Magnolol Triggers Caspase-Mediated Apoptotic Cell Death in Human Oral Cancer Cells through JNK1/2 and p38 Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Microculture Tetrazolium (MTT) Assay
2.3. Flow Cytometry Assay
2.4. Annexin V-FITC Staining Assay
2.5. Western Blot Analysis
2.6. Human Apoptosis Array
2.7. Statistical Analysis
3. Results
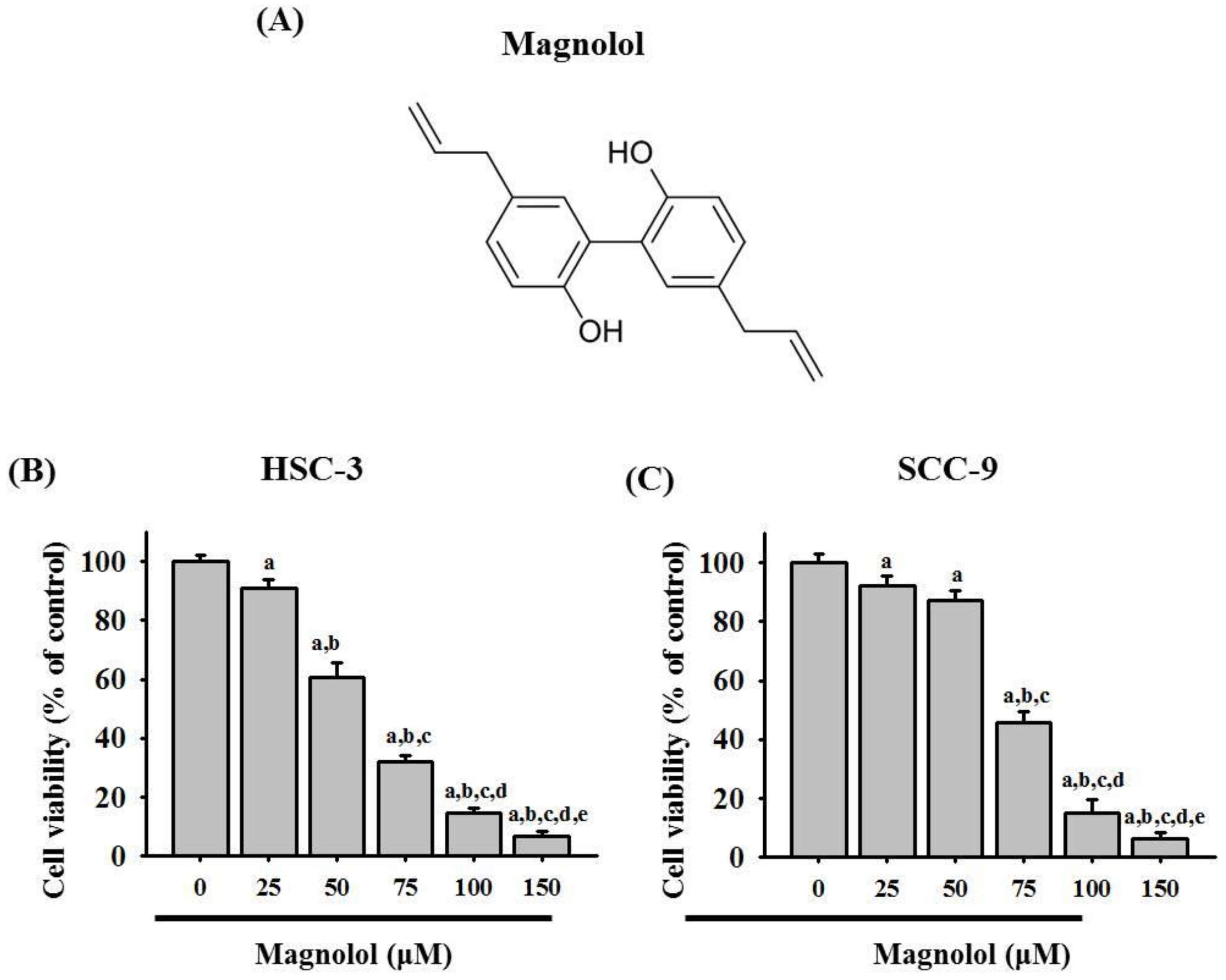
3.1. Magnolol Inhibited Cell Viability in Oral Cancer Cell Lines
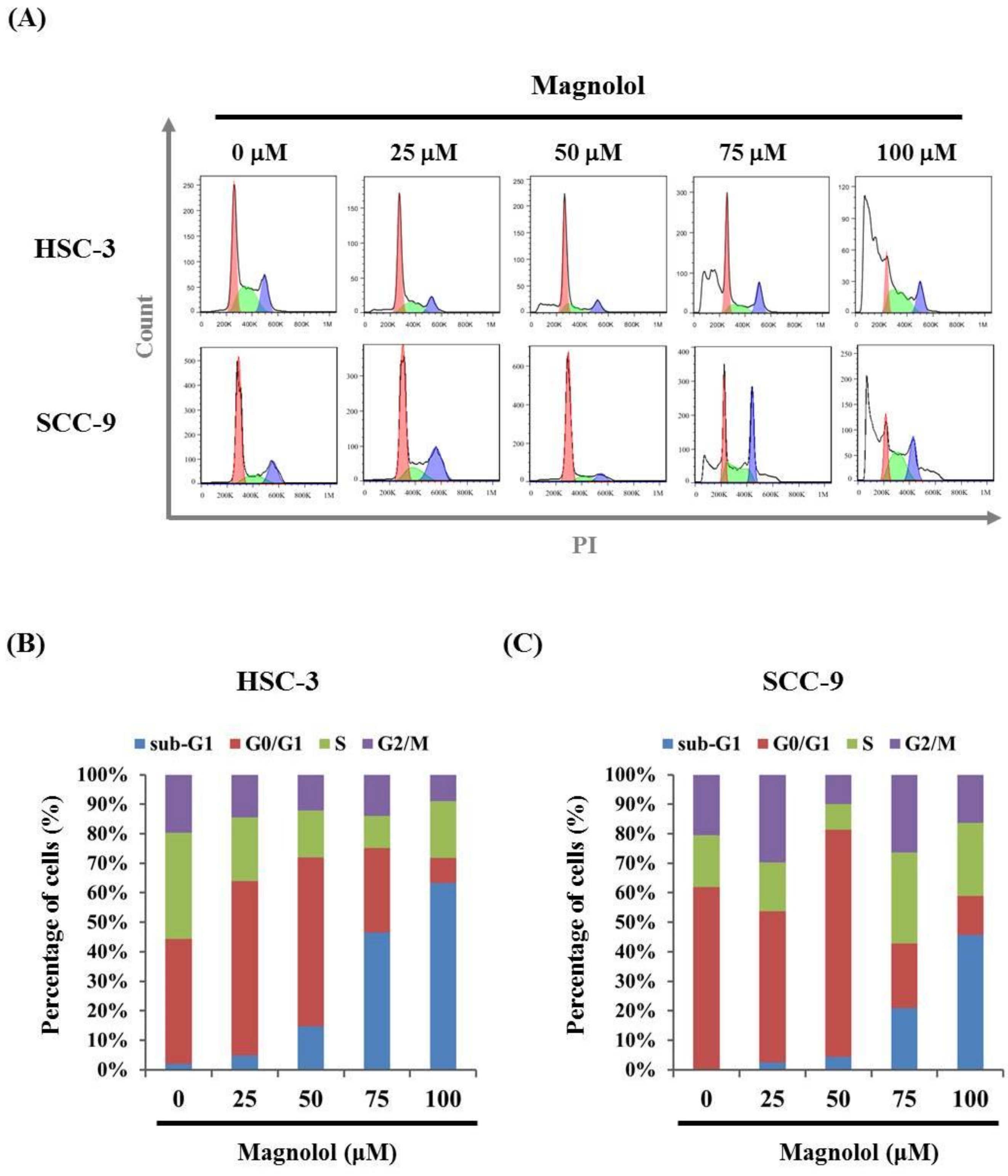
3.2. Magnolol-Induced Apoptosis in Human Oral Cancer Cell Lines
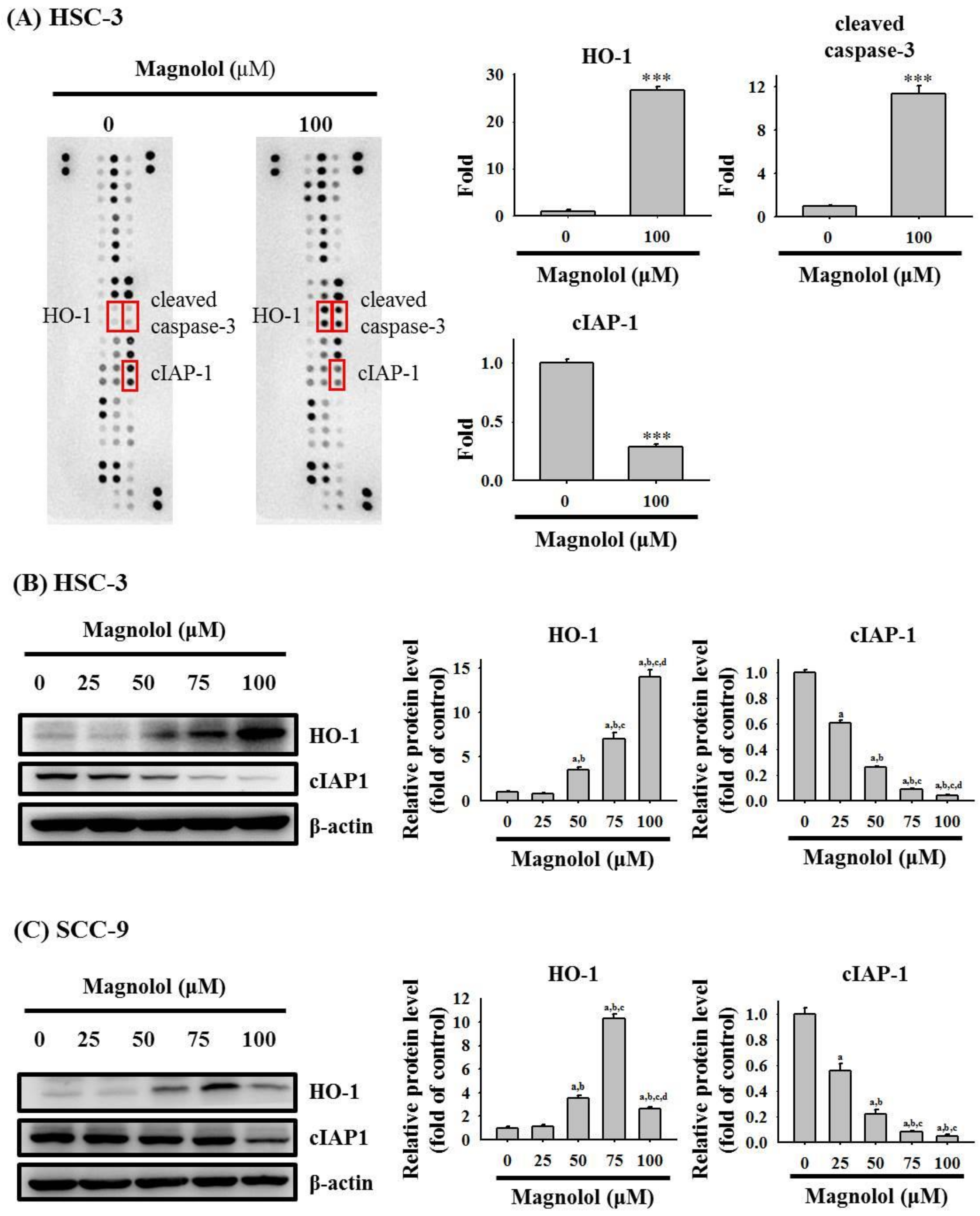
3.3. Magnolol Triggers Caspase-Mediated Apoptosis in Oral Cancer
3.4. Activation of the MAPK Signaling Pathway by Magnolol in Oral Cancer Cell
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, N.W.; Jayasekara, P.; Amarasinghe, A.A. Squamous cell carcinoma and precursor lesions of the oral cavity: Epidemiology and aetiology. Periodontology 2000 2011, 57, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Lewin, F.; Norell, S.E.; Johansson, H.; Gustavsson, P.; Wennerberg, J.; Biorklund, A.; Rutqvist, L.E. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck: A population-based case-referent study in Sweden. Cancer 1998, 82, 1367–1375. [Google Scholar] [CrossRef]
- Ram, H.; Sarkar, J.; Kumar, H.; Konwar, R.; Bhatt, M.L.; Mohammad, S. Oral cancer: Risk factors and molecular pathogenesis. J. Maxillofac. Oral Surg. 2011, 10, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Omura, K. Current status of oral cancer treatment strategies: Surgical treatments for oral squamous cell carcinoma. Int. J. Clin. Oncol. 2014, 19, 423–430. [Google Scholar] [CrossRef]
- Zhu, L.; Ge, G.; Zhang, H.; Liu, H.; He, G.; Liang, S.; Zhang, Y.; Fang, Z.; Dong, P.; Finel, M.; et al. Characterization of hepatic and intestinal glucuronidation of magnolol: Application of the relative activity factor approach to decipher the contributions of multiple udp-glucuronosyltransferase isoforms. Drug Metab. Dispos. 2012, 40, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.H.; Hsu, W.L.; Chen, T.H.; Chou, T.C. Activation of nrf2/ho-1signaling pathway involves the anti-inflammatory activity of magnolol in porphyromonas gingivalis lipopolysaccharide-stimulated mouse raw 264.7 macrophages. Int. Immunopharmacol. 2015, 29, 770–778. [Google Scholar] [CrossRef]
- Sakaue, Y.; Domon, H.; Oda, M.; Takenaka, S.; Kubo, M.; Fukuyama, Y.; Okiji, T.; Terao, Y. Anti-biofilm and bactericidal effects of magnolia bark-derived magnolol and honokiol on streptococcus mutans. Microbiol. Immunol. 2016, 60, 10–16. [Google Scholar] [CrossRef]
- Shen, Y.C.; Sung, Y.J.; Chen, C.F. Magnolol inhibits mac-1 (cd11b/cd18)-dependent neutrophil adhesion: Relationship with its antioxidant effect. Eur. J. Pharmacol. 1998, 343, 79–86. [Google Scholar] [CrossRef]
- Akagi, M.; Matsui, N.; Akae, H.; Hirashima, N.; Fukuishi, N.; Fukuyama, Y.; Akagi, R. Nonpeptide neurotrophic agents useful in the treatment of neurodegenerative diseases such as alzheimer’s disease. J. Pharmacol. Sci. 2015, 127, 155–163. [Google Scholar] [CrossRef]
- Ho, J.H.; Hong, C.Y. Cardiovascular protection of magnolol: Cell-type specificity and dose-related effects. J. Biomed. Sci. 2012, 19, 70. [Google Scholar] [CrossRef]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef]
- Mottaghi, S.; Abbaszadeh, H. Natural lignans honokiol and magnolol as potential anticarcinogenic and anticancer agents. A comprehensive mechanistic review. Nutr. Cancer 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, X.; Zeng, Y.; Lan, Y.; Wang, X. Magnolol suppressed cell migration and invasion and induced cell apoptosis via inhibition of the nf-κb signaling pathway by upregulating microrna-129 in multiple myeloma. Neoplasma 2021, 68, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-M.; Weng, Y.-S.; Kuan, L.-Y.; Chen, J.-H.; Hsu, F.-T. Suppression of PKCδ/NF-κB Signaling and Apoptosis Induction through Extrinsic/Intrinsic Pathways Are Associated with Magnolol-Inhibited Tumor Progression in Colorectal Cancer In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 3527. [Google Scholar] [CrossRef]
- Peng, C.Y.; Yu, C.C.; Huang, C.C.; Liao, Y.W.; Hsieh, P.L.; Chu, P.M.; Yu, C.H.; Lin, S.S. Magnolol inhibits cancer stemness and il-6/stat3 signaling in oral carcinomas. J. Formos. Med. Assoc. 2021, in press. [Google Scholar] [CrossRef]
- Lin, C.W.; Yang, W.E.; Lee, W.J.; Hua, K.T.; Hsieh, F.K.; Hsiao, M.; Chen, C.C.; Chow, J.M.; Chen, M.K.; Yang, S.F.; et al. Lipocalin 2 prevents oral cancer metastasis through carbonic anhydrase ix inhibition and is associated with favourable prognosis. Carcinogenesis 2016, 37, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Lin, C.W.; Hsieh, Y.S.; Cheng, H.L.; Lue, K.H.; Yang, S.F.; Lu, K.H. Selaginella tamariscina (beauv.) possesses antimetastatic effects on human osteosarcoma cells by decreasing mmp-2 and mmp-9 secretions via p38 and akt signaling pathways. Food Chem. Toxicol. 2013, 59, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.-H.; Yang, W.-E.; Yang, Y.-C.; Ku, C.-C.; Lee, W.-J.; Tsai, M.-Y.; Lin, C.-W.; Yang, S.-F. Dual Targeting of the p38 MAPK-HO-1 Axis and cIAP1/XIAP by Demethoxycurcumin Triggers Caspase-Mediated Apoptotic Cell Death in Oral Squamous Cell Carcinoma Cells. Cancers 2020, 12, 703. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, P.-C.; Chang, J.-H.; Lee, W.-J.; Ku, C.-C.; Tsai, M.-Y.; Yang, S.-F.; Chien, M.-H. The Curcumin Analogue, EF-24, Triggers p38 MAPK-Mediated Apoptotic Cell Death via Inducing PP2A-Modulated ERK Deactivation in Human Acute Myeloid Leukemia Cells. Cancers 2020, 12, 2163. [Google Scholar] [CrossRef]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme Oxygenase-1 Signaling and Redox Homeostasis in Physiopathological Conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef]
- Luu Hoang, K.N.; Anstee, J.E.; Arnold, J.N. The diverse roles of heme oxygenase-1 in tumor progression. Front. Immunol 2021, 12, 658315. [Google Scholar] [CrossRef]
- Nitti, M.; Ivaldo, C.; Traverso, N.; Furfaro, A.L. Clinical Significance of Heme Oxygenase 1 in Tumor Progression. Antioxidants 2021, 10, 789. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Jeong, G.-S.; Lim, H.-D.; An, R.-B.; Kim, Y.-C.; Kim, E.-C. Isoliquiritigenin 2′-methyl ether induces growth inhibition and apoptosis in oral cancer cells via heme oxygenase-1. Toxicol. In Vitro 2010, 24, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Auh, Q.S.; Lee, D.-W.; Kim, J.-Y.; Jung, H.-J.; Lee, S.-H.; Kim, E.-C. Involvement of nrf2-mediated upregulation of heme oxygenase-1 in mollugin-induced growth inhibition and apoptosis in human oral cancer cells. Biomed. Res. Int. 2013, 2013, 210604. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Yang, S.F.; Tsai, C.H.; Chou, M.C.; Chou, M.Y.; Chang, Y.C. Upregulation of heme oxygenase-1 expression in areca-quid-chewing-associated oral squamous cell carcinoma. J. Formos. Med. Assoc. 2008, 107, 355–363. [Google Scholar] [CrossRef][Green Version]
- Varfolomeev, E.; Vucic, D. (un)expected roles of c-iaps in apoptotic and nfkappab signaling pathways. Cell Cycle 2008, 7, 1511–1521. [Google Scholar] [CrossRef]
- Pang, L.; Zhao, X.; Liu, W.; Deng, J.; Tan, X.; Qiu, L. Anticancer effect of ursodeoxycholic acid in human oral squamous carcinoma hsc-3 cells through the caspases. Nutrients 2015, 7, 3200–3218. [Google Scholar] [CrossRef] [PubMed]
- Chilampalli, S.; Zhang, X.; Fahmy, H.; Kaushik, R.S.; Zeman, D.; Hildreth, M.B.; Dwivedi, C. Chemopreventive effects of honokiol on uvb-induced skin cancer development. Anticancer Res. 2010, 30, 777–783. [Google Scholar] [PubMed]
- Hahm, E.R.; Arlotti, J.A.; Marynowski, S.W.; Singh, S.V. Honokiol, a constituent of oriental medicinal herb magnolia officinalis, inhibits growth of pc-3 xenografts in vivo in association with apoptosis induction. Clin. Cancer Res. 2008, 14, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Park, S.-S.; Lee, U.-S.; Kim, W.-J.; Moon, S.-K. Signaling pathway for tnf-α-induced mmp-9 expression: Mediation through p38 map kinase, and inhibition by anti-cancer molecule magnolol in human urinary bladder cancer 5637 cells. Int. Immunopharmacol. 2008, 8, 1821–1826. [Google Scholar] [CrossRef]
- Chen, C.-H.; Hsu, F.-T.; Chen, W.-L.; Chen, J.-H. Induction of Apoptosis, Inhibition of MCL-1, and VEGF-A Expression Are Associated with the Anti-Cancer Efficacy of Magnolol Combined with Regorafenib in Hepatocellular Carcinoma. Cancers 2021, 13, 2066. [Google Scholar]
- Woo, S.M.; Min, K.-j.; Kwon, T.K. Magnolol Enhances the Therapeutic Effects of TRAIL through DR5 Upregulation and Downregulation of c-FLIP and Mcl-1 Proteins in Cancer Cells. Molecules 2020, 25, 4591. [Google Scholar] [CrossRef]
- Chen, Q.; Song, S.; Wang, Z.; Shen, Y.; Xie, L.; Li, J.; Jiang, L.; Zhao, H.; Feng, X.; Zhou, Y.; et al. Isorhamnetin induces the paraptotic cell death through ros and the erk/mapk pathway in oscc cells. Oral Dis. 2021, 27, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Hsieh, M.J.; Chen, C.J.; Lin, J.T.; Lo, Y.S.; Chuang, Y.C.; Chien, S.Y.; Chen, M.K. Polyphyllin g induce apoptosis and autophagy in human nasopharyngeal cancer cells by modulation of akt and mitogen-activated protein kinase pathways in vitro and in vivo. Oncotarget 2016, 7, 70276–70289. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Lo, Y.S.; Chuang, Y.C.; Lin, C.C.; Ho, H.Y.; Hsieh, M.J.; Lin, J.T. Dehydrocrenatidine extracted from picrasma quassioides induces the apoptosis of nasopharyngeal carcinoma cells through the jnk and erk signaling pathways. Oncol. Rep. 2021, 46, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Semlali, A.; Contant, C.; Al-Otaibi, B.; Al-Jammaz, I.; Chandad, F. The curcumin analog (pac) suppressed cell survival and induced apoptosis and autophagy in oral cancer cells. Sci. Rep. 2021, 11, 11701. [Google Scholar] [CrossRef]
- Chen, Y.S.; Sun, R.; Chen, W.L.; Yau, Y.C.; Hsu, F.T.; Chung, J.G.; Tsai, C.J.; Hsieh, C.L.; Chiu, Y.M.; Chen, J.H. The in vivo radiosensitizing effect of magnolol on tumor growth of hepatocellular carcinoma. In Vivo (Athens, Greece) 2020, 34, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, K.; Ding, X.; Tang, H.; Xu, Z. Magnolol inhibits growth and induces apoptosis in esophagus cancer kyse-150 cell lines via the map kinase pathway. J. Thorac. Dis. 2019, 11, 3030–3038. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-T.; Lin, C.-W.; Su, C.-W.; Yang, W.-E.; Chuang, C.-Y.; Su, S.-C.; Hsieh, M.-J.; Yang, S.-F. Magnolol Triggers Caspase-Mediated Apoptotic Cell Death in Human Oral Cancer Cells through JNK1/2 and p38 Pathways. Biomedicines 2021, 9, 1295. https://doi.org/10.3390/biomedicines9101295
Chen Y-T, Lin C-W, Su C-W, Yang W-E, Chuang C-Y, Su S-C, Hsieh M-J, Yang S-F. Magnolol Triggers Caspase-Mediated Apoptotic Cell Death in Human Oral Cancer Cells through JNK1/2 and p38 Pathways. Biomedicines. 2021; 9(10):1295. https://doi.org/10.3390/biomedicines9101295
Chicago/Turabian StyleChen, Yi-Tzu, Chiao-Wen Lin, Chun-Wen Su, Wei-En Yang, Chun-Yi Chuang, Shih-Chi Su, Ming-Ju Hsieh, and Shun-Fa Yang. 2021. "Magnolol Triggers Caspase-Mediated Apoptotic Cell Death in Human Oral Cancer Cells through JNK1/2 and p38 Pathways" Biomedicines 9, no. 10: 1295. https://doi.org/10.3390/biomedicines9101295
APA StyleChen, Y.-T., Lin, C.-W., Su, C.-W., Yang, W.-E., Chuang, C.-Y., Su, S.-C., Hsieh, M.-J., & Yang, S.-F. (2021). Magnolol Triggers Caspase-Mediated Apoptotic Cell Death in Human Oral Cancer Cells through JNK1/2 and p38 Pathways. Biomedicines, 9(10), 1295. https://doi.org/10.3390/biomedicines9101295







