Mitochondrial Membrane Potential Predicts 4-Hour Sperm Motility
Abstract
1. Introduction
2. Patients Selection
3. Materials and Methods Section
3.1. Sperm Analysis
3.2. Flow Cytometry Analysis
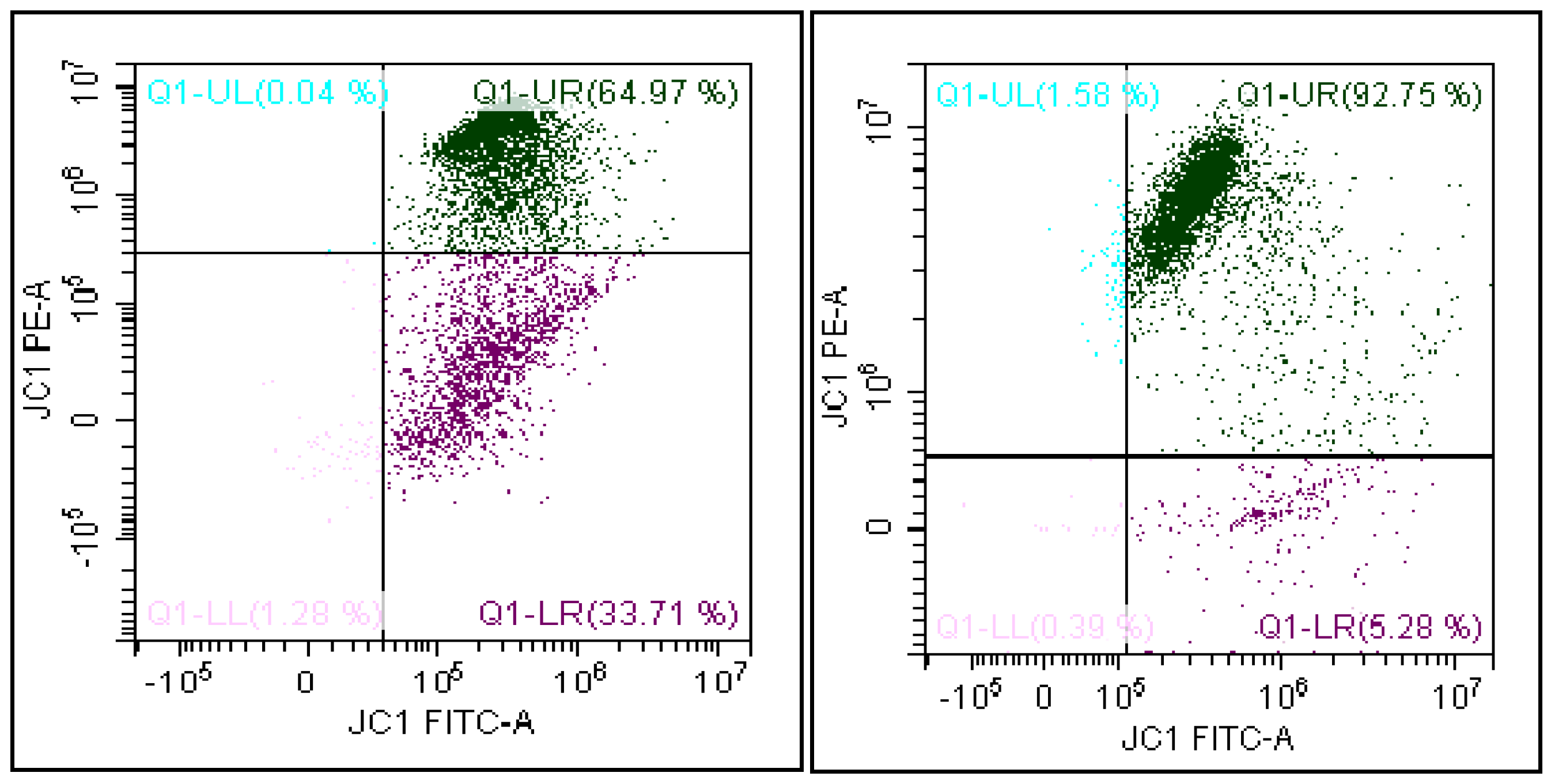
3.3. Evaluation of the Mitochondrial Membrane Potential
3.4. Statistical Analysis
4. Results
5. Discussion
Limitations of the Study
- The need to evaluate the true idiopathic cases to be differentiated from patients with secondary asthenozoospermia, to understand the real value of this flow cytometry parameter in the clinical practice;
- The difference between this study (which may seem confirmatory) with others in the literature who have not demonstrated this aspect on this specific category of patients (as mentioned above).
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharlip, I.D.; Jarow, J.P.; Belker, A.M.; Lipshultz, L.I.; Sigman, M.; Thomas, A.J.; Schlegel, P.N.; Howards, S.S.; Nehra, A.; Damewood, M.D.; et al. Best practice policies for male infertility. Fertil. Steril. 2002, 77, 873–882. [Google Scholar] [CrossRef]
- Boivin, J.; Bunting, L.; Collins, J.A.; Nygren, K.G. International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility medical care. Hum. Reprod. 2007, 22, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Rowe, P.J.; Comhaire, F.H.; Hargreave, T.B.; Mellows, H.J. WHO Manual for the Standardized Investigation and Diagnosis of the Infertile Couple; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- World Health Organization. Manual for the Examination and Processing of Human Semen, 5th ed.; WHO Laboratory: Geneva, Switzerland, 2010. [Google Scholar]
- Makler, A.; Zaidise, I.; Paldi, E.; Brandes, J.M. Factors affecting sperm motility: In vitro change in motility with time after ejaculation. Fertil. Steril. 1979, 31, 147–154. [Google Scholar] [CrossRef]
- Zollner, U.; Martin, S.; Liebermann, J.; Steck, T. Evaluation of a cut-off value for sperm motility after different hours of incubation to select the suitable reproductive technology (IVF or ICSI). Acta Obstet. Gynecol. Scand. 1999, 78, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Obert, G.; Defossez, A.; Formstecher, P.; Marchetti, P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum. Reprod. 2002, 17, 1257–1265. [Google Scholar] [CrossRef]
- Turner, R.M. Tales from the tail: What do we really know about sperm motility? J. Androl. 2003, 24, 790–803. [Google Scholar] [CrossRef]
- Wang, X.; Sharma, R.K.; Gupta, A.; George, V.; Thomas, A.J.; Falcone, T.; Agarwal, A. Alterations in mitochondria membrane potential and oxidative stress in infertile men: A prospective observational study. Fertil. Steril. 2003, 80, 844–850. [Google Scholar] [CrossRef]
- Piscopo, M.; Notariale, R.; Rabbito, D.; Ausió, J.; Olanrewaju, O.S.; Guerriero, G. Mytilus Galloprovincialis (Lamarck, 1819) Spermatozoa: hsp70 Expression and Protamine-Like Protein Property Studies. Environ. Sci. Pollut. Res. Int. 2018, 25, 12957–12966. [Google Scholar] [CrossRef]
- Piscopo, M.; Trifuoggi, M.; Scarano, C.; Gori, C.; Giarra, A.; Febbraio, F. Relevance of Arginine Residues in Cu(II)-induced DNA Breakage and Proteinase K Resistance of H1 Histones. Sci. Rep. 2018, 8, 7414. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Vicari, E.; Tumino, D.; Morgia, G.; Favilla, V.; Cimino, S.; Calogero, A.E. Markers of semen inflammation: Supplementary semen analysis? J. Reprod. Immunol. 2013, 100, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.; Polosa, R.; Perdichizzi, A.; Guarino, F.; La Vignera, S.; Scarfia, A.; Fratantonio, E.; Condorelli, R.; Bonanno, O.; Barone, N.; et al. Cigarette smoke extract immobilizes human spermatozoa and induces sperm apoptosis. Reprod. Biomed. Online 2009, 19, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.A.; Agarwal, A.; Sharma, R.K.; Nelson, D.R.; Thomas, A.J., Jr. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: A prospective study. Fertil. Steril. 2002, 78, 491–499. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol. Res. Health 2003, 27, 277–284. [Google Scholar] [PubMed]
- Latchoumycandane, C.; Mathur, P.P. Induction of oxidative stress in the rat testis after short-term exposure to the organochlorine pesticide methoxychlor. Arch. Toxicol. 2002, 76, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.A.; La Vignera, S.; Giacone, F.; Iacoviello, L.; Mongioì, L.M.; Li Volti, G.; Barbagallo, I.; Avola, R.; Calogero, A.E. Nicotine Effects and Receptor Expression on Human Spermatozoa: Possible Neuroendocrine Mechanism. Front. Physiol. 2017, 8, 177. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Di Mauro, M.; Lo Presti, D.; Mongioì, L.M.; Russo, G.; Calogero, A.E. Reproductive function in male patients with type 1 diabetes mellitus. Andrology 2015, 3, 1082–1087. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Mongioì, L.M.; Alamo, A.; Calogero, A.E. Diabetes Mellitus and Infertility: Different Pathophysiological Effects in Type 1 and Type 2 on Sperm Function. Front. Endocrinol. 2018, 9, 268. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Mongioì, L.M.; Alamo, A.; Giacone, F.; Cannarella, R.; Calogero, A.E. Thyroid Hormones and Spermatozoa: In Vitro Effects on Sperm Mitochondria, Viability and DNA Integrity. J. Clin. Med. 2019, 8, 756. [Google Scholar] [CrossRef]
- Lanzafame, F.M.; La Vignera, S.; Vicari, E.; Calogero, A.E. Oxidative stress and medical antioxidant treatment in male infertility. Reprod. Biomed. Online 2009, 19, 638–659. [Google Scholar] [CrossRef]
- Marchetti, C.; Jouy, N.; Leroy-Martin, B.; Defossez, A.; Formstecher, P.; Marchetti, P. Comparison of fourfluorochromes for the detection of the inner mitochondrial membrane potential in human spermatozoa and their correlation with sperm motility. Hum. Reprod. 2004, 19, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Krzyzosiak, J.; Molan, P.; Vishwanath, R. Measurements of bovine sperm velocities under true anaerobic and aerobic conditions. Anim. Reprod. Sci. 1999, 55, 163–173. [Google Scholar] [CrossRef]
- Ruiz-Pesini, E.; Lapeña, A.C.; Díez, C.; Alvarez, E.; Enríquez, J.A.; López-Pérez, M.J. Seminal quality correlates with mitochondrial functionality. Clin. Chim. Acta 2000, 300, 97–105. [Google Scholar] [CrossRef]
- Paoli, D.; Gallo, M.; Rizzo, F.; Baldi, E.; Francavilla, S.; Lenzi ALombardo, F.; Gandini, L. Mitochondrial membrane potential profile and its correlation with increasing sperm motility. Fertil. Steril. 2011, 95, 2315–2319. [Google Scholar] [CrossRef]
- Agnihotri, S.K.; Agrawal, A.K.; Hakim, B.A.; Vishwakarma, A.L.; Narender, T.; Sachan, R.; Sachdev, M. Mitochondrial Membrane Potential (MMP) Regulates Sperm Motility. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 953–960. [Google Scholar] [CrossRef]
- Barbagallo, F.; La Vignera, S.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of sperm mitochondrial function: A key organelle for sperm motility. J. Clin. Med. 2020, 29, 363. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Bellanca, S.; Vicari, E.; Calogero, A.E. Myoinositol: Does it improve sperm mitochondrial function and sperm motility? Urology 2012, 79, 1290–1295. [Google Scholar] [CrossRef]
- Condorelli, R.A.; Calogero, A.E.; Russo, G.I.; La Vignera, S. From spermiogram to bio-Functional sperm parameters: When and why request them? J. Clin. Med. 2020, 9, 406. [Google Scholar] [CrossRef]
- Duca, Y.; Calogero, A.E.; Cannarella, R.; Condorelli, R.A.; La Vignera, S. Current and emerging medical therapeutic agents for idiopathic male infertility. Expert Opin. Pharmacother. 2019, 20, 55–67. [Google Scholar] [CrossRef]
- Troiano, L.; Granata, A.R.; Cossarizza, A.; Kalashnikova, G.; Bianchi, R.; Pini, G.; Tropea, F.; Carani, C.; Franceschi, C. Mitochondrial membrane potential and DNA stainability in human sperm cells: A flow cytometry analysis with implication for male infertility. Exp. Cell Res. 1998, 241, 384–393. [Google Scholar] [CrossRef]
- Gallon, F.; Marchetti, C.; Jouy, N.; Marchetti, P. The functionality of mitochondria differentiates human spermatozoa with high and low fertilizing capability. Fertil. Steril. 2006, 86, 1526–1530. [Google Scholar] [CrossRef] [PubMed]


| Sperm Parameters | Values (Group A) | Values (Group B) |
|---|---|---|
| Volume (mL) | 2.7 ± 0.5 | 3.5 ± 0.7 |
| Sperm concentration (million/mL) | 47.9 ± 3.5 | 54.7 ± 5.2 |
| Total sperm count (million/ejaculate) | 170 ± 20.6 | 158 ± 37.5 |
| Normal forms (%) | 5.0 ± 0.8 | 3.9 ± 0.5 |
| Leukocyte concentration (million/mL) | 0.5 ± 0.01 | 0.5 ± 0.08 |
| Parameter | Group A (n = 18) | Group B (n = 13) |
|---|---|---|
| Sperm L-MMP (%) | 20.0–36.4 | 37.1–60.1 |
| Sperm H-MMP (%) | 63.6–79.1 | 38.7–59.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamo, A.; De Luca, C.; Mongioì, L.M.; Barbagallo, F.; Cannarella, R.; La Vignera, S.; Calogero, A.E.; Condorelli, R.A. Mitochondrial Membrane Potential Predicts 4-Hour Sperm Motility. Biomedicines 2020, 8, 196. https://doi.org/10.3390/biomedicines8070196
Alamo A, De Luca C, Mongioì LM, Barbagallo F, Cannarella R, La Vignera S, Calogero AE, Condorelli RA. Mitochondrial Membrane Potential Predicts 4-Hour Sperm Motility. Biomedicines. 2020; 8(7):196. https://doi.org/10.3390/biomedicines8070196
Chicago/Turabian StyleAlamo, Angela, Claudia De Luca, Laura M. Mongioì, Federica Barbagallo, Rossella Cannarella, Sandro La Vignera, Aldo E. Calogero, and Rosita A. Condorelli. 2020. "Mitochondrial Membrane Potential Predicts 4-Hour Sperm Motility" Biomedicines 8, no. 7: 196. https://doi.org/10.3390/biomedicines8070196
APA StyleAlamo, A., De Luca, C., Mongioì, L. M., Barbagallo, F., Cannarella, R., La Vignera, S., Calogero, A. E., & Condorelli, R. A. (2020). Mitochondrial Membrane Potential Predicts 4-Hour Sperm Motility. Biomedicines, 8(7), 196. https://doi.org/10.3390/biomedicines8070196









