Cold Saline Perfusion before Ischemia-Reperfusion Is Harmful to the Kidney and Is Associated with the Loss of Ezrin, a Cytoskeletal Protein, in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Procedures
2.2. Morphologic and Molecular Methods
2.3. Morphology
2.4. Immunoblots and Indirect Immunofluorescence
2.5. Statistical Analysis
3. Results
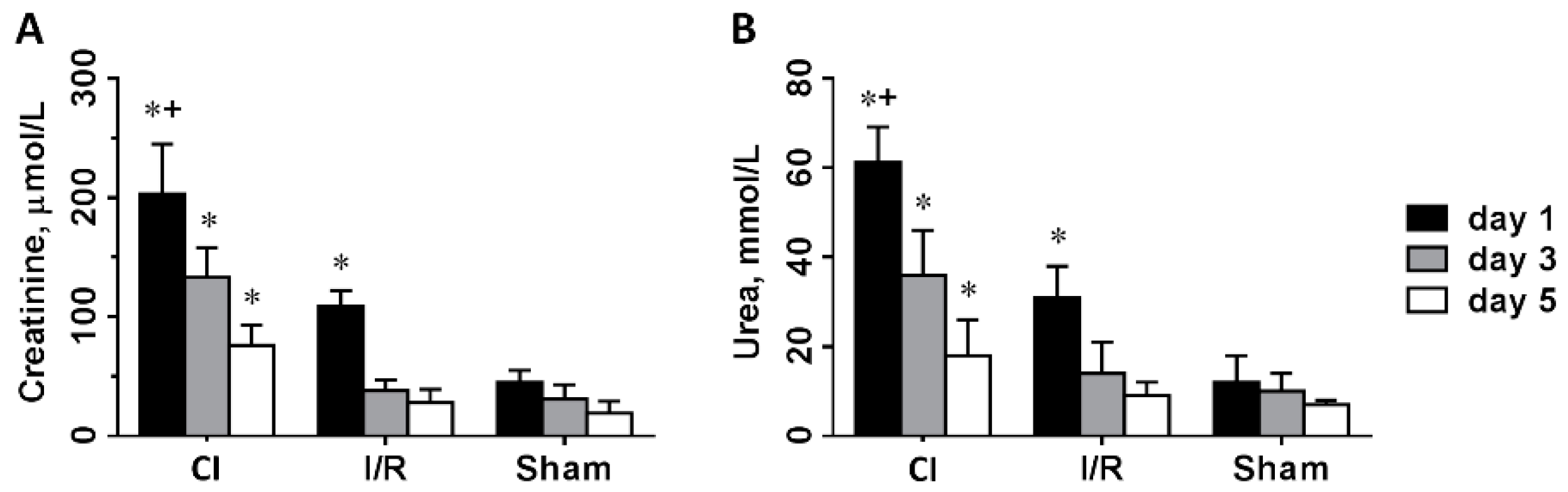
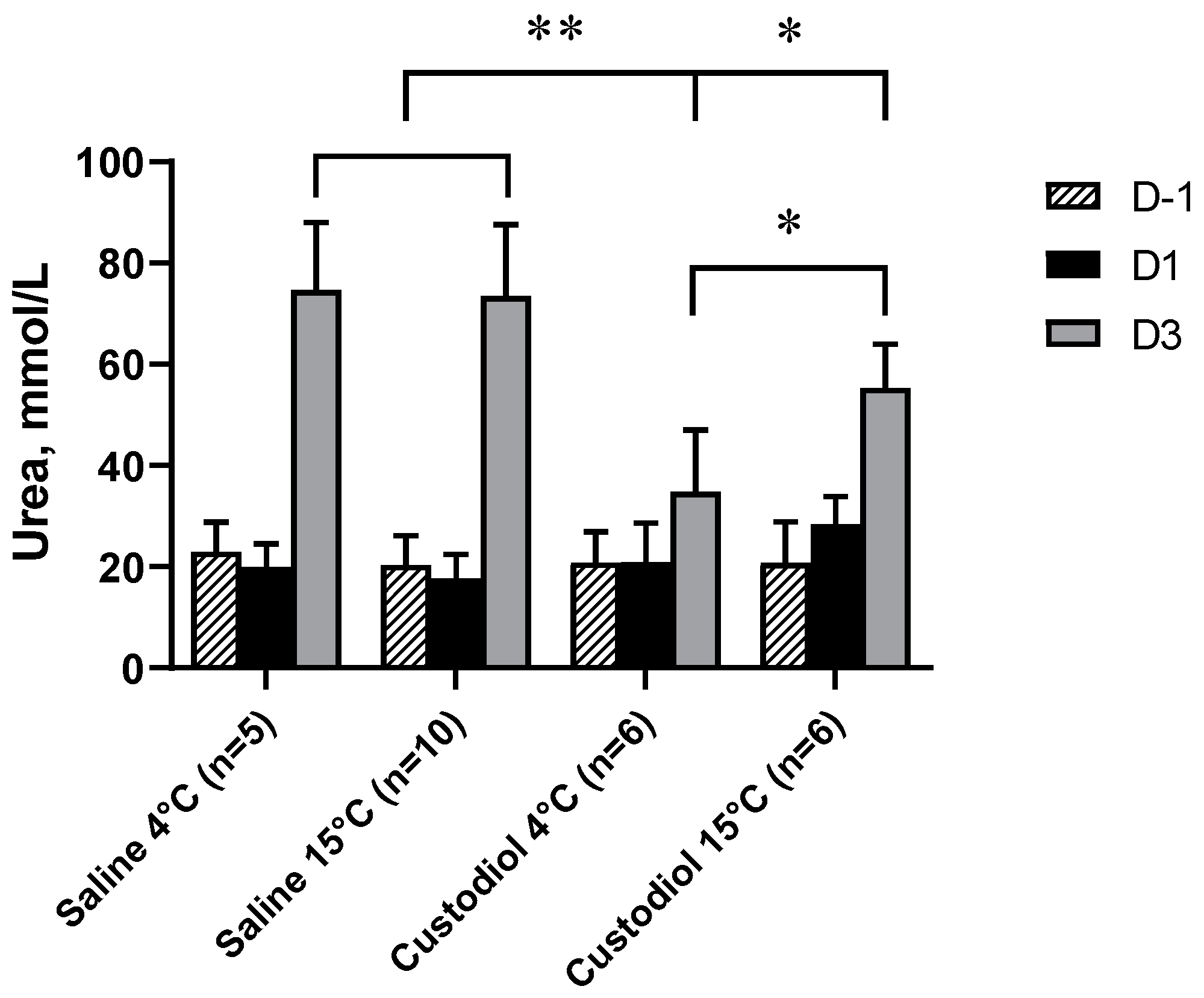
3.1. Renal Function
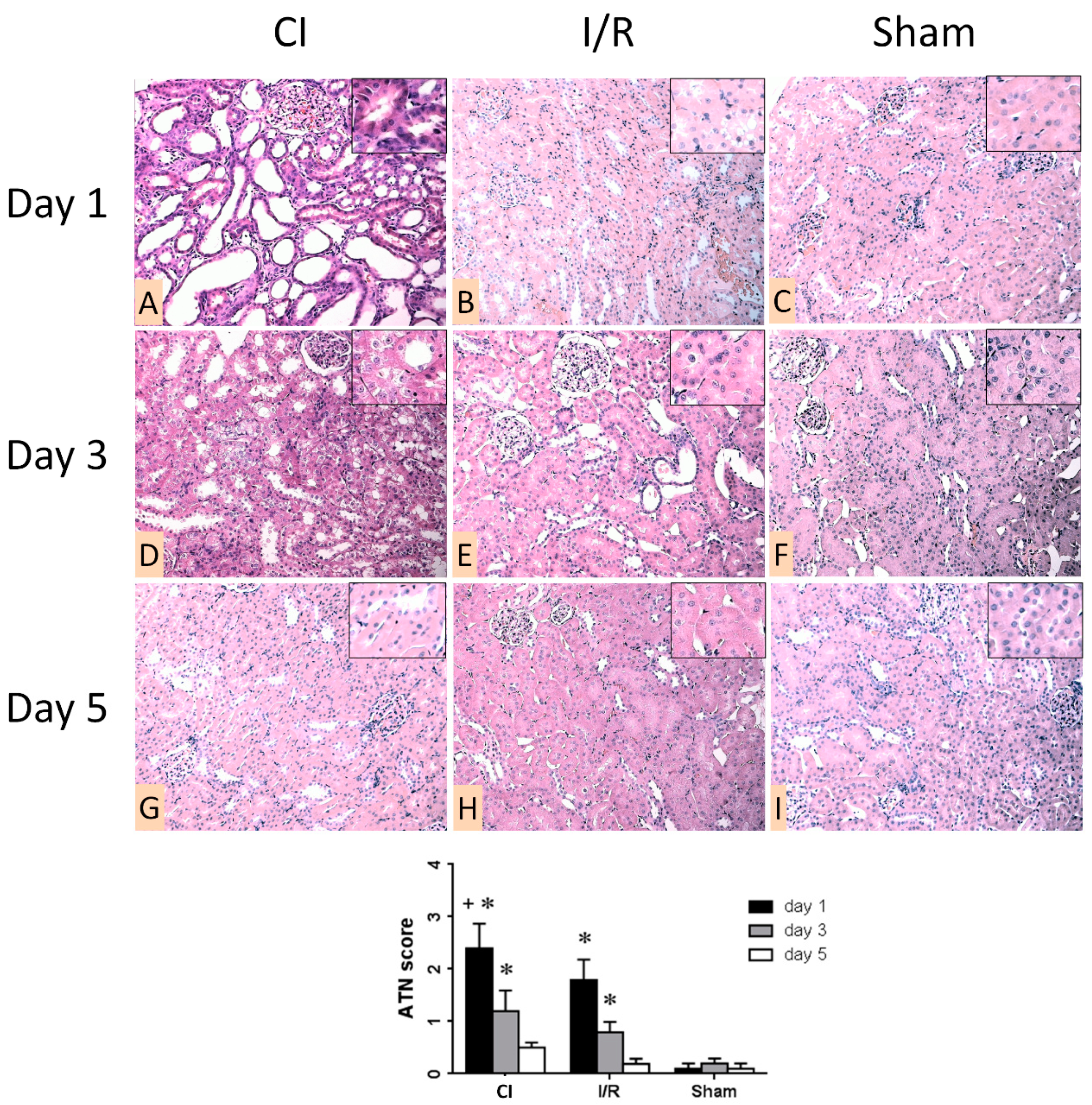
3.2. Morphologic Analysis of Kidneys
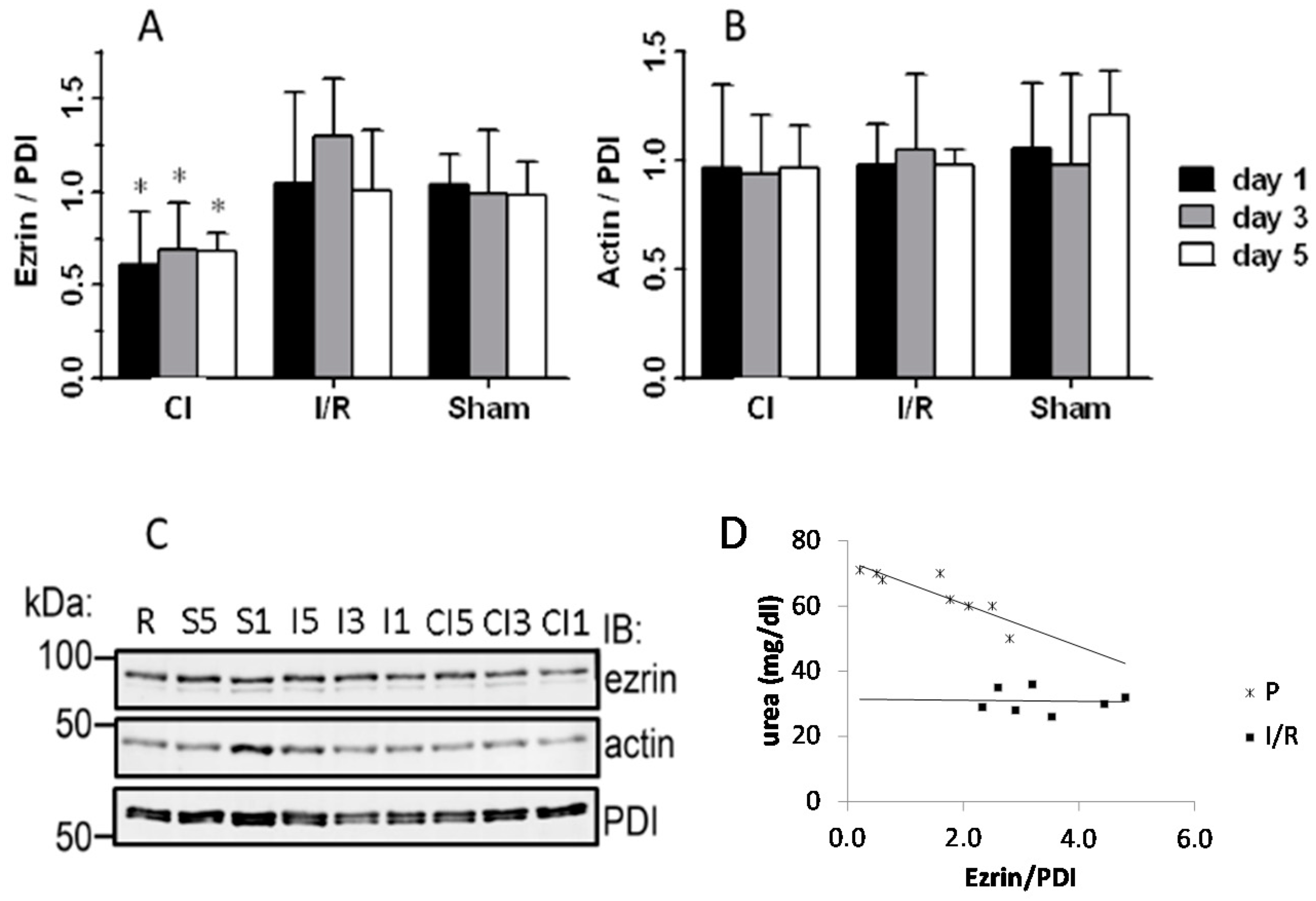
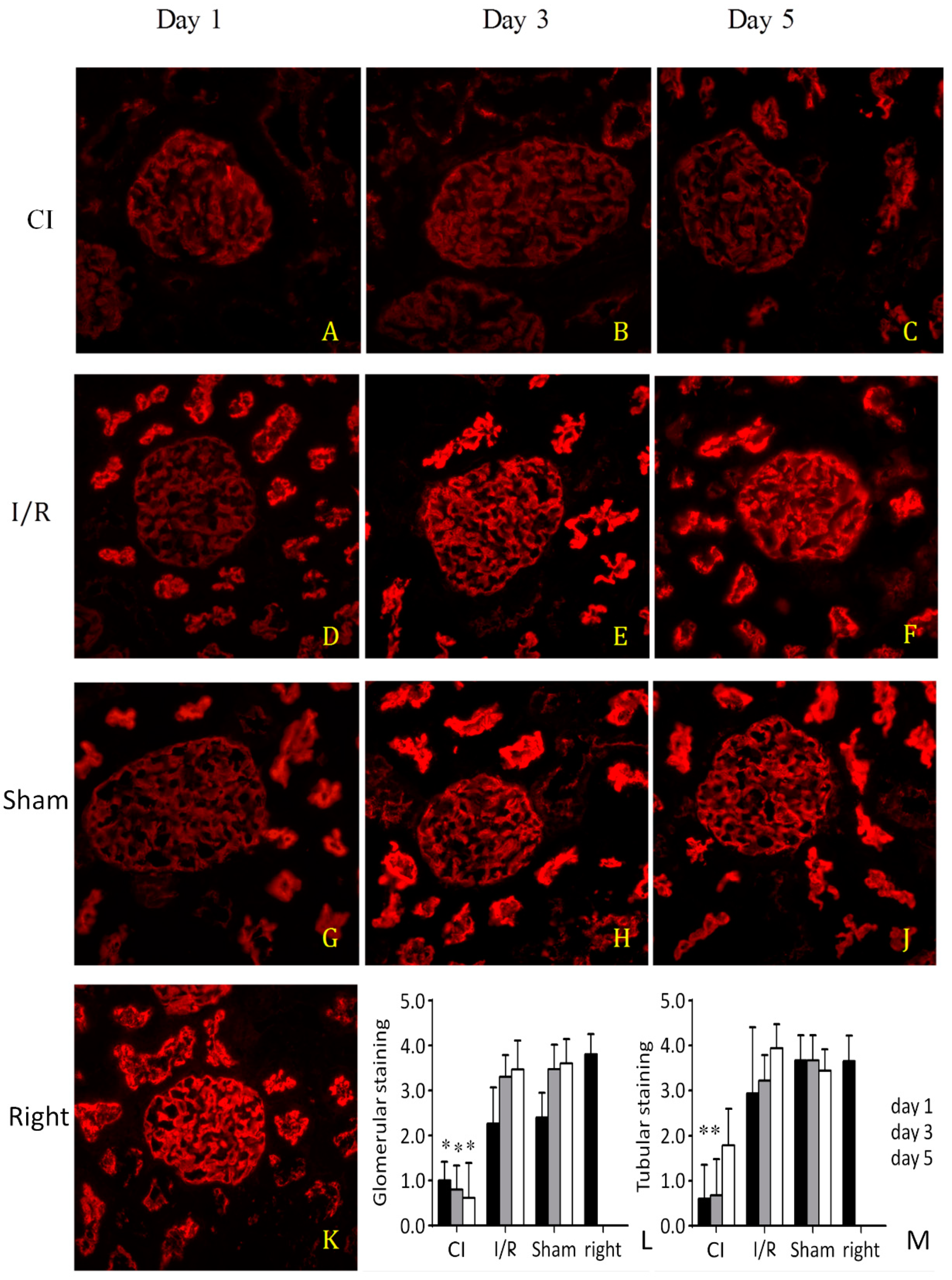
3.3. Western Blotting and Immunostaining for Ezrin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | cold-saline flushed ischemic group |
| ANOVA | analysis of variance |
| ATN | acute tubular necrosis |
| HE | hematoxylin–eosin |
| I/R | ischemia reperfusion |
| PAS | periodic-acid–Schiff |
| PDI | protein disulfide isomerase |
| SD | standard deviation |
| SHAM | sham-operated group |
| TEC | tubular epithelial cell |
References
- Guibert, E.E.; Petrenko, A.Y.; Balaban, C.L.; Somov, A.Y.; Rodriguez, J.V.; Fuller, B.J. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus. Med. Hemotherapy 2011, 38, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Fudge, J.R.; Bennett, B.L.; Simanis, J.P.; Roberts, W.O. Medical Evaluation for Exposure Extremes: Cold. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2015, 25, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Belzer, F.O.; Southard, J.H. Principles of Solid-Organ Preservation by Cold Storage. Transplantation 1988, 45, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Latchana, N.; Peck, J.R.; Whitson, B.A.; Henry, M.L.; Elkhammas, E.A.; Black, S.M. Preservation solutions used during abdominal transplantation: Current status and outcomes. World J. Transplant. 2015, 5, 154–164. [Google Scholar] [CrossRef]
- Giraud, S.; Favreau, F.; Chatauret, N.; Thuillier, R.; Maiga, S.; Hauet, T. Contribution of Large Pig for Renal Ischemia-Reperfusion and Transplantation Studies: The Preclinical Model. J. Biomed. Biotechnol. 2011, 2011, 532127. [Google Scholar] [CrossRef]
- Sapega, A.A.; Heppenstall, R.B.; Sokolow, D.P.; Graham, T.J.; Maris, J.M.; Ghosh, A.K. The bioenergetics of preservation of limbs before replantation. The rationale for intermediate hypothermia. J. Bone Joint Surg. Am. 1988, 70, 1500–1513. [Google Scholar] [CrossRef]
- Polyak, M.M.; Grosche, A.; Towl, S.; Morton, A.J. The Influence of a Novel Organ Perfusion Solution on Early Graft Function in Canine Renal Autotransplantation. Veter. Surg. 2008, 37, 383–389. [Google Scholar] [CrossRef]
- Hamar, P.; Liu, S.; Viklický, O.; Szabó, A.; Múller, V.; Heemann, U. Cyclosporine A and Azathioprine are Equipotent in Chronic Kidney Allograft Rejection. Transplantion 2000, 69, 1290–1295. [Google Scholar] [CrossRef]
- Hamar, P.; Lipták, P.; Heemann, U.; Iványi, B. Ultrastructural analysis of the Fisher to Lewis rat model of chronic allograft nephropathy. Transpl. Int. 2005, 18, 863–870. [Google Scholar] [CrossRef]
- Pahlavan, P.S.; Smallegange, C.; Adams, M.A.; Schumacher, M. Kidney transplantation procedures in rats: Assessments, complications, and management. Microsurgery 2006, 26, 404–411. [Google Scholar] [CrossRef]
- Jia, R.-P.; Luo, F.-Y.; Xie, J.-J.; Zhu, J.-G.; Fan, H.-W. Transplantation of Both Kidneys From One Donor Rat Using End-to-End Vascular Anastomosis in Normal Saline. Transplant. Proc. 2008, 40, 3728–3730. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Hockenheimer, S.; Bickerstaff, A.A.; Hadley, G.A. Murine Renal Transplantation Procedure. J. Vis. Exp. 2009, e1150. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.S.; Light, J.A.; Perloff, L.J. Perfusion-related injury in renal transplantation. Surgery 1976, 79, 440–447. [Google Scholar] [PubMed]
- Breton, S.; Brown, D. Cold-induced microtubule disruption and relocalization of membrane proteins in kidney epithelial cells. J. Am. Soc. Nephrol. 1998, 9, 155–166. [Google Scholar] [PubMed]
- Hosgood, S.A.; Nicholson, H.F.L.; Nicholson, M.L. Oxygenated Kidney Preservation Techniques. Transplantation 2012, 93, 455–459. [Google Scholar] [CrossRef]
- Collins, G.M.; Bravo-Shugarman, M.; Terasaki, P.I. Kidney preservation for transportation: Initial perfusion and 30 hours ice storage. Lancet 1969, 10, 271. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Bradley, J.A. Cold storage of deceased donor kidneys: Does the solution matter or is the solution elsewhere? Arab. Archaeol. Epigr. 2012, 12, 806–807. [Google Scholar] [CrossRef]
- Nicholson, M.L.; Hosgood, S.A. Renal Transplantation AfterEx VivoNormothermic Perfusion: The First Clinical Study. Arab. Archaeol. Epigr. 2013, 13, 1246–1252. [Google Scholar] [CrossRef]
- Mangino, M.J.; Tian, T.; Ametani, M.; Lindell, S.; Southard, J.H. Cytoskeletal Involvement in Hypothermic Renal Preservation Injury. Transplantation 2008, 85, 427–436. [Google Scholar] [CrossRef]
- Tian, T.; Lindell, S.L.; Lam, M.; Mangino, M.J. Ezrin functionality and hypothermic preservation injury in LLC-PK1 cells. Cryobiology 2012, 65, 60–67. [Google Scholar] [CrossRef]
- Hovanyecz, P.; Guibert, E.E.; Pellegrino, J.M.; Rodriguez, J.V.; Sigot, V. Extended cold storage of cultured hepatocytes impairs endocytic uptake during normothermic rewarming. Cryobiology 2013, 66, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Csipo, T.; Fulop, G.A.; Lipecz, A.; Tarantini, S.; Kiss, T.; Balasubramanian, P.; Csiszar, A.; Ungvari, Z.; Yabluchanskiy, A. Short-term weight loss reverses obesity-induced microvascular endothelial dysfunction. GeroScience 2018, 40, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, H.; Obayashi, H.; Schmieder, S.; Lee, J.; Ghosh, P.; Farquhar, M.G. Phosphorylation of Podocalyxin (Ser415) Prevents RhoA and Ezrin Activation and Disrupts Its Interaction with the Actin Cytoskeleton. Am. J. Pathol. 2011, 179, 2254–2265. [Google Scholar] [CrossRef] [PubMed]
- Wasik, A.A.; Koskelainen, S.; Hyvönen, M.E.; Musante, L.; Lehtonen, S.; Koskenniemi, K.; Tienari, J.; Vaheri, A.; Kerjaschki, N.; Szalay, C.; et al. Ezrin Is Down-Regulated in Diabetic Kidney Glomeruli and Regulates Actin Reorganization and Glucose Uptake via GLUT1 in Cultured Podocytes. Am. J. Pathol. 2014, 184, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Song, L.; Liu, M.; Mahon, M.J. Fluorescent ligand-directed co-localization of the parathyroid hormone 1 receptor with the brush-border scaffold complex of the proximal tubule reveals hormone-dependent changes in ezrin immunoreactivity consistent with inactivation. Biochim. Biophys. Acta 2012, 1823, 2243–2253. [Google Scholar] [CrossRef][Green Version]
- McRobert, E.A.; Young, A.N.; Bach, L.A. Advanced glycation end-products induce calpain-mediated degradation of ezrin. FEBS J. 2012, 279, 3240–3250. [Google Scholar] [CrossRef]
- Walker, E.M.; Slisarenko, N.; Gerrets, G.L.; Kissinger, P.J.; Didier, E.S.; Kuroda, M.J.; Veazey, R.S.; Jazwinski, M.; Rout, N. Inflammaging phenotype in rhesus macaques is associated with a decline in epithelial barrier-protective functions and increased pro-inflammatory function in CD161-expressing cells. Geroscience 2019, 41, 739–757. [Google Scholar] [CrossRef]
- Hamar, P.; Song, E.; Kökény, G.; Chen, A.; Ouyang, N.; Lieberman, J. Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2004, 101, 14883–14888. [Google Scholar] [CrossRef]
- Lee, H.J.; Feliers, D.; Barnes, J.L.; Oh, S.; Choudhury, G.G.; Diaz, V.; Galvan, V.; Strong, R.; Nelson, J.; Salmon, A.; et al. Hydrogen sulfide ameliorates aging-associated changes in the kidney. GeroScience 2018, 40, 163–176. [Google Scholar] [CrossRef]
- Heemann, U.; Szabo, A.; Hamar, P.; Muller, V.; Witzke, O.; Lutz, J.; Philipp, T. Lipopolysaccharide pretreatment protects from renal ischemia/reperfusion injury: Possible connection to an interleukin-6-dependent pathway. Am. J. Pathol. 2000, 156, 287–293. [Google Scholar] [CrossRef]
- Böhling, T.; Turunen, O.; Jääskeläinen, J.; Carpen, O.; Sainio, M.; Wahlström, T.; Vaheri, A.; Haltia, M. Ezrin expression in stromal cells of capillary hemangioblastoma. An immunohistochemical survey of brain tumors. Am. J. Pathol. 1996, 148, 367–373. [Google Scholar] [PubMed]
- Lehtonen, S.; Shah, M.; Nielsen, R.; Iino, N.; Ryan, J.J.; Zhou, H.; Farquhar, M.G. The Endocytic Adaptor Protein ARH Associates with Motor and Centrosomal Proteins and Is Involved in Centrosome Assembly and Cytokinesis. Mol. Biol. Cell 2008, 19, 2949–2961. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.-F.; Liu, Y.; Basisty, N.; Karunadharma, P.; Dastidar, S.G.; Chiao, Y.A.; Chen, T.; Beyer, R.P.; Chin, M.T.; MacCoss, M.; et al. Differential effects of various genetic mouse models of the mechanistic target of rapamycin complex I inhibition on heart failure. GeroScience 2019, 41, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Wasik, A.A.; Polianskyte-Prause, Z.; Dong, M.Q.; Shaw, A.S.; Yates, J.R., 3rd; Farquhar, M.G.; Lehtonen, S. Septin 7 forms a complex with CD2AP and nephrin and regulates glucose transporter trafficking. Mol. Biol. Cell 2012, 23, 3370–3379. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; You, Y.; Spoor, M.S.; Richer, E.J.; Kudva, V.V.; Paige, R.C.; Seiler, M.P.; Liebler, J.M.; Zabner, J.; Plopper, C.G.; et al. Foxj1 is required for apical localization of ezrin in airway epithelial cells. J. Cell Sci. 2003, 116, 4935–4945. [Google Scholar] [CrossRef]
- Gething, M.-J.; Sambrook, J. Protein folding in the cell. Nature 1992, 355, 33–45. [Google Scholar] [CrossRef]
- Lapchinsky, A.G. Recent Results of Experimental Transplantation of Preserved Limbs and Kidneys and Possible Use of This Technique in Clinical Practice. Ann. N. Y. Acad. Sci. 2006, 87, 539–571. [Google Scholar] [CrossRef]
- Palmer, R. The History of Organ Perfusion and Preservation. 2009. Available online: http://www.i-s-op.org/history.htm (accessed on 31 December 2020).
- Shrum, S.; MacMillan-Crow, L.A.; Parajuli, N. Cold Storage Exacerbates Renal and Mitochondrial Dysfunction Following Transplantation. Am. J. Kidney Dis. 2016, 2, 114. [Google Scholar]
- Li, S.; Liu, B.; Guan, Q.; Chafeeva, I.; Brooks, D.E.; Nguan, C.Y.; Kizhakkedathu, J.N.; Du, C. Cold preservation with hyperbranched polyglycerol-based solution improves kidney functional recovery with less injury at reperfusion in rats. Am. J. Transl. Res. 2017, 9, 429–441. [Google Scholar]
- Sakamuri, S.S.V.P.; Sperling, J.A.; Sure, V.N.; Dholakia, M.H.; Peterson, N.R.; Rutkai, I.; Mahalingam, P.S.; Satou, R.; Katakam, P.V.G. Measurement of respiratory function in isolated cardiac mitochondria using Seahorse XFe24 Analyzer: Applications for aging research. GeroScience 2018, 40, 347–356. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Z.; Zhong, Z.; Wang, R.; Hu, L.; Xiong, Y.; Wang, Y.; Ye, Q. Hypothermic Machine Perfusion Decreases Renal Cell Apoptosis During Ischemia/Reperfusion Injury via the Ezrin/AKT Pathway. Artif. Organs 2016, 40, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Reiermann, S.; Wörner, R.; Schröter, R.; Edemir, B.; Buck, F.; Henning, S.; Peter-Katalinić, J.; Vollenbröker, B.; Amann, K.; et al. IF/TA-related metabolic changes--proteome analysis of rat renal allografts. Nephrol. Dial. Transplant. 2010, 25, 2492–2501. [Google Scholar] [CrossRef] [PubMed]
- Moers, C.; Van Rijt, G.; Ploeg, R.J.; Leuvenink, H.G.D. The effect of normothermic recirculation before cold preservation on post-transplant injury of ischemically damaged donor kidneys. Transpl. Int. 2012, 25, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of Acute Kidney Injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-T.; Wang, J.; Wu, H.-Y.; Huang, J.-W.; Chien, K.-L. Age modifies the risk factor profiles for acute kidney injury among recently diagnosed type 2 diabetic patients: A population-based study. GeroScience 2018, 40, 201–217. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Révész, C.; Wasik, A.A.; Godó, M.; Tod, P.; Lehtonen, S.; Szénási, G.; Hamar, P. Cold Saline Perfusion before Ischemia-Reperfusion Is Harmful to the Kidney and Is Associated with the Loss of Ezrin, a Cytoskeletal Protein, in Rats. Biomedicines 2021, 9, 30. https://doi.org/10.3390/biomedicines9010030
Révész C, Wasik AA, Godó M, Tod P, Lehtonen S, Szénási G, Hamar P. Cold Saline Perfusion before Ischemia-Reperfusion Is Harmful to the Kidney and Is Associated with the Loss of Ezrin, a Cytoskeletal Protein, in Rats. Biomedicines. 2021; 9(1):30. https://doi.org/10.3390/biomedicines9010030
Chicago/Turabian StyleRévész, Csaba, Anita A. Wasik, Mária Godó, Pál Tod, Sanna Lehtonen, Gábor Szénási, and Péter Hamar. 2021. "Cold Saline Perfusion before Ischemia-Reperfusion Is Harmful to the Kidney and Is Associated with the Loss of Ezrin, a Cytoskeletal Protein, in Rats" Biomedicines 9, no. 1: 30. https://doi.org/10.3390/biomedicines9010030
APA StyleRévész, C., Wasik, A. A., Godó, M., Tod, P., Lehtonen, S., Szénási, G., & Hamar, P. (2021). Cold Saline Perfusion before Ischemia-Reperfusion Is Harmful to the Kidney and Is Associated with the Loss of Ezrin, a Cytoskeletal Protein, in Rats. Biomedicines, 9(1), 30. https://doi.org/10.3390/biomedicines9010030




