Elements of Immunoglobulin E Network Associate with Aortic Valve Area in Patients with Acquired Aortic Stenosis
Abstract
1. Introduction
2. Experimental Section
2.1. Study Subjects
2.2. Laboratory Tests
2.3. Genotyping
2.4. Statistical Analysis
3. Results
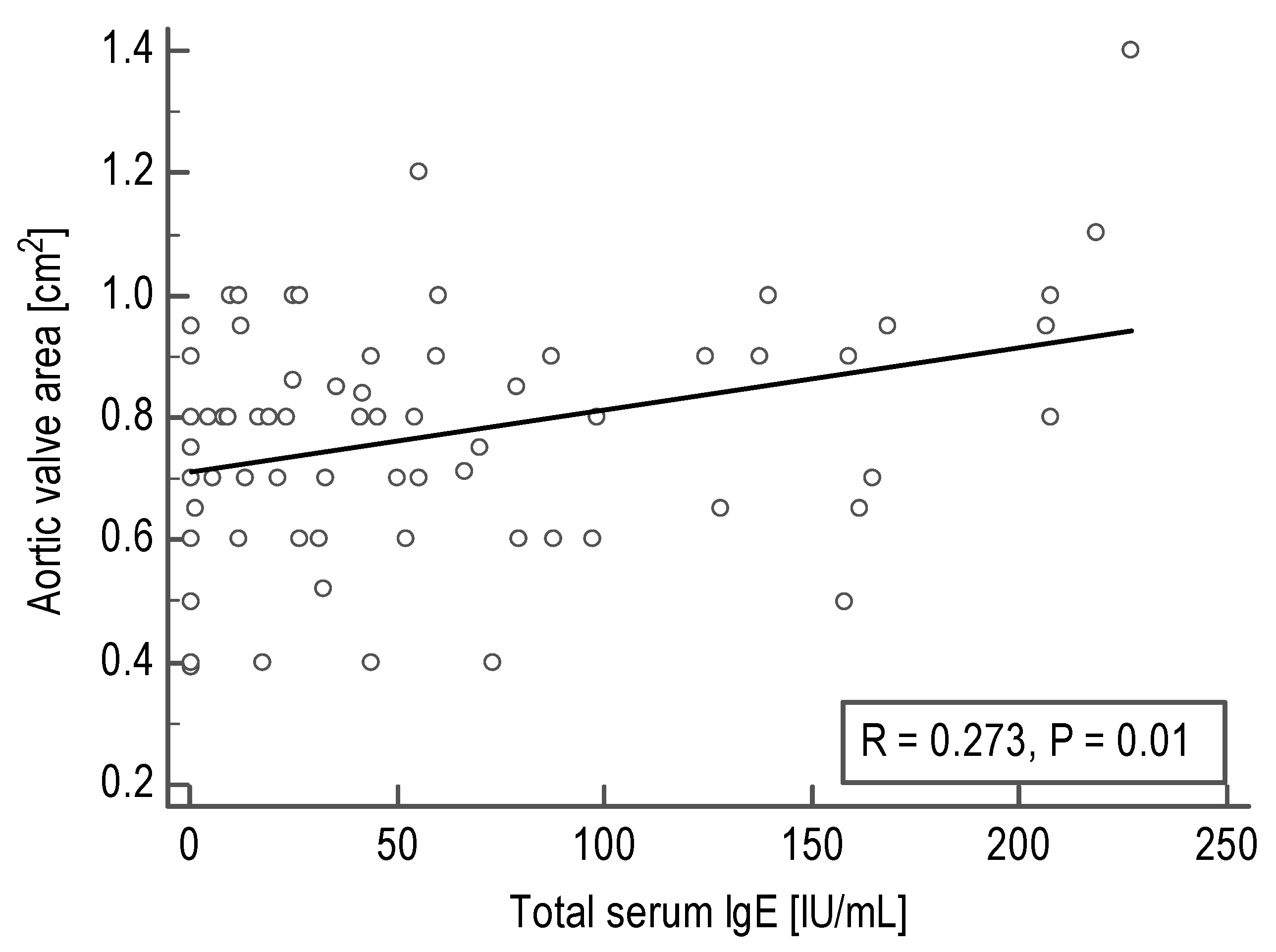
3.1. Correlations between Total Serum IgE Levels and Echocardiographic Parameters in Acquired AS Patients
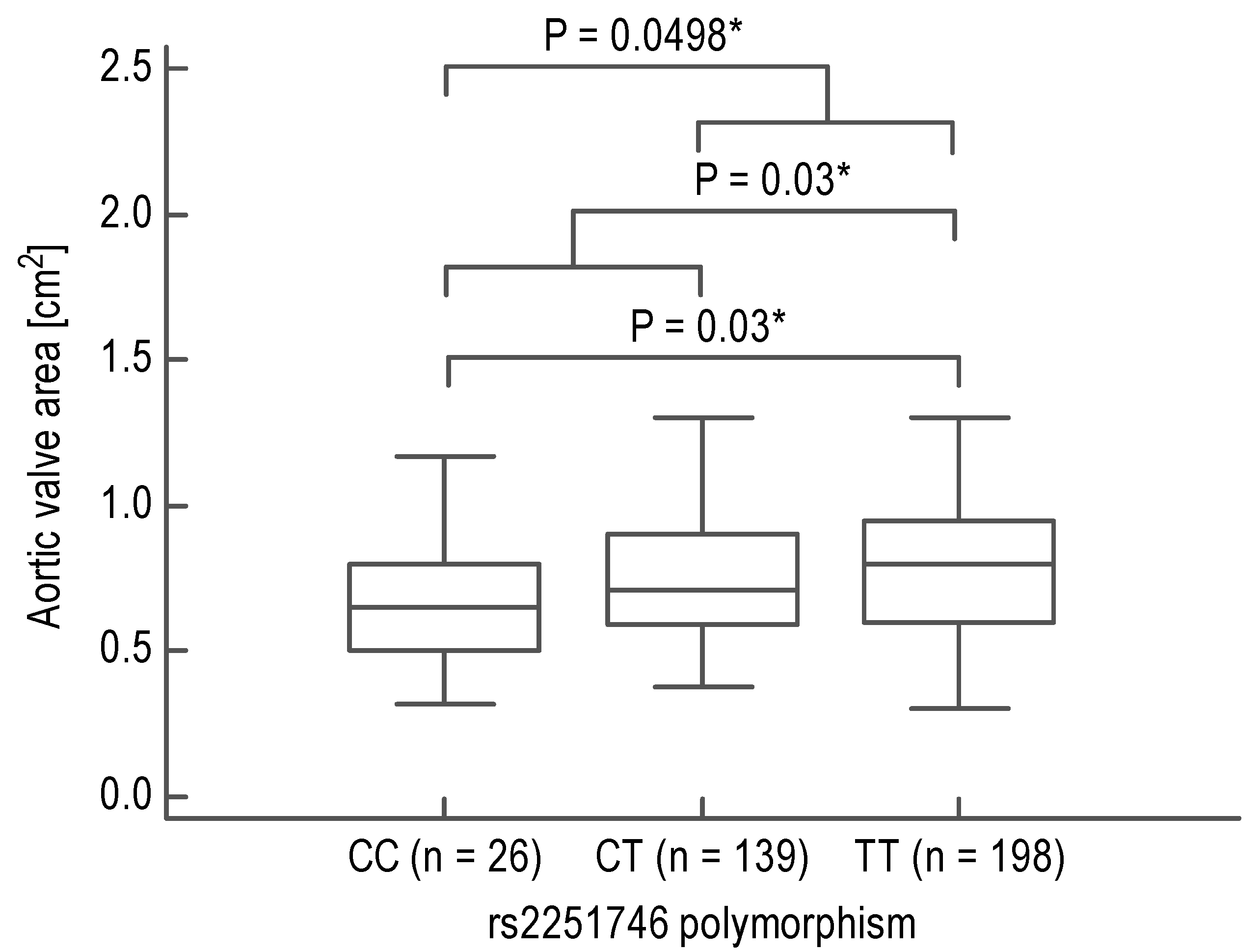
3.2. Association between FCER1A Polymorphisms and AVA in Acquired AS Patients
3.3. The Effects of FCER1A Polymorphisms on Total Serum IgE in Acquired AS Patients
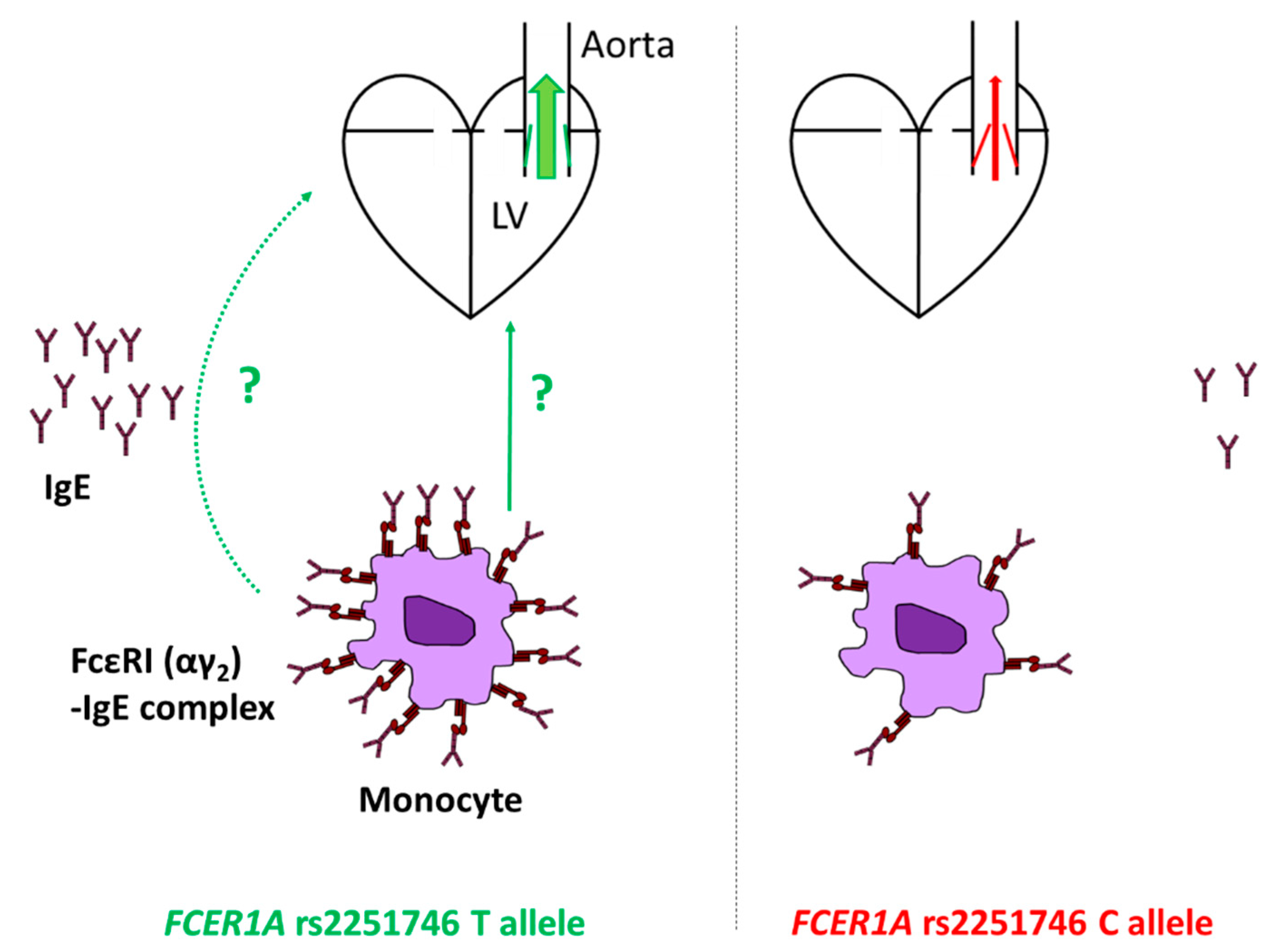
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lippi, G.; Cervellin, G.; Sanchis-Gomar, F. Immunoglobulin E (IgE) and ischemic heart disease. Which came first, the chicken or the egg? Ann. Med. 2014, 46, 456–463. [Google Scholar] [CrossRef]
- Potaczek, D.P. Links between allergy and cardiovascular or hemostatic system. Int. J. Cardiol. 2014, 170, 278–285. [Google Scholar] [CrossRef]
- Hermans, M.; Roeters van Lennep, J.; van Daele, P.; Bot, I. Mast Cells in Cardiovascular Disease: From Bench to Bedside. Int. J. Mol. Sci. 2019, 20, 3395. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Kabesch, M. Current concepts of IgE regulation and impact of genetic determinants. Clin. Exp. Allergy 2012, 42, 852–871. [Google Scholar] [CrossRef]
- Gould, H.J.; Sutton, B.J. IgE in allergy and asthma today. Nat. Rev. Immunol. 2008, 8, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yuan, S.; Liu, Y.; Zeng, Y.; Xie, H.; Liu, Z.; Zhang, S.; Fang, Q.; Wang, J.; Shen, Z. Serum IgE levels are associated with coronary artery disease severity. Atherosclerosis 2016, 251, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Hahalis, G. Serum IgE levels in coronary artery disease. Atherosclerosis 2016, 251, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, X.; Xiang, M.X.; Alanne-Kinnunen, M.; Wang, J.A.; Chen, H.; He, A.; Sun, X.; Lin, Y.; Tang, T.T.; et al. IgE stimulates human and mouse arterial cell apoptosis and cytokine expression and promotes atherogenesis in Apoe−/− mice. J. Clin. Investig. 2011, 121, 3564–3577. [Google Scholar] [CrossRef]
- Kostyunin, A.; Mukhamadiyarov, R.; Glushkova, T.; Bogdanov, L.; Shishkova, D.; Osyaev, N.; Ovcharenko, E.; Kutikhin, A. Ultrastructural Pathology of Atherosclerosis, Calcific Aortic Valve Disease, and Bioprosthetic Heart Valve Degeneration: Commonalities and Differences. Int. J. Mol. Sci. 2020, 21, 7434. [Google Scholar] [CrossRef]
- De Oliveira Sá, M.P.B.; Cavalcanti, L.R.P.; Perazzo, Á.M.; Gomes, R.A.F.; Clavel, M.A.; Pibarot, P.; Biondi-Zoccai, G.; Zhigalov, K.; Weymann, A.; Ruhparwar, A.; et al. Calcific Aortic Valve Stenosis and Atherosclerotic Calcification. Curr. Atheroscler. Rep. 2020, 22, 2. [Google Scholar] [CrossRef]
- Min, K.B.; Min, J.Y. Risk of Cardiovascular Mortality in Relation to Increased Total Serum IgE Levels in Older Adults: A Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 4350. [Google Scholar] [CrossRef]
- Donato, M.; Ferri, N.; Lupo, M.G.; Faggin, E.; Rattazzi, M. Current Evidence and Future Perspectives on Pharmacological Treatment of Calcific Aortic Valve Stenosis. Int. J. Mol. Sci. 2020, 21, 8263. [Google Scholar] [CrossRef]
- Granada, M.; Wilk, J.B.; Tuzova, M.; Strachan, D.P.; Weidinger, S.; Albrecht, E.; Gieger, C.; Heinrich, J.; Himes, B.E.; Hunninghake, G.M.; et al. A genome-wide association study of plasma total IgE concentrations in the Framingham Heart Study. J. Allergy Clin. Immunol. 2012, 129, 840–845. [Google Scholar] [CrossRef]
- Sharma, V.; Michel, S.; Gaertner, V.; Franke, A.; Vogelberg, C.; von Berg, A.; Bufe, A.; Heinzmann, A.; Laub, O.; Rietschel, E.; et al. Fine-mapping of IgE-associated loci 1q23, 5q31, and 12q13 using 1000 Genomes Project data. Allergy 2014, 69, 1077–1084. [Google Scholar] [CrossRef]
- Weidinger, S.; Gieger, C.; Rodriguez, E.; Baurecht, H.; Mempel, M.; Klopp, N.; Gohlke, H.; Wagenpfeil, S.; Ollert, M.; Ring, J.; et al. Genome-wide scan on total serum IgE levels identifies FCER1A as novel susceptibility locus. PLoS Genet. 2008, 4, e1000166. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Michel, S.; Sharma, V.; Zeilinger, S.; Vogelberg, C.; von Berg, A.; Bufe, A.; Heinzmann, A.; Laub, O.; Rietschel, E.; et al. Different FCER1A polymorphisms influence IgE levels in asthmatics and non-asthmatics. Pediatr. Allergy Immunol. 2013, 24, 441–449. [Google Scholar] [CrossRef]
- Wypasek, E.; Potaczek, D.P.; Lamplmayr, M.; Sadowski, J.; Undas, A. Interleukin-6 receptor Asp358Ala gene polymorphism is associated with plasma C-reactive protein levels and severity of aortic valve stenosis. Clin. Chem. Lab. Med. 2014, 52, 1049–1056. [Google Scholar] [CrossRef]
- Wypasek, E.; Potaczek, D.P.; Undas, A. Association of the C-Reactive Protein Gene (CRP) rs1205 C>T Polymorphism with Aortic Valve Calcification in Patients with Aortic Stenosis. Int. J. Mol. Sci. 2015, 16, 23745–23759. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Przytulska-Szczerbik, A.; Bazan-Socha, S.; Nastałek, M.; Wojas-Pelc, A.; Okumura, K.; Nishiyama, C.; Jurczyszyn, A.; Undas, A.; Wypasek, E. Interaction between functional polymorphisms in FCER1A and TLR2 and the severity of atopic dermatitis. Hum. Immunol. 2020, 81, 709–713. [Google Scholar] [CrossRef]
- Teirstein, P.; Yeager, M.; Yock, P.G.; Popp, R.L. Doppler echocardiographic measurement of aortic valve area in aortic stenosis: A noninvasive application of the Gorlin formula. J. Am. Coll. Cardiol. 1986, 8, 1059–1065. [Google Scholar] [CrossRef]
- Hellman, L. Regulation of IgE homeostasis, and the identification of potential targets for therapeutic intervention. Biomed. Pharm. 2007, 61, 34–49. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Nishiyama, C.; Sanak, M.; Szczeklik, A.; Okumura, K. Genetic variability of the high-affinity IgE receptor α-subunit (FcepsilonRIalpha). Immunol. Res. 2009, 45, 75–84. [Google Scholar] [CrossRef]
- Hasegawa, M.; Nishiyama, C.; Nishiyama, M.; Akizawa, Y.; Mitsuishi, K.; Ito, T.; Kawada, H.; Furukawa, S.; Ra, C.; Okumura, K.; et al. A novel -66T/C polymorphism in Fc epsilon RI α-chain promoter affecting the transcription activity: Possible relationship to allergic diseases. J. Immunol. 2003, 171, 1927–1933. [Google Scholar] [CrossRef]
- Kanada, S.; Nakano, N.; Potaczek, D.P.; Maeda, K.; Shimokawa, N.; Niwa, Y.; Fukai, T.; Sanak, M.; Szczeklik, A.; Yagita, H.; et al. Two different transcription factors discriminate the -315C>T polymorphism of the Fc epsilon RIα gene: Binding of Sp1 to -315C and of a high mobility group-related molecule to -315T. J. Immunol. 2008, 180, 8204–8210. [Google Scholar] [CrossRef]
- Wypasek, E.; Natorska, J.; Grudzień, G.; Filip, G.; Sadowski, J.; Undas, A. Mast cells in human stenotic aortic valves are associated with the severity of stenosis. Inflammation 2013, 36, 449–456. [Google Scholar] [CrossRef]
- Kounis, N.G.; Mazarakis, A.; Tsigkas, G.; Giannopoulos, S.; Goudevenos, J. Kounis syndrome: A new twist on an old disease. Future Cardiol. 2011, 7, 805–824. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; Velissaris, D.; Tzanis, G.; Hahalis, G. Kounis Syndrome—Not a Single-organ Arterial Disorder but a Multisystem and Multidisciplinary Disease. Balkan Med. J. 2019, 36, 212–221. [Google Scholar] [CrossRef]
- Varricchi, G.; de Paulis, A.; Marone, G.; Galli, S.J. Future Needs in Mast Cell Biology. Int. J. Mol. Sci. 2019, 20, 4397. [Google Scholar] [CrossRef]
- Babina, M.; Guhl, S.; Artuc, M.; Zuberbier, T. Allergic FcεRI- and pseudo-allergic MRGPRX2-triggered mast cell activation routes are independent and inversely regulated by SCF. Allergy 2018, 73, 256–260. [Google Scholar] [CrossRef]
- Wang, Z.; Guhl, S.; Franke, K.; Artuc, M.; Zuberbier, T.; Babina, M. IL-33 and MRGPRX2-Triggered Activation of Human Skin Mast Cells-Elimination of Receptor Expression on Chronic Exposure, but Reinforced Degranulation on Acute Priming. Cells 2019, 8, 341. [Google Scholar] [CrossRef]
- Sraeyes, S.; Pham, D.H.; Gee, T.W.; Hua, J.; Butcher, J.T. Monocytes and Macrophages in Heart Valves: Uninvited Guests or Critical Performers? Curr. Opin. Biomed. Eng. 2018, 5, 82–89. [Google Scholar] [CrossRef]
- Pyle, D.M.; Yang, V.S.; Gruchalla, R.S.; Farrar, J.D.; Gill, M.A. IgE cross-linking critically impairs human monocyte function by blocking phagocytosis. J. Allergy Clin. Immunol. 2013, 131, 491–500. [Google Scholar] [CrossRef]
- Rowe, R.K.; Pyle, D.M.; Tomlinson, A.R.; Lv, T.; Hu, Z.; Gill, M.A. IgE cross-linking impairs monocyte antiviral responses and inhibits influenza-driven TH1 differentiation. J. Allergy Clin. Immunol. 2017, 140, 294–298. [Google Scholar] [CrossRef]
- Shimoni, S.; Meledin, V.; Bar, I.; Fabricant, J.; Gandelman, G.; George, J. Circulating CD14+ monocytes in patients with aortic stenosis. J. Geriatr. Cardiol. 2016, 13, 81–87. [Google Scholar] [CrossRef]
- Ferreira, M.A.R.; Vonk, J.M.; Baurecht, H.; Marenholz, I.; Tian, C.; Hoffman, J.D.; Helmer, Q.; Tillander, A.; Ullemar, V.; Lu, Y.; et al. Eleven loci with new reproducible genetic associations with allergic disease risk. J. Allergy Clin. Immunol. 2019, 143, 691–699. [Google Scholar] [CrossRef]
| Echocardiographic Parameter | Number of Data Pairs | Spearman’s Rank Correlation Coefficient (R) | P-Value |
|---|---|---|---|
| Mean aortic gradient, mm Hg | 100 | −0.11 | 0.28 |
| Maximum aortic gradient, mm Hg | 95 | −0.06 | 0.55 |
| Left ventricular ejection fraction, % | 101 | −0.03 | 0.75 |
| Aortic valve area, cm2 | 82 | 0.27 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potaczek, D.P.; Przytulska-Szczerbik, A.; Bazan-Socha, S.; Jurczyszyn, A.; Okumura, K.; Nishiyama, C.; Undas, A.; Wypasek, E. Elements of Immunoglobulin E Network Associate with Aortic Valve Area in Patients with Acquired Aortic Stenosis. Biomedicines 2021, 9, 23. https://doi.org/10.3390/biomedicines9010023
Potaczek DP, Przytulska-Szczerbik A, Bazan-Socha S, Jurczyszyn A, Okumura K, Nishiyama C, Undas A, Wypasek E. Elements of Immunoglobulin E Network Associate with Aortic Valve Area in Patients with Acquired Aortic Stenosis. Biomedicines. 2021; 9(1):23. https://doi.org/10.3390/biomedicines9010023
Chicago/Turabian StylePotaczek, Daniel P., Aleksandra Przytulska-Szczerbik, Stanisława Bazan-Socha, Artur Jurczyszyn, Ko Okumura, Chiharu Nishiyama, Anetta Undas, and Ewa Wypasek. 2021. "Elements of Immunoglobulin E Network Associate with Aortic Valve Area in Patients with Acquired Aortic Stenosis" Biomedicines 9, no. 1: 23. https://doi.org/10.3390/biomedicines9010023
APA StylePotaczek, D. P., Przytulska-Szczerbik, A., Bazan-Socha, S., Jurczyszyn, A., Okumura, K., Nishiyama, C., Undas, A., & Wypasek, E. (2021). Elements of Immunoglobulin E Network Associate with Aortic Valve Area in Patients with Acquired Aortic Stenosis. Biomedicines, 9(1), 23. https://doi.org/10.3390/biomedicines9010023








