Heart-Ankle Pulse Wave Velocity Is Superior to Brachial-Ankle Pulse Wave Velocity in Detecting Aldosterone-Induced Arterial Stiffness
Abstract
:1. Introduction
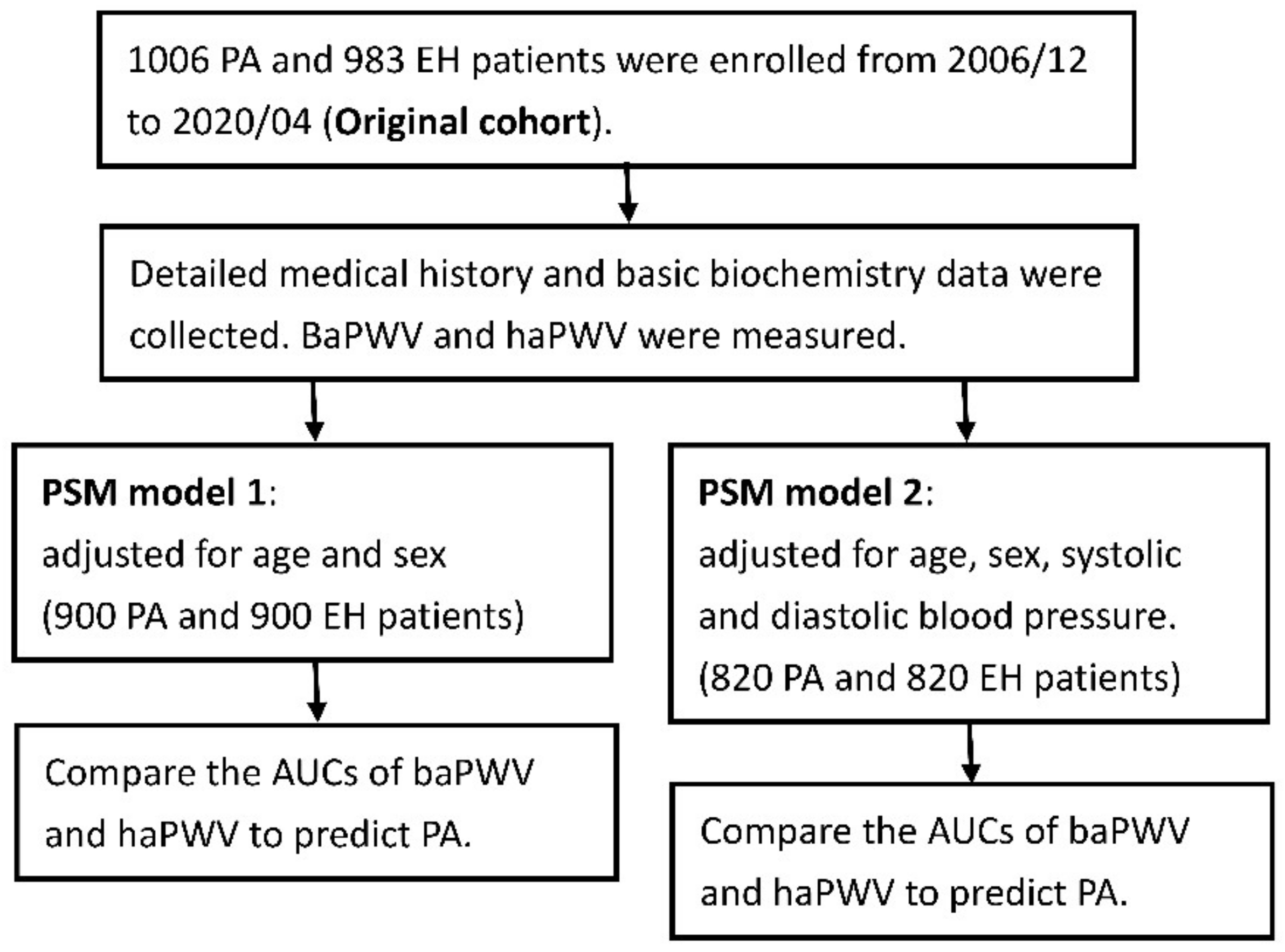
2. Materials and Methods
2.1. Patients
2.2. Diagnostic Criteria for Primary Aldosteronism
2.3. PWV Measurements
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.1.1. Overall Patients before Propensity Score Matching (PSM)
3.1.2. Propensity Score Matching for Age and Sex (PSM Model 1)
3.1.3. Propensity Score Matching for Age, Sex, Systolic and Diastolic Blood Pressures (PSM Model 2)
3.2. PWV Data
3.2.1. Original Overall Patients before PSM
3.2.2. PSM Model 1
3.2.3. PSM Model 2
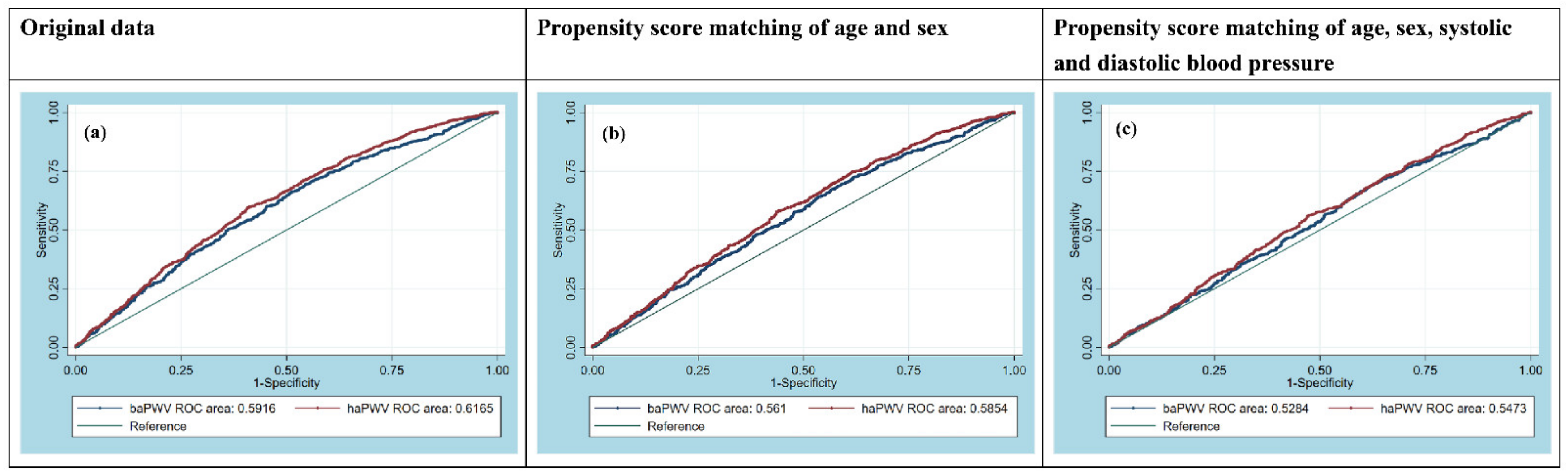
3.3. Receiver Operating Characteristic (ROC) Curve Analysis
3.3.1. Original Overall Patients before PSM
3.3.2. PSM Model 1
3.3.3. PSM Model 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Rossi, G.P.; Bisogni, V.; Rossitto, G.; Maiolino, G.; Cesari, M.; Zhu, R.; Seccia, T.M. Practice Recommendations for Diagnosis and Treatment of the Most Common Forms of Secondary Hypertension. High. Blood Press. Cardiovasc. Prev. Off. J. Ital. Soc. Hypertens. 2020, 27, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Hundemer, G.L.; Kline, G.A.; Leung, A.A. How common is primary aldosteronism? Curr. Opin. Nephrol. Hypertens. 2021. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P. Prevalence and diagnosis of primary aldosteronism. Curr. Hypertens. Rep. 2010, 12, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Monticone, S.; Burrello, J.; Tizzani, D.; Bertello, C.; Viola, A.; Buffolo, F.; Gabetti, L.; Mengozzi, G.; Williams, T.A.; Rabbia, F.; et al. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J. Am. Coll. Cardiol. 2017, 69, 1811–1820. [Google Scholar] [CrossRef]
- Chen, Z.W.; Huang, K.C.; Lee, J.K.; Lin, L.C.; Chen, C.W.; Chang, Y.Y.; Liao, C.W.; Wu, V.C.; Hung, C.S.; Lin, Y.H. Aldosterone induces left ventricular subclinical systolic dysfunction: A strain imaging study. J. Hypertens. 2018, 36, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.; Hung, C.S.; Wu, V.C.; Lin, Y.H. Primary Aldosteronism and Cerebrovascular Diseases. Endocrinol. Metab. 2018, 33, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.T.; Tsai, C.H.; Chen, Z.W.; Chang, Y.Y.; Wu, V.C.; Hung, C.S.; Lin, Y.H. Atrial Fibrillation in Primary Aldosteronism. Horm. Metab. Res. Horm. Und Stoffwechs. Horm. Et Metab. 2020, 52, 357–365. [Google Scholar] [CrossRef]
- Tsai, C.H.; Pan, C.T.; Chang, Y.Y.; Chen, Z.W.; Wu, V.C.; Hung, C.S.; Lin, Y.H. Left ventricular remodeling and dysfunction in primary aldosteronism. J. Hum. Hypertens. 2021, 35, 131–147. [Google Scholar] [CrossRef]
- Milliez, P.; Girerd, X.; Plouin, P.F.; Blacher, J.; Safar, M.E.; Mourad, J.J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 2005, 45, 1243–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catena, C.; Colussi, G.; Nadalini, E.; Chiuch, A.; Baroselli, S.; Lapenna, R.; Sechi, L.A. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch. Intern. Med. 2008, 168, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Reincke, M.; Fischer, E.; Gerum, S.; Merkle, K.; Schulz, S.; Pallauf, A.; Quinkler, M.; Hanslik, G.; Lang, K.; Hahner, S.; et al. Observational study mortality in treated primary aldosteronism: The German Conn’s registry. Hypertension 2012, 60, 618–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savard, S.; Amar, L.; Plouin, P.F.; Steichen, O. Cardiovascular complications associated with primary aldosteronism: A controlled cross-sectional study. Hypertension 2013, 62, 331–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulatero, P.; Monticone, S.; Bertello, C.; Viola, A.; Tizzani, D.; Iannaccone, A.; Crudo, V.; Burrello, J.; Milan, A.; Rabbia, F.; et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J. Clin. Endocrinol. Metab. 2013, 98, 4826–4833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, V.C.; Wang, S.M.; Chang, C.H.; Hu, Y.H.; Lin, L.Y.; Lin, Y.H.; Chueh, S.C.; Chen, L.; Wu, K.D. Long term outcome of Aldosteronism after target treatments. Sci. Rep. 2016, 6, 32103. [Google Scholar] [CrossRef]
- Monticone, S.; D’Ascenzo, F.; Moretti, C.; Williams, T.A.; Veglio, F.; Gaita, F.; Mulatero, P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018, 6, 41–50. [Google Scholar] [CrossRef]
- Chen, Z.W.; Tsai, C.H.; Pan, C.T.; Chou, C.H.; Liao, C.W.; Hung, C.S.; Wu, V.C.; Lin, Y.H. Endothelial Dysfunction in Primary Aldosteronism. Int. J. Mol. Sci. 2019, 20, 5214. [Google Scholar] [CrossRef] [Green Version]
- Asmar, R.; Benetos, A.; Topouchian, J.; Laurent, P.; Pannier, B.; Brisac, A.-M.; Target, R.; Levy, B.I. Assessment of Arterial Distensibility by Automatic Pulse Wave Velocity Measurement. Hypertension 1995, 26, 485–490. [Google Scholar] [CrossRef]
- Bonarjee, V.V.S. Arterial Stiffness: A Prognostic Marker in Coronary Heart Disease. Available Methods and Clinical Application. Front. Cardiovasc Med. 2018, 5, 64. [Google Scholar] [CrossRef] [Green Version]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Kim, E.D.; Tanaka, H.; Ballew, S.H.; Sang, Y.; Heiss, G.; Coresh, J.; Matsushita, K. Associations between Kidney Disease Measures and Regional Pulse Wave Velocity in a Large Community-Based Cohort: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2018, 72, 682–690. [Google Scholar] [CrossRef]
- Strauch, B.; Petrák, O.; Wichterle, D.; Zelinka, T.; Holaj, R.; Widimský, J. Increased Arterial Wall Stiffness in Primary Aldosteronism in Comparison With Essential Hypertension. Am. J. Hypertens. 2006, 19, 909–914. [Google Scholar] [CrossRef] [Green Version]
- Strauch, B.; Petrak, O.; Zelinka, T.; Wichterle, D.; Holaj, R.; Kasalicky, M.; Safarik, L.; Rosa, J.; Widimsky, J., Jr. Adrenalectomy improves arterial stiffness in primary aldosteronism. Am. J. Hypertens. 2008, 21, 1086–1092. [Google Scholar] [CrossRef] [Green Version]
- Bernini, G.; Galetta, F.; Franzoni, F.; Bardini, M.; Taurino, C.; Bernardini, M.; Ghiadoni, L.; Bernini, M.; Santoro, G.; Salvetti, A. Arterial stiffness, intima-media thickness and carotid artery fibrosis in patients with primary aldosteronism. J. Hypertens. 2008, 26, 2399–2405. [Google Scholar] [CrossRef]
- Rosa, J.; Somlóová, Z.; Petrák, O.; Strauch, B.; Indra, T.; Senitko, M.; Zelinka, T.; Holaj, R.; Widimský, J., Jr. Peripheral arterial stiffness in primary aldosteronism. Physiol. Res. 2012, 61, 461–468. [Google Scholar] [CrossRef]
- Wu, V.C.; Lo, S.C.; Chen, Y.L.; Huang, P.H.; Tsai, C.T.; Liang, C.J.; Kuo, C.C.; Kuo, Y.S.; Lee, B.C.; Wu, E.L.; et al. Endothelial progenitor cells in primary aldosteronism: A biomarker of severity for aldosterone vasculopathy and prognosis. J. Clin. Endocrinol. Metab. 2011, 96, 3175–3183. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.H.; Lin, L.Y.; Chen, A.; Wu, X.M.; Lee, J.K.; Su, T.C.; Wu, V.C.; Chueh, S.C.; Lin, W.C.; Lo, M.T.; et al. Adrenalectomy improves increased carotid intima-media thickness and arterial stiffness in patients with aldosterone producing adenoma. Atherosclerosis 2012, 221, 154–159. [Google Scholar] [CrossRef]
- Liao, C.W.; Lin, L.Y.; Hung, C.S.; Lin, Y.T.; Chang, Y.Y.; Wang, S.M.; Wu, V.C.; Wu, K.D.; Ho, Y.L.; Satoh, F.; et al. Time course and factors predicting arterial stiffness reversal in patients with aldosterone-producing adenoma after adrenalectomy: Prospective study of 102 patients. Sci. Rep. 2016, 6, 20862. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.H.; Hu, Y.H.; Tsai, Y.C.; Wu, C.H.; Wang, S.M.; Lin, L.Y.; Lin, Y.H.; Satoh, F.; Wu, K.D.; Wu, V.C. Arterial stiffness and blood pressure improvement in aldosterone-producing adenoma harboring KCNJ5 mutations after adrenalectomy. Oncotarget 2017, 8, 29984–29995. [Google Scholar] [CrossRef]
- Chan, C.K.; Yang, W.S.; Lin, Y.H.; Huang, K.H.; Lu, C.C.; Hu, Y.H.; Wu, V.C.; Chueh, J.S.; Chu, T.S.; Chen, Y.M. Arterial Stiffness Is Associated with Clinical Outcome and Cardiorenal Injury in Lateralized Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2020, 105, e3950–e3960. [Google Scholar] [CrossRef]
- Pan, C.T.; Wu, X.M.; Tsai, C.H.; Chang, Y.Y.; Chen, Z.W.; Chang, C.C.; Lee, B.C.; Liao, C.W.; Chen, Y.L.; Lin, L.C.; et al. Hemodynamic and Non-Hemodynamic Components of Cardiac Remodeling in Primary Aldosteronism. Front. Endocrinol. 2021, 12, 646097. [Google Scholar] [CrossRef]
- Wu, V.C.; Yang, S.Y.; Lin, J.W.; Cheng, B.W.; Kuo, C.C.; Tsai, C.T.; Chu, T.S.; Huang, K.H.; Wang, S.M.; Lin, Y.H.; et al. Kidney impairment in primary aldosteronism. Clin. Chim. Acta Int. J. Clin. Chem. 2011, 412, 1319–1325. [Google Scholar] [CrossRef]
- Chao, C.T.; Wu, V.C.; Kuo, C.C.; Lin, Y.H.; Chang, C.C.; Chueh, S.J.; Wu, K.D.; Pimenta, E.; Stowasser, M. Diagnosis and management of primary aldosteronism: An updated review. Ann. Med. 2013, 45, 375–383. [Google Scholar] [CrossRef]
- Harvey, A.; Montezano, A.C.; Lopes, R.A.; Rios, F.; Touyz, R.M. Vascular Fibrosis in Aging and Hypertension: Molecular Mechanisms and Clinical Implications. Can. J. Cardiol. 2016, 32, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.C.; Chuang, S.Y.; Lin, Y.P.; Chen, C.H. Brachial-ankle vs. carotid-femoral pulse wave velocity as a determinant of cardiovascular structure and function. J. Hum. Hypertens. 2008, 22, 24–31. [Google Scholar] [CrossRef]
- Rizzoni, D.; Paiardi, S.; Rodella, L.; Porteri, E.; De Ciuceis, C.; Rezzani, R.; Boari, G.E.M.; Zani, F.; Miclini, M.; Tiberio, G.A.M.; et al. Changes in Extracellular Matrix in Subcutaneous Small Resistance Arteries of Patients with Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2006, 91, 2638–2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzamou, V.; Kyvelou, S.M.; Karpanou, E.; Petras, D.; Vyssoulis, G. Aldosterone Levels, Aortic Stiffness, and Wave Reflection in Essential Hypertensive Patients. Am. J. Hypertens. 2015, 28, 852–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barde, M.P.; Barde, P.J. What to use to express the variability of data: Standard deviation or standard error of mean? Perspect. Clin. Res. 2012, 3, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.K.; Chen, C.Y.; Liu, H.M.; Yen, C.J.; Chang, K.J.; Chang, C.C.; Yu, Y.H.; Lin, L.Y.; Hwang, J.J. Metabolic risks, white matter hyperintensities, and arterial stiffness in high-functioning healthy adults. Int. J. Cardiol. 2010, 143, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.R.; Wilkinson, I.B.; Schiffrin, E.L.; Avolio, A.P.; Chirinos, J.A.; Cockcroft, J.R.; Heffernan, K.S.; Lakatta, E.G.; McEniery, C.M.; Mitchell, G.F.; et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension 2015, 66, 698–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munakata, M. Brachial-Ankle Pulse Wave Velocity: Background, Method, and Clinical Evidence. Pulse 2016, 3, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Ohkuma, T.; Ninomiya, T.; Tomiyama, H.; Kario, K.; Hoshide, S.; Kita, Y.; Inoguchi, T.; Maeda, Y.; Kohara, K.; Tabara, Y.; et al. Brachial-Ankle Pulse Wave Velocity and the Risk Prediction of Cardiovascular Disease: An Individual Participant Data Meta-Analysis. Hypertension 2017, 69, 1045–1052. [Google Scholar] [CrossRef]
- Shirai, K.; Utino, J.; Otsuka, K.; Takata, M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J. Atheroscler Thromb 2006, 13, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Yamamoto, T.; Tsuda, S.; Okabe, F.; Shimose, T.; Tsuji, Y.; Suzuki, K.; Otsuka, K.; Takata, M.; Shimizu, K.; et al. Coefficients in the CAVI Equation and the Comparison between CAVI With and Without the Coefficients Using Clinical Data. J. Atheroscler Thromb 2019, 26, 465–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namba, T.; Masaki, N.; Takase, B.; Adachi, T. Arterial Stiffness Assessed by Cardio-Ankle Vascular Index. Int. J. Mol. Sci. 2019, 20, 3664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyoshi, T.; Doi, M.; Hirohata, S.; Sakane, K.; Kamikawa, S.; Kitawaki, T.; Kaji, Y.; Kusano, K.F.; Ninomiya, Y.; Kusachi, S. Cardio-ankle vascular index is independently associated with the severity of coronary atherosclerosis and left ventricular function in patients with ischemic heart disease. J. Atheroscler Thromb 2010, 17, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, J.; Sakakibara, R.; Tomaru, T.; Tateno, F.; Kishi, M.; Ogawa, E.; Kurosu, T.; Shirai, K. Stroke and cardio-ankle vascular stiffness index. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2013, 22, 171–175. [Google Scholar] [CrossRef]
- Miyashita, Y.; Saiki, A.; Endo, K.; Ban, N.; Yamaguchi, T.; Kawana, H.; Nagayama, D.; Ohira, M.; Oyama, T.; Shirai, K. Effects of olmesartan, an angiotensin II receptor blocker, and amlodipine, a calcium channel blocker, on Cardio-Ankle Vascular Index (CAVI) in type 2 diabetic patients with hypertension. J. Atheroscler Thromb 2009, 16, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Ibata, J.; Sasaki, H.; Kakimoto, T.; Matsuno, S.; Nakatani, M.; Kobayashi, M.; Tatsumi, K.; Nakano, Y.; Wakasaki, H.; Furuta, H.; et al. Cardio-ankle vascular index measures arterial wall stiffness independent of blood pressure. Diabetes Res. Clin. Pract. 2008, 80, 265–270. [Google Scholar] [CrossRef]
- Horinaka, S.; Yabe, A.; Yagi, H.; Ishimura, K.; Hara, H.; Iemua, T.; Matsuoka, H. Comparison of atherosclerotic indicators between cardio ankle vascular index and brachial ankle pulse wave velocity. Angiology 2009, 60, 468–476. [Google Scholar] [CrossRef]
- Takaki, A.; Ogawa, H.; Wakeyama, T.; Iwami, T.; Kimura, M.; Hadano, Y.; Matsuda, S.; Miyazaki, Y.; Hiratsuka, A.; Matsuzaki, M. Cardio-ankle vascular index is superior to brachial-ankle pulse wave velocity as an index of arterial stiffness. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2008, 31, 1347–1355. [Google Scholar] [CrossRef] [Green Version]
| Original Data | Propensity Score Matching of Age and Sex | Propensity Score Matching of Age, Sex, Systolic and Diastolic Blood Pressure | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient characteristics | PA (n = 1006) | EH (n = 983) | p value | PA (n = 900) | EH (n = 900) | p value | PA (n = 820) | EH (n = 820) | p value |
| Sex (Male), n (%) | 449 (45) | 514 (52) | 0.001 | 426 (47) | 436 (48) | 0.637 | 380 (46) | 390 (48) | 0.621 |
| Age, years | 54 ± 12 | 50 ± 15 | <0.001 | 53 ± 12 | 52 ± 14 | 0.104 | 53 ± 12 | 53 ± 14 | 0.880 |
| Body height, cm | 163 ± 8 | 164 ± 9 | 0.001 | 163 ± 8 | 163 ± 9 | 0.781 | 163 ± 8 | 163 ± 9 | 0.760 |
| Body weight, Kg | 68 ± 14 | 69 ± 14 | 0.144 | 68 ± 14 | 68 ± 13 | 0.252 | 68 ± 14 | 68 ± 14 | 0.834 |
| Body mass index, kg m−2 | 25 ± 4 | 25 ± 4 | 0.707 | 26 ± 4 | 25 ± 4 | 0.118 | 26 ± 4 | 25 ± 4 | 0.974 |
| SBP, mmHg | 153 ± 21 | 145 ± 21 | <0.001 | 153 ± 22 | 145 ± 21 | <0.001 | 149 ± 20 | 147 ± 20 | 0.066 |
| DBP, mmHg | 91 ± 14 | 86 ± 13 | <0.001 | 91 ± 14 | 86 ± 14 | <0.001 | 89 ± 13 | 88 ± 13 | 0.097 |
| Serum creatinine level, mg dL−1 | 0.9 ± 0.5 | 1.0 ± 0.9 | 0.132 | 0.9 ± 0.4 | 1.0 ± 1.0 | 0.169 | 0.9 ± 0.5 | 1.0 ± 1.0 | 0.091 |
| Serum potassium level, mmol L−1 | 3.7 ± 0.6 | 4.1 ± 0.4 | <0.001 | 3.7 ± 0.6 | 4.1 ± 0.4 | <0.001 | 3.7 ± 0.6 | 4.1 ± 0.4 | <0.001 |
| APA, n (%) | 636 (63) | - | - | 575 (64) | - | - | 521 (64) | ||
| PAC, ng dL−1 | 42 (29–61) | 34 (23–50) | <0.001 | 42 (29–61) | 33 (22–50) | <0.001 | 42 (30–61) | 33 (22–50) | <0.001 |
| PRA, ng mL−1 h−1 | 0.3 (0.1–0.6) | 1.7 (0.5–4.6) | <0.001 | 0.3 (0.1–0.6) | 1.6 (0.5–4.4) | <0.001 | 0.3 (0.1–0.6) | 1.5 (0.4–4.1) | <0.001 |
| ARR | 169 (58–463) | 21 (9–63) | <0.001 | 169 (60–479) | 22 (9–67) | <0.001 | 162 (61–464) | 22 (9–71) | <0.001 |
| Log-transformed PAC | 1.6 ± 0.3 | 1.5 ± 0.3 | <0.001 | 1.6 ± 0.3 | 1.5 ± 0.3 | <0.001 | 1.6 ± 0.3 | 1.5 ± 0.3 | <0.001 |
| Log-transformed PRA | −0.6 ± 0.7 | 0.1 ± 0.7 | <0.001 | −0.6 ± 0.7 | 0.1 ± 0.7 | <0.001 | −0.6 ± 0.7 | 0.1 ± 0.7 | <0.001 |
| Log-transformed ARR | 2.3 ± 0.7 | 1.4 ± 0.7 | <0.001 | 2.3 ± 0.7 | 1.4 ± 0.7 | <0.001 | 2.3 ± 0.7 | 1.5 ± 0.7 | <0.001 |
| Number of antihypertensive medication type | 2.0 ± 1.3 | 1.4 ± 1.1 | <0.001 | 2.0 ± 1.3 | 1.4 ± 1.1 | <0.001 | 1.9 ± 1.3 | 1.5 ± 1.1 | <0.001 |
| Hypertension history, years | 7.8 ± 8.1 | 5.1 ± 6.8 | <0.001 | 7.6 ± 8.0 | 5.2 ± 6.9 | <0.001 | 7.3 ± 7.7 | 5.3 ± 7.0 | <0.001 |
| Hypertension medication | |||||||||
| ACEI, n (%) | 18 (2) | 17 (2) | 0.919 | 16 (2) | 17 (2) | 0.861 | 18 (2) | 17 (2) | 0.864 |
| ARB, n (%) | 377 (38) | 403 (41) | 0.108 | 345 (38) | 376 (42) | 0.136 | 301 (37) | 344 (42) | 0.030 |
| Alpha-blocker, n (%) | 206 (21) | 112 (11) | <0.001 | 190 (21) | 98 (11) | <0.001 | 156 (19) | 95 (12) | <0.001 |
| Beta-blocker, n (%) | 353 (35) | 202 (21) | <0.001 | 313 (35) | 193 (21) | <0.001 | 280 (34) | 175 (21) | <0.001 |
| CCB, n (%) | 649 (65) | 543 (55) | <0.001 | 573 (64) | 499 (55) | <0.001 | 531 (65) | 456 (56) | <0.001 |
| Vasodilator, n (%) | 63 (6) | 19 (2) | <0.001 | 54 (6) | 19 (2) | <0.001 | 49 (6) | 18 (2) | <0.001 |
| Spironolactone, n (%) | 201 (20) | 36 (4) | <0.001 | 155 (20) | 32 (4) | <0.001 | 161 (20) | 33 (4) | <0.001 |
| Diuretics, n (%) | 110 (11) | 55 (6) | <0.001 | 80 (10) | 48 (6) | 0.003 | 84 (10) | 49 (6) | 0.002 |
| Original Data | Propensity Score Matching of Age and Sex | Propensity Score Matching of Age, Sex, Systolic and Diastolic Blood Pressure | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pulse wave analysis | PA (n = 1006) | EH (n = 983) | p value | PA (n = 900) | EH (n = 900) | p value | PA (n = 820) | EH (n = 820) | p value |
| baPWV (cm/s) | 1637 (1452–1868) | 1527 (1362–1756) | <0.001 | 1616 (1440–1844) | 1544 (1371–1776) | <0.001 | 1602 (1434–1816) | 1574 (1394–1797) | 0.047 |
| haPWV (cm/s) | 1103 (1008–1218) | 1040 (945–1150) | <0.001 | 1093 (1003–1208) | 1052 (957–1158) | <0.001 | 1089 (995–1198) | 1062 (974–1167) | 0.001 |
| Original Data | Propensity Score Matching of Age and Sex | Propensity Score Matching of Age, Sex, Systolic and Diastolic Blood Pressure | |||||||
|---|---|---|---|---|---|---|---|---|---|
| baPWV | haPWV | p value | baPWV | haPWV | p value | baPWV | haPWV | p value | |
| AUC [95% CI] | 0.5916 [0.5667–0.6165] | 0.6165 [0.5920–0.6411] | 0.0001 | 0.5610 [0.5346–0.5875] | 0.5854 [0.5592–0.6116] | 0.0001 | 0.5284 [0.5004–0.5563] | 0.5473 [0.5195–0.5751] | 0.0046 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.-W.; Pan, C.-T.; Tsai, C.-H.; Chang, Y.-Y.; Chang, C.-C.; Lee, B.-C.; Chiu, Y.-W.; Huang, W.-C.; Lin, Y.-L.; Wu, V.-C.; et al. Heart-Ankle Pulse Wave Velocity Is Superior to Brachial-Ankle Pulse Wave Velocity in Detecting Aldosterone-Induced Arterial Stiffness. Biomedicines 2021, 9, 1285. https://doi.org/10.3390/biomedicines9101285
Chen Z-W, Pan C-T, Tsai C-H, Chang Y-Y, Chang C-C, Lee B-C, Chiu Y-W, Huang W-C, Lin Y-L, Wu V-C, et al. Heart-Ankle Pulse Wave Velocity Is Superior to Brachial-Ankle Pulse Wave Velocity in Detecting Aldosterone-Induced Arterial Stiffness. Biomedicines. 2021; 9(10):1285. https://doi.org/10.3390/biomedicines9101285
Chicago/Turabian StyleChen, Zheng-Wei, Chien-Ting Pan, Cheng-Hsuan Tsai, Yi-Yao Chang, Chin-Chen Chang, Bo-Ching Lee, Yu-Wei Chiu, Wei-Chieh Huang, Yu-Li Lin, Vin-Cent Wu, and et al. 2021. "Heart-Ankle Pulse Wave Velocity Is Superior to Brachial-Ankle Pulse Wave Velocity in Detecting Aldosterone-Induced Arterial Stiffness" Biomedicines 9, no. 10: 1285. https://doi.org/10.3390/biomedicines9101285
APA StyleChen, Z.-W., Pan, C.-T., Tsai, C.-H., Chang, Y.-Y., Chang, C.-C., Lee, B.-C., Chiu, Y.-W., Huang, W.-C., Lin, Y.-L., Wu, V.-C., Hung, C.-S., Liao, C.-W., Lin, Y.-H., & on behalf of TAIPAI Study Group. (2021). Heart-Ankle Pulse Wave Velocity Is Superior to Brachial-Ankle Pulse Wave Velocity in Detecting Aldosterone-Induced Arterial Stiffness. Biomedicines, 9(10), 1285. https://doi.org/10.3390/biomedicines9101285






