Splenectomy Prior to Experimental Induction of Autoimmune Hepatitis Promotes More Severe Hepatic Inflammation, Production of IL-17 and Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Mice
2.3. Adenovirus Construction
2.4. Flow Cytometry
2.5. Histology
2.6. Protein Detection in the Serum
2.7. Statistics
3. Results
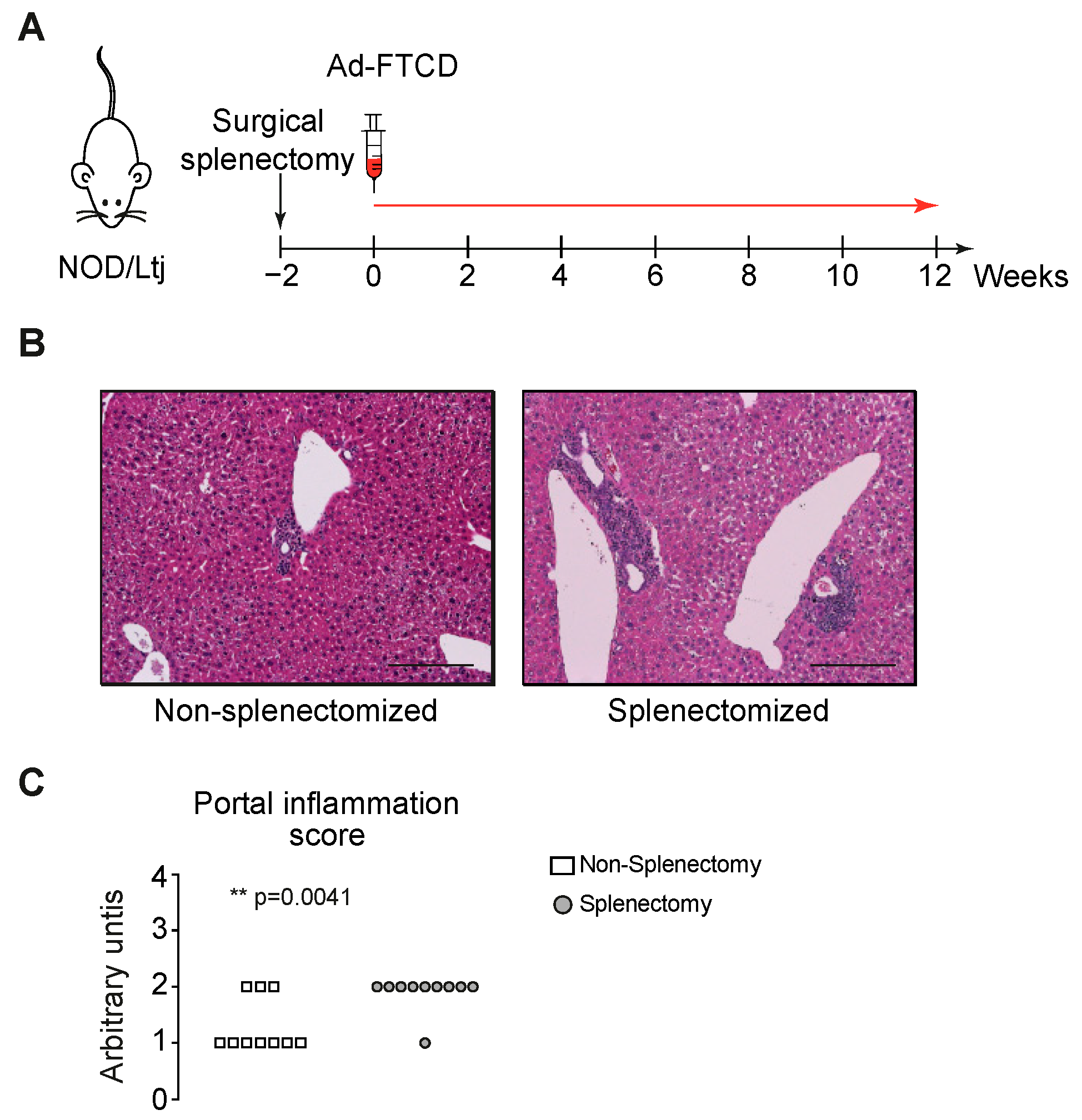
3.1. The Spleen Has a Protective Role in the Induction Phase of Autoimmune Hepatitis
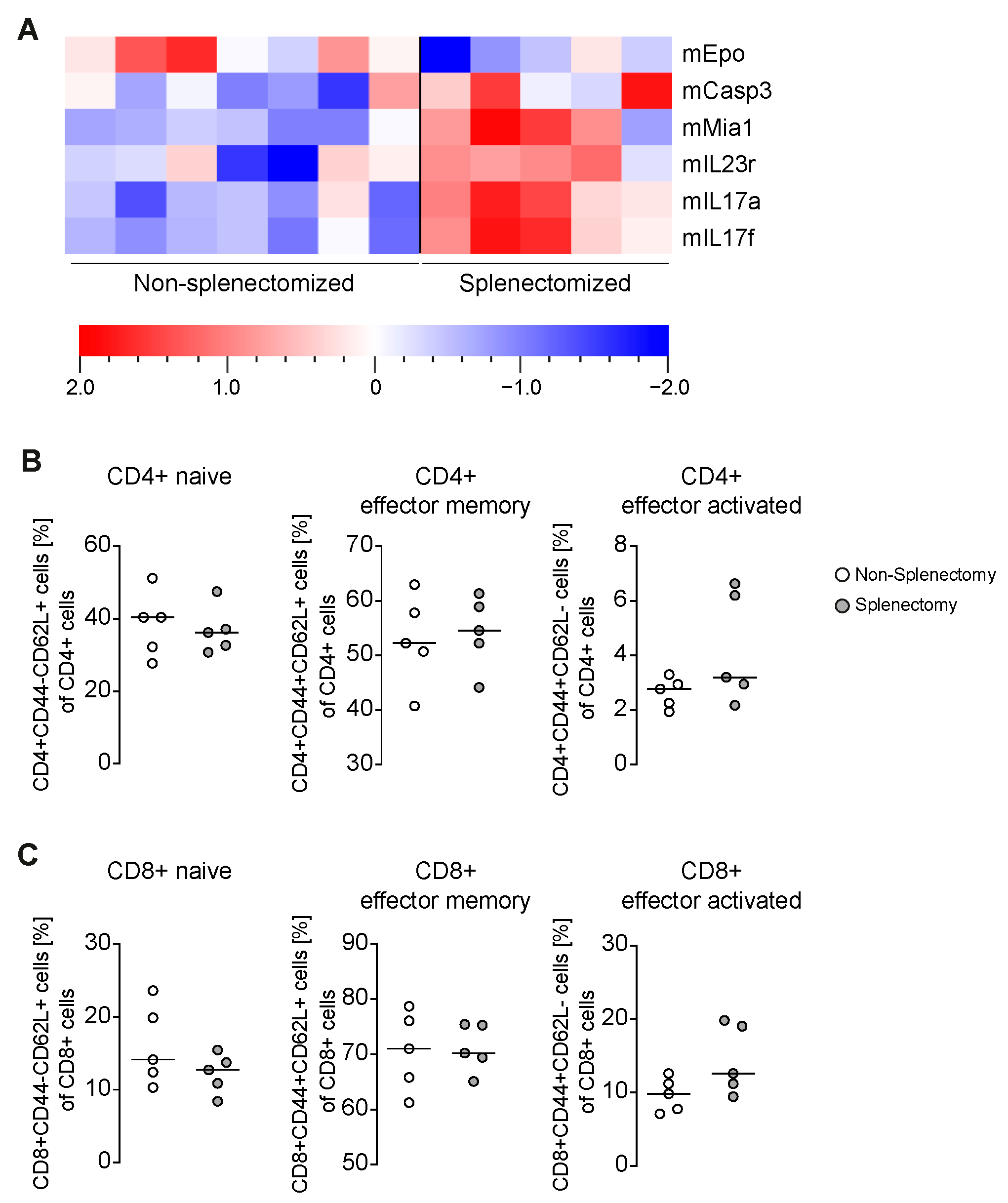
3.2. Increased Portal Inflammation Correlates with Amplified IL-17
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ad | adenovirus |
| AIH | autoimmune hepatitis |
| emAIH | experimental murine autoimmune hepatitis |
| Epo | erythropoietin |
| FTCD | formiminotransferase cyclodeaminase |
| mHAI | modified hepatitis activity index |
References
- Manns, M.P.; Czaja, A.J.; Gorham, J.D.; Krawitt, E.L.; Mieli-Vergani, G.; Vergani, D.; Vierling, J.M.; American Association for the Study of Liver. Diagnosis and management of autoimmune hepatitis. Hepatology 2010, 51, 2193–2213. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J. Hepatol. 2015, 63, 971–1004. [Google Scholar] [CrossRef]
- Hardtke-Wolenski, M.; Fischer, K.; Noyan, F.; Schlue, J.; Falk, C.S.; Stahlhut, M.; Woller, N.; Kuehnel, F.; Taubert, R.; Manns, M.P.; et al. Genetic predisposition and environmental danger signals initiate chronic autoimmune hepatitis driven by CD4(+) T cells. Hepatology 2013, 58, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Hardtke-Wolenski, M.; Taubert, R.; Noyan, F.; Sievers, M.; Dywicki, J.; Schlue, J.; Falk, C.S.; Lundgren, B.A.; Scott, H.S.; Pich, A.; et al. Autoimmune hepatitis in a murine autoimmune polyendocrine syndrome type 1 model is directed against multiple autoantigens. Hepatology 2015, 61, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Dywicki, J.; Noyan, F.; Misslitz, A.C.; Hapke, M.; Galla, M.; Schlue, J.; Liblau, R.S.; Taubert, R.; Manns, M.P.; Jaeckel, E.; et al. Hepatic T Cell Tolerance Induction in An Inflammatory Environment. Dig. Dis. 2018, 36, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, P.; Djilali-Saiah, I.; Vitozzi, S.; Alvarez, F. A murine model of type 2 autoimmune hepatitis: Xenoimmunization with human antigens. Hepatology 2004, 39, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Holdener, M.; Hintermann, E.; Bayer, M.; Rhode, A.; Rodrigo, E.; Hintereder, G.; Johnson, E.F.; Gonzalez, F.J.; Pfeilschifter, J.; Manns, M.P.; et al. Breaking tolerance to the natural human liver autoantigen cytochrome P450 2D6 by virus infection. J. Exp. Med. 2008, 205, 1409–1422. [Google Scholar] [CrossRef]
- Lohse, A.W.; Obermayer-Straub, P.; Gerken, G.; Brunner, S.; Altes, U.; Dienes, H.P.; Manns, M.P.; Buschenfelde, K.H.M.Z. Development of cytochrome P450 2D6-specific LKM-autoantibodies following liver transplantation for Wilson’s disease—Possible association with a steroid-resistant transplant rejection episode. J. Hepatol. 1999, 31, 149–155. [Google Scholar] [CrossRef]
- Backer, R.; Schwandt, T.; Greuter, M.; Oosting, M.; Jungerkes, F.; Tuting, T.; Boon, L.; O’Toole, T.; Kraal, G.; Limmer, A.; et al. Effective collaboration between marginal metallophilic macrophages and CD8+ dendritic cells in the generation of cytotoxic T cells. Proc. Natl. Acad. Sci. USA 2010, 107, 216–221. [Google Scholar] [CrossRef]
- Aoki, N.; Kido, M.; Iwamoto, S.; Nishiura, H.; Maruoka, R.; Tanaka, J.; Watanabe, T.; Tanaka, Y.; Okazaki, T.; Chiba, T.; et al. Dysregulated generation of follicular helper T cells in the spleen triggers fatal autoimmune hepatitis in mice. Gastroenterology 2011, 140, 1322–1333.e5. [Google Scholar] [CrossRef]
- Bowen, D.G.; Zen, M.; Holz, L.; Davis, T.; McCaughan, G.W.; Bertolino, P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J. Clin. Investig. 2004, 114, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Derkow, K.; Loddenkemper, C.; Mintern, J.; Kruse, N.; Klugewitz, K.; Berg, T.; Wiedenmann, B.; Ploegh, H.L.; Schott, E. Differential priming of CD8 and CD4 T-cells in animal models of autoimmune hepatitis and cholangitis. Hepatology 2007, 46, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Dywicki, J.; Buitrago-Molina, L.E.; Pietrek, J.; Lieber, M.; Broering, R.; Khera, T.; Schlue, J.; Manns, M.P.; Wedemeyer, H.; Jaeckel, E.; et al. Autoimmune hepatitis induction can occur in the liver. Liver Int. 2020, 40, 377–381. [Google Scholar] [PubMed]
- Romermann, D.; Ansari, N.; Schultz-Moreira, A.R.; Michael, A.; Marhenke, S.; Hardtke-Wolenski, M.; Longerich, T.; Manns, M.P.; Wedemeyer, H.; Vogel, A.; et al. Absence of Atg7 in the liver disturbed hepatic regeneration after liver injury. Liver Int. 2020. [Google Scholar] [CrossRef]
- Assarsson, E.; Lundberg, M.; Holmquist, G.; Bjorkesten, J.; Thorsen, S.B.; Ekman, D.; Eriksson, A.; Dickens, E.R.; Ohlsson, S.; Edfeldt, G.; et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE 2014, 9, e95192. [Google Scholar]
- Hardtke-Wolenski, M.; Dywicki, J.; Fischer, K.; Hapke, M.; Sievers, M.; Schlue, J.; Anderson, M.S.; Taubert, R.; Noyan, F.; Manns, M.P.; et al. The influence of genetic predisposition and autoimmune hepatitis inducing antigens in disease development. J. Autoimmun. 2017, 78, 39–45. [Google Scholar]
- Paulson, R.F. Epo receptor marks the spot. Blood 2019, 134, 413–414. [Google Scholar] [CrossRef]
- Nairz, M.; Sonnweber, T.; Schroll, A.; Theurl, I.; Weiss, G. The pleiotropic effects of erythropoietin in infection and inflammation. Microbes Infect. 2012, 14, 238–246. [Google Scholar]
- Ghezzi, P.; Brines, M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004, 11 (Suppl. 1), S37–S44. [Google Scholar]
- Czaja, A.J. Targeting apoptosis in autoimmune hepatitis. Dig. Dis. Sci. 2014, 59, 2890–2904. [Google Scholar] [CrossRef]
- Bantel, H.; Lugering, A.; Poremba, C.; Lugering, N.; Held, J.; Domschke, W.; Schulze-Osthoff, K. Caspase activation correlates with the degree of inflammatory liver injury in chronic hepatitis C virus infection. Hepatology 2001, 34 Pt 1, 758–767. [Google Scholar] [CrossRef]
- Wang, M.C.; Wandrer, F.; Schlue, J.; Voigtlander, T.; Lankisch, T.O.; Manns, M.P.; Bantel, H.; von Hahn, T. Transjugular diagnostics in acute liver failure including measurements of hepatocentral venous biomarker gradients. Hepatol. Res. 2018, 48, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Thapaliya, S.; Wree, A.; Povero, D.; Inzaugarat, M.E.; Berk, M.; Dixon, L.; Papouchado, B.G.; Feldstein, A.E. Caspase 3 inactivation protects against hepatic cell death and ameliorates fibrogenesis in a diet-induced NASH model. Dig. Dis. Sci. 2014, 59, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Bogdanos, D.P.; Hussain, M.J.; Underhill, J.; Bansal, S.; Longhi, M.S.; Cheeseman, P.; Mieli-Vergani, G.; Vergani, D. Polyclonal T-cell responses to cytochrome P450IID6 are associated with disease activity in autoimmune hepatitis type 2. Gastroenterology 2006, 130, 868–882. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, A. Autoreactive T cells in type 1 diabetes. J. Clin. Investig. 2017, 127, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Martinuzzi, E.; Novelli, G.; Scotto, M.; Blancou, P.; Bach, J.M.; Chaillous, L.; Bruno, G.; Chatenoud, L.; van Endert, P.; Mallone, R. The frequency and immunodominance of islet-specific CD8+ T-cell responses change after type 1 diabetes diagnosis and treatment. Diabetes 2008, 57, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Tang, Y.; You, Z.; Wang, Q.; Liang, S.; Han, X.; Qiu, D.; Wei, J.; Liu, Y.; Shen, L.; et al. Interleukin-17 contributes to the pathogenesis of autoimmune hepatitis through inducing hepatic interleukin-6 expression. PLoS ONE 2011, 6, e18909. [Google Scholar] [CrossRef]
- Abe, M.; Hiasa, Y.; Onji, M. T helper 17 cells in autoimmune liver diseases. Clin. Dev. Immunol. 2013, 2013, 607073. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buitrago-Molina, L.E.; Dywicki, J.; Noyan, F.; Trippler, M.; Pietrek, J.; Schlue, J.; Manns, M.P.; Wedemeyer, H.; Jaeckel, E.; Hardtke-Wolenski, M. Splenectomy Prior to Experimental Induction of Autoimmune Hepatitis Promotes More Severe Hepatic Inflammation, Production of IL-17 and Apoptosis. Biomedicines 2021, 9, 58. https://doi.org/10.3390/biomedicines9010058
Buitrago-Molina LE, Dywicki J, Noyan F, Trippler M, Pietrek J, Schlue J, Manns MP, Wedemeyer H, Jaeckel E, Hardtke-Wolenski M. Splenectomy Prior to Experimental Induction of Autoimmune Hepatitis Promotes More Severe Hepatic Inflammation, Production of IL-17 and Apoptosis. Biomedicines. 2021; 9(1):58. https://doi.org/10.3390/biomedicines9010058
Chicago/Turabian StyleBuitrago-Molina, Laura Elisa, Janine Dywicki, Fatih Noyan, Martin Trippler, Julia Pietrek, Jerome Schlue, Michael P. Manns, Heiner Wedemeyer, Elmar Jaeckel, and Matthias Hardtke-Wolenski. 2021. "Splenectomy Prior to Experimental Induction of Autoimmune Hepatitis Promotes More Severe Hepatic Inflammation, Production of IL-17 and Apoptosis" Biomedicines 9, no. 1: 58. https://doi.org/10.3390/biomedicines9010058
APA StyleBuitrago-Molina, L. E., Dywicki, J., Noyan, F., Trippler, M., Pietrek, J., Schlue, J., Manns, M. P., Wedemeyer, H., Jaeckel, E., & Hardtke-Wolenski, M. (2021). Splenectomy Prior to Experimental Induction of Autoimmune Hepatitis Promotes More Severe Hepatic Inflammation, Production of IL-17 and Apoptosis. Biomedicines, 9(1), 58. https://doi.org/10.3390/biomedicines9010058





