Ilimaquinone Induces Apoptosis and Autophagy in Human Oral Squamous Cell Carcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Chemicals, Antibodies
2.2. Cell Culture
2.3. Cell Viability Analysis
2.4. Flow Cytometric Analysis
2.5. Immunoblotting
2.6. Detection of Autophagosome Formation with Acridine Orange
2.7. Transient Transfection
2.8. Statistical Analysis
3. Results
3.1. IQ Decreases the Viability of OSCC
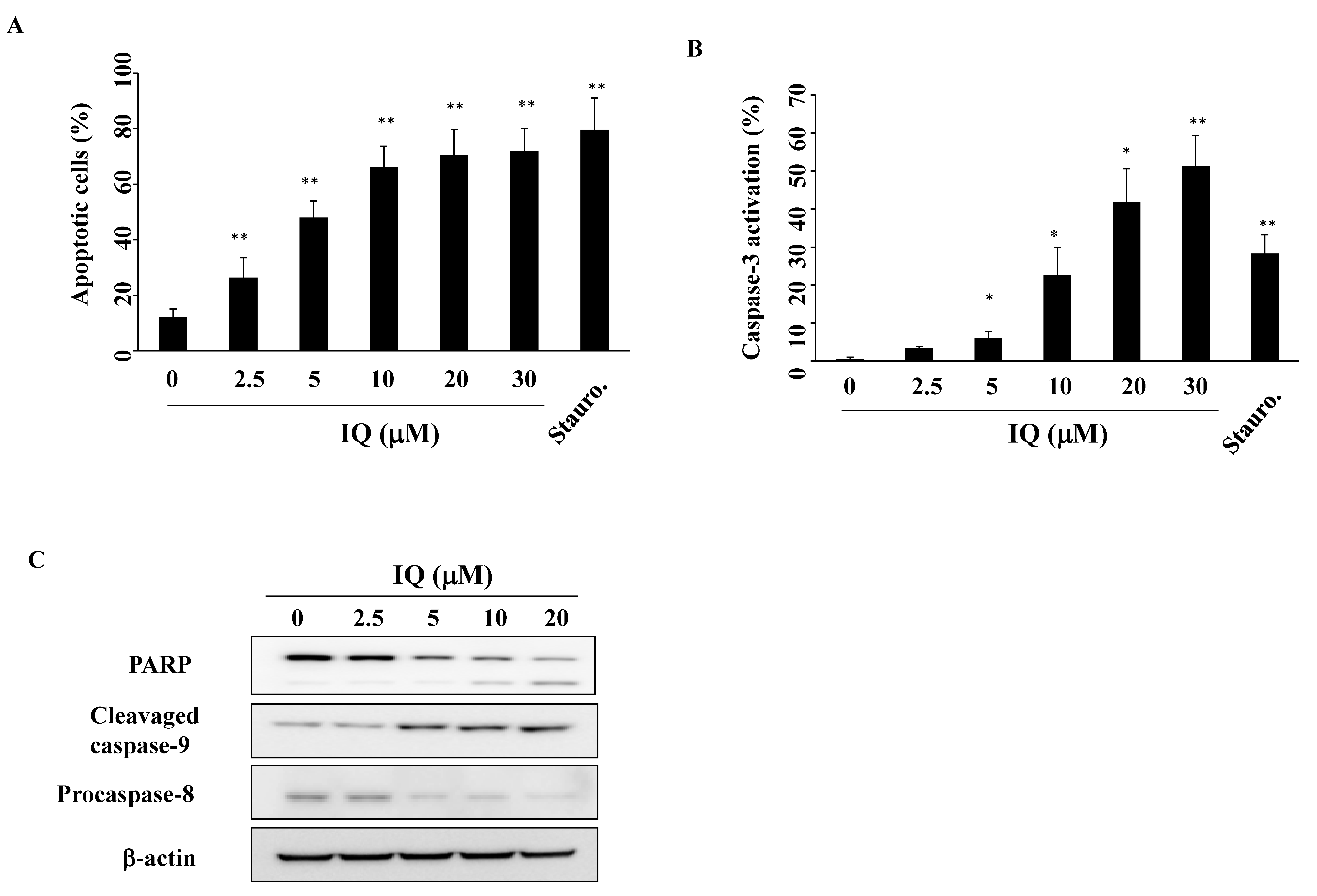
3.2. IQ Induces Caspase-Dependent Apoptosis in SCC4 Cells
3.3. IQ Modulates Akt and p53 Expression in SCC4 Cells
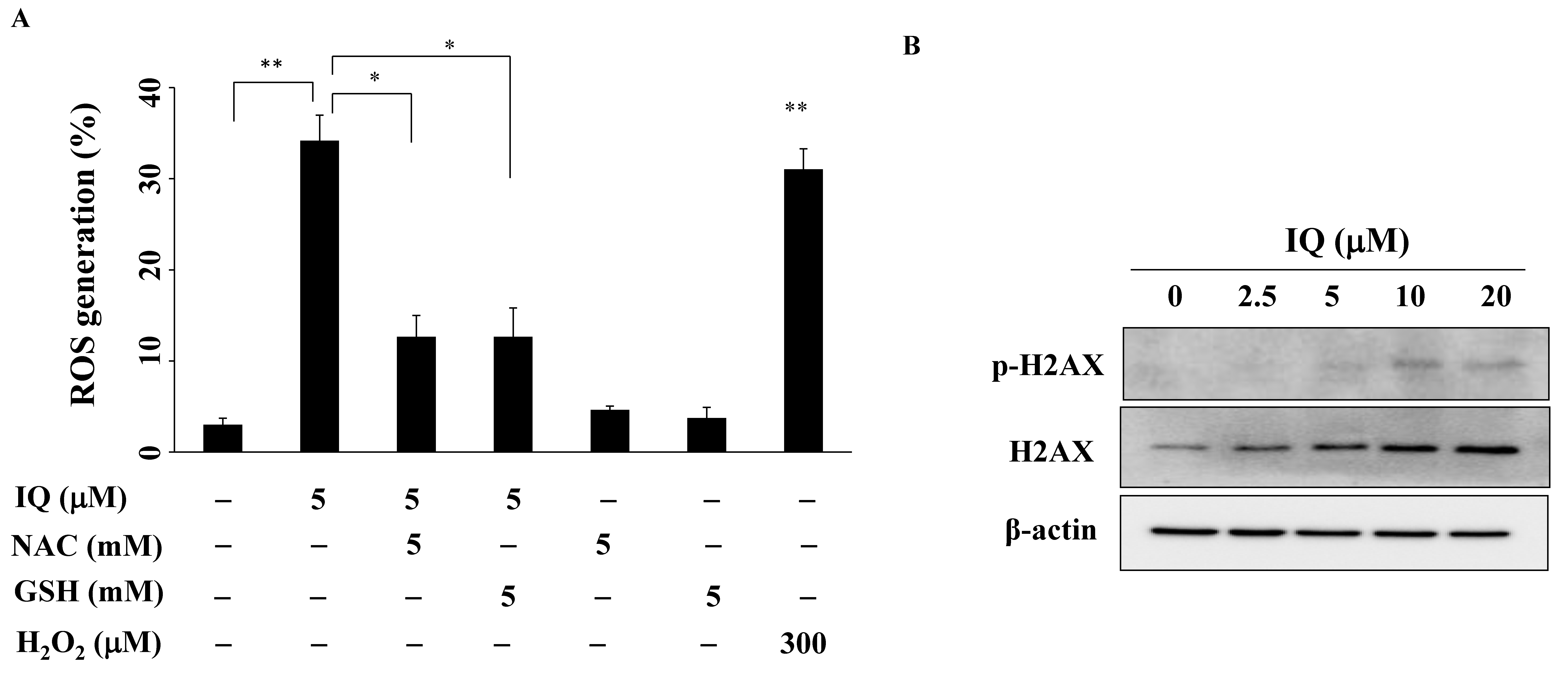
3.4. IQ Increases Reactive Oxygen Species (ROS) Generation in SCC4 Cells
3.5. IQ induces Autophagy in SCC4 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, Y.; Abu Elnaaj, I. Global incidence and risk factors of oral cancer. Harefuah 2017, 156, 645–649. [Google Scholar] [PubMed]
- Kumar, M.; Nanavati, R.; Modi, T.G.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayana, S.; Augustine, D.; Rao, R.S.; Patil, S.; Awan, K.H.; Venkatesiah, S.S.; Haragannavar, V.C.; Nambiar, S.; Prasad, K. Molecular pathways of oral cancer that predict prognosis and survival: A systematic review. J. Carcinog. 2018, 17, 7. [Google Scholar] [CrossRef]
- Chatzistefanou, I.; Lubek, J.; Markou, K.; Ord, R.A. The role of perineural invasion in treatment decisions for oral cancer patients: A review of the literature. J. Cranio-Maxillo-Facial Surg. 2017, 45, 821–825. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Wijesekara, I.; Kim, S.K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef]
- Rangel, M.; Falkenberg, M. An overview of the marine natural products in clinical trials and on the market. J. Coast. Life Med. 2015, 3, 471–478. [Google Scholar]
- Aftimos, P.; Awada, A. Survival benefit of eribulin mesylate in heavily pretreated metastatic breast cancer: What next? Adv. Ther. 2011, 28, 973–985. [Google Scholar] [CrossRef]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine sponge derived natural products between 2001 and 2010: Trends and opportunities for discovery of bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef]
- Kiem, P.V.; Huyen, L.T.; Hang, D.T.; Nhiem, N.X.; Tai, B.H.; Anh, H.L.; Cuong, P.V.; Quang, T.H.; Minh, C.V.; Dau, N.V.; et al. Sesquiterpene derivatives from marine sponge smenospongia cerebriformis and their anti-inflammatory activity. Bioorganic Med. Chem. Lett. 2017, 27, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Simithy, J.; Fuanta, N.R.; Hobrath, J.V.; Kochanowska-Karamyan, A.; Hamann, M.T.; Goodwin, D.C.; Calderon, A.I. Mechanism of irreversible inhibition of mycobacterium tuberculosis shikimate kinase by ilimaquinone. Biochim. Et. Biophys. Acta. Proteins Proteom. 2018, 1866, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Loya, S.; Hizi, A. The interaction of illimaquinone, a selective inhibitor of the rnase h activity, with the reverse transcriptases of human immunodeficiency and murine leukemia retroviruses. J. Biol. Chem. 1993, 268, 9323–9328. [Google Scholar] [PubMed]
- Do, M.T.; Na, M.; Kim, H.G.; Khanal, T.; Choi, J.H.; Jin, S.W.; Oh, S.H.; Hwang, I.H.; Chung, Y.C.; Kim, H.S.; et al. Ilimaquinone induces death receptor expression and sensitizes human colon cancer cells to trail-induced apoptosis through activation of ros-erk/p38 mapk-chop signaling pathways. Food Chem. Toxicol. 2014, 71, 51–59. [Google Scholar] [CrossRef]
- Sonoda, H.; Okada, T.; Jahangeer, S.; Nakamura, S. Requirement of phospholipase d for ilimaquinone-induced golgi membrane fragmentation. J. Biol. Chem. 2007, 282, 34085–34092. [Google Scholar] [CrossRef]
- Lu, P.H.; Chueh, S.C.; Kung, F.L.; Pan, S.L.; Shen, Y.C.; Guh, J.H. Ilimaquinone, a marine sponge metabolite, displays anticancer activity via gadd153-mediated pathway. Eur. J. Pharmacol. 2007, 556, 45–54. [Google Scholar] [CrossRef]
- Park, S.; Yun, E.; Hwang, I.H.; Yoon, S.; Kim, D.E.; Kim, J.S.; Na, M.; Song, G.Y.; Oh, S. Ilimaquinone and ethylsmenoquinone, marine sponge metabolites, suppress the proliferation of multiple myeloma cells by down-regulating the level of beta-catenin. Mar. Drugs 2014, 12, 3231–3244. [Google Scholar] [CrossRef]
- Lee, H.Y.; Chung, K.J.; Hwang, I.H.; Gwak, J.; Park, S.; Ju, B.G.; Yun, E.; Kim, D.E.; Chung, Y.H.; Na, M.; et al. Activation of p53 with ilimaquinone and ethylsmenoquinone, marine sponge metabolites, induces apoptosis and autophagy in colon cancer cells. Mar. Drugs 2015, 13, 543–557. [Google Scholar] [CrossRef]
- Ribeiro, M.; Teixeira, S.; Azevedo, M.N.; Fraga, A.C., Jr.; Gontijo, A.P.; Vêncio, E.F. Ilimaquinone, a sesquiterpenoid quinone, induces apoptosis and autophagy. Tetrahedron 1979, 35, 609–612. [Google Scholar]
- Weng, J.R.; Bai, L.Y.; Chiu, S.J.; Chiu, C.F.; Lin, W.Y.; Hu, J.L.; Shieh, T.M. Divaricoside exerts antitumor effects, in part, by modulating mcl-1 in human oral squamous cell carcinoma cells. Comput. Struct. Biotechnol. J. 2019, 17, 151–159. [Google Scholar] [CrossRef]
- Bai, L.Y.; Chiu, C.F.; Chiu, S.J.; Chu, P.C.; Weng, J.R. Fty720 induces autophagy-associated apoptosis in human oral squamous carcinoma cells, in part, through a reactive oxygen species/mcl-1-dependent mechanism. Sci. Rep. 2017, 7, 5600. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Kim, J.H.; Paeng, J.Y.; Kim, M.J.; Hong, S.D.; Lee, J.I.; Hong, S.P. Prognostic value of activated akt expression in oral squamous cell carcinoma. J. Clin. Pathol. 2005, 58, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Brusevold, I.J.; Aasrum, M.; Bryne, M.; Christoffersen, T. Migration induced by epidermal and hepatocyte growth factors in oral squamous carcinoma cells in vitro: Role of mek/erk, p38 and pi-3 kinase/akt. J. Oral Pathol. Med. 2012, 41, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Nieh, S.; Jao, S.W.; Liu, C.L.; Wu, C.H.; Chang, Y.C.; Yang, C.Y.; Lin, Y.S. Quercetin suppresses drug-resistant spheres via the p38 mapk-hsp27 apoptotic pathway in oral cancer cells. PLoS ONE 2012, 7, e49275. [Google Scholar] [CrossRef]
- Smolensky, D.; Rathore, K.; Bourn, J.; Cekanova, M. Inhibition of the pi3k/akt pathway sensitizes oral squamous cell carcinoma cells to anthracycline-based chemotherapy in vitro. J. Cell. Biochem. 2017, 118, 2615–2624. [Google Scholar] [CrossRef]
- Qian, J.; Wenguang, X.; Zhiyong, W.; Yuntao, Z.; Wei, H. Hypoxia inducible factor: A potential prognostic biomarker in oral squamous cell carcinoma. Tumour Biol. 2016, 37, 10815. [Google Scholar] [CrossRef]
- Poomsawat, S.; Punyasingh, J.; Vejchapipat, P. Overexpression of survivin and caspase 3 in oral carcinogenesis. Appl. Immunohistochem. Mol. Morphol. 2014, 22, 65–71. [Google Scholar] [CrossRef]
- Selvakumaran, M.; Lin, H.K.; Miyashita, T.; Wang, H.G.; Krajewski, S.; Reed, J.C.; Hoffman, B.; Liebermann, D. Immediate early up-regulation of bax expression by p53 but not tgf beta 1: A paradigm for distinct apoptotic pathways. Oncogene 1994, 9, 1791–1798. [Google Scholar]
- Sinevici, N.; O’Sullivan, J. Oral cancer: Deregulated molecular events and their use as biomarkers. Oral Oncol. 2016, 61, 12–18. [Google Scholar] [CrossRef]
- Ji, W.T.; Yang, S.R.; Chen, J.Y.; Cheng, Y.P.; Lee, Y.R.; Chiang, M.K.; Chen, H.R. Arecoline downregulates levels of p21 and p27 through the reactive oxygen species/mtor complex 1 pathway and may contribute to oral squamous cell carcinoma. Cancer Sci. 2012, 103, 1221–1229. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, W.; Zhang, Y.; Wu, S.; Liu, Y.; Deng, X.; Xie, L.; Yang, J.; Yu, H.; Su, J.; et al. Abt737 reverses cisplatin resistance by targeting glucose metabolism of human ovarian cancer cells. Int. J. Oncol. 2018, 53, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.; Carracedo, A.; Salanueva, I.J.; Hernandez-Tiedra, S.; Lorente, M.; Egia, A.; Vazquez, P.; Blazquez, C.; Torres, S.; Garcia, S.; et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of er stress in human glioma cells. J. Clin. Investig. 2009, 119, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Paglin, S.; Hollister, T.; Delohery, T.; Hackett, N.; McMahill, M.; Sphicas, E.; Domingo, D.; Yahalom, J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001, 61, 439–444. [Google Scholar] [PubMed]
- Liu, Y.; Shi, Q.F.; Ye, Y.C.; Tashiro, S.; Onodera, S.; Ikejima, T. Activated o2(*-) and h2o2 mediated cell survival in su11274-treated non-small-cell lung cancer a549 cells via c-met-pi3k-akt and c-met-grb2/sos-ras-p38 pathways. J. Pharmacol. Sci. 2012, 119, 150–159. [Google Scholar] [CrossRef]
- Miller, B.C.; Zhao, Z.; Stephenson, L.M.; Cadwell, K.; Pua, H.H.; Lee, H.K.; Mizushima, N.N.; Iwasaki, A.; He, Y.W.; Swat, W.; et al. The autophagy gene atg5 plays an essential role in b lymphocyte development. Autophagy 2008, 4, 309–314. [Google Scholar] [CrossRef]
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2005-6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Et. Biophys. Acta 2009, 1790, 283–308. [Google Scholar]
- Kuo, Y.Y.; Jim, W.T.; Su, L.C.; Chung, C.J.; Lin, C.Y.; Huo, C.; Tseng, J.C.; Huang, S.H.; Lai, C.J.; Chen, B.C.; et al. Caffeic acid phenethyl ester is a potential therapeutic agent for oral cancer. Int. J. Mol. Sci. 2015, 16, 10748–10766. [Google Scholar] [CrossRef]
- Wu, H.; Li, L.; Ai, Z.; Yin, J.; Chen, L. Pristimerin induces apoptosis of oral squamous cell carcinoma cells via g1 phase arrest and mapk/erk1/2 and akt signaling inhibition. Oncol. Lett. 2019, 17, 3017–3025. [Google Scholar] [CrossRef]
- Herr, I.; Debatin, K.M. Cellular stress response and apoptosis in cancer therapy. Blood 2001, 98, 2603–2614. [Google Scholar] [CrossRef]
- Popovic, B.; Jekic, B.; Novakovic, I.; Lukovic, L.J.; Tepavcevic, Z.; Jurisic, V.; Vukadinovic, M.; Milasin, J. Bcl-2 expression in oral squamous cell carcinoma. Ann. New York Acad. Sci. 2007, 1095, 19–25. [Google Scholar] [CrossRef]
- Kashyap, T.; Pramanik, K.K.; Nath, N.; Mishra, P.; Singh, A.K.; Nagini, S.; Rana, A.; Mishra, R. Crosstalk between raf-mek-erk and pi3k-akt-gsk3beta signaling networks promotes chemoresistance, invasion/migration and stemness via expression of cd44 variants (v4 and v6) in oral cancer. Oral Oncol. 2018, 86, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Neoh, C.A.; Wu, W.T.; Dai, G.F.; Su, J.H.; Liu, C.I.; Su, T.R.; Wu, Y.J. Flaccidoxide-13-acetate extracted from the soft coral cladiella kashmani reduces human bladder cancer cell migration and invasion through reducing activation of the fak/pi3k/akt/mtor signaling pathway. Molecules 2017, 23, 58. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Huang, S.; Wang, L.; Yuan, X.; Dong, Q.; Zhang, D.; Wang, X. Clinical and prognostic significance of hif-1alpha overexpression in oral squamous cell carcinoma: A meta-analysis. World J. Surg. Oncol. 2017, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef]
- Verma, R.; Singh, A.; Jaiswal, R.; Chandra, A.; Verma, R.; Tak, J. Association of ki-67 antigen and p53 protein at invasive tumor front of oral squamous cell carcinoma. Indian J. Pathol. Microbiol. 2014, 57, 553–557. [Google Scholar]
- Agha-Hosseini, F.; Mirzaii-Dizgah, I.; Miri-Zarandi, N. Unstimulated salivary p53 in patients with oral lichen planus and squamous cell carcinoma. Acta Med. Iran. 2015, 53, 439–443. [Google Scholar]
- Zou, Z.; Chang, H.; Li, H.; Wang, S. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef]
- Gwangwa, M.V.; Joubert, A.M.; Visagie, M.H. Crosstalk between the warburg effect, redox regulation and autophagy induction in tumourigenesis. Cell. Mol. Biol. Lett. 2018, 23, 20. [Google Scholar] [CrossRef]
- Adhauliya, N.; Kalappanavar, A.N.; Ali, I.M.; Annigeri, R.G. Autophagy: A boon or bane in oral cancer. Oral Oncol. 2016, 61, 120–126. [Google Scholar] [CrossRef]
- Ratovitski, E.A. Tumor protein (tp)-p53 members as regulators of autophagy in tumor cells upon marine drug exposure. Mar. Drugs 2016, 14, 154. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-W.; Bai, L.-Y.; Su, J.-H.; Chiu, C.-F.; Lin, W.-Y.; Huang, W.-T.; Shih, M.-C.; Huang, Y.-T.; Hu, J.-L.; Weng, J.-R. Ilimaquinone Induces Apoptosis and Autophagy in Human Oral Squamous Cell Carcinoma Cells. Biomedicines 2020, 8, 296. https://doi.org/10.3390/biomedicines8090296
Lin C-W, Bai L-Y, Su J-H, Chiu C-F, Lin W-Y, Huang W-T, Shih M-C, Huang Y-T, Hu J-L, Weng J-R. Ilimaquinone Induces Apoptosis and Autophagy in Human Oral Squamous Cell Carcinoma Cells. Biomedicines. 2020; 8(9):296. https://doi.org/10.3390/biomedicines8090296
Chicago/Turabian StyleLin, Cheng-Wen, Li-Yuan Bai, Jui-Hsin Su, Chang-Fang Chiu, Wei-Yu Lin, Wei-Ting Huang, Ming-Cheng Shih, Yu-Ting Huang, Jing-Lan Hu, and Jing-Ru Weng. 2020. "Ilimaquinone Induces Apoptosis and Autophagy in Human Oral Squamous Cell Carcinoma Cells" Biomedicines 8, no. 9: 296. https://doi.org/10.3390/biomedicines8090296
APA StyleLin, C.-W., Bai, L.-Y., Su, J.-H., Chiu, C.-F., Lin, W.-Y., Huang, W.-T., Shih, M.-C., Huang, Y.-T., Hu, J.-L., & Weng, J.-R. (2020). Ilimaquinone Induces Apoptosis and Autophagy in Human Oral Squamous Cell Carcinoma Cells. Biomedicines, 8(9), 296. https://doi.org/10.3390/biomedicines8090296





