Non-pharmacological Treatment of Refractory Angina and Microvascular Angina
Abstract
:1. Introduction
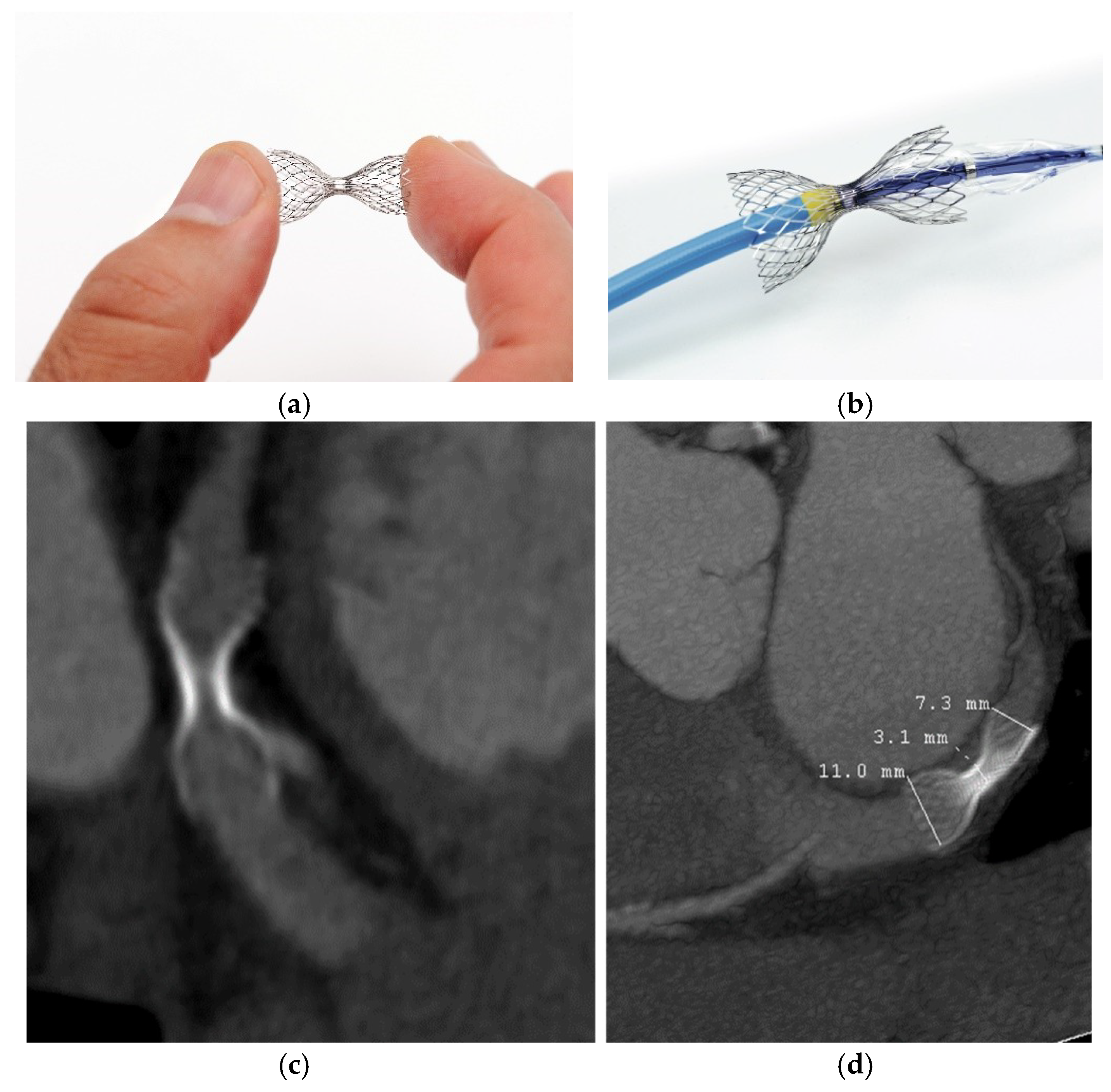
2. Coronary Sinus Reducer
3. Revascularization of Chronic Total Occlusions
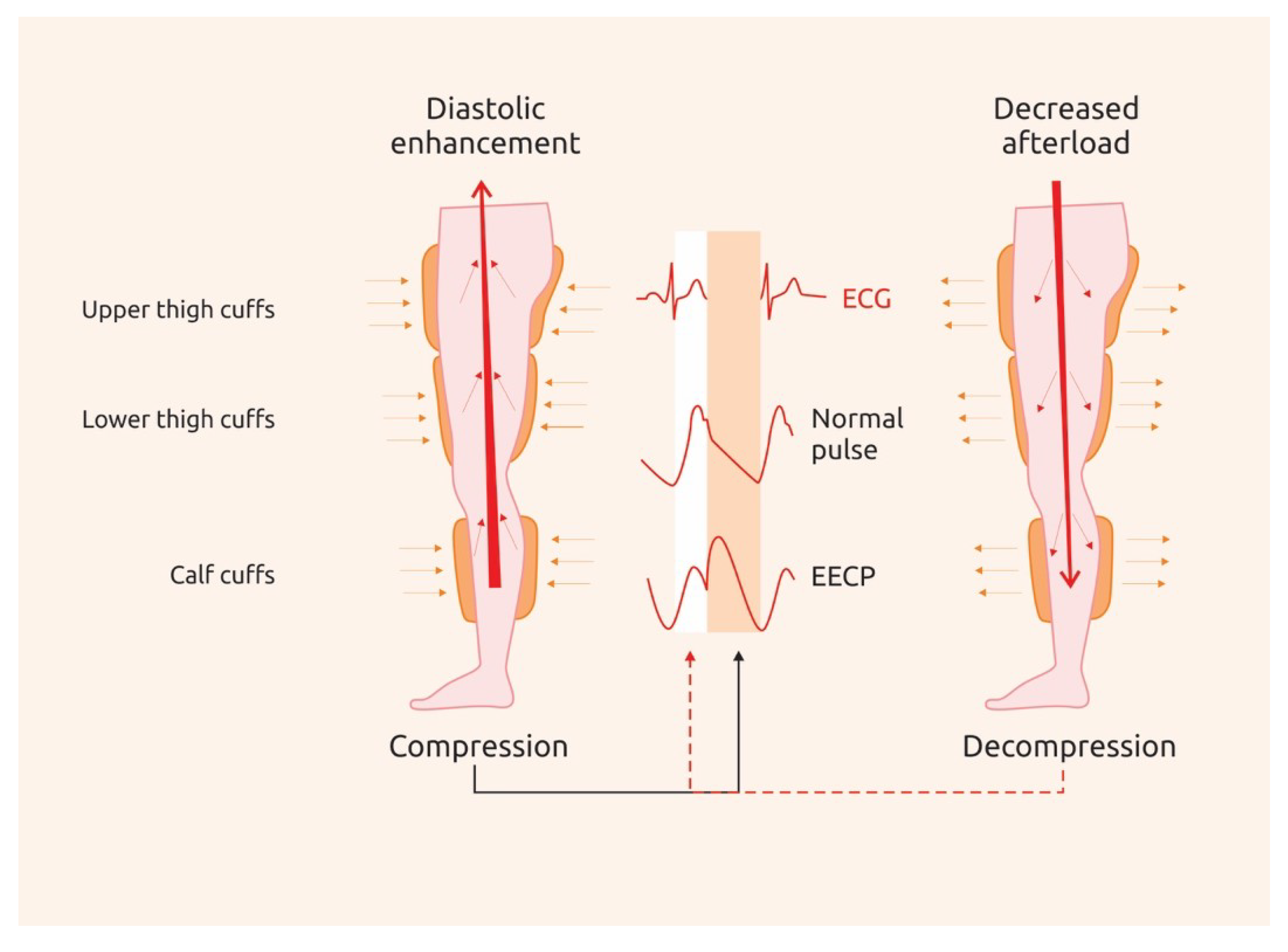
4. Enhanced External Counterpulsation
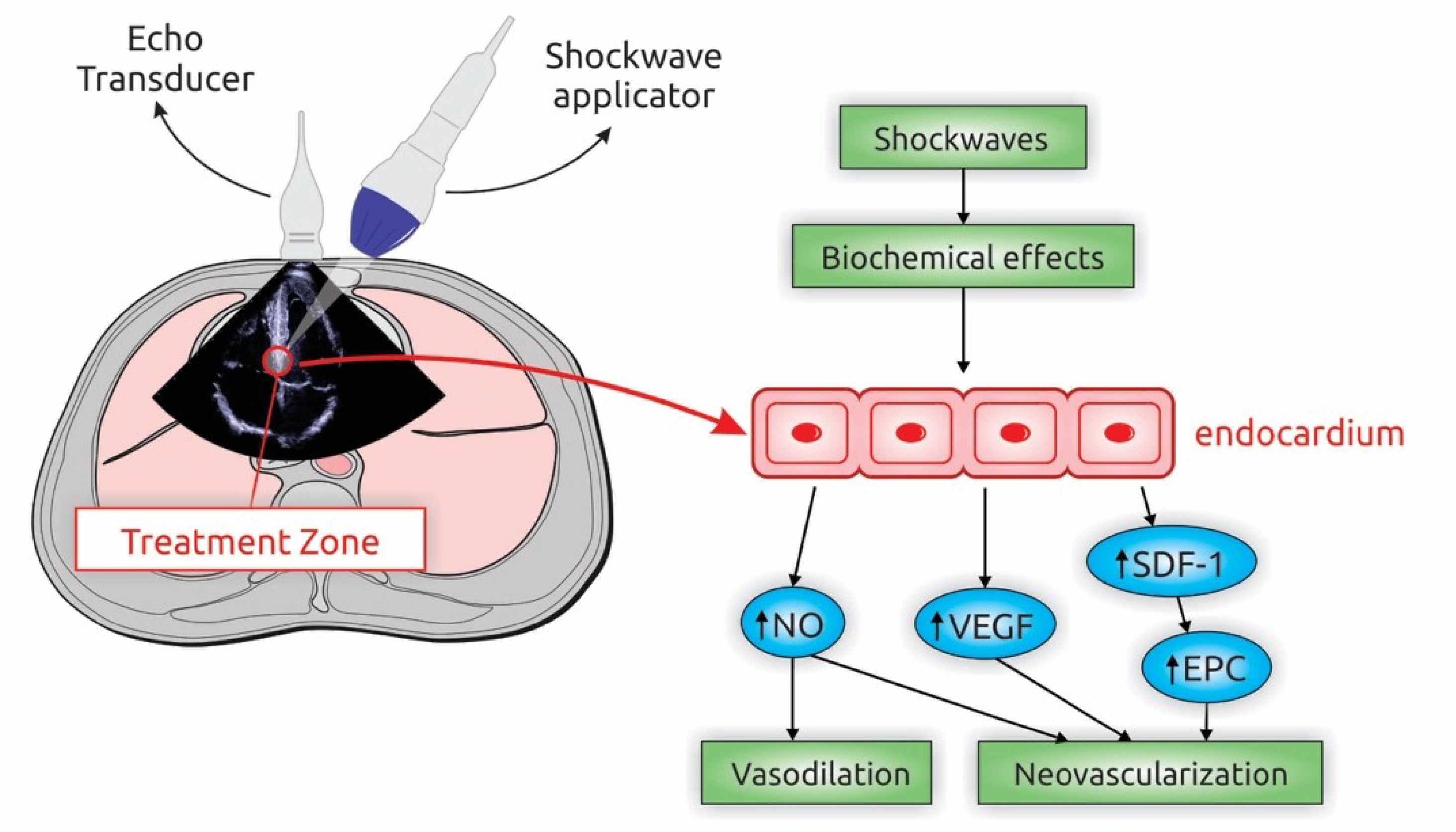
5. Extracorporeal Shockwave Myocardial Revascularization
6. Stem Cell Therapy
7. Spinal Cord Stimulation and Transcutaneous Electrical Nerve Stimulation (TENS)
8. Transmyocardial Laser Revascularization
9. Refractory Microvascular Angina
10. What Do Guidelines Say?
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mannheimer, C.; Camici, P.; Chester, M.R.; Collins, A.; DeJongste, M.; Eliasson, T. The problem of chronic refractory angina Report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur. Heart J. 2002, 23, 355–370. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Jolicoeur, E.M.; Cartier, R.; Henry, T.D.; Barsness, G.W.; Bourassa, M.G.; McGillion, M.; L’Allier, P.L. Patients with Coronary Artery Disease Unsuitable for Revascularization: Definition, General Principles and a Classification. Can. J. Cardiol. 2012, 28, S50–S59. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.D.; Satran, D.; Jolicoeur, E.M. Treatment of refractory angina in patients not suitable for revascularization. Nat. Rev. Cardiol. 2013, 11, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.; Widmer, R.J.; Matsuzawa, Y. Prevalence of Coronary Microvascular Dysfunction Among Patients with Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc. Interv. 2015, 8, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, D.; Young, R.; Adlam, D.; McConnachie, A.; Mangion, K.; Ripley, D.; Cairns, D.; Brown, J.; Bucciarelli-Ducci, C.; Baumbach, A.; et al. Coronary microvascular dysfunction in patients with stable coronary artery disease: The CE-MARC 2 coronary physiology sub-study. Int. J. Cardiol. 2018, 266, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safdar, B.; D’Onofrio, G.; Dziura, J.; Russell, R.R.; Johnson, C.; Sinusas, A.J. Prevalence and characteristics of coronary microvascular dysfunction among chest pain patients in the emergency department. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 5–13. [Google Scholar] [CrossRef]
- Henry, T.D.; Satran, D.; Hodges, J.S. Long-term survival in patients with refractory angina. Eur. Heart J. 2013, 34, 2683–2688. [Google Scholar] [CrossRef]
- Weisz, G.; Généreux, P.; Iñiguez, A.; Zurakowski, A.; Shechter, M.; Alexander, K.P.; Dressler, O.; Osmukhina, A.; James, S.; Ohman, E.M.; et al. Ranolazine in patients with incomplete revascularisation after percutaneous coronary intervention (RIVER-PCI): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015, 6736, 1–10. [Google Scholar] [CrossRef]
- Calcagno, S.; Infusino, F.; Salvi, N.; Taccheri, T.; Colantonio, R.; Bruno, E.; Birtolo, L.I.; Severino, P.; Lavalle, C.; Pucci, M.; et al. The Role of Ranolazine for the Treatment of Residual Angina beyond the Percutaneous Coronary Revascularization. J. Clin. Med. 2020, 9, 2110. [Google Scholar] [CrossRef]
- Ido, A.; Hasebe, N.; Matsuhashi, H.; Kikuchi, K. Coronary sinus occlusion enhances coronary collateral flow and reduces subendocardial ischemia. Am. J. Physiol Heart Circ. Physiol. 2001, 280, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, M.L. Cardiac shock wave therapy: An alternative non-invasive therapy for refractory angina. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5402–5410. [Google Scholar] [CrossRef] [PubMed]
- Verheye, S.; Jolicœur, E.M.; Behan, M.W.; Pettersson, P.; Sainsbury, P.; Hill, J.; Vrolix, M.; Agostoni, P.; Engstrom, T.; Labinaz, M. Efficacy of a device to narrow the coronary sinus in refractory Angina. N. Engl. J. Med. 2015, 372, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.R.; Chou, T.M.; Jain, D.; Fleishman, B.; Crawford, L.; McKiernan, T.; Nesto, R.W. The multicenter study of enhanced external counterpulsation (MUST-EECP): Effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J. Am. Coll. Cardiol. 1999, 33, 1833–1840. [Google Scholar] [CrossRef] [Green Version]
- Losordo, D.W.; Schatz, R.A.; White, C.J.; White, C.J.; Udelson, J.E.; Veereshwarayya, V.; Durgin, M.; Poh, K.K.; Weinstein, R.; Kearney, M.; et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: A phase I/IIa double-blind, randomized controlled trial. Circulation 2007, 115, 3165–3172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Povsic, T.J.; Henry, T.D.; Traverse, J.H.; Fortuin, F.D.; Schaer, G.L.; Kereiakes, D.J.; Schatz, R.A. The RENEW Trial: Efficacy and Safety of Intramyocardial Autologous CD34+ Cell Administration in Patients with Refractory Angina. JACC Cardiovasc. Interv. 2016, 9, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Zipes, D.P.; Svorkdal, N.; Berman, D.; Boortz-Marx, R.; Henry, T.; Lerman, A.; Ross, E.; Turner, M.; Irwin, C. Spinal cord stimulation therapy for patients with refractory angina who are not candidates for revascularization. Neuromodulation 2012, 15, 550–559. [Google Scholar] [CrossRef]
- Leon, M.B.; Kornowski, R.; Downey, W.E.; Weisz, G.; Baim, S.; Bonow, R.O.; Hendel, R.C.; Cohen, D.J.; Gervino, E.; Laham, R.; et al. A blinded, randomized, placebo-controlled trial of percutaneous laser myocardial revascularization to improve angina symptoms in patients with severe coronary disease. J. Am. Coll. Cardiol. 2005, 46, 1812–1819. [Google Scholar] [CrossRef] [Green Version]
- Beck, C.S.; Leighninger, D.S. Scientific basis for the surgical treatment of coronary artery disease. J. Am. Med. Assoc. 1955, 159, 1264–1271. [Google Scholar] [CrossRef]
- Banai, S.; Ben Muvhar, S.; Parikh, K.H.; Medina, A.; Sievert, H.; Seth, A.; Tsehori, J.; Paz, Y.; Sheinfeld, A.; Keren, G. Coronary Sinus Reducer Stent for the Treatment of Chronic Refractory Angina Pectoris. A Prospective, Open-Label, Multicenter, Safety Feasibility First-in-Man Study. J. Am. Coll. Cardiol. 2007, 49, 1783–1789. [Google Scholar] [CrossRef] [Green Version]
- Giannini, F.; Tzanis, G.; Ponticelli, F.; Baldetti, L.; Demir, O.M.; Mitomo, S.; Gallone, G.; Banai, S.; Colombo, A. Technical aspects in coronary sinus Reducer implantation. EuroIntervention 2020, 15, 1269–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camici, P.G.; Crea, F. Coronary microvascular dysfunction. N. Eng. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaski, J.C.; Crea, F.; Gersh, B.J.; Camici, P. Reappraisal of ischemic heart disease: Fundamental role of coronary microvascular dysfunction in the pathogenesis of angina pectoris. Circulation 2018, 138, 1463–1480. [Google Scholar] [CrossRef]
- Stoller, M.; Traupe, T.; Khattab, A.A.; De Marchi, S.F.; Steck, H.; Seiler, C. Effects of coronary sinus occlusion on myocardial ischaemia in humans: Role of coronary collateral function. Heart 2013, 99, 548–555. [Google Scholar] [CrossRef]
- Giannini, F.; Baldetti, L.; Ielasi, A.; Ruparelia, N.; Ponticelli, F.; Latib, A.; Mitomo, S. First Experience with the Coronary Sinus Reducer System for the Management of Refractory Angina in Patients Without Obstructive Coronary Artery Disease. JACC Cardiovasc. Interv. 2017, 10, 1901–1903. [Google Scholar] [CrossRef] [PubMed]
- Gori, T. Coronary Sinus Reducer for Microvascular Angina. Available online: https://www.pcronline.com/Cases-resources-images/Images-interventional-cardiology/EuroIntervention-images/Coronary-sinus-reducer-for-microvascular-angina (accessed on 11 August 2020).
- Parikh, P.; Bhatt, P.; Shah, D.; Thakar, P.; Naik, A.; Baxi, H.; Banai, S.; Parikh, K. First-in-Human Use of Coronary Sinus Reducer in Patients with Refractory Angina. J. Am. Coll. Cardiol. 2018, 72, 3227–3228. [Google Scholar] [CrossRef]
- Abawi, M.; Nijhoff, F.; Stella, P.R.; Voskuil, M.; Benedetto, D.; Doevendans, P.A.; Agostoni, P. Safety and efficacy of a device to narrow the coronary sinus for the treatment of refractory angina: A single-centre real-world experience. Neth. Heart J. 2016, 24, 544–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannini, F.; Baldetti, L.; Ponticelli, F.; Ruparelia, N.; Mitomo, S.; Latib, A.; Montorfano, M.; Jabbour, R.J.; Aurelio, A.; Ferri, L.; et al. Coronary Sinus Reducer Implantation for the Treatment of Chronic Refractory Angina: A Single-Center Experience. JACC Cardiovasc. Interv. 2018, 11, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Konigstein, M.; Bazan, S.; Revivo, M.; Banai, S. Coronary Sinus Reducer implantation improves symptoms, ischaemia and physical capacity in patients with refractory angina unsuitable for myocardial revascularisation: A single-centre experience. EuroIntervention 2018, 14, e452–e458. [Google Scholar] [CrossRef]
- Giannini, F.; Baldetti, L.; Konigstein, M.; Rosseel, L.; Ruparelia, N.; Gallone, G.; Colombo, A.; Banai, S.; Verheye, S. Safety and efficacy of the reducer: A multi-center clinical registry–REDUCE study. Int. J. Cardiol. 2018, 269, 40–44. [Google Scholar] [CrossRef]
- Ponticelli, F.; Tzanis, G.; Gallone, G. Safety and efficacy of Coronary Sinus Reducer implantation at 2-year follow-up. Int. J. Cardiol. 2019, 292, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Zivelonghi, C.; Konigstein, M.; Azzano, A.; Agostoni, P.; Topilski, Y.; Banai, S.; Verheye, S. Coronary sinus Reducer implantation results in improved oxygen kinetics at cardiopulmonary exercise test in patients with refractory angina. EuroIntervention 2020. [Google Scholar] [CrossRef]
- Tzanis, G.; Palmisano, A.; Gallone, G.; Ponticelli, F.; Baldetti, L.; Esposito, A.; Colombo, A.; Giannini, F. The impact of the coronary sinus reducer upon left ventricular function in patients with refractory angina pectoris. Catheter. Cardiovasc. Interv. 2019, 95, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Giannini, F.; Palmisano, A.; Baldetti, L.; Benedetti, G.; Ponticelli, F.; Rancoita, P.M.V.; Ruparelia, N.; Gallone, G.; Ancona, M.; Mangieri, A.; et al. Patterns of Regional Myocardial Perfusion Following Coronary Sinus Reducer Implantation: Insights by Stress Cardiac Magnetic Resonance. Circ. Cardiovasc. Imaging. 2019, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Gallone, G.; Baldetti, L.; Palmisano, A.; Ponticelli, F.; Tzanis, G.; Colombo, A.; Esposito, A.; Giannini, F. Coronary Sinus Reducer Implantation to Reduce the Ischemic Burden in Refractory Angina. JACC Cardiovasc. Interv. 2019, 12, e11–e13. [Google Scholar] [CrossRef]
- Gallone, G.; Palmisano, A.; Baldetti, L.; Monti, C.B.; Ponticelli, F.; Tzanis, G.; Colombo, A.; Esposito, A.; Giannini, F. Improved Myocardial Function with Coronary Sinus Reducer in a Patient with Refractory Angina and Heart Failure With Reduced Ejection Fraction. Can. J. Cardiol. 2020, 36, 589. [Google Scholar] [CrossRef]
- Szekely, Y.; Topilsky, Y.; Bazan, S.; Revivo, M.; Banai, S.; Konigstein, M. The impact of coronary sinus narrowing on diastolic function in patients with refractory angina. Int. J. Cardiol. 2019, 291, 8–12. [Google Scholar] [CrossRef]
- Konigstein, M.; Giannini, F.; Banai, S. The Reducer device in patients with angina pectoris: Mechanisms, indications, and perspectives. Eur. Heart J. 2018, 39, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Galassi, A.R.; Brilakis, E.S.; Boukhris, M.; Tomasello, S.D.; Sianos, G.; Karmpaliotis, D.; Di Mario, C.; Strauss, B.H.; Rinfret, S.; Yamane, M.; et al. Appropriateness of percutaneous revascularization of coronary chronic total occlusions: An overview. Eur. Heart J. 2016, 37, 2692–2700. [Google Scholar] [CrossRef] [Green Version]
- Brilakis, E.S.; Mashayekhi, K.; Burke, M.N. How DECISION-CTO Can Help Guide the Decision to Perform Chronic Total Occlusion Percutaneous Coronary Intervention. Circulation 2019, 139, 1684–1687. [Google Scholar] [CrossRef]
- Safley, D.M.; Grantham, J.A.; Hatch, J.; Jones, P.G.; Spertus, J.A. Quality of life benefits of percutaneous coronary intervention for chronic occlusions. Catheter. Cardiovasc. Interv. 2014, 84, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grantham, J.A.; Jones, P.G.; Cannon, L.; Spertus, J.A. Quantifying the early health status benefits of successful chronic total occlusion Recanalization results from the FlowCardia’s Approach to chronic total occlusion Recanalization (FACTOR) trial. Circ. Cardiovasc. Qual Outcomes 2010, 3, 284–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoebers, L.P.; Claessen, B.E.; Elias, J.; Dangas, G.D.; Mehran, R.; Henriques, J.P.S. Meta-analysis on the impact of percutaneous coronary intervention of chronic total occlusions on left ventricular function and clinical outcome. Int. J. Cardiol. 2015, 187, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Lee, P.H.; Ahn, J.M.; Park, D.W.; Yun, S.C.; Han, S.; Kang, H. Randomized Trial Evaluating Percutaneous Coronary Intervention for the Treatment of Chronic Total Occlusion: The DECISION-CTO Trial. Circulation 2019, 139, 1683. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.S.; Martin-Yuste, V.; Hildick-Smith, D.; Boudou, N.; Sianos, G.; Gelev, V.; Rumoroso, J.R. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur. Heart J. 2018, 39, 2484–2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Zivelonghi, C.; Verheye, S.; Timmers, L.; Van Kuijk, J.P.; Giannini, F.; Dekker, M.; Silvis, M. Efficacy of Coronary Sinus Reducer in Patients with Non–revascularized Chronic Total Occlusions. J. Interv. Cardiol. 2020, 31, 775–779. [Google Scholar] [CrossRef]
- Masuda, D.; Nohara, R.; Hirai, T.; Kataoka, K.; Chen, L.G.; Hosokawa, R.; Inubushi, M.; Tadamura, E.; Fujita, M.; Sasayama, S. Enhanced external counterpulsation improved myocardial perfusion and coronary flow reserve in patients with chronic stable angina: Evaluation by 13N-ammonia positron emission tomography. Eur. Heart J. 2001, 22, 1451–1458. [Google Scholar] [CrossRef]
- Buschmann, E.E.; Utz, W.; Pagonas, N.; Schulz-Menger, J.; Busjahn, A.; Monti, J.; Maerz, W. Improvement of fractional flow reserve and collateral flow by treatment with external counterpulsation (Art.Net.-2 Trial). Eur. J. Clin. Investig. 2009, 39, 866–875. [Google Scholar] [CrossRef]
- Gloekler, S.; Meier, P.; De Marchi, S.F.; Rutz, T.; Traupe, T.; Rimoldi, S.F.; Wustmann, K. Coronary collateral growth by external counterpulsation: A randomised controlled trial. Heart 2010, 96, 202–207. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Barsness, G.W.; Keelan, P.C.; Schnell, T.I.; Pumper, G.M.; Kuvin, J.T.; Schnall, R.P.; Holmes, D.R.; Higano, S.T.; Lerman, A. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J. Am. Coll. Cardiol. 2003, 41, 1761–1768. [Google Scholar] [CrossRef] [Green Version]
- Nichols, W.W.; Estrada, J.C.; Braith, R.W.; Owens, K.; Conti, C.R. Enhanced External Counterpulsation Treatment Improves Arterial Wall Properties and Wave Reflection Characteristics in Patients with Refractory Angina. J. Am. Coll. Cardiol. 2006, 48, 1208–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, D.P.; Conti, C.R.; Nichols, W.W.; Choi, C.Y.; Khuddus, M.A.; Braith, R.W. Effect of Enhanced External Counterpulsation on Inflammatory Cytokines and Adhesion Molecules in Patients with Angina Pectoris and Angiographic Coronary Artery Disease. Am. J. Cardiol. 2008, 101, 300–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braith, R.W.; Conti, C.R.; Nichols, W.W.; Choi, C.Y.; Khuddus, M.A.; Beck, D.T.; Casey, D.P. Enhanced external counterpulsation improves peripheral artery flow-mediated dilation in patients with chronic angina: A randomized sham-controlled study. Circulation 2010, 122, 1612–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiernan, T.J.; Boilson, B.A.; Tesmer, L.; Harbuzariu, A.; Simari, R.D.; Barsness, G.W. Effect of enhanced external counterpulsation on circulating CD34+ progenitor cell subsets. Int. J. Cardiol. 2011, 153, 202–206. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Liu, X.; Wang, X.; Wang, Q.; Zhang, Y.; Ge, Z. Efficacy of enhanced external counterpulsation in patients with chronic refractory angina on Canadian cardiovascular society (CCS) angina class. Medicine 2015, 94, e2002. [Google Scholar] [CrossRef]
- Shah, S.A.; Shapiro, R.J.; Mehta, R.; Snyder, J.A. Impact of enhanced external counterpulsation on Canadian Cardiovascular Society angina class in patients with chronic stable angina: A meta-analysis. Pharmacotherapy 2010, 30, 639–645. [Google Scholar] [CrossRef]
- Qin, X.; Deng, Y.; Wu, D.; Yu, L.; Huang, R. Does enhanced external counterpulsation (EECP) significantly affect myocardial perfusion? A systematic review & meta-analysis. PLoS ONE 2016, 11, e151822. [Google Scholar] [CrossRef] [Green Version]
- McKenna, C.; Hawkins, N.; Claxton, K.; McDaid, C.; Suekarran, S.; Light, K.; Chester, M.; Cleland, J.G.F.; Woolacott, N.; Sculpher, M. Cost-effectiveness of enhanced external counterpulsation (EECP) for the treatment of stable angina in the United Kingdom. Int. J. Technol. Assess. Health Care 2010, 26, 175–182. [Google Scholar] [CrossRef]
- Cassar, A.; Prasad, M.; Rodriguez-Porcel, M.; Reeder, G.S.; Karia, D.; DeMaria, A.N.; Lerman, A. Safety and efficacy of extracorporeal shock wave myocardial revascularization therapy for refractory angina pectoris. Mayo Clin. Proc. 2014, 89, 346–354. [Google Scholar] [CrossRef]
- Nishida, T.; Shimokawa, H.; Oi, K.; Tatewaki, H.; Uwatoku, T.; Abe, K.; Matsumoto, Y. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation 2004, 110, 3055–3061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, J.; Ohmori, K.; Takeuchi, H.; Shinomiya, K.; Namba, T.; Kondo, I.; Kiyomoto, H.; Kohno, M. Treatment of ischemic limbs based on local recruitment of vascular endothelial growth factor-producing inflammatory cells with ultrasonic microbubble destruction. J. Am. Coll. Cardiol. 2005, 46, 899–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariotto, S.; Cavalieri, E.; Amelio, E.; Ciampa, A.R.; De Prati, A.C.; Marlinghaus, E.; Russo, S.; Suzuki, H. Extracorporeal shock waves: From lithotripsy to anti-inflammatory action by NO production. Nitric Oxide Biol. Chem. 2005, 12, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Aicher, A.; Heeschen, C.; Sasaki, K.I.; Urbich, C.; Zeiher, A.M.; Dimmeler, S. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: A new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation 2006, 114, 2823–2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, M.; Wan Ahmad, W.A.; Sukmawan, R.; Magsombol, E.B.L.; Cassar, A.; Vinshtok, Y.; Ismail, M.D. Extracorporeal shockwave myocardial therapy is efficacious in improving symptoms in patients with refractory angina pectoris—A multicenter study. Coron. Artery Dis. 2015, 26, 194–200. [Google Scholar] [CrossRef]
- Alunni, G.; Marra, S.; Meynet, I.; D’Amico, S.; Pianelli, M.; Zema, D.; Bongiovanni, F.; Gaita, F. The beneficial effect of extracorporeal shockwave myocardial revascularization in patients with refractory angina. Cardiovasc. Revascularization Med. 2015, 16, 6–11. [Google Scholar] [CrossRef]
- Alunni, G.; Barbero, U.; Vairo, A.; D’Amico, S.; Pianelli, M.; Zema, D.; Bongiovanni, F.; Gaita, F. The beneficial effect of extracorporeal shockwave myocardial revascularization: Two years of follow-up. Cardiovasc. Revascularization Med. 2017, 18, 572–576. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Ito, K.; Shindo, T.; Hao, K.; Shiroto, T.; Matsumoto, Y.; Takahashi, J. A multicenter trial of extracorporeal cardiac shock wave therapy for refractory angina pectoris: Report of the highly advanced medical treatment in Japan. Heart Vessels 2019, 34, 104–113. [Google Scholar] [CrossRef]
- Vainer, J.; Habets, J.H.M.; Schalla, S.; Lousberg, H.P.; de Pont, C.D.J.M.; Vöö, S.A.; Brans, B.T.; Hoorntje, J.C.A.; Waltenberger, J. Cardiac shockwave therapy in patients with chronic refractory angina pectoris. Neth. Heart J. 2016, 24, 343–349. [Google Scholar] [CrossRef]
- Schmid, J.P.; Capoferri, M.; Wahl, A.; Eshtehardi, P.; Hess, O.M. Cardiac Shock Wave Therapy for Chronic Refractory Angina Pectoris. A Prospective Placebo-Controlled Randomized Trial. Cardiovasc. Ther. 2013, 31, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Burneikaite, G.; Shkolnik, E.; Čelutkiene, J.; Zuoziene, G.; Butkuviene, I.; Petrauskiene, B.; Šerpytis, P.; Laucevičius, A.; Lerman, A. Cardiac shock-wave therapy in the treatment of coronary artery disease: Systematic review and meta-analysis. Cardiovasc. Ultrasound 2017, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Corban, M.T.; Henry, T.D.; Dietz, A.B.; Lerman, L.O.; Lerman, A. Promise of Autologous CD34 + Stem/Progenitor Cell Therapy for Treatment of Cardiovascular Disease. Cardiovasc. Res. 2020, 116, 1423–1424. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.S.; Li, Q.; Ghasemzadeh, N.; Eapen, D.J.; Moss, L.D.; Janjua, A.U.; Manocha, P. Circulating CD34+ progenitor cells and risk of mortality in a +population with coronary artery disease. Circ. Res. 2015, 116, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinman, M.E.; Blei, F.; Gurtner, G.C. Circulating endothelial progenitor cells and vascular anomalies. Lymphat. Res. Biol. 2005, 3, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, A.; Iwasaki, H.; Kusano, K.; Murayama, T.; Oyamada, A.; Silver, M.; Hulbert, C. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation 2006, 114, 2163–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losordo, D.W.; Henry, T.D.; Davidson, C.; Lee, J.S.; Costa, M.A.; Bass, T.; Mendelsohn, F.; Fortuin, F.D.; Pepine, C.J.; Traverse, J.H.; et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ. Res. 2011, 109, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Henry, T.D.; Schaer, G.L.; Traverse, J.H.; Traverse, J.H.; Povsic, T.J.; Davidson, C.; Lee, J.C.; Costa, M.A. Autologous CD34+ cell therapy for refractory angina: 2-year outcomes from the ACT34-CMI study. Cell Transplant. 2016, 25, 1701–1711. [Google Scholar] [CrossRef] [Green Version]
- Henry, T.D.; Losordo, D.W.; Traverse, J.H.; Schatz, R.A.; Joliceur, E.M.; Schaer, G.L.; Clare, R.; Chiswell, K.; White, C.J.; Fortuin, F.D.; et al. Autologous CD34+ cell therapy improves exercise capacity, angina frequency and reduces mortality in no-option refractory angina: A patient-level pooled analysis of randomized double-blinded trials. Eur. Heart J. 2018, 39, 2208–2216. [Google Scholar] [CrossRef] [Green Version]
- Lee, F.Y.; Chen, Y.L.; Sung, P.H.; Ma, M.C.; Pei, S.N.; Wu, C.J.; Yang, C.H. Intracoronary transfusion of circulation-derived CD34+ cells improves left ventricular function in patients with end-stage diffuse coronary artery disease unsuitable for coronary intervention. Crit. Care Med. 2015, 43, 2117–2132. [Google Scholar] [CrossRef]
- Sung, P.H.; Lee, F.Y.; Tong, M.S.; Chiang, J.Y.; Pei, S.N.; Ma, M.C.; Li, Y.C. The five-year clinical and angiographic follow-up outcomes of intracoronary transfusion of circulation-derived cd34+ cells for patients with end-stage diffuse coronary artery disease unsuitable for coronary intervention–Phase i clinical trial. Crit. Care Med. 2018, 46, e411–e418. [Google Scholar] [CrossRef]
- Jimenez-Quevedo, P.; Gonzalez-Ferrer, J.J.; Sabate, M.; Garcia-Moll, X.; Delgado-Bolton, R.; Llorente, L.; Bernardo, E. Selected CD133+ progenitor cells to promote angiogenesis in patients with refractory angina final results of the PROGENITOR randomized trial. Circ. Res. 2014, 115, 950–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojakowski, W.; Jadczyk, T.; Michalewska-Włudarczyk, A.; Parma, Z.; Markiewicz, M.; Rychlik, W.; Kostkiewicz, M. Effects of transendocardial delivery of bone marrow-derived CD133+ cells on left ventricle perfusion and function in patients with refractory angina. Circ. Res. 2017, 120, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, B.; Carbucicchio, C.; Catto, V.; Gambini, E.; Rurali, E.; Bestetti, A.; Gaipa, G. Linking cell function with perfusion: Insights from the transcatheter delivery of bone marrow-derived CD133 + cells in ischemic refractory cardiomyopathy trial (RECARDIO) 11 Medical and Health Sciences 1103 Clinical Sciences 11 Medical and Health Sciences. Stem Cell Res. Ther. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Kucia, M.; Dawn, B.; Hunt, G. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ. Res. 2004, 95, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Urbich, C.; Aicher, A.; Heeschen, C.; Dernbach, E.; Hofmann, W.K.; Zeiher, A.M.; Dimmeler, S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J. Mol. Cell. Cardiol. 2005, 39, 733–742. [Google Scholar] [CrossRef]
- Jones, D.A.; Weeraman, D.; Colicchia, M.; Hussain, M.A.; Veerapen, D.; Andiapen, M.; Rathod, K.S.; Baumbach, A.; Mathur, A. The Impact of Cell Therapy on Cardiovascular Outcomes in Patients with Refractory Angina: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Circ. Res. 2019, 124, 1786–1795. [Google Scholar] [CrossRef]
- Khan, A.R.; Farid, T.A.; Pathan, A.; Tripathi, A.; Ghafghazi, S.; Wysoczynski, M.; Bolli, R. Impact of cell therapy on myocardial perfusion and cardiovascular outcomes in patients with angina refractory to medical therapy: A systematic review and meta-analysis. Circ. Res. 2016, 118, 984–993. [Google Scholar] [CrossRef]
- Gallone, G.; Baldetti, L.; Tzanis, G.; Gramegna, M.; Latib, A.; Colombo, A.; Henry, T.D.; Giannini, F. Refractory Angina: From Pathophysiology to New Therapeutic Nonpharmacological Technologies. JACC Cardiovasc. Interv. 2020, 13, 1–19. [Google Scholar] [CrossRef]
- Rosen, S.D.; Paulesu, E.; Wise, R.J.S.; Camici, P.G. Central neural contribution to the perception of chest pain in cardiac syndrome X. Heart 2002, 87, 513–519. [Google Scholar] [CrossRef] [Green Version]
- De Vries, J.; Anthonio, R.L.; De Jongste, M.J.L.; Jessurun, G.A.; Tan, E.S.; de Smet, B.J.G.L.; van den Heuvel, A.F.M.; Staal, M.J.; Zijlstra, F. The effect of electrical neurostimulation on collateral perfusion during acute coronary occlusion. BMC Cardiovasc. Disord. 2007, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Eldabe, S.; Thomson, S.; Duarte, R.; Brookes, M.; Debelder, M.; Raphael, J.; Davies, E.; Taylor, R. The effectiveness and cost-effectiveness of spinal cord stimulation for refractory angina (RASCAL study): A pilot randomized controlled trial. Neuromodulation 2016, 19, 60–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraste, A.; Ukkonen, H.; Varis, A.; Vasankari, T.; Tunturi, S.; Taittonen, M.; Rautakorpi, P.; Luotolahti, M.; Airaksinen, K.E.J.; Knuuti, J. Effect of spinal cord stimulation on myocardial perfusion reserve in patients with refractory angina pectoris. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.A.; Sestito, A.; Sgueglia, G.A.; Infusino, F.; Papacci, F.; Visocchi, M.; Ierardi, C.; Meglio, M.; Bellocci, F.; Crea, F. Effect of spinal cord stimulation on spontaneous and stress-induced angina and “ischemia-like” ST-segment depression in patients with cardiac syndrome X. Eur. Heart J. 2005, 26, 983–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eddicks, S.; Maier-Hauff, K.; Schenk, M.; Müller, A.; Baumann, G.; Theres, H. Thoracic spinal cord stimulation improves functional status and relieves symptoms in patients with refractory angina pectoris: The first placebo-controlled randomised study. Heart 2007, 93, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Imran, T.F.; Malapero, R.; Qavi, A.H.; Hasan, Z.; de la Torre, B.; Patel, Y.R.; Yong, R.J.; Djousse, L.; Gaziano, J.M.; Gerhard-Herman, M.D. Efficacy of spinal cord stimulation as an adjunct therapy for chronic refractory angina pectoris. Int. J. Cardiol. 2017, 227, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Bao, H.; Si, Y.; Xu, C.; Chen, H.; Gao, X.; Xie, X.; Xu, Y.; Sun, F.; Zeng, L. Spinal Cord Stimulation for Refractory Angina Pectoris. Clin. J. Pain. 2017, 33, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Briones, E.; Lacalle, J.R.; Marin-Leon, I. Transmyocardial laser revascularization versus medical therapy for refractory angina. Cochrane Database Syst. Rev. 2015, 2. [Google Scholar] [CrossRef]
- Schofield, P.M.; McNab, D. NICE evaluation of transmyocardial laser revascularisation and percutaneous laser revascularisation for refractory angina. Heart 2010, 96, 312–313. [Google Scholar] [CrossRef]
- Lanza, G.A.; De Vita, A.; Kaski, J.C. “Primary” microvascular angina: Clinical characteristics, pathogenesis and management. Interv. Cardiol. Rev. 2018, 13, 108–111. [Google Scholar] [CrossRef]
- Taqueti, V.R.; Di Carli, M.F. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2625–2641. [Google Scholar] [CrossRef]
- Bairey Merz, C.N.; Pepine, C.J.; Shimokawa, H.; Berry, C. Treatment of coronary microvascular dysfunction. Cardiovasc. Res. 2020, 116, 856–870. [Google Scholar] [CrossRef] [PubMed]
- Vermeltfoort, I.A.C.; Teule, G.J.J.; Van Dijk, A.B. Long-term prognosis of patients with cardiac syndrome X: A review. Neth. Heart J. 2012, 20, 365–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; Robertson, K.; et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J. Am. Coll Cardiol. 2018, 72, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Xiao-Ming, W.; Gui-Fu, W. Expert consensus on the clinical application of enhanced external counterpulsation in elderly people (2019). Aging Med. 2020, 3, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Kronhaus, K.D.; Lawson, W.E. Enhanced external counterpulsation is an effective treatment for Syndrome, X. Int. J. Cardiol. 2009, 135, 256–257. [Google Scholar] [CrossRef]
- Luo, C.; Liu, D.; Wu, G.; Hu, C.; Zhang, Y.; Du, Z.; Dong, Y. Effect of enhanced external counterpulsation on coronary slow flow and its relation with endothelial function and inflammation: A mid-term follow-up study. Cardiology 2012, 122, 260–268. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Wu, F.; Zhang, H.; Wu, G. GW29-e0861 Effect of Enhanced External Counterpulsation on Microvascular Dysfunction in Patients with Coronary Artery Disease after Coronary Stent Implantation. J. Am. Coll Cardiol. 2018, 72, C236. [Google Scholar] [CrossRef]
- Orlic, D.; Kajstura, J.; Chimenti, S.; Orlic, D.; Kajstura, J.; Chimenti, S.; Jakoniuk, I.; Anderson, S.M.; Li, B.; Pickel, J.; et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001, 410, 701–705. [Google Scholar] [CrossRef]
- Erbs, S.; Linke, A.; Schächinger, V.; Assmus, B.; Thiele, H.; Diederich, K.W.; Hoffmann, C. Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: The Doppler substudy of the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial. Circulation 2007, 116, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Schächinger, V.; Assmus, B.; Honold, J.; Lehmann, R.; Hofmann, W.K.; Martin, H.; Dimmeler, S.; Zeiher, A.M. Normalization of coronary blood flow in the infarct-related artery after intracoronary progenitor cell therapy: Intracoronary Doppler substudy of the TOPCARE-AMI trial. Clin. Res. Cardiol. 2006, 95, 13–22. [Google Scholar] [CrossRef]
- Fihn, S.D.; Gardin, J.M.; Abrams, J.; Berra, K.; Blankenship, J.C.; Dallas, A.P.; Douglas, P.S.; Foody, J.M.; Gerber, T.C.; Hinderliter, A.L.; et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. J. Am. Coll. Cardiol. 2012, 60, e44–e164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Mechanism of Action | Invasive-ness | Complications | Cost | RCTs | Guideline Recommendations | |
|---|---|---|---|---|---|---|
| CSR |
| + | Device migration (rare) Coronary sinus perforation, dissection, thrombotic occlusion (rare) Access site bleeding | Similar to CABG | COSIRA [13] | ESC (IIb/B), ACC/AHA (NA) |
| EECP |
| - | Paresthesia Leg pain Skin abrasion | Low | MUST-EECP [14] | ESC (IIb/B), ACC/AHA (IIb/B) |
| ESMR |
| - | None | Low | NA | NA |
| Cell Therapy |
| + | Ventricular Tachycardia Access site bleeding Stroke (rare) | NA | ACT-34 [15], RENEW [16] | NA |
| SCS |
| + | Lead migration Lead fracture Infection Headache Neurological damage | Similar to CABG | STARTSTIM [17] | ESC (IIa/B), ACC/AHA (IIb/B) |
| TMLR |
| ++ | Myocardial infarction Cardiac tamponade Heart failure Death | High | [18] | ESC (III/A), ACC/AHA (IIb/B) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakhimov, K.; Gori, T. Non-pharmacological Treatment of Refractory Angina and Microvascular Angina. Biomedicines 2020, 8, 285. https://doi.org/10.3390/biomedicines8080285
Rakhimov K, Gori T. Non-pharmacological Treatment of Refractory Angina and Microvascular Angina. Biomedicines. 2020; 8(8):285. https://doi.org/10.3390/biomedicines8080285
Chicago/Turabian StyleRakhimov, Kudrat, and Tommaso Gori. 2020. "Non-pharmacological Treatment of Refractory Angina and Microvascular Angina" Biomedicines 8, no. 8: 285. https://doi.org/10.3390/biomedicines8080285
APA StyleRakhimov, K., & Gori, T. (2020). Non-pharmacological Treatment of Refractory Angina and Microvascular Angina. Biomedicines, 8(8), 285. https://doi.org/10.3390/biomedicines8080285





