholar] [CrossRef]
- Sourdet, V.; Russier, M.; Daoudal, G.; Ankri, N.; Debanne, D. Long-Term Enhancement of Neuronal Excitability and Temporal Fidelity Mediated by Metabotropic Glutamate Receptor Subtype 5. J. Neurosci. 2003, 23, 10238–10248. [Google Scholar] [CrossRef]
- Desai, N.S.; Rutherford, L.C.; Turrigiano, G.G. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat. Neurosci. 1999, 2, 515–520. [Google Scholar] [CrossRef]
- Cudmore, R.; Turrigiano, G.G. Long-Term Potentiation of Intrinsic Excitability in LV Visual Cortical Neurons. J. Neurophysiol. 2004, 92, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.; Yaari, Y. Plasticity of intrinsic neuronal properties in CNS disorders. Nat. Rev. Neurosci. 2008, 9, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Mitterdorfer, J.; Bean, B.P. Potassium Currents during the Action Potential of Hippocampal CA3 Neurons. J. Neurosci. 2002, 22, 10106–10115. [Google Scholar] [CrossRef] [PubMed]
- Blümcke, I.; Thom, M.; Aronica, E.; Armstrong, D.D.; Bartolomei, F.; Bernasconi, A.; Bernasconi, N.; Bien, C.G.; Cendes, F.; Coras, R.; et al. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: A Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia 2013, 54, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Codadu, N.K.; Graham, R.T.; Burman, R.J.; Jackson-Taylor, R.T.; Raimondo, J.V.; Trevelyan, A.J.; Parrish, R.R. Divergent paths to seizure-like events. Physiol. Rep. 2019, 7, e14226. [Google Scholar] [CrossRef] [PubMed]
- Verma-Ahuja, S.; Pencek, T.L. Hippocampal CA1 neuronal properties in genetically epilepsyprone rats: Evidence for increased excitation. Epilepsy Res. 1994, 18, 205–215. [Google Scholar] [CrossRef]
- Ghotbedin, Z.; Janahmadi, M.; Mirnajafi-Zadeh, J.; Behzadi, G.; Semnanian, S. Electrical Low Frequency Stimulation of the Kindling Site Preserves the Electrophysiological Properties of the Rat Hippocampal CA1 Pyramidal Neurons From the Destructive Effects of Amygdala Kindling: The Basis for a Possible Promising Epilepsy Therapy. Brain Stimul. 2013, 6, 515–523. [Google Scholar] [CrossRef]
- Minge, D.; Bähring, R. Acute Alterations of Somatodendritic Action Potential Dynamics in Hippocampal CA1 Pyramidal Cells after Kainate-Induced Status Epilepticus in Mice. PLoS ONE 2011, 6, e26664. [Google Scholar] [CrossRef]
- Pelletier, M.R.; Carlen, P.L. Repeated tetanic stimulation in piriform cortex in vitro: Epileptogenesis and pharmacology. J. Neurophysiol. 1996, 76, 4069–4079. [Google Scholar] [CrossRef]
- Niesen, C.E.; Ge, S. Chronic epilepsy in developing hippocampal neurons: Electrophysiologic and morphologic features. Dev. Neurosci. 1999, 21, 328–338. [Google Scholar] [CrossRef]
- Shah, M.; Anderson, A.E.; Leung, V.; Lin, X.; Johnston, D. Seizure-Induced Plasticity of h Channels in Entorhinal Cortical Layer III Pyramidal Neurons. Neuron 2004, 44, 495–508. [Google Scholar] [CrossRef]
- Marcelin, B.; Chauviere, L.; Becker, A.; Migliore, M.; Esclapez, M.; Bernard, C. h channel-dependent deficit of theta oscillation resonance and phase shift in temporal lobe epilepsy. Neurobiol. Dis. 2009, 33, 436–447. [Google Scholar] [CrossRef]
- Sanabria, E.R.G.; Su, H.; Yaari, Y. Initiation of network bursts by Ca 2+ -dependent intrinsic bursting in the rat pilocarpine model of temporal lobe epilepsy. J. Physiol. 2001, 532, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Yaari, Y.; Yue, C.; Su, H. Recruitment of apical dendritic T-type Ca2+channels by backpropagating spikes underliesde novointrinsic bursting in hippocampal epileptogenesis. J. Physiol. 2007, 580, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Royeck, M.; Kelly, T.; Opitz, T.; Otte, D.-M.; Rennhack, A.; Woitecki, A.; Pitsch, J.; Becker, A.; Schoch, S.; Kaupp, U.B.; et al. Downregulation of Spermine Augments Dendritic Persistent Sodium Currents and Synaptic Integration after Status Epilepticus. J. Neurosci. 2015, 35, 15240–15253. [Google Scholar] [CrossRef] [PubMed]
- Peña-Ortega, F.; Bargas, J.; Tapia, R. Paired pulse facilitation is turned into paired pulse depression in hippocampal slices after epilepsy induced by 4-aminopyridine in vivo. Neuropharmacology 2002, 42, 807–812. [Google Scholar] [CrossRef]
- Smirnova, E.Y.; Chizhov, A.; Zaitsev, A.V. Presynaptic GABAB receptors underlie the antiepileptic effect of low-frequency electrical stimulation in the 4-aminopyridine model of epilepsy in brain slices of young rats. Brain Stimul. 2020, 13, 1387–1395. [Google Scholar] [CrossRef]
- Abegg, M.H.; Savić, N.; Ehrengruber, M.U.; McKinney, R.A.; Gähwiler, B.H. Epileptiform activity in rat hippocampus strengthens excitatory synapses. J. Physiol. 2004, 554, 439–448. [Google Scholar] [CrossRef]
- Liao, D.; Hessler, N.A.; Malinow, R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nat. Cell Biol. 1995, 375, 400–404. [Google Scholar] [CrossRef]
- Choquet, D.; Triller, A. The Dynamic Synapse. Neuron 2013, 80, 691–703. [Google Scholar] [CrossRef]
- Newpher, T.M.; Ehlers, M.D. Glutamate Receptor Dynamics in Dendritic Microdomains. Neuron 2008, 58, 472–497. [Google Scholar] [CrossRef] [PubMed]
- Opazo, P.; Sainlos, M.; Choquet, D. Regulation of AMPA receptor surface diffusion by PSD-95 slots. Curr. Opin. Neurobiol. 2012, 22, 453–460. [Google Scholar] [CrossRef]
- Lisman, J.E.; Raghavachari, S.; Tsien, R.W. The sequence of events that underlie quantal transmission at central glutamatergic synapses. Nat. Rev. Neurosci. 2007, 8, 597–609. [Google Scholar] [CrossRef]
- Tang, A.-H.; Chen, H.; Li, T.P.; Metzbower, S.R.; MacGillavry, H.; Blanpied, T.A. A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature 2016, 536, 210–214. [Google Scholar] [CrossRef]
- Rajasekaran, K.; Todorovic, M.; Kapur, J. Calcium-permeable AMPA receptors are expressed in a rodent model of status epilepticus. Ann. Neurol. 2012, 72, 91–102. [Google Scholar] [CrossRef]
- Rakhade, S.N.; Zhou, C.; Aujla, P.K.; Fishman, R.; Sucher, N.J.; Jensen, F.E. Early Alterations of AMPA Receptors Mediate Synaptic Potentiation Induced by Neonatal Seizures. J. Neurosci. 2008, 28, 7979–7990. [Google Scholar] [CrossRef]
- Joshi, S.; Rajasekaran, K.; Sun, H.; Williamson, J.; Kapur, J. Enhanced AMPA receptor-mediated neurotransmission on CA1 pyramidal neurons during status epilepticus. Neurobiol. Dis. 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Russo, I.; Bonini, D.; La Via, L.; Barlati, S.; Barbon, A. AMPA Receptor Properties are Modulated in the Early Stages Following Pilocarpine-induced Status Epilepticus. Neuromol. Med. 2013, 15, 324–338. [Google Scholar] [CrossRef]
- Peled, E.S.; Newman, Z.L.; Isacoff, E.Y. Evoked and Spontaneous Transmission Favored by Distinct Sets of Synapses. Curr. Biol. 2014, 24, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Kaeser, P.S.; Regehr, W.G. Molecular Mechanisms for Synchronous, Asynchronous, and Spontaneous Neurotransmitter Release. Annu. Rev. Physiol. 2014, 76, 333–363. [Google Scholar] [CrossRef] [PubMed]
- Kavalali, E.T. The mechanisms and functions of spontaneous neurotransmitter release. Nat. Rev. Neurosci. 2015, 16, 5–16. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ergina, J.L.; Amakhin, D.V.; Postnikova, T.Y.; Soboleva, E.B.; Zaitsev, A.V. Short-Term Epileptiform Activity Potentiates Excitatory Synapses but Does Not Affect Intrinsic Membrane Properties of Pyramidal Neurons in the Rat Hippocampus In Vitro. Biomedicines 2021, 9, 1374. https://doi.org/10.3390/biomedicines9101374
Ergina JL, Amakhin DV, Postnikova TY, Soboleva EB, Zaitsev AV. Short-Term Epileptiform Activity Potentiates Excitatory Synapses but Does Not Affect Intrinsic Membrane Properties of Pyramidal Neurons in the Rat Hippocampus In Vitro. Biomedicines. 2021; 9(10):1374. https://doi.org/10.3390/biomedicines9101374
Chicago/Turabian StyleErgina, Julia L., Dmitry V. Amakhin, Tatyana Y. Postnikova, Elena B. Soboleva, and Aleksey V. Zaitsev. 2021. "Short-Term Epileptiform Activity Potentiates Excitatory Synapses but Does Not Affect Intrinsic Membrane Properties of Pyramidal Neurons in the Rat Hippocampus In Vitro" Biomedicines 9, no. 10: 1374. https://doi.org/10.3390/biomedicines9101374
APA StyleErgina, J. L., Amakhin, D. V., Postnikova, T. Y., Soboleva, E. B., & Zaitsev, A. V. (2021). Short-Term Epileptiform Activity Potentiates Excitatory Synapses but Does Not Affect Intrinsic Membrane Properties of Pyramidal Neurons in the Rat Hippocampus In Vitro. Biomedicines, 9(10), 1374. https://doi.org/10.3390/biomedicines9101374





