Abstract
Background. A major concern in the care of common variable immunodeficiency (CVID) patients is the persistence of subclinical or recurrent respiratory tract infections (RRTI) despite adequate trough IgG levels, which impacts the quality of life (QoL) and morbidity. Therefore, the development of new approaches to prevent and treat infection, especially RRTI, is necessary. Objectives. We conducted a clinical observational study from May, 2016 to December, 2017 in 20 CVID patients; ten of these patients had a history of RRTI and received the polybacterial preparation MV130, a trained immunity-based vaccine (TIbV) to assess its impact on their QoL and prognosis. Methods. Subjects with RRTI received MV130 for 3 months and were followed up to 12 months after initiation of the treatment. The primary endpoint was a reduction in RRTI at the end of the study. We analyzed the pharmacoeconomic impact on the RRTI group before and after immunotherapy by estimating the direct and indirect costs, and assessed CVID-QoL and cytokine profile. Specific antibody responses to the bacteria contained in MV130 were measured. Results. The RRTI-group treated with TIbV MV130 showed a significant decrease in infection rate (p = 0.006) throughout the 12 months after initiation of the treatment. A decrease in antibiotic use and unscheduled outpatient visits was observed (p = 0.005 and p = 0.002, respectively). Significant increases in anti-pneumococcus and anti-MV130 IgA antibodies (p = 0.039 both) were detected after 12 months of MV130. Regarding the CVID QoL questionnaire, an overall decrease in the score by more than 50% was observed (p < 0.05) which demonstrated that patients experienced an improvement in their QoL. The pharmacoeconomic analysis showed that the real annual direct costs decreased up to 4 times per patient with the prophylactic intervention (p = 0.005). Conclusion. The sublingual administration of the TIbV MV130 significantly reduced the rate of respiratory infections, antibiotic use and unscheduled visits, while increasing specific IgA responses in CVID patients. Additionally, the CVID population felt that their QoL was improved, and a decrease in expenses derived from health care was predicted.
1. Introduction
Common variable immunodeficiency (CVID) is one of the most common symptomatic and heterogenous primary immune disorders (PID) [1,2,3,4]. The hallmark of the disease is a defect in specific antibody production and hypogammaglobulinemia of at least two isotypes (IgG and IgA/IgM). The pathogenesis of CVID remains unknown for most patients, with 15% of cases pointing to T-B co-stimulation and BCR-dependent gene lesions [2,5,6,7]. Over 90% of CVID patients suffer from increased susceptibility to infections by different pathogens that affect several systems [1,8,9]. Additionally, the prevalence of bacterial infections might be falsely decreased due to antibiotic prophylaxis in patients with specific conditions [10]. Recurrent respiratory tract infections (RRTI) are very frequent in CVID [1,11]. Recurring infections and subclinical infection are common despite adequate immunoglobulin replacement therapy (IgRT) and can predispose patients to develop chronic lung diseases, like bronchiectasis, interstitial lung disease, granulomatous inflammation, and obstructive/restrictive lung disease, which are a leading cause of both morbidity and mortality in CVID patients [12,13,14,15,16].
Antibiotic prophylaxis in patients with CVID has been shown to improve lung function, reduce the risk of infection-related hospitalization and improve patients’ QoL in a recent double-blind placebo-controlled trial [17,18,19]. However, the risk of bacterial colonization, development of complicated pneumonia, invasive infections or infections by atypical microorganisms was not fully studied [17,18,20]. Antimicrobial resistance (AMR) represents a significant health concern worldwide. It is unclear whether PID patients are more susceptible to developing AMR [17,20,21], even though the use of long-term antibiotic prophylaxis is common in the management of PID patients. Indeed, prolonged administration of antibiotics can result in AMR to Gram positive and Gram negative pathogens, such as Group B streptococcus or Klebsiella pneumoniae, respectively [21]. In addition, antibiotics have limitations against several infections, mainly due to viruses, are detrimental to the already altered microbiota in PID, and are not free of adverse effects in the long-term. In this setting, complementary or alternative strategies for preventing infections in this highly susceptible population is a priority.
Immunotherapy with trained-immunity based vaccines (TIbV) are meant to enhance innate cross-protection to heterologous pathogens and to induce more efficient adaptive responses against the specific pathogens contained in the vaccine [22,23,24]. Their use in PID has not been previously reported. Sublingual administration of fully inactivated bacteria has been proposed as a safe and clinically beneficial strategy of preventing upper and lower respiratory tract infections [23,25,26], although it requires continued, daily administration. MV130 is a TIbV formulated with a combination of whole cell inactivated Gram-positive (90%) and Gram-negative bacteria (10%) that has previously been demonstrated to be a safe and effective immunotherapy against RRTI and induces long-lasting protection [23,25,26]. Bacterial preparations have been shown to elicit systemic and mucosal antigen-specific humoral and cell-mediated immunity [22,27,28,29,30]. Sublingual immunization with MV130 has been shown to up-regulate Th1, Th17 and IL-10 antigen-specific, as well as non-specific memory CD4+ T cell responses in vitro and ex vivo [25,26]. Concomitant up-regulation of IL-10 by MV130 may add immunomodulatory potential in the setting of autoimmune or inflammatory imbalance in CVID [25]. Finally, a recent clinical trial in children with recurrent wheezing, a disease mostly triggered by respiratory viruses, demonstrated that MV130 significantly decreased wheezing attacks in children. MV130 induced trained immunity and elicited an antiviral effect in experimental models with vaccinia and flu viruses [31,32].
We used a proof-of-concept study to determine the beneficial effects of a novel adjuvant therapeutic strategy with a TIbV in CVID patients suffering RRTI despite adequate trough IgG levels and antibiotic prophylaxis.
2. Methods
The present study is a single-institution retrospective observational study conducted at the Clinical Immunology Department of the Hospital Clínico San Carlos, Madrid, Spain, from May 2016 to December 2017.
Approval for the study was obtained from the Hospital Clinico San Carlos institutional research Ethics Committee (16/511-E). Written informed consent was obtained from all patients for inclusion in the study protocol.
2.1. Population
Data from a cohort of 20 patients with a definite diagnosis of CVID, aged between 18 to 65 years, and serum IgG above 600 mg/dL on IVIg therapy were recorded. The exclusion criteria were: patients with lymphoproliferative disorders, asthma treated with inhaled or systemic corticosteroids, inability or unwillingness to provide written informed consent or significant medical or psychiatric illness that, in the opinion of the treating clinician, precluded participation.
Subsequently, the cohort was subdivided into two groups according to whether or not the patient presented with RRTI, which was defined by ≥3 episodes of upper (rhinitis, sinusitis, otitis, pharyngitis, tonsillitis) or lower respiratory infection (bronchitis), ≥2 episodes of severe respiratory infection (pneumonia), or the need for antibiotics for almost two months/year in the previous 12 months as described elsewhere [9,10]. This group was referred to as the RRTI-Group. According to our clinical routine protocols at the time, the RRTI-group (n = 10) had received a TIbV MV130 (Bactek®, Inmunotek, Madrid, Spain) daily for 3 months. According to the drug data sheet, the MV130 sublingual immunotherapy was indicated for recurrent infections. MV130 is composed of whole complete inactivated bacteria, as follows: 90% Gram-positive bacteria such as Staphylococcus spp., Streptococcus pneumoniae, and 10% Gram-negative bacteria such as Klebsiella pneumoniae, Moraxella catarrhalis, Haemophilus influenzae.
As part of our regular medical follow-up, RRTI-group was evaluated before and at 12 months of sublingual prophylaxis, with a complete medical history, physical exam and immunological profile. The sera were stored at −80 ºC. The remaining patients (n = 10) that did not fulfil the RRTI-Group criteria were designated as the Non-RRTI-Group. This group was considered as a control group for the comparison of the immunological CVID profile (Figure 1).
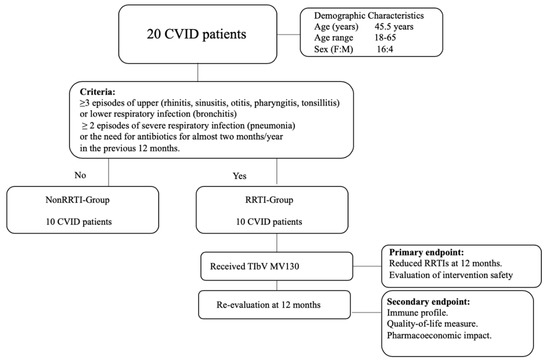
Figure 1.
Flow chart of the classification of common variable immunodeficiency (CVID) patients according to clinical criteria.
2.2. Immunological Assessment
We measured the presence of specific serum IgA and IgG against MV130 and S. pneumoniae (the main Gram- positive component) in the RRTI group by ELISA. The samples were stored frozen at −80 °C before processing. Briefly, 96-well non-tissue culture-treated plates were pretreated with 100 µL of poly-L-lysine (stock at 0.01%, 1:1000 dilution) (Sigma-Aldrich) for 1 h under UV light and coated with the appropriate whole-cell heat-inactivated bacteria or polybacterial mixture (300 nephelometric turbidity units (NTU), ~109 bacteria) overnight at 4 °C, and subsequently incubated with human sera for 2 h at room temperature. IgA and IgG antibodies were detected using the following reagents: biotin rat anti-human IgA or IgG (both from Sigma-Aldrich) and streptavidin horseradish peroxidase (HRP) (Sigma-Aldrich). Peroxidase activity was revealed by the addition of o-phenylenediamine dihydrochloride (Sigma-Aldrich) and the reaction was stopped with HCl 1N. Plates were read on an ELISA reader at 490 nm (Triturus Elisa, Grifols).
Likewise, an extensive panel of Th1/Th2/Th17/Treg cytokine profiles, growth factor, and chemokines was carried out (Pro human cytokine 27-plex kit, Bio-Plex™ from BioRad Inc) at baseline and after treatment (12 months) in the RRTI group and was compared with the Non-RRTI-Group in sera samples, following the manufacturer’s instructions. The samples were stored frozen at −80 °C before analysis.
2.3. Endpoints
The primary endpoint of the study was to evaluate the percentage of CVID patients on MV130 who had reduced RRTI in terms of frequency of infections, as well as safety issues. The secondary endpoints were the reduction in the number of antibiotic cycles and in the unscheduled outpatient visits due to infections.
Each CVID subject in the RRTI-group was given the CVID-QoL assessment, before and 12 months after treatment. This was measured by the Health-Related Quality of Life questionnaire for adults with common variable immunodeficiency proposed by Quinti et al. [33]. The questionnaire comprises 32 items with responses given on a 4-point scale, with 0 = “never” and 4 = “always,” with higher values generally indicating increasing disability (range, 0 to 160 points).
We evaluated the pharmacoeconomic impact of this prophylactic approach by comparing the characteristics of the RRTI-group before and after vaccination measured by direct and indirect costs. The direct costs estimation was comprised of the medical direct costs and non-medical direct costs. The medical direct costs included those costs attributable to health care visits for the treatment of the disease including primary and secondary care consultations, prescriptions, hospital-based procedures, nature and length of inpatient stay, surgery, and over-the-counter purchases by patients [34]. Non-medical direct costs included travel expenses, home care, etc. The information was collected from the CRF (patient self-reported information) and completed with medical records. These costs were assessed using the official rates most recently published for the public health service for a follow-up period of 12 months. The direct costs were calculated according to the following formula: Direct Costs = Medical direct costs + non-medical direct costs. The evaluation of indirect costs included those derived from work suspension related to the disease. For working patients, an average hourly wage (€14.04) was applied to all absentee time, based on salary cost per productive hour, type of working day, and activity sectors in the National Statistics Institute data [35].
2.4. Statistical Analysis
The integration of all obtained data (from laboratory to clinical data) was done by using bivariate correlations and multivariate analysis in order to detect associations between different variables. Patients were stratified according to the obtained results (different clinical and immunological responses). Normal distribution and testing of outliers were analyzed by means of Shapiro–Wilk and Tukey’s range tests, respectively. Data were analyzed by Chi-squared, Fisher’s exact, Pearson, and Spearman correlation coefficient, Mann Whitney U-tests, paired Student’s t test and Wilcoxon signed-rank test, and one-sample t test using SPSS (Chicago, Illinois) and GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). A p-value below 0.05 was considered as statistically significant.
3. Results
3.1. MV130 Significantly Decreased Respiratory Infection Rate, Antibiotic Use and Unscheduled Outpatients’ Visits in CVID Patients
All patients suffering from RRTI completed the daily treatment with MV130 for 3 months and were followed up for 12 months. The RRTI-Group treated with TIbV MV130 significantly decreased the median (range min-max) infection rate from 3.00 (1–7) to 0.00 (0–2) (p = 0.006) post-treatment. All of the patients had a reduced infection rate in the average follow-up time of 12 months (Figure 2). Thirty percent maintained upper respiratory tract infections. Antibiotic consumption significantly decreased from 5.00 cycles (3–7) to 1.00 (0–1) cycle (p = 0.005); the number of infectious-related unscheduled outpatient visits significantly declined from 5.00 (2–6) to 1.00 (0–3) (p = 0.002); and work absenteeism decreased from 2.00 (0–3) to 0.00 (0–2) days (p = 0.005) in the 12 months after initiation of MV130 administration (Figure 3, Supplementary Materials, Table S1). None of the patients reported any side effects regarding MV130, either local at the site of administration or systemic and no adverse effects were noted during the 12-month observational period.
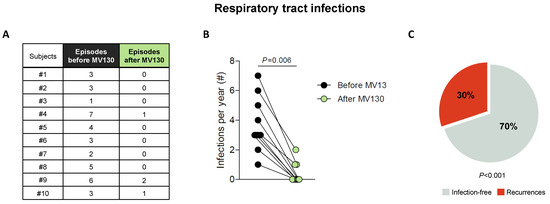
Figure 2.
MV130 significantly reduces the incidence of respiratory infections (A–C). (A,B) Number of respiratory tract infectious episodes scored 1 year prior to immunization (black) and in the 12 months after the initiation of immunotherapy with MV130 (green). (C) Percentage of subjects that remained free of infection (grey) or suffered recurrences (red) in the 12 months following MV130 administration. Data from 10 subjects are shown. (B) Lines link paired values. Normal distribution was evaluated using the Shapiro–Wilk test, p value was calculated using Wilcoxon signed-rank test. (C) p value was calculated using Fischer’s exact test, compared with rates prior to MV130 initiation (100% of subjects suffering infections).
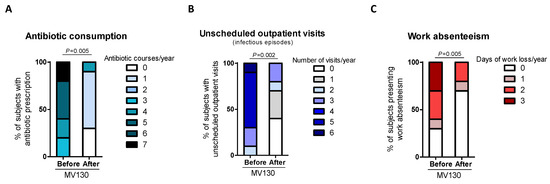
Figure 3.
Prophylaxis with MV130 significantly decreases the rate of healthcare resources consumption and work absenteeism. (A–C) Antibiotic consumption (A), visits to emergency unit (B) and work absenteeism (C) during the year before and after the initiation of MV130 treatment. Bars show the relative number of antibiotic courses (A), emergency unit visits (B) or days of work lost (C) in the total of subjects recorded. Data from 10 subjects are shown. Normal distribution was evaluated using the Shapiro–Wilk test. p values were calculated using Wilcoxon signed-rank test.
3.2. Immune Profile
As a preliminary approach, to assess whether sublingual immunotherapy with MV130 triggers a systemic humoral response as well, blood samples were assayed for specific IgG and IgA at baseline and after 12-months of initiating MV130. A significant increase in both anti-S. pneumoniae and anti-MV130 IgA (p = 0.039) but not IgG (p = 0.094) serum antibodies following MV130 immunization were observed (Figure 4). As S. pneumoniae represents the main Gram-positive component in MV130, these results point to the induction of a humoral specific response upon bacterial immunotherapy.
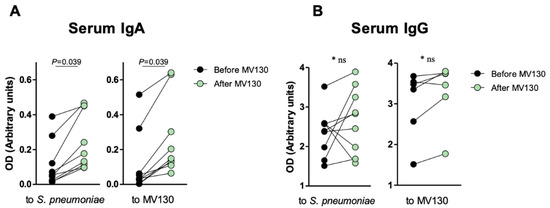
Figure 4.
Specific anti-MV130 IgA and IgG antibodies. Prophylaxis with MV130 increases serum IgA antibody production. (A,B) Serum IgA (A) and IgG (B) antibodies against S. pneumoniae (left panels) or the bacterial mixture (right panels), collected from subjects before and after 12 months following MV130 immunotherapy analyzed by ELISA. Data from 6–10 individuals are shown. Lines show paired values. Normal distribution was evaluated using the Shapiro–Wilk test. p values were calculated using Paired Student’s t-test or Wilcoxon signed-rank test.
We then analyzed the baseline expression of an extensive panel of serum cytokines, chemokines and growth factors in both cohorts (RRTI-group and Non-RRTI group). The RRTI-group showed higher baseline expression of IL-4, IL-17, IFN-γ, MCP-1 (MCAF) and TNF-α, with respect to Non-RRTI group, without statistical differences. Interestingly, when comparing the RRTI-group at baseline with respect to 12 months after MV130, a significantly lower concentration of serum IL-17 (49.49; 0–90.04 vs. 26.85; 0–70.27, p < 0.05) with a significant fold reduction in serum IL-17 (0.649, p < 0.05); and IL-4 (23.01; 7.03–27.44 vs. 16.92; 5.13–26.35, p < 0.05), and fold reduction of IL-4 (0.780, p < 0.05) were observed. This decrease in serum IL-17 restored cytokine levels to those found in non-RRTI individuals (data not shown). The cytokine expression measured pre- and post-immunization in the RRTI-group can be seen in Figure 5.
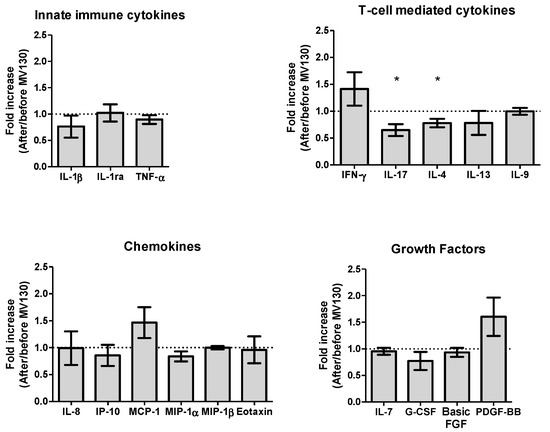
Figure 5.
Prophylaxis with MV130 modulates serum cytokine, chemokine and growth factor secretion pattern. Cytokines (upper panels), chemokines (left bottom panel) and growth factors (right bottom panel) secreted in serum one year following MV130 administration determined by Luminex technique. Fold induction relative to basal level (before MV130 treatment) is shown. Undetectable values were found for IL-2, IL-5, IL-10, IL-12(p70), IL-15, GM-CSF, RANTES and VEGF. Data from 9–10 subjects are shown as mean ± SEM of fold increase. Outliers were identified by means of Tukey’s range test on represented values. Normal distribution was evaluated using the Shapiro–Wilk test. p values were calculated using one-sample t-test with a theoretical value of 1 (no fold induction). * p < 0.05.
3.3. Perceived QoL Improved in CVID Patients after MV130
We evaluated the self-reported quality of life of each RRTI-group patient at baseline and 12 months after receiving prophylaxis with MV130 based on the adapted CVID-QoL questionnaire proposed by Quinti et al. [33]. An overall significant decrease in score was observed (the median (range) of the global score decreased from 47.0 (27–86) to 39.5 (13–86), p < 0.05), reflecting an improvement in QoL for patients (Supplementary Materials, Figure S1). The median percentage of pre to post MV130 CVID-QoL improvement was 16.87% (range, 0% to 51.85%). Only one item, “Run out of medications” showed an increase in perception post-treatment while the item that decreased the most was “Difficulty in usual activities”.
3.4. Pharmacoeconomic Impact
The economic impact of this prophylactic approach (pre/post-MV130 immunization) was evaluated in terms of direct costs, which were comprised of medical and non-medical direct costs. The indirect costs evaluation included those derived from work suspension related to the disease (Supplementary Materials, Table S2).
Regarding the direct costs, the total average cost for ambulatory care for patients with RRTI is 1656 €/patient, according to the official report of the Spanish Ministry of Health, Consumer Affairs and Social Welfare in 2017 [36]. This amount can increase to 3962 €/patient when hospital admission is required. The estimated real median annual direct cost is approximately 18,600 €/patient in the RRTI group (Table 1). Taking into account the cost of the intervention, our results showed that the real annual median direct cost decreases up to 4-fold per patient with the prophylaxis intervention of TIbV MV130 (from €18,600 to €4500, p < 0.005).

Table 1.
Costs of the most frequent conditions affecting CVID patients with RRTI—comparison of the year before and the year after the prophylaxis with TIbV (Euros). (#: number)
With regard to indirect costs, 14 euros per hour was defined as the cost of absenteeism due to illness, as well as presenteeism due to the reduction in the percentage of productivity [36]. A median of 2 days of absenteeism (the daily workday is 8 h), equivalent to €28, added up to €308 for a cumulative 22 h of labor absenteeism, giving a total of 336 €/patient of annual indirect costs in the pre-immunization RRTI group. These costs are only imperceptibly different (median work absenteeism post-immunization was 0 days) to those calculated after the prophylaxis intervention of MV130 (p < 0.005).
4. Discussion
Subclinical and recurrent infections remain a considerable healthcare concern in a subgroup of CVID patients, and they affect the risk of complications and hence the prognosis [10,37]. Bronchiectasis is a consequence of recurrent lower respiratory tract infections and the most common chronic pulmonary complication at CVID patients. It is also the most important cause of morbidity in these patients [11,38,39]. The lung damage could contribute to the high recurrence of infections in a specific group of CVID patients. To our knowledge, this is the first study that shows the beneficial effects of an adjuvant TIbV on the control of recurrent upper respiratory tract infections in CVID patients.
IgRT has been demonstrated to be the best intervention to change the course of CVID by preventing infections [40]. Still, despite adequate serum trough IgG levels, some CVID patients on IVIg/SCIg replacement suffer from subclinical infections and RRTI that require constant or frequent antibiotics and/or they may develop bronchiectasis, which negatively impacts their vital prognosis [40,41,42]. Therefore, new adjuvant prophylactic approaches to infection, especially in this CVID group are greatly needed.
Most pathogens affecting CVID patients enter the body by the oropharyngeal mucosa. In this proof-of concept observational study, MV130 significantly decreased the rate of respiratory tract infections, the frequency of antibiotic consumption and the frequency of unscheduled outpatient visits. Moreover, specific IgA responses were observed despite the intrinsic antibody defect in CVID. IgA secretion has been strongly associated with decreased rates of respiratory infections, and thus overall mortality risk [43,44]. Significantly higher specific anti-pneumococcal and anti-MV130 IgA antibodies is highly relevant in the context of CVID, especially because these patients are usually low antibody producers. Specific, significant differences in serum IgA may be explained by the mucosal route of administration of the treatment. MV130 has been shown to increase mucosal IgA secretion [45]. IgA synthesis occurs in mucosal-associated lymphoid follicles through B cell hypermutation and class switch recombination, and is secreted by plasma cells. Secretory IgA (sIgA) is exclusively present on mucosal surfaces and consists of dimeric IgA linked via the J-chain to the secretory components [46,47]. Circulating sIgA constitutes a natural key systemic anti-inflammatory factor due to its ability to interact with DCs through receptors such as ICAM-3, DC-SIGN, favoring the generation of Ag-specific regulatory CD4+ T cells [46,48], which further suggests that IgA has a homeostatic role between commensal microorganisms and pathogens.
Even though the main strength of MV130 as a TIbV may rely on the induction of broad-spectrum, non-specific protection, the data support the hypothesis that specific adaptive responses are also generated that may also correlate with the clinical benefits that result from MV130 administration.
The MV130 polybacterial mixture is prepared by using whole complete inactivated bacteria (90% Gram positive and 10% Gram negative bacteria). This formula was initially standardized according to the ad hoc prevalence of isolated pathogens for the preparation of individualized vaccines for patients with recurrent respiratory tract infections. The Gram positive and Gram negative bacteria act in a synergistic and complementary way in the activation of innate immunity [25]. MV130 has been shown to activate trained immunity in vitro and ex vivo [26,32] by inducing epigenetic and metabolic changes. Regarding the route of administration, mucosal routes for vaccines against respiratory pathogens have been shown to be effective and safe compared to other routes [26,47,48,49,50,51], and they induce a greater immune response in the respiratory tract mucosa.
MV130 may enhance innate immunity at the entry portal of the pathogen. Several studies have highlighted the important role of mucosal preparations formulated with inactivated bacteria in controlling respiratory exacerbations in the setting of chronic obstructive pulmonary disease [52] and preliminary results in patients with RRTI without a defined PID [31,51]. This strategy might provide a potential solution to clinical problems not fully covered by other treatments.
Trained immunity is based on biological processes derived from innate immune memory associated with intracellular signals driving deep metabolic changes and epigenetic modifications that result in reprogramming in cells of innate immunity [53,54]. These effects occur synergistically where various metabolites and metabolic enzymes act as cofactors in the process of epigenetic modification such as transcriptional changes, activation/inhibition of histones, or modulation of cytokine expression [55]. Several therapeutic strategies have been designed to modulate trained immunity [23,24,25,26,56]. TIbVs have the potential to improve immune responses by protecting against secondary related or bystander infections, and reversing immunotolerance states as well as chronic inflammatory conditions [22,24,27,29,51].
The main goal of this proof-of-concept study is to generate preliminary data on the beneficial effects and potential risks of these polybacterial mucosal vaccines in order to support the design a larger randomized double-blind clinical trial. The present study has provided an initial evaluation of the safety of the intervention in the target population, which is a major clinical endpoint. The main weakness of this study is the observational retrospective design, which may result in missing variables not recorded in routine clinical practice. However, due to the promising results obtained, this study could help in the design of further studies.
MV130 induces the activation of TLR and NLR signaling pathways in human dendritic cells (DCs), which secrete cytokines such as TNF-α, IL-6 and IL-1β related to trained immunity. These DCs also possess the capacity to promote Th1 and Th17 responses through the RIPK2 and MyD88 signaling pathway under the control of IL-10. In addition, DCs have a large number of pattern recognition receptors that facilitate the synergistic action of the innate and adaptive immune systems [25].
Several studies have identified abnormalities in cytokine secretion in CVID patients, including decreased production of IFN-γ, IL-2, IL-5, IL-7, IL 4, IL-10 or IL-12 [57,58,59,60,61,62]. IL-10 is essential to avoid excessive deleterious responses, to enhance pathogen clearance, and to keep tissue homeostasis [6,63,64,65,66]. No significant changes in serum IL-1β or IL-10 with MV130 were observed. However, we consider that this may be because the cytokine measurement was performed 12-months after MV130 administration, and thus, is reflects the long-term regulation of the proinflammatory status due to the decrease in recurrent infections in this group of patients. The high baseline expression of IL-17 observed in our cohort has already been described in previous studies [58]. However, their clinical significance and the correlation with other biomarkers such as IFN-γ tend to be heterogeneous. Nevertheless, the correct way to observe the real behavior and significance of these results is to carry out in vitro activation of peripheral blood cells of our patients that is stimulated by various ligands, and measure the different expression of cytokines during and at the end of the treatment.
QoL is a multidimensional concept that encompasses several elements such as the physical, psychological and social well-being of individuals. The main point of QoL is to assess an individual’s perception of the impact of illness on his/her life at a similar level to that of the clinical factors in the disease’s prognosis [67]. RRTI has a critical effect on the QoL of CVID patients. The prophylaxis intervention of TIbV MV130 improved the global perception of QoL in more than 50% of the CVID patients (p < 0.005). “Difficulty in usual activities” was the component with the highest post treatment decrease in score. The only element that scored higher after the prophylactic intervention was “Run out of medications”. These findings further highlight the positive impact of the prophylaxis with a TIbV such as MV130 on the QoL of CVID patients.
CVID treatment without complications requires IgRT, which implies an annual cost of about 15,700 €/patient. In selected patients, treatment costs may be controlled by modifying the dosage of IgRT, changing the administration route to SCIg or changing the intervals between administrations [68]. The reduced frequency of infections among CVID patients after treatment with IVIg/SCIg translates into health care cost savings [69]. However, a subgroup of CVID patients is unable to improve their infection profile in spite of IVIg/SCIg treatment, consequently, the implementation of an effective alternative treatment can have a direct impact on the abovementioned health care costs of such patients.
Despite the limitations inherent to this observational small study, we show that the clinical benefits of intervention by TIbV are accompanied by healthcare cost savings of approximately 9000–14,000 €/patient per year in terms of hospital, healthcare and lost wages. Considering the high cumulative cost of the treatment of this disease, bacterial immune-stimulation could be an effective strategy to control subclinical infection and respiratory tract exacerbations, and to reduce costs in CVID patients.
5. Conclusions
There is a need to develop alternative or adjuvant strategies to prevent infections in selected CVID patients with subclinical and/or recurrent infections. The preliminary data in our small cohort of CVID patients with recurrent infections despite adequate trough IgG levels show that mucosal vaccines are a potentially useful strategy for preventing infections at the actual point of entry for most pathogens. The study shows enough promise to be repeated in a double-blind clinical trial. The prophylaxis with sublingual TIbV MV130 based on whole cell inactivated bacteria resulted in significant clinical benefits in terms of recurrence and severity of respiratory tract infections, with a concomitant improvement in QoL perception as well as a decrease in health care expenses.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9059/8/7/203/s1, Table S1. MV130 significantly reduces consumption of healthcare resources and work absenteeism. Antibiotic consumption (left columns), visits to emergency unit (middle columns) and work absenteeism (right columns) during the year before and after the initiation of MV130. Absolute numbers from 10 subjects are shown. Figure S1. Analysis of the CVID-QoL, before and after treatment with the prophylaxis intervention of TIbV MV130, using the CVID_QoL questionnaire proposed by Quinti et al. [33]. Table S2. Cost assessed, source of resources, source of costs per “unit” and cost calculation used for direct and indirect costs associated with recurrent respiratory infections.
Author Contributions
K.G.-H. and S.S.-R. designed the study, analyzed the data and wrote the manuscript. M.F.-A., J.O.-G. and R.P.d.D. contributed to the design of the methodology. P.S.-L. contributed to the writing of the manuscript and statistical analysis of data and figures. C.M.D.-R. contributed to the measurement of specific antibodies and writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Inmunotek Laboratories funded part of the laboratory kits necessary to carry out the research.
Acknowledgments
We sincerely thank all the patients that participated in the study.
Conflicts of Interest
P.S.-L. and C.M.D.-R. belong to the Inmunotek R&D research team.
Abbreviations
| CVID | Common variable immunodeficiency. |
| RRTI | Recurrent respiratory tract infections, defined by ≥3 episodes of upper (rhinitis, sinusitis, otitis, pharyngitis, tonsillitis) or lower respiratory infection (bronchitis), ≥2 episode of severe respiratory infection (pneumonia), or the need for antibiotics for almost two months/year in the previous 12 months [9,10]. |
| IVIg | Intravenous immunoglobulin. |
| SCIg | Subcutaneous immunoglobulin. |
| QoL | Quality of life. |
| IP-10 | Interferon gamma-induced protein 10. |
| VEGF | Vascular endothelial growth factor. |
| TIbV | Trained immunity-based vaccines. |
| DCs | Dendritic cells |
References
- Fried, A.J.; Bonilla, F.A. Pathogenesis, Diagnosis, and Management of Primary Antibody Deficiencies and Infections. Clin. Microbiol. Rev. 2009, 22, 396–414. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ramón, S.; Radigan, L.; Yu, J.E.; Bard, S.; Cunningham-Rundles, C. Memory B cells in common variable immunodeficiency: Clinical associations and sex differences. Clin. Immunol. 2008, 128, 314–321. [Google Scholar] [CrossRef]
- Cunningham-Rundles, C. The many faces of common variable immunodeficiency. Hematol. Am. Soc. Hematol. Educ. Program 2012, 2012, 301–305. [Google Scholar] [CrossRef]
- Jolles, S. The variable in common variable immunodeficiency: A disease of complex phenotypes. J. Allergy Clin. Immunol. Pract. 2013, 1, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Warnatz, K.; Denz, A.; Dräger, R.; Braun, M.; Groth, C.; Wolff-Vorbeck, G.; Eibel, H.; Schlesier, M.; Peter, H.H. Severe deficiency of switched memory B cells (CD27(+)IgM(-)IgD(-)) in subgroups of patients with common variable immunodeficiency: A new approach to classify a heterogeneous disease. Blood 2002, 99, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Arandi, N.; Mirshafiey, A.; Jeddi-Tehrani, M.; Abolhassani, H.; Sadeghi, B.; Mirminachi, B.; Shaghaghi, M.; Aghamohammadi, A. Evaluation of CD4+CD25+FOXP3+ regulatory T cells function in patients with common variable immunodeficiency. Cell. Immunol. 2013, 281, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Siachoque, H.; Satisteban, N.; Iglesias-Gamarra, A. T regulatory lymphocytes: Subpopulations, mechanism of action and importance in the control of autoimmunity. Rev. Colomb. Reumatol. 2011, 18, 203–220. [Google Scholar]
- Cunningham-Rundles, C.; Bodian, C. Common variable immunodeficiency: Clinical and immunological features of 248 patients. Clin. Immunol. 1999, 92, 34–48. [Google Scholar] [CrossRef]
- Ballow, M. Approach to the patient with recurrent infections. Clin. Rev. Allergy Immunol. 2008, 34, 129–140. [Google Scholar] [CrossRef]
- Kainulainen, L.; Vuorinen, T.; Rantakokko-Jalava, K.; Osterback, R.; Ruuskanen, O. Recurrent and persistent respiratory tract viral infections in patients with primary hypogammaglobulinemia. J. Allergy Clin. Immunol. 2010, 126, 120–126. [Google Scholar] [CrossRef]
- Bazregari, S.; Azizi, G.; Tavakol, M.; Asgardoon, M.H.; Kiaee, F.; Tavakolinia, N.; Valizadeh, A.; Abolhassani, H.; Aghamohammadi, A. Evaluation of infectious and non-infectious complications in patients with primary immunodeficiency. Cent. Eur. J. Immunol. 2017, 42, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Cunningham-Rundles, C. Common variable immune deficiency: Dissection of the variable. Immunol. Rev. 2019, 287, 145–161. [Google Scholar] [CrossRef]
- Chapel, H.; Lucas, M.; Lee, M.; Bjorkander, J.; Webster, D.; Grimbacher, B.; Fieschi, C.; Thon, V.; Abedi, M.R.; Hammarstrom, L. Common variable immunodeficiency disorders: Division into distinct clinical phenotypes. Blood 2008, 112, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Geha, R.S.; Notarangelo, L.D.; Casanova, J.-L.; Chapel, H.; Conley, M.E.; Fischer, A.; Hammarström, L.; Nonoyama, S.; Ochs, H.D.; Puck, J.; et al. The International Union of Immunological Societies (IUIS) Primary Immunodeficiency Diseases (PID) Classification Committee. J. Allergy Clin. Immunol. 2007, 120, 776–794. [Google Scholar] [CrossRef] [PubMed]
- Vivas-Rosales, I.J.; Hernández-Ojeda, M.; O’Farrill-Romanillos, P.M.; Herrera-Sánchez, D.A.; Maciel-Fierro, A.E.; Núñez-Enríquez, J.C. Bronchiectasis severity in adult patients with common variable immunodeficiency. Rev. Alerg. Mex. 2018, 65, 242–249. [Google Scholar] [CrossRef]
- Sherani, K.; Upadhyay, H.; Vakil, A.; Cervellione, K.; Thurm, C. Common Variable Immunodeficiency and Bronchiectasis: An Easily Missed Common Association. CHEST 2014, 145, 123A. [Google Scholar] [CrossRef]
- Milito, C.; Pulvirenti, F.; Cinetto, F.; Lougaris, V.; Soresina, A.; Pecoraro, A.; Vultaggio, A.; Carrabba, M.; Lassandro, G.; Plebani, A.; et al. Double-blind, placebo-controlled, randomized trial on low-dose azithromycin prophylaxis in patients with primary antibody deficiencies. J. Allergy Clin. Immunol. 2019, 144, 584–593.e7. [Google Scholar] [CrossRef]
- Kuruvilla, M.; de la Morena, M.T. Antibiotic prophylaxis in primary immune deficiency disorders. J. Allergy Clin. Immunol. Pract. 2013, 1, 573–582. [Google Scholar] [CrossRef]
- Sperlich, J.M.; Grimbacher, B.; Workman, S.; Haque, T.; Seneviratne, S.L.; Burns, S.O.; Reiser, V.; Vach, W.; Hurst, J.R.; Lowe, D.M. Respiratory Infections and Antibiotic Usage in Common Variable Immunodeficiency. J. Allergy Clin. Immunol. Pract. 2018, 6, 159–168.e3. [Google Scholar] [CrossRef]
- Mohammadinejad, P.; Ataeinia, B.; Kaynejad, K.; Zeinoddini, A.; Sadeghi, B.; Hosseini, M.; Rezaei, N.; Aghamohammadi, A. Antibiotic resistance in patients with primary immunodeficiency disorders versus immunocompetent patients. Expert Rev. Clin. Immunol. 2015, 11, 1163–1172. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Spotlight—Emerging Infectious Diseases Journal—CDC. Available online: https://wwwnc.cdc.gov/eid/spotlight/antimicrobial-resistance (accessed on 25 May 2020).
- Bellanti, J.A.; Settipane, R.A. Bacterial vaccines and the innate immune system: A journey of rediscovery for the allergist-immunologist and all health care providers. Allergy Asthma Proc. 2009, 30 (Suppl. 1), S3–S4. [Google Scholar] [CrossRef] [PubMed]
- García González, L.-A.; Arrutia Díez, F. Mucosal bacterial immunotherapy with MV130 highly reduces the need of tonsillectomy in adults with recurrent tonsillitis. Hum. Vaccines Immunother. 2019, 15, 2150–2153. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ramón, S.; Conejero, L.; Netea, M.G.; Sancho, D.; Palomares, Ó.; Subiza, J.L. Trained Immunity-Based Vaccines: A New Paradigm for the Development of Broad-Spectrum Anti-infectious Formulations. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Cirauqui, C.; Benito-Villalvilla, C.; Sánchez-Ramón, S.; Sirvent, S.; Diez-Rivero, C.M.; Conejero, L.; Brandi, P.; Hernández-Cillero, L.; Ochoa, J.L.; Pérez-Villamil, B.; et al. Human dendritic cells activated with MV130 induce Th1, Th17 and IL-10 responses via RIPK2 and MyD88 signalling pathways. Eur. J. Immunol. 2018, 48, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Alecsandru, D.; Valor, L.; Sánchez-Ramón, S.; Gil, J.; Carbone, J.; Navarro, J.; Rodríguez, J.J.; Rodríguez-Sainz, C.; Fernández-Cruz, E. Sublingual therapeutic immunization with a polyvalent bacterial preparation in patients with recurrent respiratory infections: Immunomodulatory effect on antigen-specific memory CD4+ T cells and impact on clinical outcome. Clin. Exp. Immunol. 2011, 164, 100–107. [Google Scholar] [CrossRef]
- Tejera-Alhambra, M.; Palomares, O.; Perez de Diego, R.; Diaz-Lezcano, I.; Sanchez-Ramon, S. New Biological Insights in the Immunomodulatory Effects of Mucosal Polybacterial Vaccines in Clinical Practice. Curr. Pharm. Des. 2016, 22, 6283–6293. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Shapiro, S.; Ernst, P.; Renzi, P.; Ducruet, T.; Robinson, A. Effects of an Immunostimulating Agent on Acute Exacerbations and Hospitalizations in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1997, 156, 1719–1724. [Google Scholar] [CrossRef]
- Roży, A.; Chorostowska-Wynimko, J. Bacterial immunostimulants-mechanism of action and clinical application in respiratory diseases. Adv. Respir. Med. 2008, 76, 353–359. [Google Scholar]
- Lusuardi, M. Challenging mucosal immunity with bacterial extracts to prevent respiratory infections: An old therapy revisited. Monaldi Arch. Chest Dis. 2004, 61, 4–5. [Google Scholar]
- Quinti, I.; Pulvirenti, F.; Giannantoni, P.; Hajjar, J.; Canter, D.L.; Milito, C.; Abeni, D.; Orange, J.S.; Tabolli, S. Development and Initial Validation of a Questionnaire to Measure Health-Related Quality of Life of Adults with Common Variable Immune Deficiency: The CVID_QoL Questionnaire. J. Allergy Clin. Immunol. Pract. 2016, 4, 1169–1179.e4. [Google Scholar] [CrossRef]
- Gasto farmacéutico por unidades. Available online: https://www.comunidad.madrid/servicios/salud/gasto-farmaceutico-unidades (accessed on 28 June 2020).
- Coste salarial por hora efectiva, tipo de jornada, sectores de actividad (6040). Available online: https://www.ine.es/jaxiT3/Tabla.htm?t=6040 (accessed on 28 June 2020).
- Boletín Estadístico del Personal al Servicio de la Comunidad de Madrid. Available online: https://www.comunidad.madrid/gobierno/transparencia/boletin-estadistico-personal-servicio-comunidad-madrid (accessed on 2 May 2020).
- Hampson, F.A.; Chandra, A.; Screaton, N.J.; Condliffe, A.; Kumararatne, D.S.; Exley, A.R.; Babar, J.L. Respiratory disease in common variable immunodeficiency and other primary immunodeficiency disorders. Clin. Radiol. 2012, 67, 587–595. [Google Scholar] [CrossRef] [PubMed]
- do Amor Divino, P.H.; de Carvalho Basilio, J.H.; Fabbri, R.M.A.; Bastos, I.P.; Forte, W.C.N. Bronchiectasis caused by common variable immunodeficiency. J. Bras. Pneumol. 2015, 41, 482–483. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Erdem, S.B.; Gulez, N.; Genel, F.; Karaman, S.; Nacaroglu, H.T. Characteristics of the patients followed with the diagnosis of common variable immunodeficiency and the complications. Cent. Eur. J. Immunol. 2019, 44, 119–126. [Google Scholar] [CrossRef]
- Lucas, M.; Lee, M.; Lortan, J.; Lopez-Granados, E.; Misbah, S.; Chapel, H. Infection outcomes in patients with common variable immunodeficiency disorders: Relationship to immunoglobulin therapy over 22 years. J. Allergy Clin. Immunol. 2010, 125, 1354–1360.e4. [Google Scholar] [CrossRef] [PubMed]
- Orange, J.S.; Grossman, W.J.; Navickis, R.J.; Wilkes, M.M. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: A meta-analysis of clinical studies. Clin. Immunol. 2010, 137, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Orange, J.S.; Belohradsky, B.H.; Berger, M.; Borte, M.; Hagan, J.; Jolles, S.; Wasserman, R.L.; Baggish, J.S.; Saunders, R.; Grimbacher, B. Evaluation of correlation between dose and clinical outcomes in subcutaneous immunoglobulin replacement therapy. Clin. Exp. Immunol. 2012, 169, 172–181. [Google Scholar] [CrossRef]
- Phillips, A.C.; Carroll, D.; Drayson, M.T.; Der, G. Salivary Immunoglobulin A Secretion Rate Is Negatively Associated with Cancer Mortality: The West of Scotland Twenty-07 Study. PLoS ONE 2015, 10, e0145083. [Google Scholar] [CrossRef]
- Rodríguez, A.; Tjärnlund, A.; Ivanji, J.; Singh, M.; García, I.; Williams, A.; Marsh, P.D.; Troye-Blomberg, M.; Fernández, C. Role of IgA in the defense against respiratory infections IgA deficient mice exhibited increased susceptibility to intranasal infection with Mycobacterium bovis BCG. Vaccine 2005, 23, 2565–2572. [Google Scholar] [CrossRef]
- Boyaka, P.N. Inducing mucosal IgA: A challenge for vaccine adjuvants and delivery systems. J. Immunol. 2017, 199, 9–16. [Google Scholar] [CrossRef]
- Diana, J.; Moura, I.C.; Vaugier, C.; Gestin, A.; Tissandie, E.; Beaudoin, L.; Corthésy, B.; Hocini, H.; Lehuen, A.; Monteiro, R.C. Secretory IgA induces tolerogenic dendritic cells through SIGNR1 dampening autoimmunity in mice. J. Immunol. 2013, 191, 2335–2343. [Google Scholar] [CrossRef]
- Gutzeit, C.; Magri, G.; Cerutti, A. Intestinal IgA production and its role in host-microbe interaction. Immunol. Rev. 2014, 260, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, H.; Ohteki, T. Regulation of IgA Production by Intestinal Dendritic Cells and Related Cells. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, C.S.; Blazevic, A.; Killoran, E.A.; Morris, M.S.; Hoft, D.F. Induction of mycobacterial protective immunity by sublingual BCG vaccination. Vaccine 2019, 37, 5364–5370. [Google Scholar] [CrossRef]
- Gallorini, S.; Taccone, M.; Bonci, A.; Nardelli, F.; Casini, D.; Bonificio, A.; Kommareddy, S.; Bertholet, S.; O’Hagan, D.T.; Baudner, B.C. Sublingual immunization with a subunit influenza vaccine elicits comparable systemic immune response as intramuscular immunization, but also induces local IgA and TH17 responses. Vaccine 2014, 32, 2382–2388. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ramón, S.; Pérez de Diego, R.; Dieli-Crimi, R.; Subiza, J.-L. Extending the clinical horizons of mucosal bacterial vaccines: Current evidence and future prospects. Curr. Drug Targets 2014, 15, 1132–1143. [Google Scholar] [CrossRef]
- Varkey, J.B.; Varkey, A.B.; Varkey, B. Prophylactic vaccinations in chronic obstructive pulmonary disease: Current status. Curr. Opin. Pulm. Med. 2009, 15, 90–99. [Google Scholar] [CrossRef]
- Selva, B.; Nieto, M.; Bartoll, E.; Mazon, A.; Calaforra, S.; Calderón, R.; Palau, M.J.; Nieto, A.; Caballero, R.; Guzmán-Fulgencio, M.; et al. Sublingual therapeutic immunotherapy with a polyvalent bacterial preparation in preschool children with recurrent respiratory tract infections [abstract]. Allergy 2017, 72, 196. [Google Scholar]
- van der Meer, J.W.M.; Joosten, L.A.B.; Riksen, N.; Netea, M.G. Trained immunity: A smart way to enhance innate immune defence. Mol. Immunol. 2015, 68, 40–44. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 1–14. [Google Scholar] [CrossRef]
- van der Heijden, C.D.C.C.; Noz, M.P.; Joosten, L.A.B.; Netea, M.G.; Riksen, N.P.; Keating, S.T. Epigenetics and Trained Immunity. Antioxid. Redox Signal. 2018, 29, 1023–1040. [Google Scholar] [CrossRef]
- Mourits, V.P.; Wijkmans, J.C.; Joosten, L.A.; Netea, M.G. Trained immunity as a novel therapeutic strategy. Curr. Opin. Pharmacol. 2018, 41, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Brandi, P.; Conejero, L.; Cueto, F.J.; Martínez-Cano, S.; Saz-Leal, P.; Enamorado, M.; Amores-Iniesta, J.; Subiza, J.L.; Sancho, D. MV130, a polybacterial mucosal preparation, protects mice against experimental viral infections inducing trained immunity [abstract]. Allergy 2019, 74, 111. [Google Scholar]
- Rezaei, N.; Amirzargar, A.A.; Shakiba, Y.; Mahmoudi, M.; Moradi, B.; Aghamohammadi, A. Proinflammatory cytokine gene single nucleotide polymorphisms in common variable immunodeficiency. Clin. Exp. Immunol. 2009, 155, 21–27. [Google Scholar] [CrossRef]
- Holm, A.M.; Aukrust, P.; Damås, J.K.; Müller, F.; Halvorsen, B.; Frøland, S.S. Abnormal interleukin-7 function in common variable immunodeficiency. Blood 2005, 105, 2887–2890. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holm, A.M.; Aukrust, P.; Aandahl, E.M.; Müller, F.; Taskén, K.; Frøland, S.S. Impaired secretion of IL-10 by T cells from patients with common variable immunodeficiency—involvement of protein kinase A type I. J. Immunol. Baltim. Md 1950 2003, 170, 5772–5777. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.B.; Hauber, I.; Vogel, E.; Wolf, H.M.; Mannhalter, J.W.; Eibl, M.M. Defective interleukin-2 and interferon-gamma gene expression in response to antigen in a subgroup of patients with common variable immunodeficiency. J. Allergy Clin. Immunol. 1993, 92, 340–352. [Google Scholar] [CrossRef]
- North, M.E.; Webster, A.D.; Farrant, J. Role of interleukin-2 and interleukin-6 in the mitogen responsiveness of T cells from patients with “common-variable” hypogammaglobulinaemia. Clin. Exp. Immunol. 1990, 81, 412–416. [Google Scholar] [CrossRef]
- Del Vecchio, G.C.; Martire, B.; Lassandro, G.; Cecinati, V.; De Mattia, D.; Ciccarelli, M.; Piacente, L.; Giordano, P. Reduced interleukin-5 production by peripheral CD4+ T cells in common variable immunodeficiency patients. Immunopharmacol. Immunotoxicol. 2008, 30, 679–686. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, R.; Danon, M.; Mayer, L.; Cunningham-Rundles, C. IL-10 production in common variable immunodeficiency. Clin. Immunol. Immunopathol. 1998, 86, 298–304. [Google Scholar] [CrossRef]
- Kasztalska, K.; Ciebiada, M.; Cebula-Obrzut, B.; Górski, P. Intravenous immunoglobulin replacement therapy in the treatment of patients with common variable immunodeficiency disease: An open-label prospective study. Clin. Drug Investig. 2011, 31, 299–307. [Google Scholar] [CrossRef]
- Isgrò, A.; Marziali, M.; Mezzaroma, I.; Luzi, G.; Mazzone, A.M.; Guazzi, V.; Andolfi, G.; Cassani, B.; Aiuti, A.; Aiuti, F. Bone marrow clonogenic capability, cytokine production, and thymic output in patients with common variable immunodeficiency. J. Immunol. 2005, 174, 5074–5081. [Google Scholar] [CrossRef] [PubMed]
- Pons, J.; Ferrer, J.M.; Martínez-Pomar, N.; Iglesias-Alzueta, J.; Matamoros, N. Costimulatory molecules and cytokine production by T lymphocytes in common variable immunodeficiency disease. Scand. J. Immunol. 2006, 63, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.B.; Midttun, K.; Feragen, K.J.B. Measuring quality of life of primary antibody deficiency patients using a disease-specific health-related quality of life questionnaire for common variable immunodeficiency (CVID_QoL). J. Patient-Rep. Outcomes 2019, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- EconPapers: Financial Integration, International Portfolio Choice and the European Monetary Union. Available online: https://econpapers.repec.org/paper/ecbecbwps/2006626.htm (accessed on 28 June 2020).
- Modell, V.; Orange, J.S.; Quinn, J.; Modell, F. Global report on primary immunodeficiencies: 2018 update from the Jeffrey Modell Centers Network on disease classification, regional trends, treatment modalities, and physician reported outcomes. Immunol. Res. 2018, 66, 367–380. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).