Changes in Circulating Extracellular Vesicles in Patients with ST-Elevation Myocardial Infarction and Potential Effects of Remote Ischemic Conditioning—A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Study Interventions
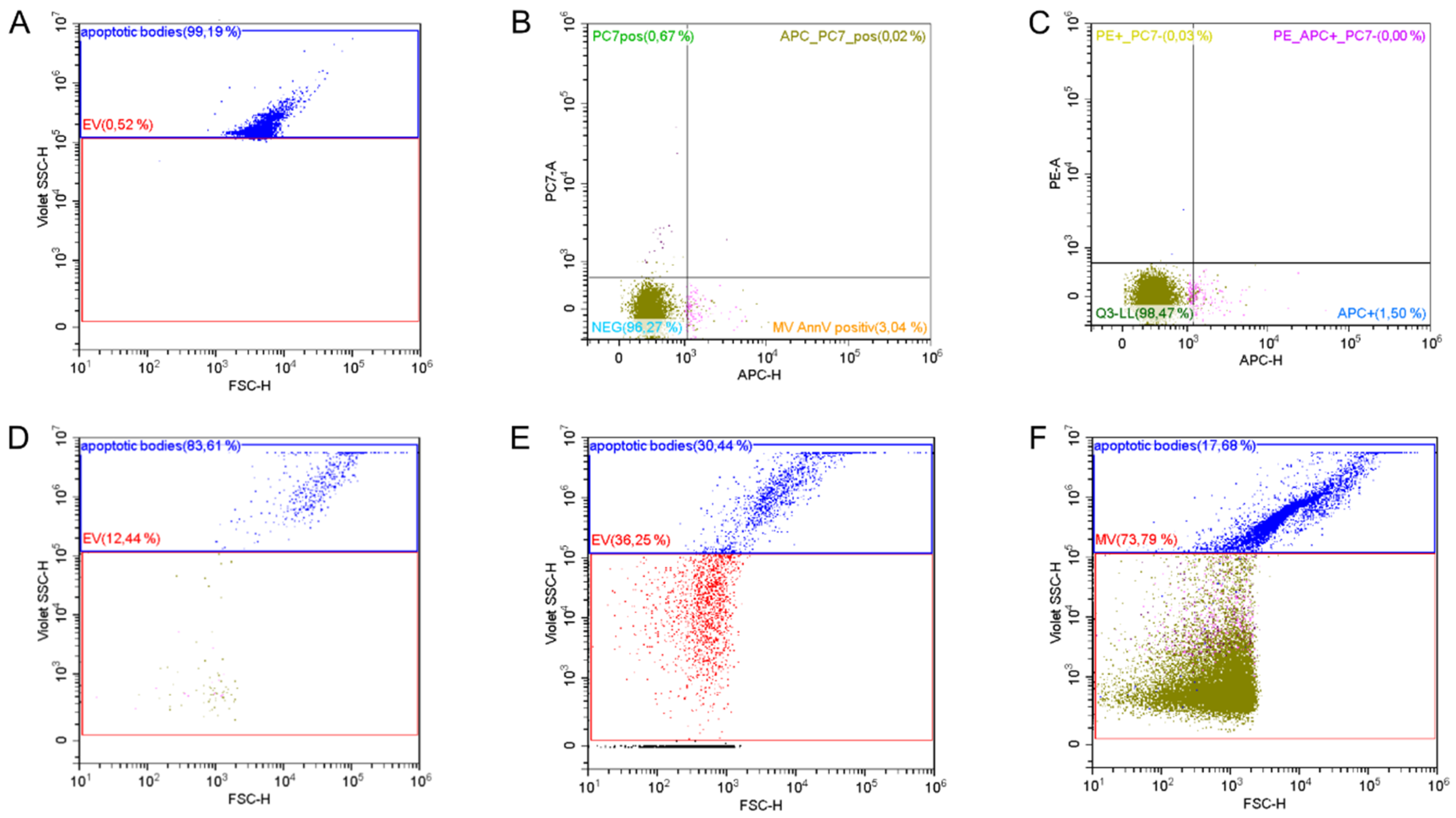
2.3. Study Parameters
2.4. Statistics
3. Results
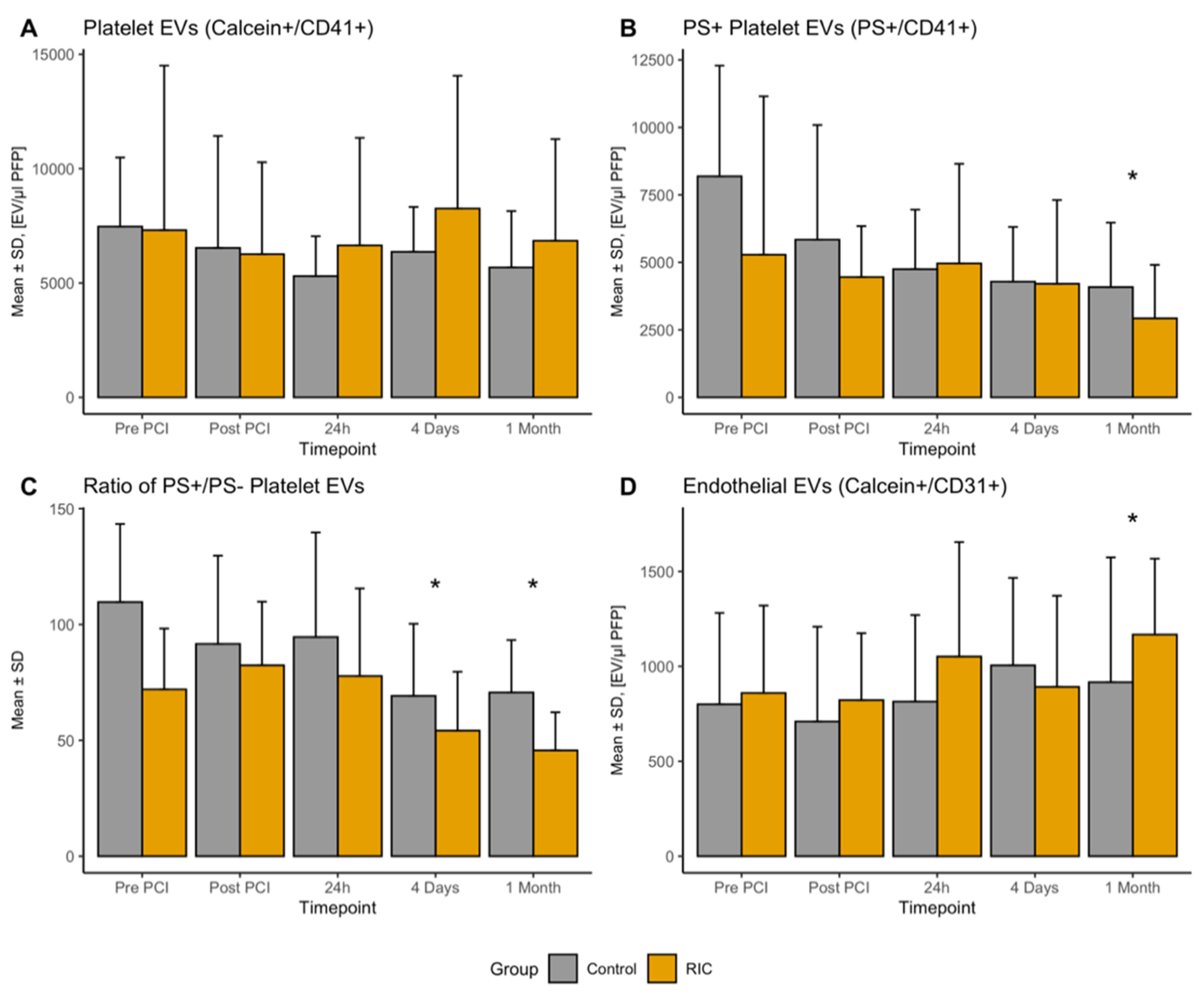
3.1. Platelet-Derived EVs
3.2. Endothelial-Derived EVs
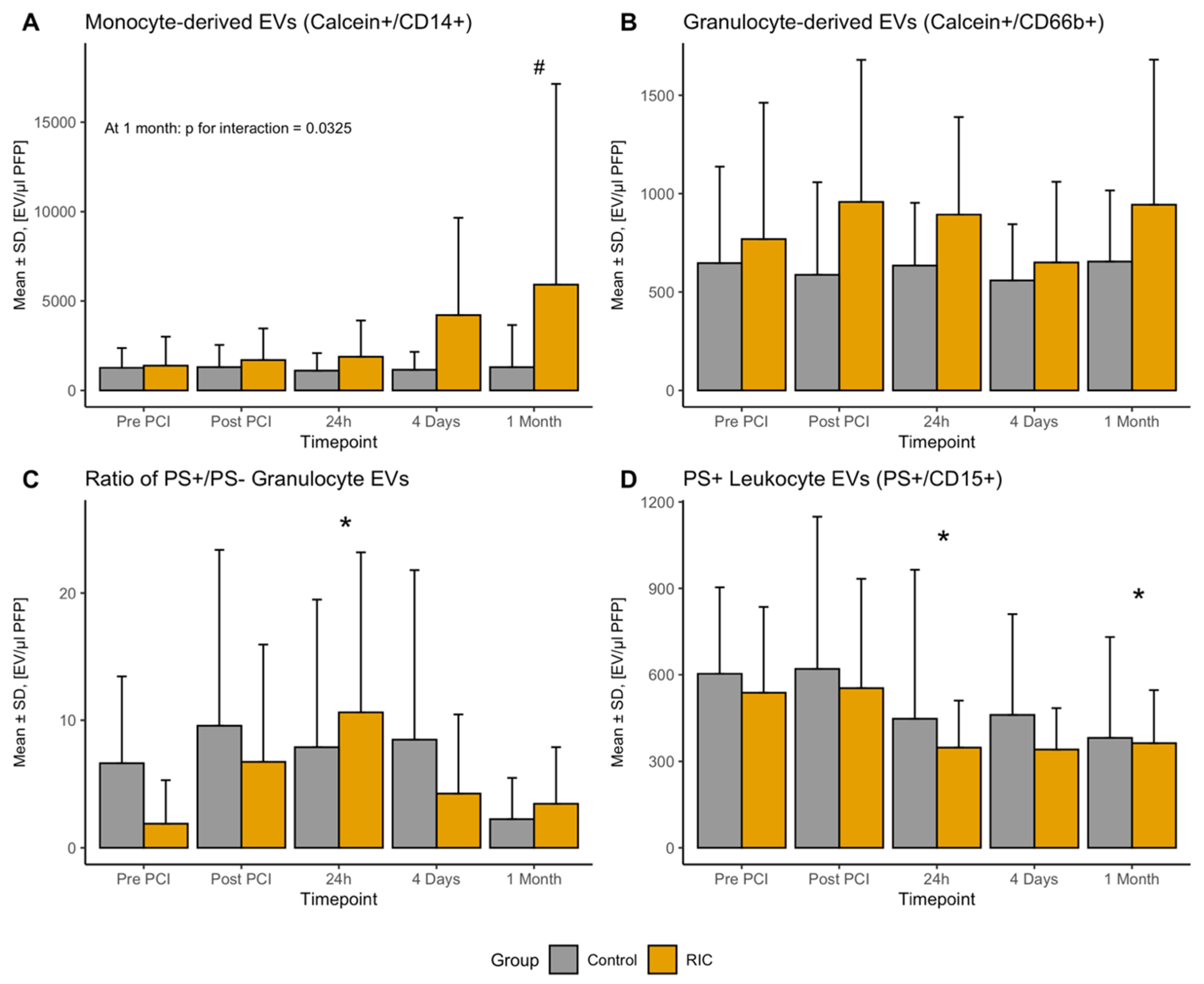
3.3. Leukocyte-Derived EVs
3.4. EVs after RIC in Healthy Volunteers
4. Discussion
4.1. Strengths and Limitations
4.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EEV | Endothelial-derived extracellular vesicles |
| EV | Extracellular vesicle |
| GEV | Granulocyte-derived extracellular vesicles |
| LEV | Leucocyte-derived extracellular vesicles |
| IRI | Ischemia-reperfusion injury |
| MEV | Monocyte-derived extracellular vesicles |
| PCI | Percutaneous coronary intervention |
| PEV | Platelet-derived extracellular vesicles |
| PS | Phosphatidyl-serine |
| RIC | Remote ischemic conditioning |
| STEMI | ST-elevation myocardial infarction |
References
- McManus, D.D.; Gore, J.; Yarzebski, J.; Spencer, F.; Lessard, D.; Goldberg, R.J. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am. J. Med. 2011, 124, 40–47. [Google Scholar] [CrossRef]
- Widimsky, P.; Wijns, W.; Fajadet, J.; de Belder, M.; Knot, J.; Aaberge, L.; Andrikopoulos, G.; Baz, J.A.; Betriu, A.; Claeys, M.; et al. Reperfusion therapy for ST elevation acute myocardial infarction in Europe: Description of the current situation in 30 countries. Eur. Heart J. 2010, 31, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Haller, P.M.; Jager, B.; Farhan, S.; Christ, G.; Schreiber, W.; Weidinger, F.; Stefenelli, T.; Delle-Karth, G.; Kaff, A.; Maurer, G.; et al. Impact of age on short- and long-term mortality of patients with ST-elevation myocardial infarction in the VIENNA STEMI network. Wien. Klin. Wochenschr. 2018, 130, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Lonborg, J.; Vejlstrup, N.; Kelbaek, H.; Holmvang, L.; Jorgensen, E.; Helqvist, S.; Saunamaki, K.; Ahtarovski, K.A.; Botker, H.E.; Kim, W.Y.; et al. Final infarct size measured by cardiovascular magnetic resonance in patients with ST elevation myocardial infarction predicts long-term clinical outcome: An observational study. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E.; Kloner, R.A. Myocardial reperfusion: A double-edged sword? J. Clin. Investig. 1985, 76, 1713–1719. [Google Scholar] [CrossRef]
- Heusch, G.; Gersh, B.J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: A continual challenge. Eur. Heart J. 2017, 38, 774–784. [Google Scholar] [CrossRef]
- Gyongyosi, M.; Wojakowski, W.; Lemarchand, P.; Lunde, K.; Tendera, M.; Bartunek, J.; Marban, E.; Assmus, B.; Henry, T.D.; Traverse, J.H.; et al. Meta-Analysis of Cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ. Res. 2015, 116, 1346–1360. [Google Scholar] [CrossRef] [PubMed]
- Gyongyosi, M.; Haller, P.M.; Blake, D.J.; Martin Rendon, E. Meta-Analysis of Cell Therapy Studies in Heart Failure and Acute Myocardial Infarction. Circ. Res. 2018, 123, 301–308. [Google Scholar] [CrossRef]
- Przyklenk, K.; Bauer, B.; Ovize, M.; Kloner, R.A.; Whittaker, P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993, 87, 893–899. [Google Scholar] [CrossRef]
- Bromage, D.I.; Pickard, J.M.; Rossello, X.; Ziff, O.J.; Burke, N.; Yellon, D.M.; Davidson, S.M. Remote ischaemic conditioning reduces infarct size in animal in vivo models of ischaemia-reperfusion injury: A systematic review and meta-analysis. Cardiovasc. Res. 2017, 113, 288–297. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. 2016, 13, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Haller, P.M.; Vargas, K.G.; Haller, M.C.; Piackova, E.; Wojta, J.; Gyongyosi, M.; Gersh, B.J.; Kiss, A.; Podesser, B.K.; Huber, K. Remote ischaemic conditioning for myocardial infarction or elective PCI: Systematic review and meta-analyses of randomised trials. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 82–92. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Kharbanda, R.K.; Møller, U.K.; Ramlall, M.; Aarøe, J.; Butler, R.; Bulluck, H.; Clayton, T.; Dana, A.; Dodd, M.; et al. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): A single-blind randomised controlled trial. Lancet 2019, 394, 1415–1424. [Google Scholar] [CrossRef]
- Jeanneteau, J.; Hibert, P.; Martinez, M.C.; Tual-Chalot, S.; Tamareille, S.; Furber, A.; Andriantsitohaina, R.; Prunier, F. Microparticle release in remote ischemic conditioning mechanism. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, 871–877. [Google Scholar] [CrossRef][Green Version]
- Giricz, Z.; Varga, Z.V.; Baranyai, T.; Sipos, P.; Paloczi, K.; Kittel, A.; Buzas, E.I.; Ferdinandy, P. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J. Mol. Cell. Cardiol. 2014, 68, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Vicencio, J.M.; Yellon, D.M.; Sivaraman, V.; Das, D.; Boi-Doku, C.; Arjun, S.; Zheng, Y.; Riquelme, J.A.; Kearney, J.; Sharma, V.; et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Sluijter, J.P.G.; Davidson, S.M.; Boulanger, C.M.; Iren Buzas, E.; de Kleijn, D.P.V.; Engel, F.B.; Giricz, Z.; Hausenloy, D.J.; Kishore, R.; Lecour, S.; et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2018, 114, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Karakas, M.; Schulte, C.; Appelbaum, S.; Ojeda, F.; Lackner, K.J.; Munzel, T.; Schnabel, R.B.; Blankenberg, S.; Zeller, T. Circulating microRNAs strongly predict cardiovascular death in patients with coronary artery disease-results from the large AtheroGene study. Eur. Heart J. 2017, 38, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Navickas, R.; Gal, D.; Laucevicius, A.; Taparauskaite, A.; Zdanyte, M.; Holvoet, P. Identifying circulating microRNAs as biomarkers of cardiovascular disease: A systematic review. Cardiovasc. Res. 2016, 111, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Diehl, P.; Fricke, A.; Sander, L.; Stamm, J.; Bassler, N.; Htun, N.; Ziemann, M.; Helbing, T.; El-Osta, A.; Jowett, J.B.; et al. Microparticles: Major transport vehicles for distinct microRNAs in circulation. Cardiovasc. Res. 2012, 93, 633–644. [Google Scholar] [CrossRef]
- Boulanger, C.M.; Loyer, X.; Rautou, P.E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed]
- Wisgrill, L.; Lamm, C.; Hartmann, J.; Preissing, F.; Dragosits, K.; Bee, A.; Hell, L.; Thaler, J.; Ay, C.; Pabinger, I.; et al. Peripheral blood microvesicles secretion is influenced by storage time, temperature, and anticoagulants. Cytom. A 2016, 89, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Haller, P.M.; Stojkovic, S.; Piackova, E.; Andric, T.; Wisgrill, L.; Spittler, A.; Wojta, J.; Huber, K.; Jäger, B. The association of P2Y12 inhibitors with pro-coagulatory extracellular vesicles and microRNAs in stable coronary artery disease. Platelets 2020, 18, 497–504. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundatin for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.R-project.org/ (accessed on 16 July 2020).
- Jung, C.; Sorensson, P.; Saleh, N.; Arheden, H.; Ryden, L.; Pernow, J. Circulating endothelial and platelet derived microparticles reflect the size of myocardium at risk in patients with ST-elevation myocardial infarction. Atherosclerosis 2012, 221, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Min, P.K.; Kim, J.Y.; Chung, K.H.; Lee, B.K.; Cho, M.; Lee, D.L.; Hong, S.Y.; Choi, E.Y.; Yoon, Y.W.; Hong, B.K.; et al. Local increase in microparticles from the aspirate of culprit coronary arteries in patients with ST-segment elevation myocardial infarction. Atherosclerosis 2013, 227, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Montoro-Garcia, S.; Shantsila, E.; Tapp, L.D.; Lopez-Cuenca, A.; Romero, A.I.; Hernandez-Romero, D.; Orenes-Pinero, E.; Manzano-Fernandez, S.; Valdes, M.; Marin, F.; et al. Small-size circulating microparticles in acute coronary syndromes: Relevance to fibrinolytic status, reparative markers and outcomes. Atherosclerosis 2013, 227, 313–322. [Google Scholar] [CrossRef]
- Abbas, M.; Jesel, L.; Auger, C.; Amoura, L.; Messas, N.; Manin, G.; Rumig, C.; Leon-Gonzalez, A.J.; Ribeiro, T.P.; Silva, G.C.; et al. Endothelial Microparticles From Acute Coronary Syndrome Patients Induce Premature Coronary Artery Endothelial Cell Aging and Thrombogenicity: Role of the Ang II/AT1 Receptor/NADPH Oxidase-Mediated Activation of MAPKs and PI3-Kinase Pathways. Circulation 2017, 135, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Laake, K.; Myhre, P.; Bratseth, V.; Arnesen, H.; Solheim, S.; Badimon, L.; Seljeflot, I. Platelet-, monocyte-derived and tissue factor-carrying circulating microparticles are related to acute myocardial infarction severity. PLoS ONE 2017, 12, e0172558. [Google Scholar] [CrossRef] [PubMed]
- Westman, P.C.; Lipinski, M.J.; Luger, D.; Waksman, R.; Bonow, R.O.; Wu, E.; Epstein, S.E. Inflammation as a Driver of Adverse Left Ventricular Remodeling After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 67, 2050–2060. [Google Scholar] [CrossRef]
- Loyer, X.; Zlatanova, I.; Devue, C.; Yin, M.; Howangyin, K.Y.; Klaihmon, P.; Guerin, C.L.; Kheloufi, M.; Vilar, J.; Zannis, K.; et al. Intra-Cardiac Release of Extracellular Vesicles Shapes Inflammation Following Myocardial Infarction. Circ. Res. 2018, 123, 100–106. [Google Scholar] [CrossRef]
- Suades, R.; Padro, T.; Crespo, J.; Ramaiola, I.; Martin-Yuste, V.; Sabate, M.; Sans-Rosello, J.; Sionis, A.; Badimon, L. Circulating microparticle signature in coronary and peripheral blood of ST elevation myocardial infarction patients in relation to pain-to-PCI elapsed time. Int. J. Cardiol. 2016, 202, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.B.; Ci, H.B.; Li, Y.; Cheng, T.P.; Liu, D.H.; Wang, Y.S.; Xu, J.; Yuan, H.X.; Li, H.M.; Chen, J.; et al. Endothelial microparticles are increased in congenital heart diseases and contribute to endothelial dysfunction. J. Transl. Med. 2017, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Botker, H.E. Why did remote ischaemic conditioning not improve clinical outcomes in acute myocardial infarction in the CONDI-2/ERIC-PPCI trial? Cardiovasc. Res. 2019, 115, 161–163. [Google Scholar] [CrossRef]
- Pickard, J.M.; Davidson, S.M.; Hausenloy, D.J.; Yellon, D.M. Co-dependence of the neural and humoral pathways in the mechanism of remote ischemic conditioning. Basic. Res. Cardiol. 2016, 111, 50. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Yellon, D.M. Exosomes and cardioprotection—A critical analysis. Mol. Asp. Med. 2018, 60, 104–114. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Arraud, N.; Gounou, C.; Turpin, D.; Brisson, A.R. Fluorescence triggering: A general strategy for enumerating and phenotyping extracellular vesicles by flow cytometry. Cytom. A 2016, 89, 184–195. [Google Scholar] [CrossRef]
- van der Pol, E.; Coumans, F.; Varga, Z.; Krumrey, M.; Nieuwland, R. Innovation in detection of microparticles and exosomes. J. Thromb. Haemost. 2013, 11 (Suppl. 1), 36–45. [Google Scholar] [CrossRef]
| Total (n = 32) | Control (n = 16) | RIC (n = 16) | p-Value | |
|---|---|---|---|---|
| Age, mean (SD) | 61.4 (13.7) | 59.6 (13.1) | 63.2 (14.5) | 0.463 |
| BMI, mean (SD) | 28.4 (5.1) | 27.3 (3.5) | 29.6 (6.2) | 0.194 |
| Hypertension, n (%) | 18 (56.2%) | 6 (37.5%) | 12 (75.0%) | 0.033 |
| Hyperlipoproteinemia, n (%) | 9 (28.1%) | 4 (25.0%) | 13 (56.2%) | 0.066 |
| Diabetes mellitus, n (%) | 1 (3.1%) | 1 (6.2%) | 0 (0.0%) | 0.310 |
| Smoking, n (%) | 0.195 | |||
| Previously | 13 (40.6%) | 4 (25.0%) | 9 (56.2%) | |
| Continued | 14 (43.8%) | 9 (56.2%) | 5 (31.2%) | |
| History of coronary artery disease, n (%) | 2 (6.2%) | 0 (0.0%) | 2 (12.5%) | 0.144 |
| Ambulatory medication, n (%) | ||||
| Acetylsalicylic acid | 7 (21.9%) | 2 (12.5%) | 5 (31.2%) | 0.200 |
| ACE/ARB | 15 (48.4%) | 4 (26.7%) | 11 (68.8%) | 0.019 |
| Statin | 2 (6.2%) | 0 (0.0%) | 2 (12.5%) | 0.144 |
| Beta blocker | 7 (22.6%) | 1 (6.7%) | 6 (37.5%) | 0.040 |
| Culprit lesion, n (%) | 0.686 | |||
| Left main | 1 (3.1%) | 1 (6.2%) | 0 (0.0%) | |
| Left anterior descending artery | 17 (53.1%) | 8 (50.0%) | 9 (56.2%) | |
| Right coronary artery | 11 (34.4%) | 6 (37.5%) | 5 (31.2%) | |
| Circumflex artery | 3 (9.4%) | 1 (6.2%) | 2 (12.5%) | |
| P2Y12 Inhibitor, n (%) | 0.388 | |||
| Prasugrel | 23 (71.9%) | 13 (81.2%) | 10 (62.5%) | |
| Ticagrelor | 8 (25.0%) | 3 (18.8%) | 5 (31.2%) | |
| Clopidogrel | 1 (3.1%) | 0 (0.0%) | 1 (6.2%) | |
| PCI with stenting, n (%) | 31 (96.9%) | 16 (100.0%) | 15 (93.8%) | 0.31 |
| Initial TIMI flow 0/I, n (%) | 23 (74.2%) | 11 (68.8%) | 12 (80.0%) | 0.712 |
| Final TIMI flow III, n (%) | 32 (100%) | 16 (100%) | 16 (100%) | 1.0 |
| Peak cardiac troponin, median (IQR) | 75 (34; 160) | 46,6 (26; 142) | 99 (59; 183) | 0.214 |
| Peak creatine kinase, median (IQR) | 1865 (1022; 3400) | 1465 (563; 2557) | 2678 (1503; 3729) | 0.152 |
| Before | After | p-Value | |
|---|---|---|---|
| PS+ EVs | 1227.5 (512.2) | 1380.0 (625.6) | 0.73 |
| Platelet EVs | 2805.0 (2105.7) | 3305.0 (1757.3) | 0.34 |
| PS+ platelet EVs | 1115.0 (864.5) | 1190.0 (614.6) | 0.87 |
| Endothelial EVs | 1075.0 (846.5) | 895.0 (430.6) | 0.42 |
| PS+ endothelial EVs | 105.0 (165.3) | 35.0 (41.8) | 0.29 |
| Leukocytes EVs | 1285.0 (957.3) | 905.0 (575.4) | 0.14 |
| PS+ leukocyte EVs | 230.0 (221.8) | 260.0 (85.9) | 0.76 |
| Monocyte EVs | 1150.0 (1262.1) | 1060.0 (651.6) | 0.88 |
| PS+ Monocyte EVs | 225.0 (211.4) | 205.0 (157.5) | 0.87 |
| Granulocyte EVs | 315.0 (255.3) | 485.0 (351.6) | 0.19 |
| PS+ granulocyte EVs | 0.0 (0.0) | 5.0 (11.2) | 0.37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haller, P.M.; Jäger, B.; Piackova, E.; Sztulman, L.; Wegberger, C.; Wojta, J.; Gyöngyösi, M.; Kiss, A.; Podesser, B.K.; Spittler, A.; et al. Changes in Circulating Extracellular Vesicles in Patients with ST-Elevation Myocardial Infarction and Potential Effects of Remote Ischemic Conditioning—A Randomized Controlled Trial. Biomedicines 2020, 8, 218. https://doi.org/10.3390/biomedicines8070218
Haller PM, Jäger B, Piackova E, Sztulman L, Wegberger C, Wojta J, Gyöngyösi M, Kiss A, Podesser BK, Spittler A, et al. Changes in Circulating Extracellular Vesicles in Patients with ST-Elevation Myocardial Infarction and Potential Effects of Remote Ischemic Conditioning—A Randomized Controlled Trial. Biomedicines. 2020; 8(7):218. https://doi.org/10.3390/biomedicines8070218
Chicago/Turabian StyleHaller, Paul M., Bernhard Jäger, Edita Piackova, Larissa Sztulman, Claudia Wegberger, Johann Wojta, Mariann Gyöngyösi, Attila Kiss, Bruno K. Podesser, Andreas Spittler, and et al. 2020. "Changes in Circulating Extracellular Vesicles in Patients with ST-Elevation Myocardial Infarction and Potential Effects of Remote Ischemic Conditioning—A Randomized Controlled Trial" Biomedicines 8, no. 7: 218. https://doi.org/10.3390/biomedicines8070218
APA StyleHaller, P. M., Jäger, B., Piackova, E., Sztulman, L., Wegberger, C., Wojta, J., Gyöngyösi, M., Kiss, A., Podesser, B. K., Spittler, A., & Huber, K. (2020). Changes in Circulating Extracellular Vesicles in Patients with ST-Elevation Myocardial Infarction and Potential Effects of Remote Ischemic Conditioning—A Randomized Controlled Trial. Biomedicines, 8(7), 218. https://doi.org/10.3390/biomedicines8070218










