HBeAg Levels Vary across the Different Stages of HBV Infection According to the Extent of Immunological Pressure and Are Associated with Therapeutic Outcome in the Setting of Immunosuppression-Driven HBV Reactivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- 20 patients with acute HBV infection;
- 20 patients with HBeAg-positive chronic infection;
- 32 patients with HBeAg-positive chronic hepatitis (with elevated ALT);
- 29 patients undergoing immunosuppression-driven HBV reactivation.
2.2. HBV Laboratory Evaluation
2.2.1. Quantification of Serum HBV-DNA
2.2.2. Quantification of HBsAg
2.2.3. Quantification of HBeAg
2.2.4. Qualitative Detection of HBeAg and HBsAg
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
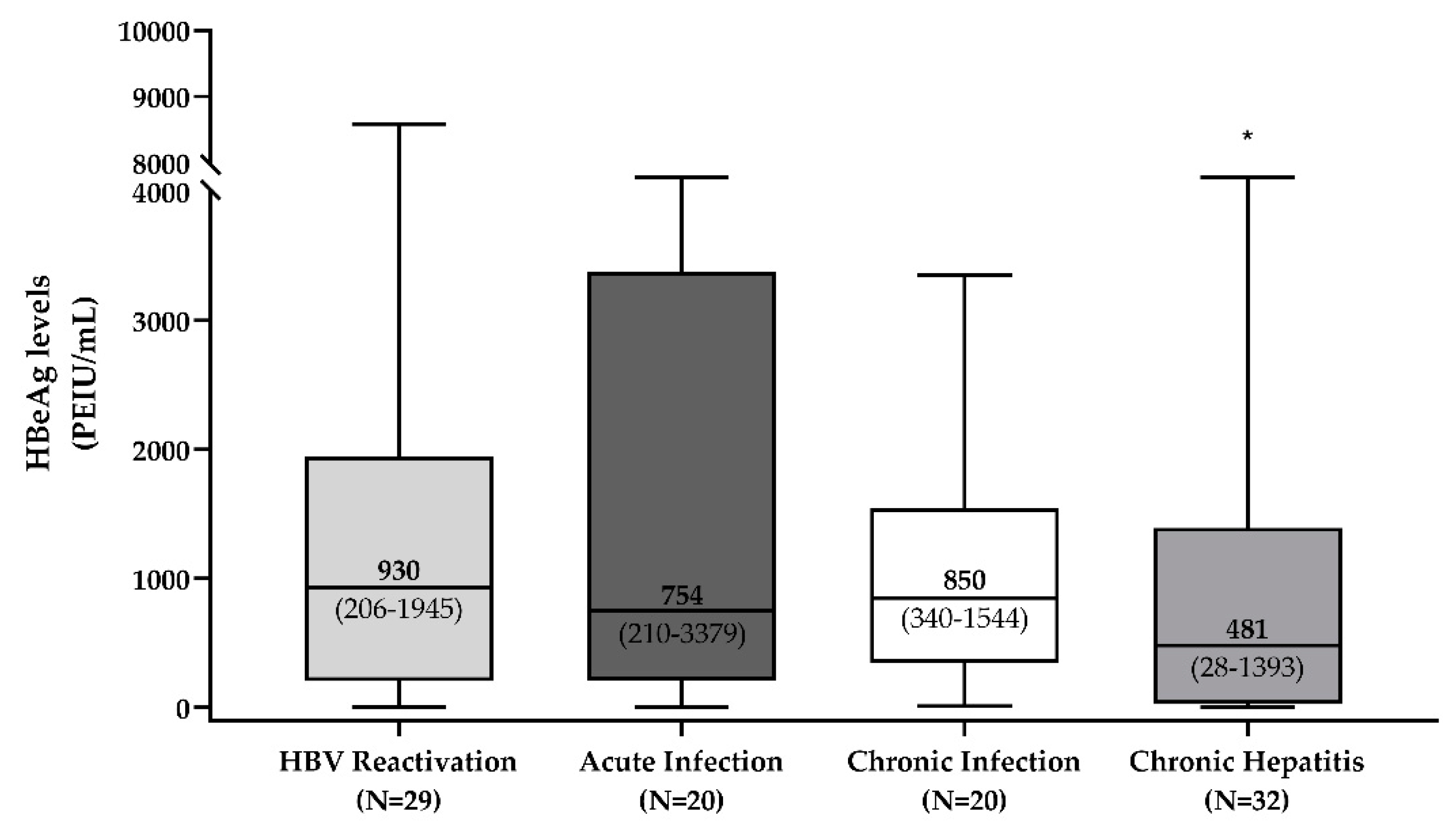
3.2. HBeAg Levels across the Different HBeAg-Positive Phases of HBV Infection
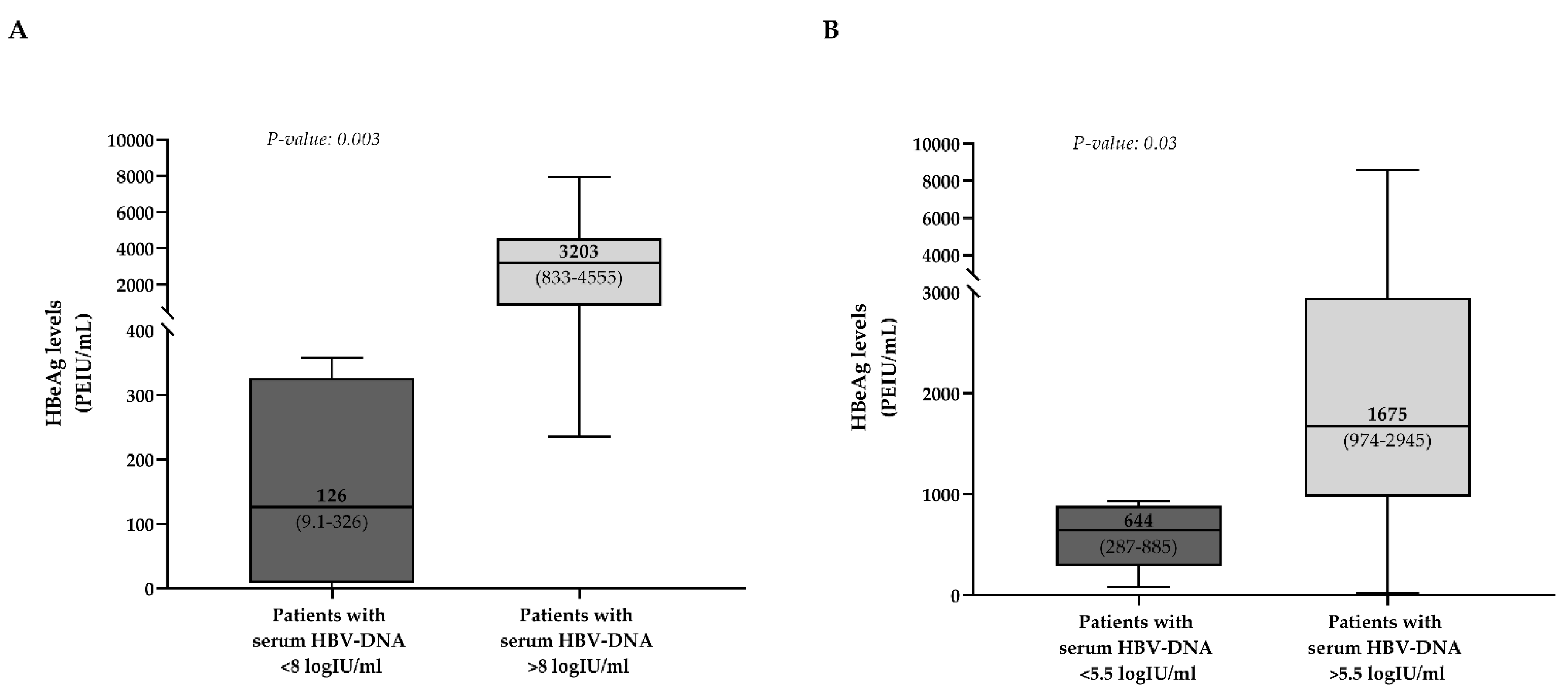
3.3. Correlation of HBeAg Levels with the Other HBV Biomarkers
3.3.1. Acute Infection
3.3.2. HBeAg-Positive Chronic Infection and Hepatitis
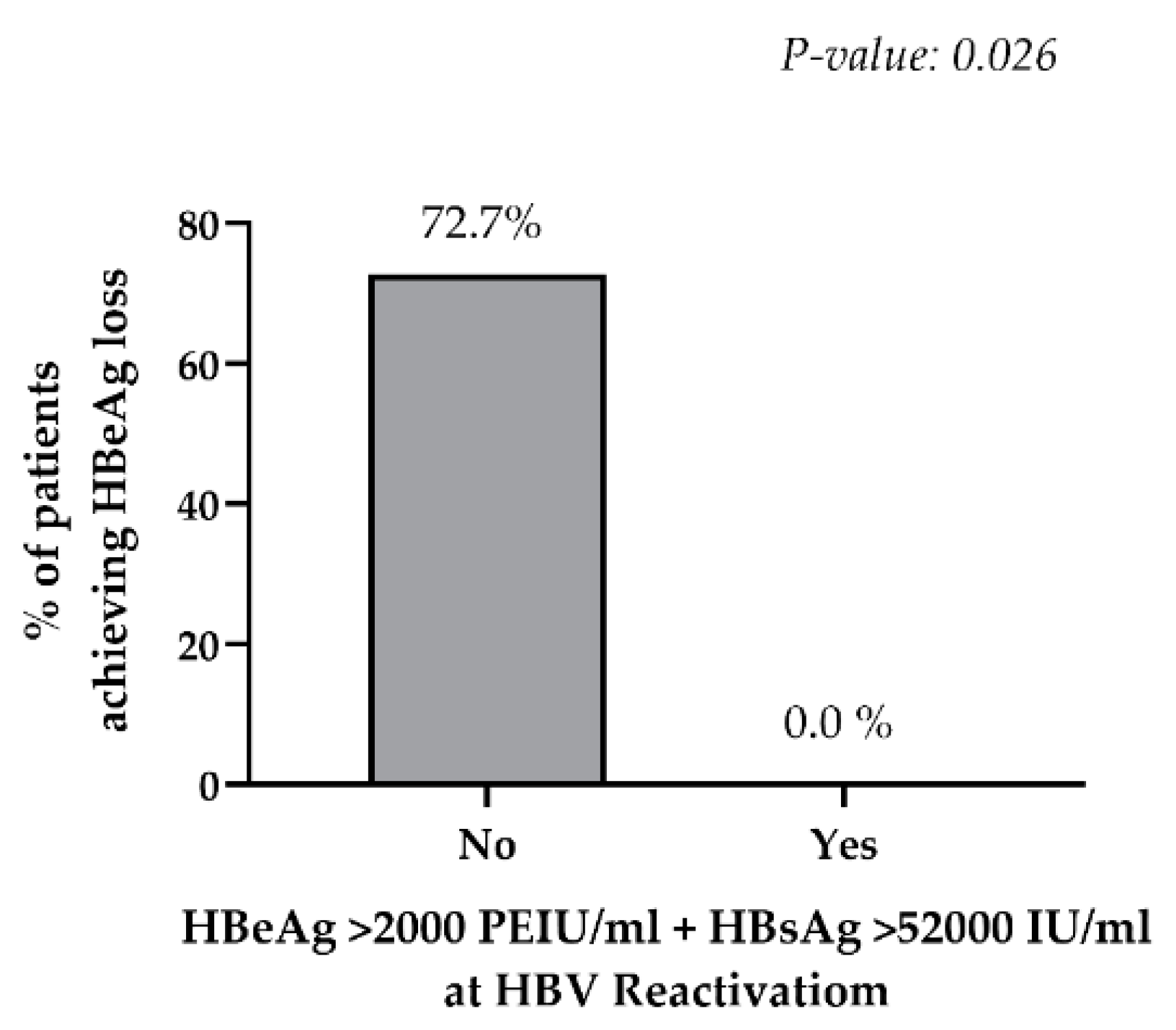
3.3.3. Immunosuppression-Driven HBV Reactivation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Hepatitis Report, 2017; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Revill, P.; Yuen, L.; Walsh, R.; Perrault, M.; Locarnini, S.; Kramvis, A. Bioinformatic analysis of the hepadnavirus e-antigen and its precursor identifies remarkable sequence conservation in all orthohepadnaviruses. J. Med. Virol. 2010, 82, 104–115. [Google Scholar] [CrossRef]
- Kramvis, A.; Kostaki, E.G.; Hatzakis, A.; Paraskevis, D. Immunomodulatory function of HBeAg related to short-sighted evolution, transmissibility, and clinical manifestation of Hepatitis B virus. Front. Microbiol. 2018, 9, 2521. [Google Scholar] [CrossRef]
- Quarleri, J. Core promoter: A critical region where the hepatitis B virus makes decisions. World J. Gastroenterol. 2014, 20, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Milich, D.R. Is the function of the HBeAg really unknown? Hum. Vaccines Immunother. 2019, 15, 2187–2191. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Sällberg, M.; Hughes, J.; Jones, J.; Guidotti, L.G.; Chisari, F.V.; Billaud, J.-N.; Milich, D.R. Immune Tolerance Split between Hepatitis B Virus Precore and Core Proteins. J. Virol. 2005, 79, 3016–3027. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.T.; Billaud, J.N.; Sällberg, M.; Guidotti, L.G.; Chisari, F.V.; Jones, J.; Hughes, J.; Milich, D.R. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc. Natl. Acad. Sci. USA 2004, 101, 14913–14918. [Google Scholar] [CrossRef] [PubMed]
- Lampertico, P.; Agarwal, K.; Berg, T.; Buti, M.; Janssen, H.L.A.; Papatheodoridis, G.; Zoulim, F.; Tacke, F. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Liaw, Y.F.; Brunetto, M.R.; Hadziyannis, S. The natural history of chronic HBV infection and geographical differences. Antivir. Ther. 2010, 15, 25. [Google Scholar] [CrossRef]
- Likhitsup, A.; Lok, A.S. Understanding the Natural History of Hepatitis B Virus Infection and the New Definitions of Cure and the Endpoints of Clinical Trials. Clin. Liver Dis. 2019, 23, 401–416. [Google Scholar] [CrossRef]
- Hoofnagle, J.H. Reactivation of hepatitis B. Hepatology 2009, 49, S156–S165. [Google Scholar] [CrossRef]
- Salpini, R.; Battisti, A.; Colagrossi, L.; Di Carlo, D.; Fabeni, L.; Piermatteo, L.; Cerva, C.; Lichtner, M.; Mastroianni, C.; Marignani, M.; et al. A snapshot of virological presentation and outcome of immunosuppression-driven HBV reactivation from real clinical practice: Evidence of a relevant risk of death and evolution from silent to chronic infection. J. Viral Hepat. 2019, 26, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, P.H.; Beames, M.P.; Kuhns, M.C.; Hoofnagle, J.H.; Di Bisceglie, A.M. Use of quantitative assays for hepatitis B e antigen and IgM antibody to hepatitis B core antigen to monitor therapy in chronic hepatitis B. Am. J. Gastroenterol. 1996, 91, 2323–2328. [Google Scholar] [PubMed]
- Heijtink; Janssen; Hop; Osterhaus; Schalm Interferon-alpha therapy in chronic hepatitis B: Early monitoring of hepatitis B e antigen may help to decide whether to stop or to prolong therapy. J. Viral Hepat. 2000, 7, 382–386. [CrossRef] [PubMed]
- Perrillo, R.; Mimms, L.; Schechtman, K.; Robbins, D.; Campbell, C. Monitoring of antiviral therapy with quantitative evaluation of hbeag: A comparison with HBV DNA testing. Hepatology 1993, 18, 1306–1312. [Google Scholar] [CrossRef]
- Fried, M.W.; Piratvisuth, T.; Lau, G.K.K.; Marcellin, P.; Chow, W.-C.; Cooksley, G.; Luo, K.-X.; Paik, S.W.; Liaw, Y.-F.; Button, P.; et al. HBeAg and hepatitis B virus DNA as outcome predictors during therapy with peginterferon alfa-2a for HBeAg-positive chronic hepatitis B. Hepatology 2008, 47, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Buster, E.H.C.J.; Flink, H.J.; Simsek, H.; Heathcote, E.J.; Sharmila, S.; Kitis, G.E.; Gerken, G.; Buti, M.; de Vries, R.A.; Verhey, E.; et al. Early HBeAg Loss During Peginterferon α-2b Therapy Predicts HBsAg Loss: Results of a Long-Term Follow-up Study in Chronic Hepatitis B Patients. Am. J. Gastroenterol. 2009, 104, 2449–2457. [Google Scholar] [CrossRef]
- Wang, C.-C.; Liu, C.-J.; Lai, M.-Y.; Kao, J.-H.; Chen, D.-S. Dynamics of hepatitis B e antigen index ratio correlate with treatment response in chronic hepatitis B patients. Liver Int. 2007, 27, 235–239. [Google Scholar] [CrossRef]
- Da Silva, L.C.; da Nova, M.L.; Ono-Nita, S.K.; Pinho, J.R.R.; Sitnik, R.; dos Santos, V.A.; Carrilho, F.J. Simultaneous quantitation of serum HBV DNA and HBeAg can distinguish between slow and fast viral responses to antiviral therapy in patients with chronic hepatitis B. Rev. Inst. Med. Trop. Sao Paulo 2009, 51, 261–268. [Google Scholar] [CrossRef][Green Version]
- Park, N.H.; Shin, J.W.; Park, J.H.; Bang, S.-J.; Kim, D.-H.; Joo, K.R.; Kim, D.H. Monitoring of HBeAg levels may help to predict the outcomes of lamivudine therapy for HBeAg positive chronic hepatitis B. J. Viral Hepat. 2005, 12, 216–221. [Google Scholar] [CrossRef]
- Shin, J.W.; Park, N.H.; Jung, S.W.; Kim, B.C.; Kwon, S.H.; Park, J.S.; Du Jeong, I.; Bang, S.-J.; Kim, D.H. Clinical significance of hepatitis B e antigen level easurement during long-term lamivudine therapy in chronic hepatitis B patients with e antigen positive. World J. Gastroenterol. WJG 2006, 12, 6693–6698. [Google Scholar] [CrossRef]
- Wooddell, C.I.; Yuen, M.F.; Chan, H.L.Y.; Gish, R.G.; Locarnini, S.A.; Chavez, D.; Ferrari, C.; Given, B.D.; Hamilton, J.; Kanner, S.B.; et al. Rnai-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis b virus DNA is a source of hbsag. Sci. Transl. Med. 2017, 9, eaan0241. [Google Scholar] [CrossRef] [PubMed]
- Pollicino, T.; Caminiti, G. HBV-Integration Studies in the Clinic: Role in the Natural History of Infection. Viruses 2021, 13, 368. [Google Scholar] [CrossRef]
- Thompson, A.J.; Nguyen, T.; Iser, D.; Ayres, A.; Jackson, K.; Littlejohn, M.; Slavin, J.; Bowden, S.; Gane, E.J.; Abbott, W.; et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: Disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology 2010, 51, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Driedger, M.; Wong, D.; Haylock-Jacobs, S.; Aziz Shaheen, A.; Osiowy, C.; Fung, S.; Doucette, K.; Wong, A.; Barrett, L.; et al. Distinct Hepatitis B and HIV co-infected populations in Canada. J. Viral Hepat. 2021, 28, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Matthews, G.V.; Ali, R.J.; Avihingsanon, A.; Amin, J.; Hammond, R.; Bowden, S.; Lewin, S.R.; Sasadeusz, J.; Littlejohn, M.; Locarnini, S.L.; et al. Quantitative HBsAg and HBeAg Predict Hepatitis B Seroconversion after Initiation of HAART in HIV-HBV Coinfected Individuals. PLoS ONE 2013, 8, e61297. [Google Scholar] [CrossRef] [PubMed]
| Overall (N = 101) | Acute Infection (N = 20) | eAg+ Chronic Infection (N = 20) | eAg+ Chronic Hepatitis (N = 32) | HBV Reactivation (N = 29) | |
|---|---|---|---|---|---|
| Age, years, median (IQR) | 30 (24–54) | 40 (24–43) | 25 (21–27) | 29 (19–38) | 59 (49–72) |
| Male, N (%) | 68 (68.7) | 19 (95.0) | 9 (45.0) | 22 (73.3) | 18 (62.1) |
| Italian nationality, N (%) | 53 (54.1) | 17 (85.0) | 1 (5.0) | 9 (28.1) | 26 (89.7) |
| Serum HBV-DNA, log IU/mL, median (IQR) | 8.1 (6.8–8.4) | 8.3 (7.9–8.7) | 8.2 (8.0–8.5) | 8.2 (7.1–8.6) | 6.8 (5.6–8.0) |
| HBsAg levels, IU/mL, median (IQR) | 48,000 (12,000–60,829) | 48,000 (12,500–52,000) | 64,673 (44,049–85,219) | 35,270 (10,338–65,236) | 31,882 (9902–52,000) |
| ALT, U/L, median (IQR) | 71 (39–227) | 1670 (183–2155) | 29 (21–35) | 74 (52–137) | 143 (40–528) |
| ALT, N × ULN 1 | 1.8× (1.0×–5.7×) | 41.8× (4.6×–53.9×) | 0.7× (0.5×–0.9×) | 1.9× (1.3×–3.4×) | 3.6× (1.0×–13.2×) |
| HBV-Genotypes, N (%) | |||||
| D | 34 (44.2) | 4 (23.5) | 2 (28.6) | 7 (50.0) | 21 (72.4) |
| A | 22 (28.6) | 8 (47.1) | 1 (14.3) | 7 (50.0) | 6 (20.7) |
| E | 7 (9.1) | 0 (0.0) | 1 (14.3) | 6 (42.9) | 0 (0.0) |
| B | 4 (5.2) | 0 (0.0) | 3 (42.9) | 1 (7.1) | 0 (0.0) |
| F | 7 (9.1) | 5 (29.4) | 0 (0.0) | 1 (7.1) | 1 (3.4) |
| C | 2 (2.6) | 0 (0.0) | 0 (0.0) | 2 (14.3) | 0 (0.0) |
| G | 1 (1.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.4) |
| Not available | 25 (32.5) | 3 (15.0) | 13 (65.0) | 8 (25.0) | 0 (0.0) |
| Correlation of HBeAg Levels with | ||||
|---|---|---|---|---|
| HBsAg Levels | Serum HBV-DNA | ALT Levels | ||
| Acute infection | rhoa | 0.78 | 0.27 | −0.66 |
| p-valuea | 0.0006 | 0.9 | 0.006 | |
| Chronic infection | rhoa | 0.04 | 0.23 | −0.35 |
| p-valuea | 0.9 | 0.3 | 0.9 | |
| Chronic hepatitis | rhoa | 0.62 | 0.57 | −0.35 |
| p-valuea | 0.0002 | 0.0008 | 0.05 | |
| HBV-reactivation | rhoa | 0.59 | 0.17 | 0.04 |
| p-valuea | 0.007 | 0.5 | 0.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piermatteo, L.; Alkhatib, M.; D’Anna, S.; Malagnino, V.; Bertoli, A.; Andreassi, E.; Basile, E.; Iuvara, A.; De Cristofaro, M.; Cappiello, G.; et al. HBeAg Levels Vary across the Different Stages of HBV Infection According to the Extent of Immunological Pressure and Are Associated with Therapeutic Outcome in the Setting of Immunosuppression-Driven HBV Reactivation. Biomedicines 2021, 9, 1352. https://doi.org/10.3390/biomedicines9101352
Piermatteo L, Alkhatib M, D’Anna S, Malagnino V, Bertoli A, Andreassi E, Basile E, Iuvara A, De Cristofaro M, Cappiello G, et al. HBeAg Levels Vary across the Different Stages of HBV Infection According to the Extent of Immunological Pressure and Are Associated with Therapeutic Outcome in the Setting of Immunosuppression-Driven HBV Reactivation. Biomedicines. 2021; 9(10):1352. https://doi.org/10.3390/biomedicines9101352
Chicago/Turabian StylePiermatteo, Lorenzo, Mohammad Alkhatib, Stefano D’Anna, Vincenzo Malagnino, Ada Bertoli, Eleonora Andreassi, Elisa Basile, Alessandra Iuvara, Maria De Cristofaro, Giuseppina Cappiello, and et al. 2021. "HBeAg Levels Vary across the Different Stages of HBV Infection According to the Extent of Immunological Pressure and Are Associated with Therapeutic Outcome in the Setting of Immunosuppression-Driven HBV Reactivation" Biomedicines 9, no. 10: 1352. https://doi.org/10.3390/biomedicines9101352
APA StylePiermatteo, L., Alkhatib, M., D’Anna, S., Malagnino, V., Bertoli, A., Andreassi, E., Basile, E., Iuvara, A., De Cristofaro, M., Cappiello, G., Cerva, C., Minichini, C., Pisaturo, M., Starace, M., Coppola, N., Fontana, C., Grelli, S., Ceccherini-Silberstein, F., Andreoni, M., ... Svicher, V. (2021). HBeAg Levels Vary across the Different Stages of HBV Infection According to the Extent of Immunological Pressure and Are Associated with Therapeutic Outcome in the Setting of Immunosuppression-Driven HBV Reactivation. Biomedicines, 9(10), 1352. https://doi.org/10.3390/biomedicines9101352









